a9f1716d81e99b354e9c164da3fcab71.ppt
- Количество слайдов: 45
 Kerry S. Courneya, Ph. D Professor and Canada Research Chair in Physical Activity and Cancer University of Alberta, Edmonton, Canada Dr. Courneya indicates that he has no financial disclosures or conflicts of interest.
Kerry S. Courneya, Ph. D Professor and Canada Research Chair in Physical Activity and Cancer University of Alberta, Edmonton, Canada Dr. Courneya indicates that he has no financial disclosures or conflicts of interest.
 Physical Activity and Cancer Survivorship: Does the Data Support an Exercise Prescription? Kerry S. Courneya, Ph. D Professor and Canada Research Chair in Physical Activity and Cancer Director, Behavioral Medicine Laboratory University of Alberta, Edmonton, Canada
Physical Activity and Cancer Survivorship: Does the Data Support an Exercise Prescription? Kerry S. Courneya, Ph. D Professor and Canada Research Chair in Physical Activity and Cancer Director, Behavioral Medicine Laboratory University of Alberta, Edmonton, Canada
 (Courneya et al. Res Quart Ex Sport 2015; 86: 107 -116)
(Courneya et al. Res Quart Ex Sport 2015; 86: 107 -116)
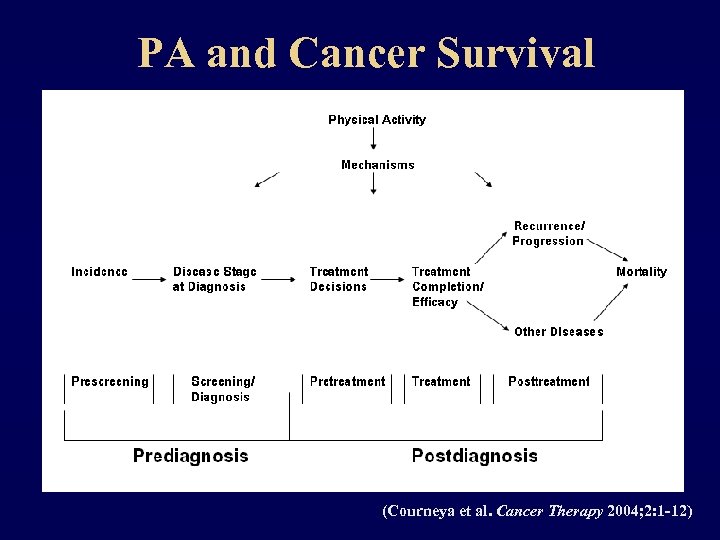 PA and Cancer Survival (Courneya et al. Cancer Therapy 2004; 2: 1 -12)
PA and Cancer Survival (Courneya et al. Cancer Therapy 2004; 2: 1 -12)
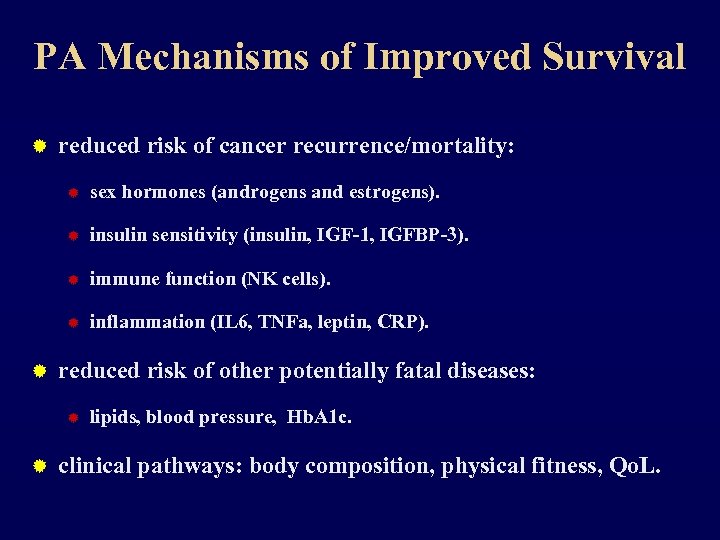 PA Mechanisms of Improved Survival ® reduced risk of cancer recurrence/mortality: ® ® insulin sensitivity (insulin, IGF-1, IGFBP-3). ® immune function (NK cells). ® ® sex hormones (androgens and estrogens). inflammation (IL 6, TNFa, leptin, CRP). reduced risk of other potentially fatal diseases: ® ® lipids, blood pressure, Hb. A 1 c. clinical pathways: body composition, physical fitness, Qo. L.
PA Mechanisms of Improved Survival ® reduced risk of cancer recurrence/mortality: ® ® insulin sensitivity (insulin, IGF-1, IGFBP-3). ® immune function (NK cells). ® ® sex hormones (androgens and estrogens). inflammation (IL 6, TNFa, leptin, CRP). reduced risk of other potentially fatal diseases: ® ® lipids, blood pressure, Hb. A 1 c. clinical pathways: body composition, physical fitness, Qo. L.
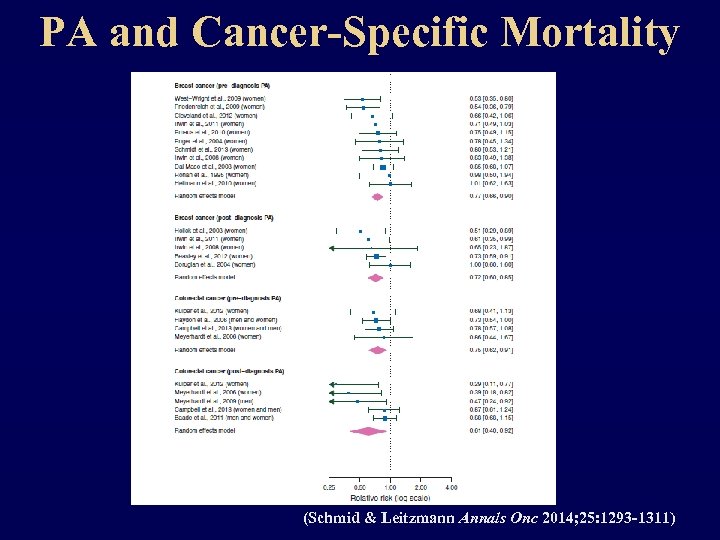 PA and Cancer-Specific Mortality (Schmid & Leitzmann Annals Onc 2014; 25: 1293 -1311)
PA and Cancer-Specific Mortality (Schmid & Leitzmann Annals Onc 2014; 25: 1293 -1311)
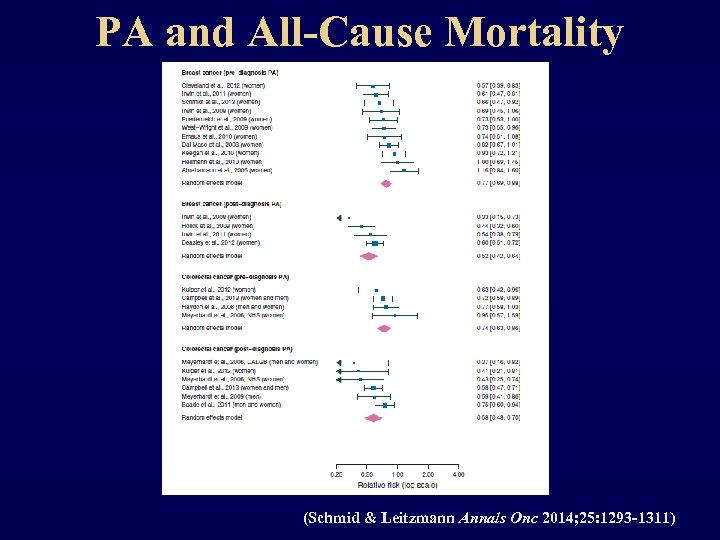 PA and All-Cause Mortality (Schmid & Leitzmann Annals Onc 2014; 25: 1293 -1311)
PA and All-Cause Mortality (Schmid & Leitzmann Annals Onc 2014; 25: 1293 -1311)
 Limitations of PA Research ® no randomized trials with survival endpoints. For observational studies: ® none had PA/fitness as primary exposure. ® basic assessment of self-reported PA. ® no objective measures of PA. ® no measures of sedentary behavior.
Limitations of PA Research ® no randomized trials with survival endpoints. For observational studies: ® none had PA/fitness as primary exposure. ® basic assessment of self-reported PA. ® no objective measures of PA. ® no measures of sedentary behavior.
 Limitations of PA Research ® few objective fitness assessments (e. g. , BMI). ® limited ® few biomarker data. designed as cancer survivor cohorts. ® variable ® limited ® few and arbitrary follow-up assessments. disease and treatment data (covariates). cancer groups have been examined.
Limitations of PA Research ® few objective fitness assessments (e. g. , BMI). ® limited ® few biomarker data. designed as cancer survivor cohorts. ® variable ® limited ® few and arbitrary follow-up assessments. disease and treatment data (covariates). cancer groups have been examined.
 Research on PA & Cancer Outcomes ® follow-up of the START Trial (breast). ® update of the AMBER Cohort Study (breast). ® update of the CHALLENGE (CO. 21) Trial (colon). ® unpublished ® update prostate cancer survival cohort data. of the GAP 4 Trial (prostate). ® follow-up of the HELP Trial (lymphoma).
Research on PA & Cancer Outcomes ® follow-up of the START Trial (breast). ® update of the AMBER Cohort Study (breast). ® update of the CHALLENGE (CO. 21) Trial (colon). ® unpublished ® update prostate cancer survival cohort data. of the GAP 4 Trial (prostate). ® follow-up of the HELP Trial (lymphoma).
 Supervised Trial of Aerobic versus Resistance Training (START) ® RCT examining AET versus RET versus UC in 242 BC patients on chemotherapy. ® multicenter trial: Edmonton, Ottawa, and Vancouver. Funded by the Canadian Breast Cancer Research Alliance.
Supervised Trial of Aerobic versus Resistance Training (START) ® RCT examining AET versus RET versus UC in 242 BC patients on chemotherapy. ® multicenter trial: Edmonton, Ottawa, and Vancouver. Funded by the Canadian Breast Cancer Research Alliance.
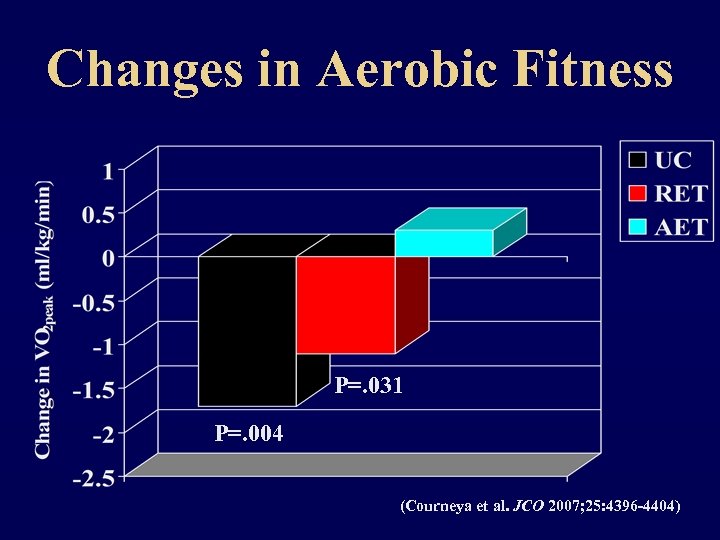 Changes in Aerobic Fitness P=. 031 P=. 004 (Courneya et al. JCO 2007; 25: 4396 -4404)
Changes in Aerobic Fitness P=. 031 P=. 004 (Courneya et al. JCO 2007; 25: 4396 -4404)
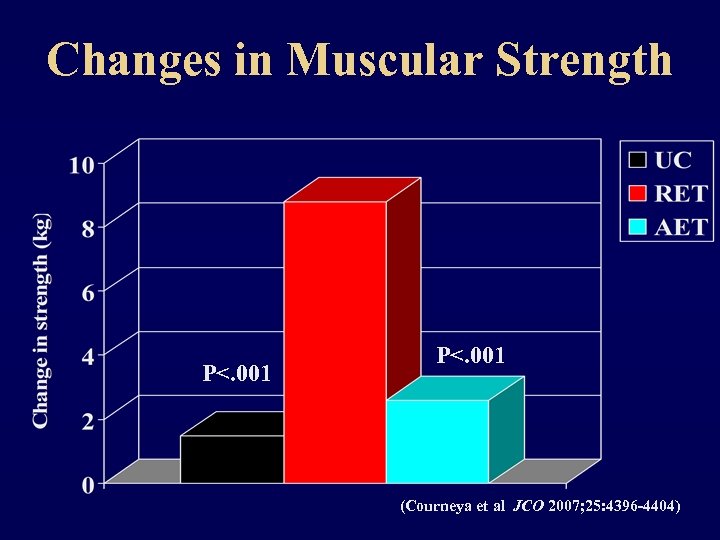 Changes in Muscular Strength P<. 001 (Courneya et al JCO 2007; 25: 4396 -4404)
Changes in Muscular Strength P<. 001 (Courneya et al JCO 2007; 25: 4396 -4404)
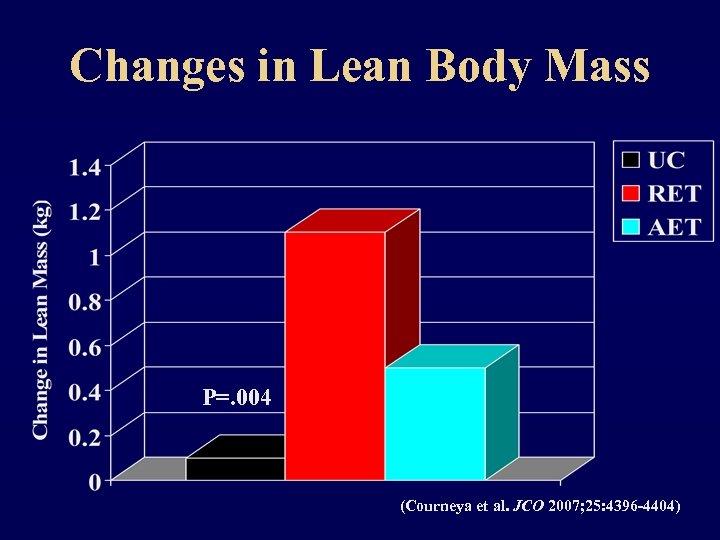 Changes in Lean Body Mass P=. 004 (Courneya et al. JCO 2007; 25: 4396 -4404)
Changes in Lean Body Mass P=. 004 (Courneya et al. JCO 2007; 25: 4396 -4404)
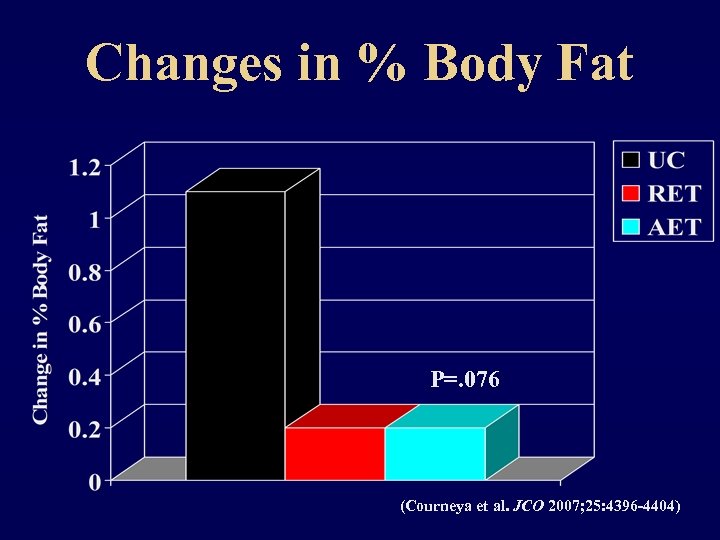 Changes in % Body Fat P=. 076 (Courneya et al. JCO 2007; 25: 4396 -4404)
Changes in % Body Fat P=. 076 (Courneya et al. JCO 2007; 25: 4396 -4404)
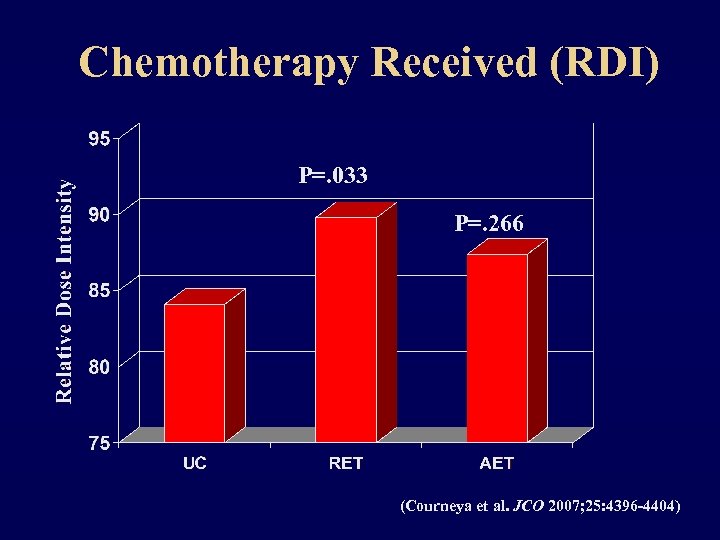 Chemotherapy Received (RDI) P=. 033 P=. 266 (Courneya et al. JCO 2007; 25: 4396 -4404)
Chemotherapy Received (RDI) P=. 033 P=. 266 (Courneya et al. JCO 2007; 25: 4396 -4404)
 START Trial Outcomes ® patients randomized between 2003 -2005. ® electronic record review in summer 2012. ® abstracted events, dates, and treatments. ® explored DFS, OS, DDFS, and RFI (STEEP definitions). ® Cox proportional hazards models and KM curves. ® combined exercise groups and explored selected subgroups. ® median follow-up of 89 months. (Courneya et al. MSSE 2014; 46: 1744 -51)
START Trial Outcomes ® patients randomized between 2003 -2005. ® electronic record review in summer 2012. ® abstracted events, dates, and treatments. ® explored DFS, OS, DDFS, and RFI (STEEP definitions). ® Cox proportional hazards models and KM curves. ® combined exercise groups and explored selected subgroups. ® median follow-up of 89 months. (Courneya et al. MSSE 2014; 46: 1744 -51)
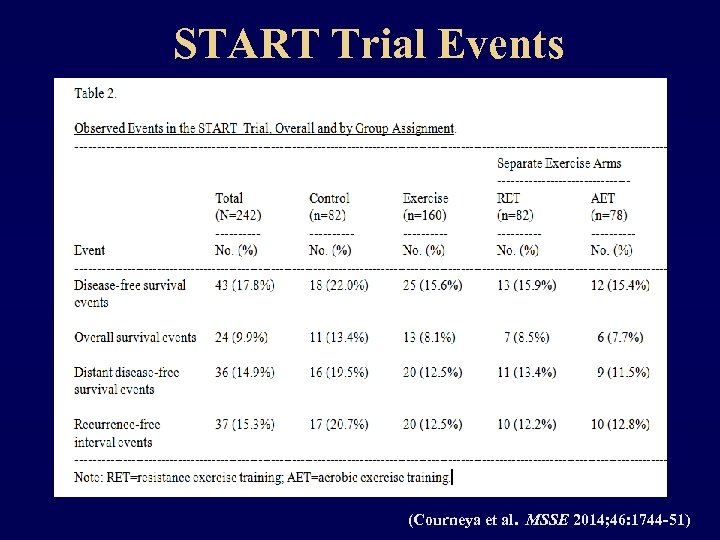 START Trial Events (Courneya et al. MSSE 2014; 46: 1744 -51)
START Trial Events (Courneya et al. MSSE 2014; 46: 1744 -51)
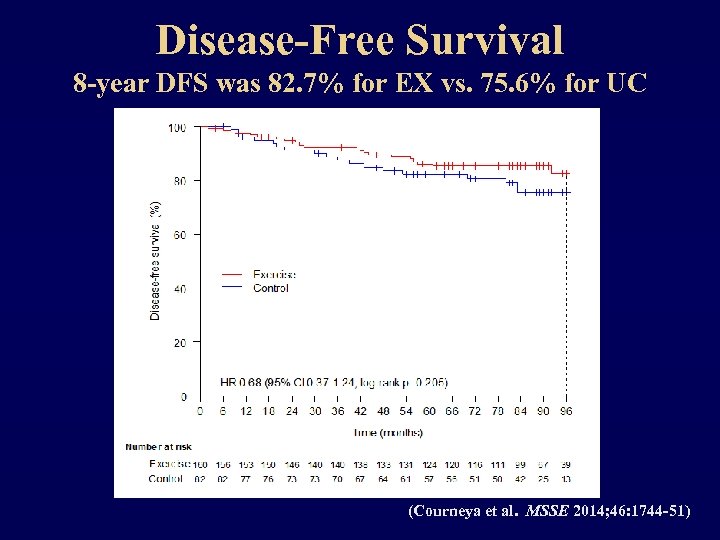 Disease-Free Survival 8 -year DFS was 82. 7% for EX vs. 75. 6% for UC (Courneya et al. MSSE 2014; 46: 1744 -51)
Disease-Free Survival 8 -year DFS was 82. 7% for EX vs. 75. 6% for UC (Courneya et al. MSSE 2014; 46: 1744 -51)
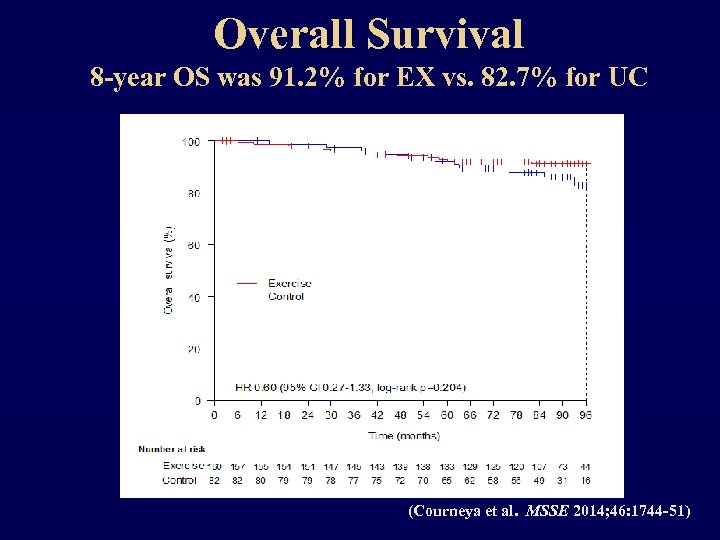 Overall Survival 8 -year OS was 91. 2% for EX vs. 82. 7% for UC (Courneya et al. MSSE 2014; 46: 1744 -51)
Overall Survival 8 -year OS was 91. 2% for EX vs. 82. 7% for UC (Courneya et al. MSSE 2014; 46: 1744 -51)
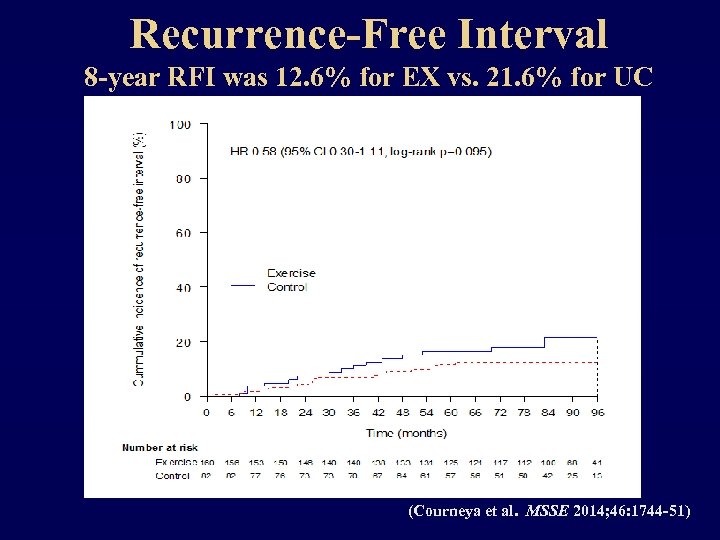 Recurrence-Free Interval 8 -year RFI was 12. 6% for EX vs. 21. 6% for UC (Courneya et al. MSSE 2014; 46: 1744 -51)
Recurrence-Free Interval 8 -year RFI was 12. 6% for EX vs. 21. 6% for UC (Courneya et al. MSSE 2014; 46: 1744 -51)
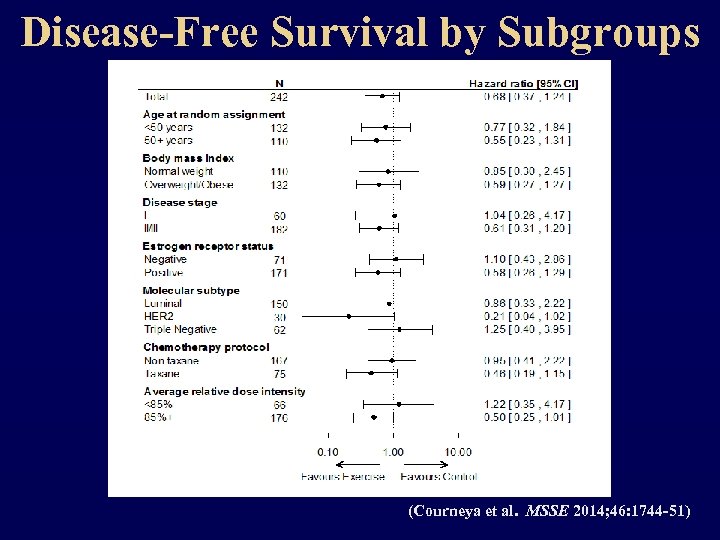 Disease-Free Survival by Subgroups (Courneya et al. MSSE 2014; 46: 1744 -51)
Disease-Free Survival by Subgroups (Courneya et al. MSSE 2014; 46: 1744 -51)
 Possible Explanations ® PA improved chemotherapy completion rate (although strongest effect was with optimal chemotherapy dosing). ® PA during chemotherapy may promote PA during survivorship (6 month follow-up data not supportive). ® PA may potentiate effects of chemotherapy: ® drug distribution, pharmacodynamics, and metabolism, ® nitric oxide-mediated peripheral blood flow, angiogenesis, endogenous antioxidant expression. (Courneya et al. MSSE 2014; 46: 1744 -51)
Possible Explanations ® PA improved chemotherapy completion rate (although strongest effect was with optimal chemotherapy dosing). ® PA during chemotherapy may promote PA during survivorship (6 month follow-up data not supportive). ® PA may potentiate effects of chemotherapy: ® drug distribution, pharmacodynamics, and metabolism, ® nitric oxide-mediated peripheral blood flow, angiogenesis, endogenous antioxidant expression. (Courneya et al. MSSE 2014; 46: 1744 -51)
 Why Not a Randomized Trial? ® no phase III randomized exercise trials with survival endpoints are ongoing in early stage breast cancer. ® Excellent survival rate >90% means that: ® sample size is too large (several thousand). ® length of intervention is too long (>5 years). ® length of follow-up is too long (>10 years). ® logistical and financial challenges.
Why Not a Randomized Trial? ® no phase III randomized exercise trials with survival endpoints are ongoing in early stage breast cancer. ® Excellent survival rate >90% means that: ® sample size is too large (several thousand). ® length of intervention is too long (>5 years). ® length of follow-up is too long (>10 years). ® logistical and financial challenges.
 Funded by the Canadian Institutes of Health Research.
Funded by the Canadian Institutes of Health Research.
 AMBER Methods ® prospective cohort design with breast cancer patients being recruited soon after surgery. ® sample size of 1500 Alberta breast cancer patients diagnosed with stages I (T 1 c) to IIIc. ® assessments at baseline (<90 days of surgery), 1 year (after completion of primary therapy), 3 years (early survivorship), and 5 years (mid survivorship). ® 5 -10 year follow-up for disease outcomes. (Courneya et al BMC Cancer 2012; 12: 525)
AMBER Methods ® prospective cohort design with breast cancer patients being recruited soon after surgery. ® sample size of 1500 Alberta breast cancer patients diagnosed with stages I (T 1 c) to IIIc. ® assessments at baseline (<90 days of surgery), 1 year (after completion of primary therapy), 3 years (early survivorship), and 5 years (mid survivorship). ® 5 -10 year follow-up for disease outcomes. (Courneya et al BMC Cancer 2012; 12: 525)
 AMBER PA and HRF Measures ® comprehensive self-report of PA and sedentary. ® objective PA and sedentary (accelerometers). ® maximal cardiorespiratory fitness (gas exchange). ® maximal strength and endurance (8 RM, SLT). ® flexibility and balance. ® body composition (DXA, anthropometrics). ® lymphedema (perometers) and neuropathy. (Courneya et al BMC Cancer 2012; 12: 525)
AMBER PA and HRF Measures ® comprehensive self-report of PA and sedentary. ® objective PA and sedentary (accelerometers). ® maximal cardiorespiratory fitness (gas exchange). ® maximal strength and endurance (8 RM, SLT). ® flexibility and balance. ® body composition (DXA, anthropometrics). ® lymphedema (perometers) and neuropathy. (Courneya et al BMC Cancer 2012; 12: 525)
 AMBER Update ® accrual began July, 2012. ® >600 patients accrued as of June, 2015. ® estimated accrual completion in 2018. ® 250 patients with 1 year follow-up. ® most common reason for ineligibility is
AMBER Update ® accrual began July, 2012. ® >600 patients accrued as of June, 2015. ® estimated accrual completion in 2018. ® 250 patients with 1 year follow-up. ® most common reason for ineligibility is
 CHALLENGE Trial (CO. 21)
CHALLENGE Trial (CO. 21)
 Colon Health and Life-Long Exercise Change (CHALLENGE) Trial (CO. 21) ® primary objective is to determine whether a 3 year structured exercise program improves DFS in high risk stage II or III colon cancer survivors 2 -6 months after chemotherapy. ® first exercise trial with a survival-related outcome as primary. ® multinational in Canada (NCIC CTG) and Australia (SURG). ® funded by NCIC-CTG and NHMRC in Australia. (Courneya et al Curr Onc 2008; 15: 262 -70)
Colon Health and Life-Long Exercise Change (CHALLENGE) Trial (CO. 21) ® primary objective is to determine whether a 3 year structured exercise program improves DFS in high risk stage II or III colon cancer survivors 2 -6 months after chemotherapy. ® first exercise trial with a survival-related outcome as primary. ® multinational in Canada (NCIC CTG) and Australia (SURG). ® funded by NCIC-CTG and NHMRC in Australia. (Courneya et al Curr Onc 2008; 15: 262 -70)
 CHALLENGE Trial Update ® 20 centers in Canada. ® 26 centers in Australia. ® 380 randomized. ® US, Israel, Kor. , France ® UK expansion. ® majority of patients are adhering to program. ® most improving fitness.
CHALLENGE Trial Update ® 20 centers in Canada. ® 26 centers in Australia. ® 380 randomized. ® US, Israel, Kor. , France ® UK expansion. ® majority of patients are adhering to program. ® most improving fitness.
 PA and Prostate Cancer Survival ® 830 men diagnosed with stage I-III prostate cancer in 1997 -2000 and followed until Oct. 2014. ® PA assessed at diagnosis and every 2 years after using the LTPAQ and converted to MET hours. ® adjusted for disease and treatment variables as well as diet and body mass index. ® 577 deaths; 236 prostate deaths; 401 recurrences. (Friedenreich et al. submitted)
PA and Prostate Cancer Survival ® 830 men diagnosed with stage I-III prostate cancer in 1997 -2000 and followed until Oct. 2014. ® PA assessed at diagnosis and every 2 years after using the LTPAQ and converted to MET hours. ® adjusted for disease and treatment variables as well as diet and body mass index. ® 577 deaths; 236 prostate deaths; 401 recurrences. (Friedenreich et al. submitted)
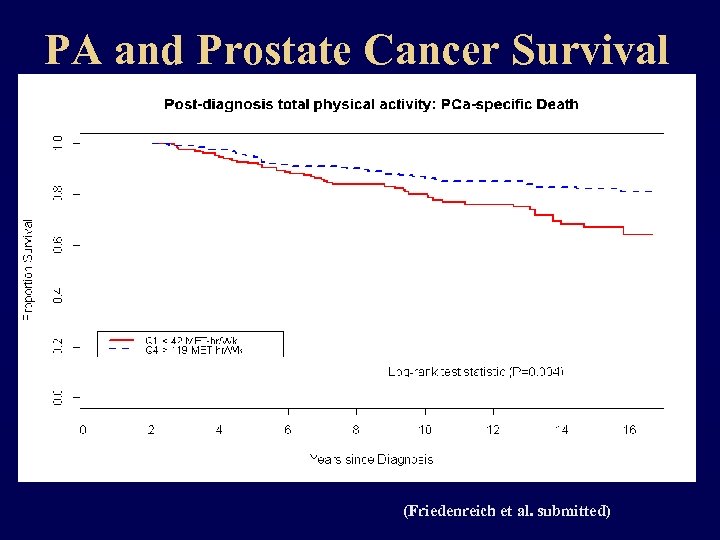 PA and Prostate Cancer Survival (Friedenreich et al. submitted)
PA and Prostate Cancer Survival (Friedenreich et al. submitted)
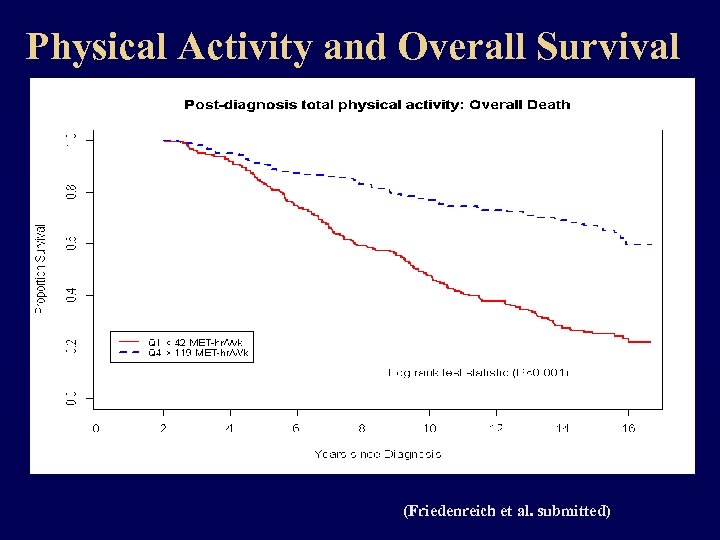 Physical Activity and Overall Survival (Friedenreich et al. submitted)
Physical Activity and Overall Survival (Friedenreich et al. submitted)
 Global Action Project (GAP 4) Trial ® phase III RCT to test the effects of exercise on overall survival in prostate cancer patients with M 1 CRPC. ® multinational ® Study Co-Chairs: Fred Saad and Rob Newton. ® currently ® plan trial supported by Movember. finalizing protocol. is to launch the trial in fall 2015.
Global Action Project (GAP 4) Trial ® phase III RCT to test the effects of exercise on overall survival in prostate cancer patients with M 1 CRPC. ® multinational ® Study Co-Chairs: Fred Saad and Rob Newton. ® currently ® plan trial supported by Movember. finalizing protocol. is to launch the trial in fall 2015.
 GAP 4 Trial ® Patients: 860 M 1 CRPC, ECOG 0 -1, medical clearance, not meeting EX guidelines (HR=0. 78). ® Intervention: tapered supervised EX for 9 months; transition to home program for 15 months. ® Comparison: ® Outcomes: no exercise; psychosocial support. OS, PFS, PSA progression, skeletal adverse events, pain, fatigue, Qo. L, mechanisms.
GAP 4 Trial ® Patients: 860 M 1 CRPC, ECOG 0 -1, medical clearance, not meeting EX guidelines (HR=0. 78). ® Intervention: tapered supervised EX for 9 months; transition to home program for 15 months. ® Comparison: ® Outcomes: no exercise; psychosocial support. OS, PFS, PSA progression, skeletal adverse events, pain, fatigue, Qo. L, mechanisms.
 Healthy Exercise for Lymphoma Patients (HELP) Trial ® RCT comparing AET to UC in 122 lymphoma patients receiving chemotherapy or off treatments. ® 12 weeks supervised exercise training. ® primary endpoint TOI-An. ® secondary endpoints of HRF, PROs, medical. (Courneya et al. J Clin Onc 2009; 27: 4605 -12)
Healthy Exercise for Lymphoma Patients (HELP) Trial ® RCT comparing AET to UC in 122 lymphoma patients receiving chemotherapy or off treatments. ® 12 weeks supervised exercise training. ® primary endpoint TOI-An. ® secondary endpoints of HRF, PROs, medical. (Courneya et al. J Clin Onc 2009; 27: 4605 -12)
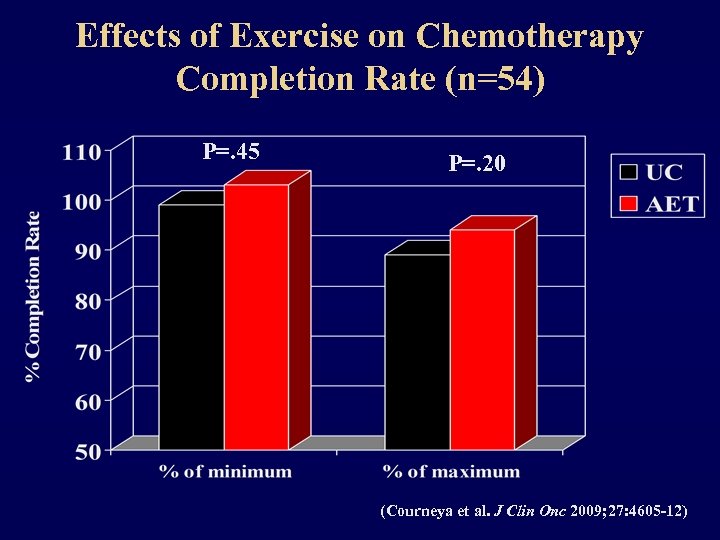 Effects of Exercise on Chemotherapy Completion Rate (n=54) P=. 45 P=. 20 (Courneya et al. J Clin Onc 2009; 27: 4605 -12)
Effects of Exercise on Chemotherapy Completion Rate (n=54) P=. 45 P=. 20 (Courneya et al. J Clin Onc 2009; 27: 4605 -12)
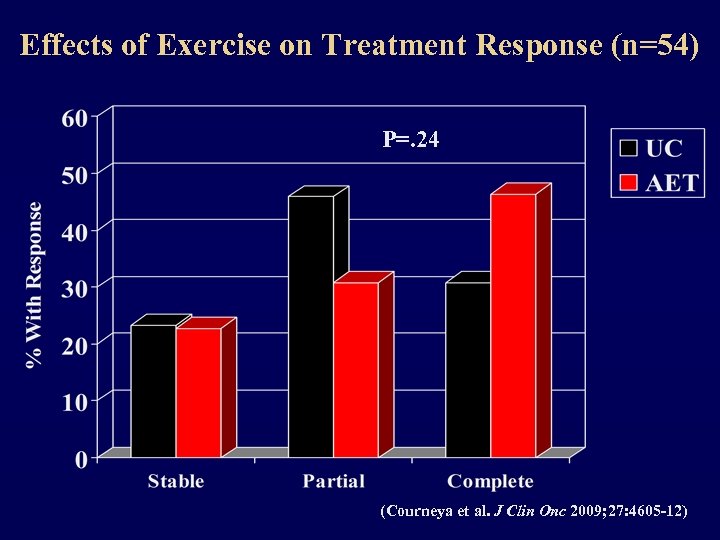 Effects of Exercise on Treatment Response (n=54) P=. 24 (Courneya et al. J Clin Onc 2009; 27: 4605 -12)
Effects of Exercise on Treatment Response (n=54) P=. 24 (Courneya et al. J Clin Onc 2009; 27: 4605 -12)
 HELP Trial Outcomes ® patients randomized between 2005 -2008. ® electronic record review in summer 2013. ® abstracted events, dates, and treatments. ® explored progression-free survival (PFS). ® adjusted analyses based on propensity scores. ® ITT analyses and explored protocol adherence (crossover). ® median follow-up of 61 months. (Courneya et al. Can Causes Con 2015; 26: 269 -76)
HELP Trial Outcomes ® patients randomized between 2005 -2008. ® electronic record review in summer 2013. ® abstracted events, dates, and treatments. ® explored progression-free survival (PFS). ® adjusted analyses based on propensity scores. ® ITT analyses and explored protocol adherence (crossover). ® median follow-up of 61 months. (Courneya et al. Can Causes Con 2015; 26: 269 -76)
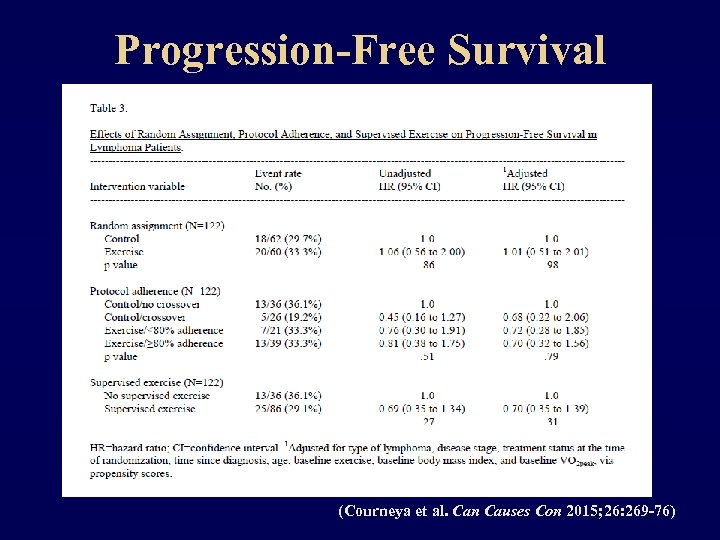 Progression-Free Survival (Courneya et al. Can Causes Con 2015; 26: 269 -76)
Progression-Free Survival (Courneya et al. Can Causes Con 2015; 26: 269 -76)
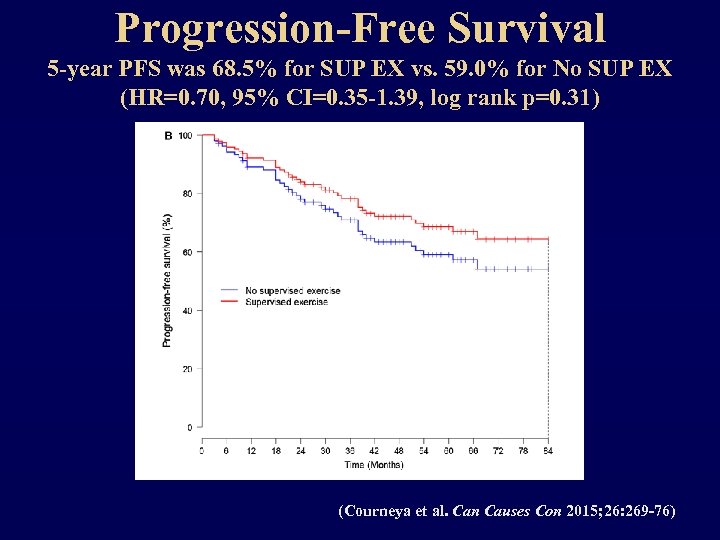 Progression-Free Survival 5 -year PFS was 68. 5% for SUP EX vs. 59. 0% for No SUP EX (HR=0. 70, 95% CI=0. 35 -1. 39, log rank p=0. 31) (Courneya et al. Can Causes Con 2015; 26: 269 -76)
Progression-Free Survival 5 -year PFS was 68. 5% for SUP EX vs. 59. 0% for No SUP EX (HR=0. 70, 95% CI=0. 35 -1. 39, log rank p=0. 31) (Courneya et al. Can Causes Con 2015; 26: 269 -76)
 Optimal Exercise Prescription? Optimal exercise prescription for improved cancer outcomes is unknown, however: ® aerobic > strength; ® volume is important; ® some better than none (biggest gains); ® more is even better (diminishing returns); ® vigorous > moderate; ® avoiding sitting may also be important.
Optimal Exercise Prescription? Optimal exercise prescription for improved cancer outcomes is unknown, however: ® aerobic > strength; ® volume is important; ® some better than none (biggest gains); ® more is even better (diminishing returns); ® vigorous > moderate; ® avoiding sitting may also be important.
 Summary and Conclusions ® self-reported PA is associated with survival in several cancer groups (breast, colon, prostate). ® phase II data suggestive of possible survival benefit of supervised exercise in breast and lymphoma. ® phase III exercise trials ongoing in colon and prostate cancer. ® lifestyle ® weight trials ongoing in several cancer groups. loss trials are also being pursued.
Summary and Conclusions ® self-reported PA is associated with survival in several cancer groups (breast, colon, prostate). ® phase II data suggestive of possible survival benefit of supervised exercise in breast and lymphoma. ® phase III exercise trials ongoing in colon and prostate cancer. ® lifestyle ® weight trials ongoing in several cancer groups. loss trials are also being pursued.
 Acknowledgements ® Many colleagues, students, staff and study participants have contributed to this research. ® K. S. Courneya is supported by the Canada Research Chairs Program.
Acknowledgements ® Many colleagues, students, staff and study participants have contributed to this research. ® K. S. Courneya is supported by the Canada Research Chairs Program.


