7edd37ad47fd8d27ba938b584c8fb043.ppt
- Количество слайдов: 27

Key Findings and Recommendations External review to TB Prevention, Control and Care in the Republic of Kazakhstan Astana, 16 May 2013 Dr Masoud Dara Programme Manager, TB and M/XDR-TB WHO Regional Office for Europe

Outline of presentation • Summary epidemiological situation in the Region and Kazakhstan • Overview of finding and recommendations of the extensive programme review 2012 • Next steps

No reason to be complacent about TB in the WHO European Region • Total of over 500 000 estimated TB patients in the Region © Carl Cordonnier • 380 000 new TB cases estimated to occur in a year • 44 000 deaths, mostly in the east © Ivan Chernichckin
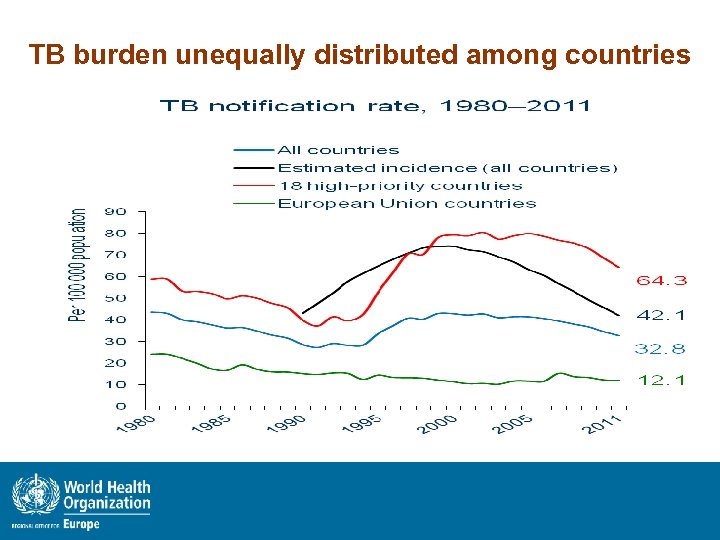
TB burden unequally distributed among countries
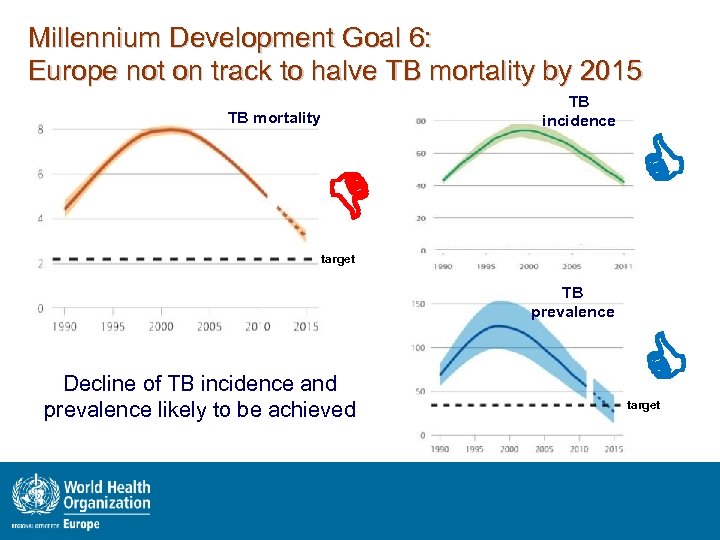
Millennium Development Goal 6: Europe not on track to halve TB mortality by 2015 TB incidence TB mortality target TB prevalence Decline of TB incidence and prevalence likely to be achieved target
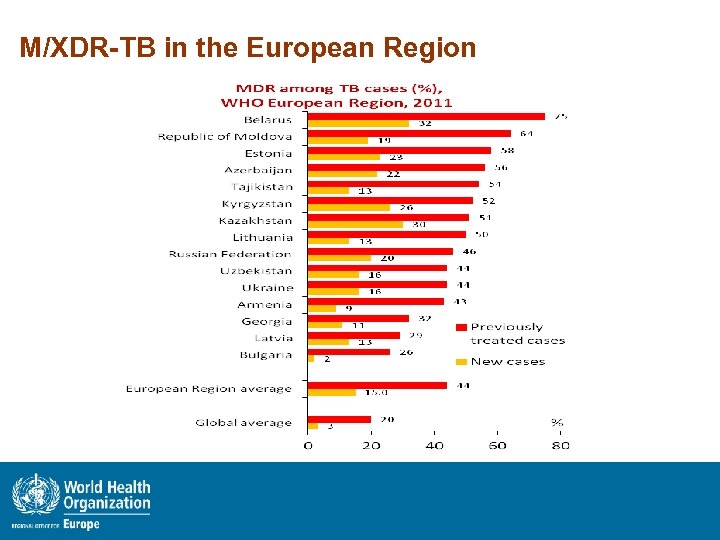
M/XDR-TB in the European Region

Consolidated Action Plan to Prevent and Combat MDR-TB (MAP) • Prompt diagnosis including newly endorsed molecular diagnostic techniques • Equitable access to adequate treatment • Health system approach to MDR-TB prevention and control • Emphasis on involvement of civil society organizations • Identifying and addressing social determinants • Working in partnership, twinning of cities/programmes • Robust monitoring framework, accountability and follow-up • Including neglected aspects (e. g. palliative care, surgery)

Overview of the Action Plan Goal • To contain the spread of drug-resistant TB by achieving universal access to prevention, diagnosis and treatment of M/XDR-TB in all Member States of the WHO European Region by 2015 Targets • To decrease by 20 percentage points the proportion of MDRTB among previously treated patients by end 2015 • To diagnose at least 85% of estimated MDR-TB patients by 2015 • To treat successfully at least 75% of notified MDR-TB patients by 2015
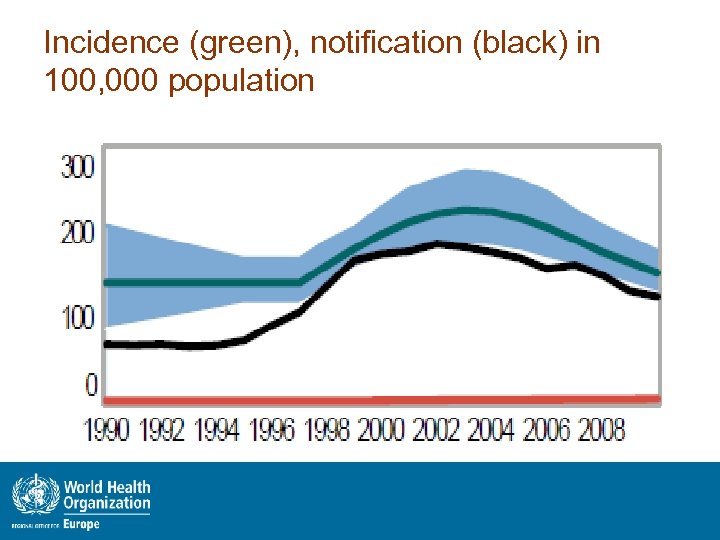
Incidence (green), notification (black) in 100, 000 population
Summary epidemiological situation • Notification 2011: 26211 (162 in 100 k). New 14396 (including SS+3289), 11815 retreatment (45% of all TB cases) • MDR-TB among new cases 30% • MDR-TB among previously treated cases 51. 3% • 1. 6% HIV prevalence among incident TB cases

Programme review in a nutshell • Previous extensive review in 2007 • 16 international experts from 11 countries including USAID, CDC and KNCV HQ • 10 -16 May 2012 • Akmolinsky Oblast, Almaty, Almatinsky Oblast and South Kazakhstan Oblast • Desk review, interviews, observation and verification • Based on six health system building blocks

Key findings • Excellent progress in all aspects of TB prevention, control and care – Through care – MDR-TB diagnosis and treatment – Infection control – Surveillance – Monitoring and supervision • TB and M/XDR-TB still a big public health challenge • Contact investigation has a low yield

Childhood TB - recommendations • Update childhood TB treatment and prophylactic treatment guidelines in accordance with latest WHO guidelines (2011) • Train TB pediatricians on new treatment guidelines • Improve diagnostic specimen collection for bacteriological confirmation of diagnosis in children • Revise criteria for hospitalization and placing children in sanatoriums

OR - recommendations • Prepare plan for building research capacity for the NTP and Oblast staff and organize trainings/coaching on OR • Increase use of routinely collected data for OR • Based on the result of programme review set research priorities (WHO and partners will provide assistance)
Prison sector - recommendations • • • Improve timely start of treatment for TB and DR-TB (start treatment when diagnosed) Increase access to rapid molecular diagnosis of DR-TB, at least to R Provide universal coverage with DST at least to FLD for all S+/C+ patients Provide access to Cat IV treatment to all patients registered with DR-TB, including XDR-TB Strengthen the IC in TB penitentiaries: Administrative separation by smear/culture and DST status Environmental measures: installation of adequate quantity of UVGIs Individual protection cough etiquette and masks for patients and respirators for personnel Address the problem of understaffing and motivation of personnel Strengthen the through-care system.
TB/HIV • Diagnosis of TB not in HIV/AIDS centres • Low case detection of TB among PLWH • Insufficient clinical management of TB/HIV patients • Late start of ART in co-infected individuals
TB/HIV recommendations – ART to all patients with active TB as soon as TB treatment is tolerated – IPT to PLHIV with LTB, monitor outcomes – Improve TB/HIV data coordination and use for managerial decisions
Findings TB laboratory diagnosis • The rate of sputum and culture confirmation is low (30% smear positivity among new cases in 2010) • Well-developed laboratory network including sample referral and laboratory infrastructure, good materials and resources, high level of performance discipline • Biosafety concerns in some facilities with regard to engineering, equipment and GLP • Methods for rapid detection of TB and anti-TB drug resistance prediction are not (yet) in place

Recommendations laboratory • Finalize and implement National Laboratory Strategic Plan for the development of laboratory services • Develop and implement a comprehensive QMS in all laboratories and develop a quality manual • Optimize biosafety and develop a biosafety manual • Implement rapid methods for the detection of (MDR) TB for all MDR-TB suspects and HIV+ patients • Optimize TB laboratory diagnosis by organizing TA and training on primary isolation and culture of TB • Develop and implement a computerized LIMS

Migration- Recommendations • To examine the recently published Minimum Package of Cross border TB Control and Care • To pilot cross border TB control and care between selected countries of CAR (WHO ready to assist) • To provide measures on infection control for the patients moving from one country to another

Governance -recommendations • In addition to supervisory visits, use teleconference calls for regular support to the Regions • Use data for improving programme performance at Oblast level • Consider establishing Kazakhstan National Stop TB partnership
TB Surveillance, Monitoring & Evaluation Main recommendations: • Further develop and strengthen the electronic TB recording and reporting system by: – a) establishing a National TB Register for Prevention and Treatment as a unified source of data/information for the NTP and the SES – b) integrating TB laboratory services and prison TB care services into electronic recording and reporting • Conduct a country-wide review of the current practice and efficiency of population screenings for TB. • Initiate monitoring of drug side effects within the surveillance system. Develop SOPs for their management. • Develop SOPs for data quality, data storage and data safety.
TB - health system issues: Key recommendations • Design and introduce patient centered approaches which will help proper strategic planning and inform decision on changes in financing, care delivery, staffing, information system, etc. • Conduct comprehensive analysis of TB hospitals’ performance using proper methodology for activities, expenditure, and outcomes • Promote outpatient TB case management (e. g. MDR-TB case management), • Start asap with a limited-scale demonstration project in few territories • Develop clear criteria for hospitalization and discharge (based on clinical but not on ‘epidemiological’ or social factors) • Scale up rapid diagnostics for TB and MDR-TB (e. g. Xpert), change diagnostic algorithm and adjust service delivery accordingly (including stopping cultures at rayon level) • Adjust payment mechanisms for TB case management for a higher impact
Recommendations – Anti-TB drugs • Mo. H/SK Pharmacia/NTP: Promote process of WHO prequalification by including it as a requirement in tender documentation for state procurement • Local Manufacturers: start the process of WHO prequalification • Mo. H/NTP: facilitate process of registration of pediatric formulations • Mo. H/NTP: Monitor side effects of the 1 st and 2 nd line anti-TB drugs • Mo. H/NTP: Plan operational research on risk factors and rates of 1 st and 2 nd line side effects among TB patients and on management of those side effects

Next steps • Developing Concept Note • High Level policy Dialogue • Continuous dialogue and sustainable technical assistance • Cross border TB control and care • Robust monitoring to document the progress

Joint launch of the MAP

Thank you for your attention Email: Tuberculosis@euro. who. int
7edd37ad47fd8d27ba938b584c8fb043.ppt