diabetes mellitus.ppt
- Количество слайдов: 91

Karazin Kharkiv National University Department of Pediatrics Diabetes mellitus in children Assistant Tatyana Golovko

DEFINITION The term diabetes mellitus describes a metabolic disorder of multiple etiologies which characterized by chronic hyperglycemia with disturbances of carbohydrate, fat and protein metabolism resulting from defects of insulin secretion, insulin action or both.

Type 1 diabetes is a chronic illness characterized by the body’s inability to produce insulin due to the autoimmune destruction of the beta cells in the pancreas. Most pediatric patients with diabetes have type 1 and a lifetime depends on exogenous insulin.

Until recently, diabetes in children was virtually synonymous with type 1 diabetes mellitus, whereas type 2 diabetes was a disease of middle age and the elderly. Changes in food consumption and exercise are forcing a worldwide increase in obesity in children and adolescents. As a consequence of this dramatic development an increasing rate of type 2 diabetes can be observed in children and adolescents in many countries.

EPIDEMIOLOGY – – – – Most common metabolic disease in childhood Annual incidence is 15 new cases per 100, 000 in children before 18 yrs Frequency increases with increasing age. 1: 1400 before 5 yrs 1: 400 before 16 yrs Males and females equally affected No correlation with socioeconomic status

Data from the SEARCH for Diabetes in Youth Study (SEARCH study), a multicenter study of diabetes among youth aged 0 -19 years, were examined. Incidence rates were calculated per 100, 000 person/year within 4 incident years (2002 -2005), and prevalence in 2001 was calculated per 1, 000 of youths.

The prevalence of type 1 diabetes (at age 0 -19 years) was 2. 00/1, 000, which was similar for male (2. 02/1, 000) and female (1. 97/1, 000) subjects. The incidence of type 1 diabetes was 23. 6/100, 000, slightly higher for male compared with female subjects (24. 5 vs. 22. 7 per 100, 000, respectively, P = 0. 04).

Few cases of type 2 diabetes in children younger than 10 years were found. The prevalence of type 2 diabetes (at age 10 -19 years) was 0. 18/1, 000, which is significantly higher for female compared with male subjects (0. 22 vs. 0. 15 per 1, 000, P = 0. 01). Incidence of type 2 diabetes was 3. 7/100, 000, with similar rates for female and male subjects (3. 9 vs. 3. 4 per 1, 000, respectively, P = 0. 3).

Type 1 diabetes mellitus has wide geographic variation in incidence and prevalence. Annual incidence varies from 0. 61 cases per 100, 000 population in China to 41. 4 cases per 100, 000 population in Finland. Substantial variations are observed between nearby countries with differing lifestyles, such as Estonia and Finland, and between genetically similar populations, such as those in Iceland Norway.
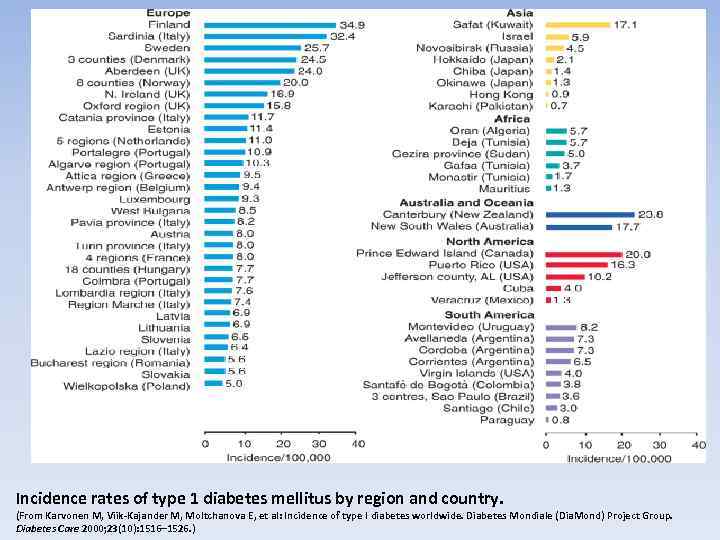
Incidence rates of type 1 diabetes mellitus by region and country. (From Karvonen M, Viik-Kajander M, Moltchanova E, et al: Incidence of type I diabetes worldwide. Diabetes Mondiale (Dia. Mond) Project Group. Diabetes Care 2000; 23(10): 1516– 1526. )

DM RISK OF INHERITANCE • Children of both DM parent: 2 -5% of risk • Children of diabetic mother: 2% of risk • • • Children of diabetic father: 5% of risk Monozygotic twins: – 50% of inheritance risk Dizygotic twins: – 20% of inheritance risk

Gender-related demographics The influence of gender varies with the overall incidence rates. Males are at greater risk in regions of high incidence, particularly older males, whose incidence rates often show seasonal variation. Females appear to be at a greater risk in low-incidence regions.

Pathophysiology Insulin is essential to process carbohydrates, fat, and protein. Insulin reduces blood glucose levels by allowing glucose to enter muscle cells and by stimulating the conversion of glucose to glycogen (glycogenesis) as a carbohydrate store. Insulin also inhibits the release of stored glucose from liver - glycogen (glycogenolysis) and slows the breakdown of fat to triglycerides, free fatty acids, and ketones. It also stimulates fat storage. Additionally, insulin inhibits the breakdown of protein and fat for glucose production (gluconeogenesis) in the liver and kidneys.

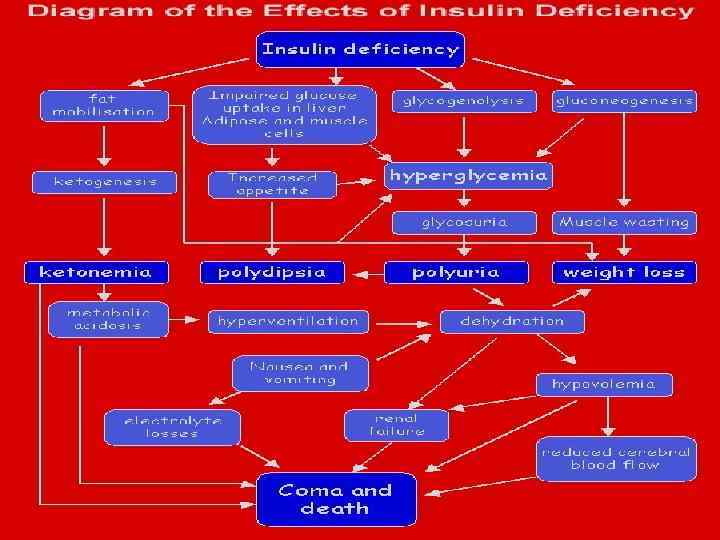
Hyperglycemia (i. e. , accidental blood glucose concentration of more than 200 mg/d. L or 11 mmol/L) results when insulin deficiency leads to uninhibited gluconeogenesis and prevents the usage and storage of circulating glucose. The kidneys cannot reabsorb the excess glucose load, causing glycosuria, osmotic diuresis, thirst, and dehydration. Increased fat and protein breakdown leads to ketone production and weight loss.
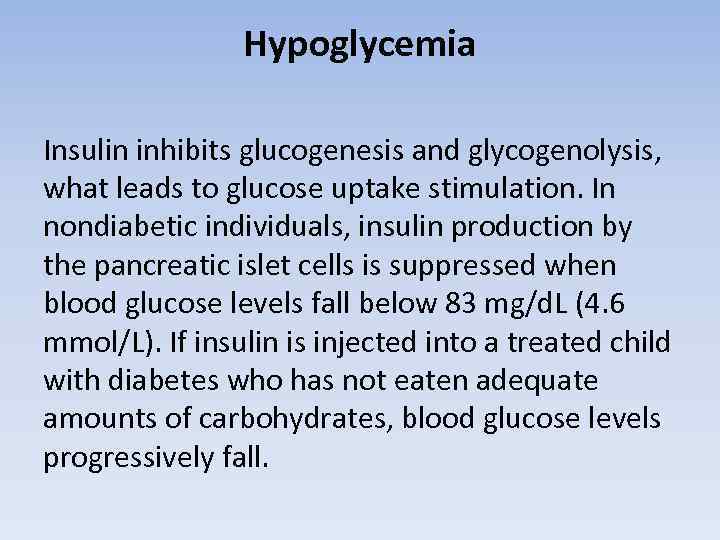
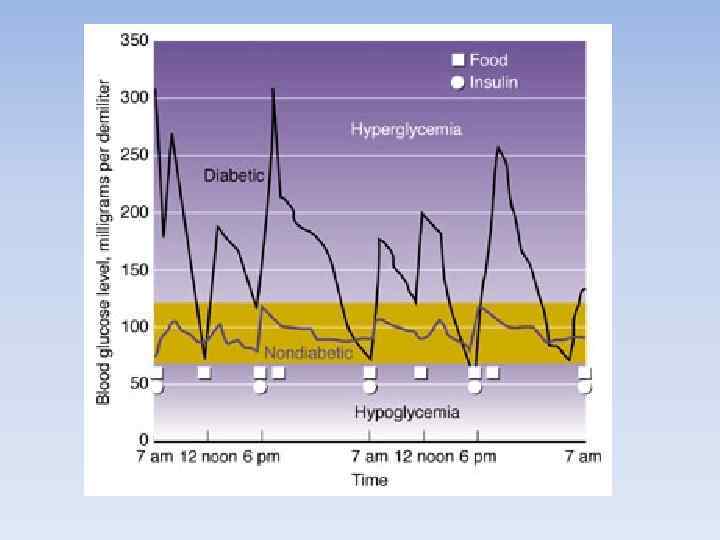
Hypoglycemia Insulin inhibits glucogenesis and glycogenolysis, what leads to glucose uptake stimulation. In nondiabetic individuals, insulin production by the pancreatic islet cells is suppressed when blood glucose levels fall below 83 mg/d. L (4. 6 mmol/L). If insulin is injected into a treated child with diabetes who has not eaten adequate amounts of carbohydrates, blood glucose levels progressively fall.

The brain depends on glucose as a fuel. As glucose levels drop below 65 mg/d. L (3. 2 mmol/L) counterregulatory hormones (e. g, glucagon, cortisol, epinephrine) are released, and symptoms of hypoglycemia develop. These symptoms include sweatiness, shivering, confused mental state, behavioral changes, and, eventually, coma when blood glucose levels fall below 30 -40 mg/d. L.

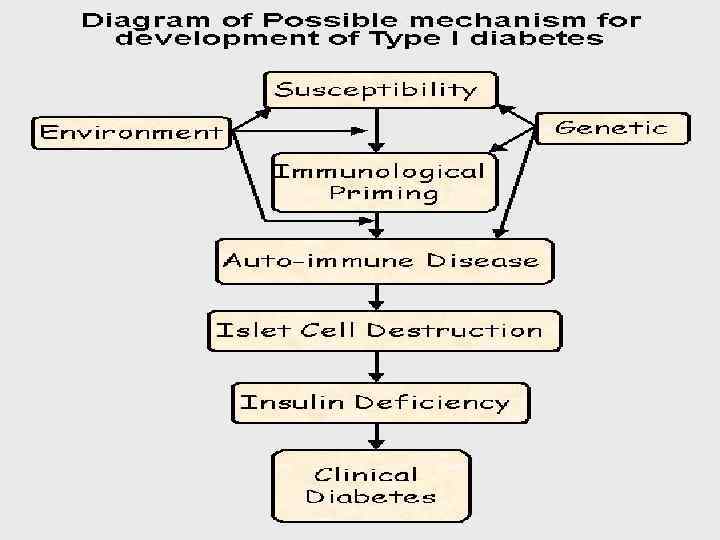
Most cases (95%) of type 1 diabetes mellitus are the result of environmental factors interacting with hereditary background. This interaction leads to the development of autoimmune disease directed at the insulin-producing cells of the pancreatic islets of Langerhans. These cells are progressively destroyed, with insulin deficiency usually developing after the destruction of 90% of islet cells.
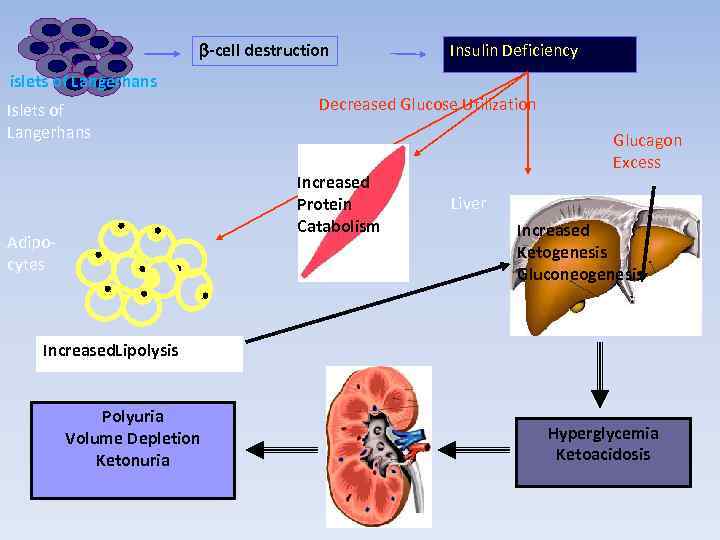
b-cell destruction Insulin Deficiency islets of Langerhans Islets of Langerhans Decreased Glucose Utilization Increased Protein Catabolism Adipocytes Glucagon Excess Liver Increased Ketogenesis Gluconeogenesis Increased. Lipolysis Polyuria Volume Depletion Ketonuria Hyperglycemia Ketoacidosis

Etiology • Viral infections may be the most important environmental factor in the development of type 1 diabetes mellitus, probably by initiating or modifying an autoimmune process. Paradoxically, type 1 diabetes mellitus incidence is higher in areas where the total incidence of infectious disease is lower. • Dietary factors are also relevant. Breastfed infants have a lower risk for type 1 diabetes. Children on cow's milk consumption have a greater risk of diabetes incidence. Some cow's milk proteins (e. g. , bovine serum albumin) have antigenic similarities to an islet cell antigen.

• The known association of increasing incidence of type 1 diabetes mellitus with distance from the equator may now have an explanation. Reduced exposure to ultraviolet (UV) light and lower vitamin D levels, both of which are more likely found in the higher latitudes, are associated with an increased risk of type 1 diabetes mellitus. • Congenital absence of the pancreas or islet cells • Pancreatectomy • Pancreatic damage (i. e. , cystic fibrosis, chronic pancreatitis, thalassemia major, hemochromatosis, hemolytic-uremic syndrome) • Wolfram syndrome (diabetes insipidus, diabetes mellitus, optic atrophy, deafness)

WHO CLASSIFICATION (2000 year) • Is based on etiology not on type of treatment or age of the patient. • Type 1 Diabetes (idiopathic or autoimmune β-cell destruction) • Type 2 Diabetes (defects in insulin secretion or its activity) • Other specific types • Gestational diabetes is a separate entity • Impaired Glucose Tolerance (IGT) indicates blood glucose levels between normal and diabetic cut off points during glucose tolerance test.
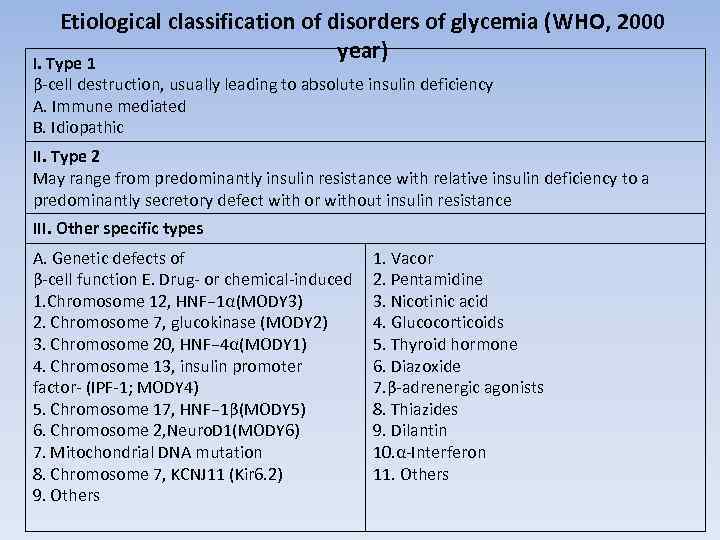
Etiological classification of disorders of glycemia (WHO, 2000 year) I. Type 1 β-cell destruction, usually leading to absolute insulin deficiency A. Immune mediated B. Idiopathic II. Type 2 May range from predominantly insulin resistance with relative insulin deficiency to a predominantly secretory defect with or without insulin resistance III. Other specific types A. Genetic defects of β-cell function E. Drug- or chemical-induced 1. Chromosome 12, HNF− 1α(MODY 3) 2. Chromosome 7, glucokinase (MODY 2) 3. Chromosome 20, HNF− 4α(MODY 1) 4. Chromosome 13, insulin promoter factor- (IPF-1; MODY 4) 5. Chromosome 17, HNF− 1β(MODY 5) 6. Chromosome 2, Neuro. D 1(MODY 6) 7. Mitochondrial DNA mutation 8. Chromosome 7, KCNJ 11 (Kir 6. 2) 9. Others 1. Vacor 2. Pentamidine 3. Nicotinic acid 4. Glucocorticoids 5. Thyroid hormone 6. Diazoxide 7. β-adrenergic agonists 8. Thiazides 9. Dilantin 10. α-Interferon 11. Others
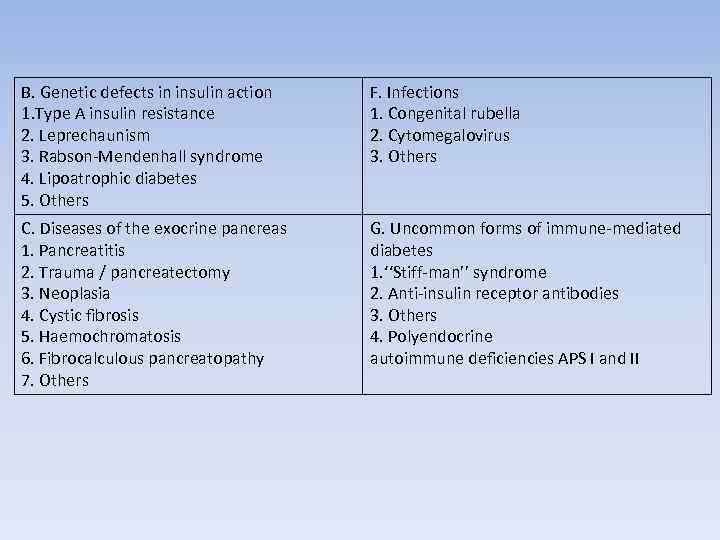
B. Genetic defects in insulin action 1. Type A insulin resistance 2. Leprechaunism 3. Rabson-Mendenhall syndrome 4. Lipoatrophic diabetes 5. Others F. Infections 1. Congenital rubella 2. Cytomegalovirus 3. Others C. Diseases of the exocrine pancreas 1. Pancreatitis 2. Trauma / pancreatectomy 3. Neoplasia 4. Cystic fibrosis 5. Haemochromatosis 6. Fibrocalculous pancreatopathy 7. Others G. Uncommon forms of immune-mediated diabetes 1. ‘‘Stiff-man’’ syndrome 2. Anti-insulin receptor antibodies 3. Others 4. Polyendocrine autoimmune deficiencies APS I and II
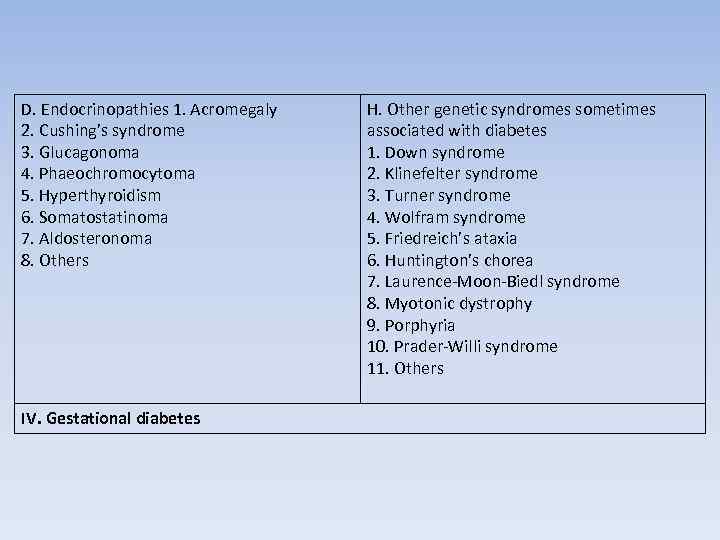
D. Endocrinopathies 1. Acromegaly 2. Cushing’s syndrome 3. Glucagonoma 4. Phaeochromocytoma 5. Hyperthyroidism 6. Somatostatinoma 7. Aldosteronoma 8. Others IV. Gestational diabetes H. Other genetic syndromes sometimes associated with diabetes 1. Down syndrome 2. Klinefelter syndrome 3. Turner syndrome 4. Wolfram syndrome 5. Friedreich’s ataxia 6. Huntington’s chorea 7. Laurence-Moon-Biedl syndrome 8. Myotonic dystrophy 9. Porphyria 10. Prader-Willi syndrome 11. Others

Types of Diabetes in Children • Type 1 diabetes mellitus accounts for > 90% of cases. • Type 2 diabetes is increasingly recognized in children more often in adults. • Transient neonatal diabetes and neonatal diabetes. • Secondary diabetes (e. g. in cystic fibrosis or Cushing syndrome).

Transient neonatal diabetes In newborns who are very underweight, blood sugar levels may be elevated transiently, usually because they are given intravenous infusions of glucose too rapidly. The infusions are given to increase the newborn's weight. This problem usually resolves without treatment.

Neonatal diabetes including diagnosis in infants younger than 6 months is most likely due to an inherited defect of the i. Kir 6. 2 subunit potassium channel of the islet beta cells and is indicated by the genetic screening. This is particularly important, because these children respond well to sulphonylurea therapy.

DIABETES MELLITUS CLINICAL MANIFESTATIONS • Classic Presentation: – Polyuria – Polydipsia – Polyphagia – Weight loss. • Gradually increasing – Onset of lethargy and weakness. – Duration of symptoms is usually < 1 month.

Hyperglycemia • Hyperglycemia alone may not cause obvious symptoms, although some children report general malaise, headache, and weakness. Children may also appear irritable and become ill-tempered. The main symptoms of hyperglycemia are secondary to osmotic diuresis and glycosuria. Glycosuria • This condition leads to frequency and volume of urinary increase (e. g. , polyuria), which is particularly troublesome at night (e. g. , nycturia) and often leads to enuresis in a previously continent child. These symptoms are easy to overlook in infants because of their naturally high fluid intake and diaper/napkin use.

Polydipsia • Increased thirst, which may be insatiable, is secondary to the osmotic diuresis causing dehydration. Weight loss • Insulin deficiency leads to uninhibited gluconeogenesis, causing breakdown of protein and fat. Weight loss may be dramatic, although the child's appetite usually remains good. Failure to thrive may be the first symptom noted in an infant or toddler and may precede frank hyperglycemia. Nonspecific malaise • Although this condition may be present before symptoms of hyperglycemia or as a separate symptom of hyperglycemia. Though more often it is recognized only retrospectively.

Symptoms in infants and toddlers may include the following: Onset in the first year of life, although unusual, can occur, so type 1 diabetes mellitus must be considered in any infant or toddler, because these children have the greatest risk for mortality if diagnosis is delayed. • Severe diaper/napkin rash • Unexplained malaise • Poor weight gain or weight loss • Increased thirst • Vomiting and dehydration, with a constantly wet napkin/diaper.
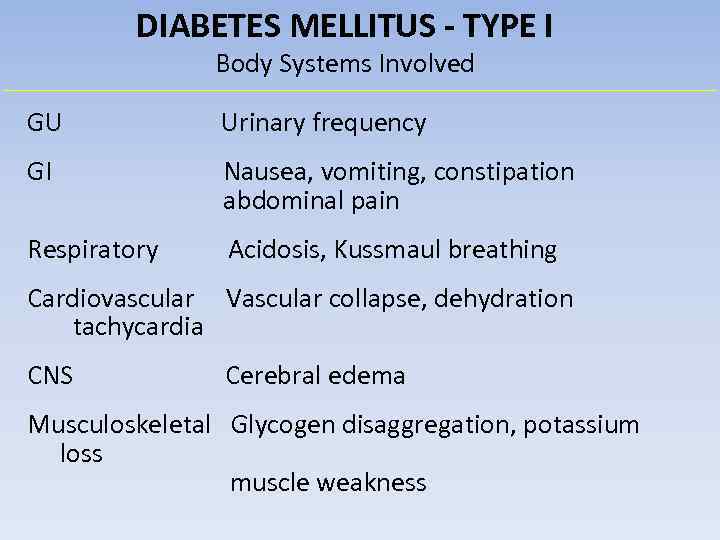
DIABETES MELLITUS - TYPE I Body Systems Involved GU Urinary frequency GI Nausea, vomiting, constipation abdominal pain Respiratory Acidosis, Kussmaul breathing Cardiovascular Vascular collapse, dehydration tachycardia CNS Cerebral edema Musculoskeletal Glycogen disaggregation, potassium loss muscle weakness

Additional symptoms Hyperglycemia impairs immunity and makes a child more susceptible to recurrent infection, particularly of the urinary tract, skin, and respiratory tract. Candidosis may develop, especially in the inguinal region and in flexural areas.

Diabetic ketoacidosis (DKA) is much less common than hypoglycemia but is potentially far more serious, creating a life-threatening medical emergency. Ketosis usually does not occur when insulin is present. In the absence of insulin, however, severe hyperglycemia, dehydration, and ketone production contribute to the development of DKA. The most serious complication of DKA is the development of cerebral edema, which increases the risk of death and long-term morbidity. Very young children at the time of first diagnosis are most likely to develop cerebral edema.

DKA usually follows increasing hyperglycemia and symptoms of osmotic diuresis. They are more likely to present with nausea, vomiting, and abdominal pain, symptoms similar to food poisoning. DKA may manifest as respiratory distress.

Symptoms of ketoacidosis These symptoms include the following: • Severe dehydration • Smell of ketones • Acidotic breathing (i. e. , Kussmaul respiration), masquerading as respiratory distress • Abdominal pain • Vomiting • Drowsiness and coma

Diagnosis Blood glucose tests using capillary blood samples, reagent sticks, and blood glucose meters are the usual methods for monitoring day-to-day diabetes control. Diagnostic criteria by the American Diabetes Association (ADA) include the following: • A fasting plasma glucose (FPG) level ≥ 126 mg/d. L (7. 0 mmol/L). Fasting is defined as no caloric intake for at least 8 h. , or • A 2 -hour plasma glucose level ≥ 200 mg/d. L (11. 1 mmol/L) during a 75 -g oral glucose tolerance test (OGTT). The test should be performed as described by WHO , using a glucose load containing the equivalent of 75 g anhydrous glucose dissolved in water or 1. 75 g/kg of body weight to a maximum of 75 g. , or • A casual plasma glucose ≥ 200 mg/d. L (11. 1 mmol/L) in a patient with classic symptoms of hyperglycemia or hyperglycemic crisis. Casual is defined as any time of day without regard to time since last meal.
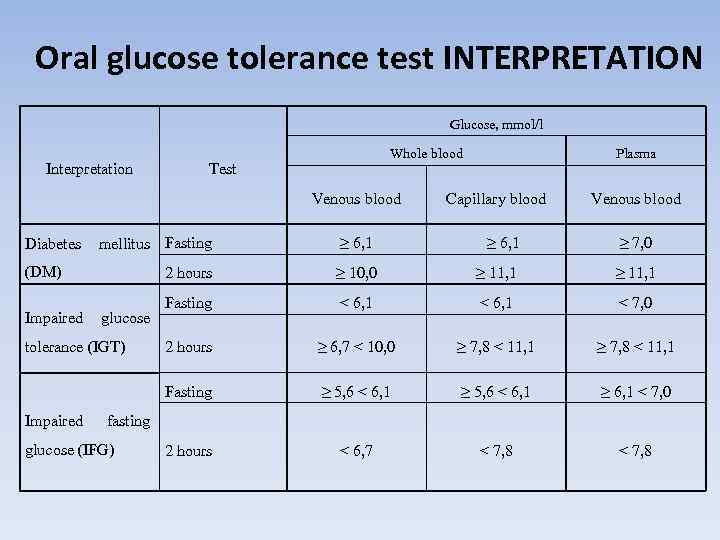
Oral glucose tolerance test INTERPRETATION Glucose, mmol/l Interpretation Whole blood Test Venous blood Plasma Capillary blood Venous blood Diabetes mellitus Fasting ≥ 6, 1 ≥ 7, 0 (DM) 2 hours ≥ 10, 0 ≥ 11, 1 Fasting < 6, 1 < 7, 0 2 hours ≥ 6, 7 < 10, 0 ≥ 7, 8 < 11, 1 Fasting ≥ 5, 6 < 6, 1 ≥ 6, 1 < 7, 0 2 hours < 6, 7 < 7, 8 Impaired glucose tolerance (IGT) Impaired fasting glucose (IFG)

Glycated hemoglobin (hemoglobin A 1 c, Hb. A 1 c, A 1 C, or Hb 1 c; sometimes also Hb. A 1 c) is a form of hemoglobin that is measured primarily to identify the average plasma glucose concentration over prolonged periods of time. It is formed in a non-enzymatic glycation pathway by hemoglobin's exposure to plasma glucose. Normal levels of glucose produce a normal amount of glycated hemoglobin. The average amount of plasma glucose increases follows after the fraction of glycated hemoglobin increases.

Amount of glycated hemoglobin indicates an average blood sugar level for the past two to three months. It works by measuring the percentage of blood sugar linked to hemoglobin, the oxygen-carrying protein in red blood cells. The higher the blood sugar level is, the more hemoglobin is that is linked with sugar. A hemoglobin A 1 C level of 6. 5 percent or higher on two separate tests indicates diabetes.
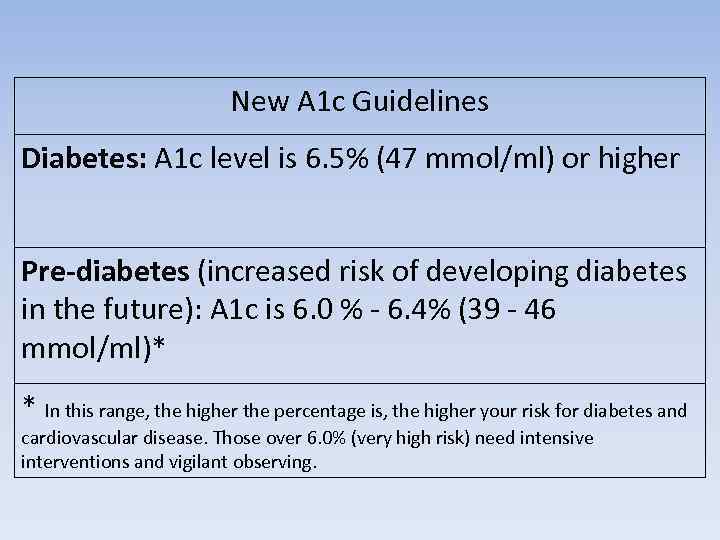
New A 1 c Guidelines Diabetes: A 1 c level is 6. 5% (47 mmol/ml) or higher Pre-diabetes (increased risk of developing diabetes in the future): A 1 c is 6. 0 % - 6. 4% (39 - 46 mmol/ml)* * In this range, the higher the percentage is, the higher your risk for diabetes and cardiovascular disease. Those over 6. 0% (very high risk) need intensive interventions and vigilant observing.
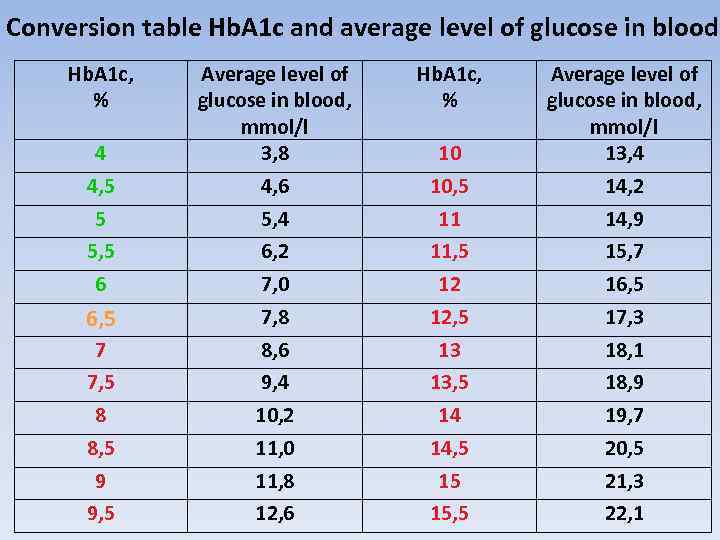
Conversion table Hb. A 1 c and average level of glucose in blood Hb. A 1 c, % 4 4, 5 5 5, 5 6 6, 5 7 7, 5 8 8, 5 9 9, 5 Average level of glucose in blood, mmol/l 3, 8 4, 6 5, 4 6, 2 7, 0 7, 8 8, 6 9, 4 10, 2 11, 0 11, 8 12, 6 Hb. A 1 c, % 10 10, 5 11 11, 5 12 12, 5 13 13, 5 14 14, 5 15 15, 5 Average level of glucose in blood, mmol/l 13, 4 14, 2 14, 9 15, 7 16, 5 17, 3 18, 1 18, 9 19, 7 20, 5 21, 3 22, 1

Morbidity Children aged 1 -4 years are particularly at risk and may die due to DKA at the time of diagnosis. Adolescents are also a high-risk group. Most deaths result from delayed diagnosis or neglected treatment and subsequent cerebral edema during treatment for DKA, although untreated hypoglycemia also causes some deaths. Unexplained death during sleep may also occur and appears more likely to affect young males.

Mortality Information on mortality rates for type 1 diabetes mellitus is difficult to ascertain without complete national registers of childhood diabetes. The complications of type 1 diabetes mellitus can be divided into 3 major categories: acute complications, long-term complications, and complications caused by associated autoimmune diseases.

Acute complications • Hypoglycemia, • Hyperglycemia, • Diabetic ketoacidosis (DKA).

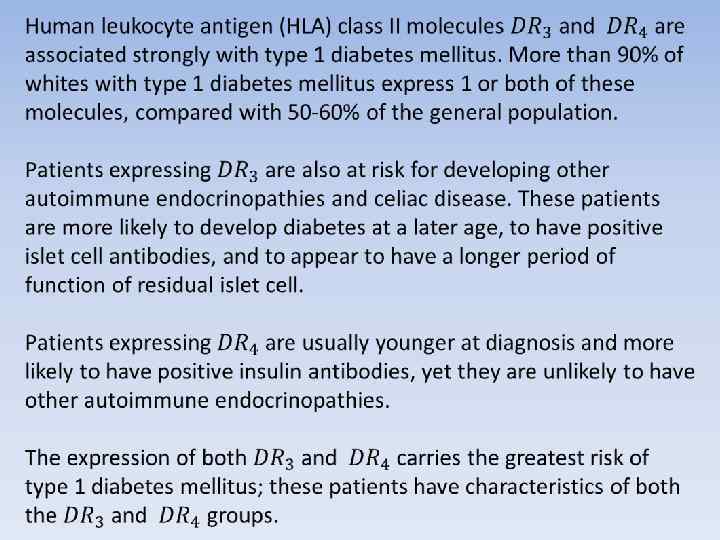
Associated autoimmune diseases are common in type 1 diabetes mellitus, particularly in children who have HLA-DR 3. Some conditions may precede the development of diabetes, and others may develop later. As many as 20% of children with diabetes have thyroid autoantibodies.

Long-term complications include the following: • • • Retinopathy Cataracts Gastroparesis Hypertension Progressive renal failure Early coronary artery disease Peripheral vascular disease Peripheral and autonomic neuropathy Increased risk of infection

Diabetic retinopathy The most common cause of acquired blindness in many developed nations, diabetic retinopathy is rare in the prepubertal child or within 5 years of onset of diabetes. The prevalence and severity of retinopathy increase with age and are greatest in patients whose diabetic control is poor. Prevalence rates seem to be declining, though an estimated 80% of people with type 1 diabetes mellitus develop retinopathy.

Injection-site hypertrophy If children persistently inject their insulin into the same area, subcutaneous tissue may develop swelling, causing induration and disturbing insulin absorption. Rotating the injection sites prevents the condition. Fat atrophy can also occur, possibly in association with insulin antibodies.
Diabetic nephropathy and hypertension The exact mechanism of diabetic nephropathy is unknown. Peak incidence is in youth, 10 -15 years after diagnosis, and it may occur in as many as 30% of people with type 1 diabetes mellitus. In a patient with nephropathy, the albumin excretion rate (AER) increases until frank proteinuria develops, and this may progress to renal failure. Blood pressure rises with increased AER, and hypertension accelerates the progression to renal failure. Having diabetic nephropathy also increases the risk of significant diabetic retinopathy. A child younger than 15 years old with persistent proteinuria may have a nondiabetic cause and should be referred to a pediatric nephrologist for further assessment.
Peripheral and autonomic neuropathy The peripheral and autonomic nerves are affected in type 1 diabetes mellitus. Hyperglycemic effects on axons and microvascular changes in endoneural capillaries are amongst the proposed mechanisms. Autonomic changes involving cardiovascular control (e. g. , heart rate, postural responses) have been described in as many as 40% of children with diabetes. Cardiovascular control changes become more likely with increasing duration and worsening control. In adults, peripheral neuropathy usually occurs as a distal sensory loss. Gastroparesis is another complication which may be caused by autonomic dysfunction. Gastric emptying is significantly delayed, leading to problems of bloating and unpredictable variations of blood glucose levels.

Macrovascular disease Although this complication is not seen in pediatric patients, it is a significant cause of morbidity and premature mortality in adults with diabetes. People with type 1 diabetes mellitus have twice the risk of fatal myocardial infarction (MI) and stroke that people unaffected with diabetes do; in women, the MI risk is 4 times greater. People with type 1 diabetes mellitus also have 4 times greater risk for atherosclerosis. The combination of peripheral vascular disease and peripheral neuropathy can cause serious foot pathology. Smoking, hypertension, hyperlipidemia, and poor diabetic control greatly increase the risk of vascular disease.

Autoimmune diseases Hyperthyroidism affects 1% of children with diabetes; the condition is usually discovered at the time of diabetes diagnosis. Although Addison disease is uncommon, affecting less than 1% of children with diabetes, it is a life-threatening condition that is easily missed. Addison disease may reduce the insulin requirement and increase the frequency of hypoglycemia. (These effects may also be the result of unrecognized hypothyroidism. ) Celiac disease, associated with an abnormal sensitivity to gluten in wheat products, is probably a form of autoimmune disease and may occur in as many as 5% of children with type 1 diabetes mellitus. Necrobiosis lipoidica is probably another form of autoimmune disease. This condition is usually, but not exclusively, found in patients with type 1 diabetes. Necrobiosis lipoidica affects 1 -2% of children and may be more common in children with poor diabetic control.

Limited joint mobility Originally described in approximately 30% of patients with type 1 diabetes mellitus, limited joint mobility occurs in 50% of patients older than age 10 years who have had diabetes for longer than 5 years. The condition restricts joint extension, making it difficult to press the hands flat against each other. The skin of patients with severe joint involvement has a thickened and waxy appearance. Limited joint mobility is associated with increased risks for diabetic retinopathy and nephropathy.

The differential diagnosis of type 1 diabetes include the following: • • Type 2 diabetes mellitus Maturity-onset diabetes of the young (MODY) Psychogenic polydipsia Nephrogenic diabetes insipidus High-output renal failure Transient hyperglycemia with illness and other stress Steroid therapy Factitious illness (Münchhausen syndrome by proxy)

The differential diagnosis of hyperglycosemia • • • Diabetes Insipidus Hyperthyroidism Pheochromocytoma Renal Glucosuria Toxicity, Salicylate

TREATMENT GOALS • Prevent death and alleviate symptoms • Achieve biochemical control • Achieve normal growth and physical development and psychological maturation • Prevent acute complications • Prevent or delay late-onset complications
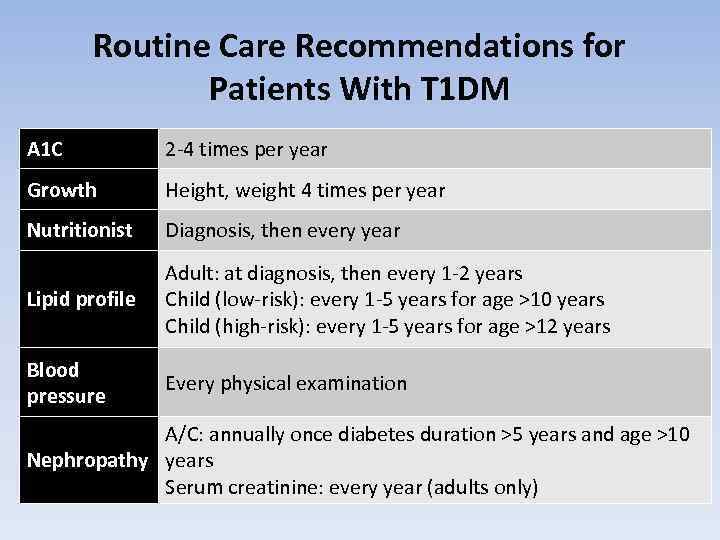
Routine Care Recommendations for Patients With T 1 DM A 1 C 2 -4 times per year Growth Height, weight 4 times per year Nutritionist Diagnosis, then every year Lipid profile Adult: at diagnosis, then every 1 -2 years Child (low-risk): every 1 -5 years for age >10 years Child (high-risk): every 1 -5 years for age >12 years Blood pressure Every physical examination A/C: annually once diabetes duration >5 years and age >10 Nephropathy years Serum creatinine: every year (adults only)

Recent Advances in the Care of Persons With T 1 DM • Development of insulin analogues • Insulin pump therapy • Home continuous glucose monitoring (CGM)

TREATMENT ELEMENTS • • Education Insulin therapy Diet and meal planning Monitoring üHb. A 1 c every 2 -months üHome regular BG monitoring üHome urine ketones tests

Continuous Glucose Monitoring • Professional CGM • Personal CGM

Pediatric Diabetes Consensus Conference: Use of CGM • Frequent, nearly daily use of CGM: – Can lower A 1 C levels in children and adolescents who are not well-controlled, irrespective of the treatment regimen – Can reduce exposure to hypoglycemia and maintain target A 1 C levels in well-controlled patients • Intermittent use of CGM: – Maybe used to detect post-meal hyperglycemia, nocturnal hypoglycemia, and the dawn phenomenon • The development of smaller, more accurate and easier devices allowed to improve CGM in youth with T 1 DM.

American Association of Clinical Endocrinologists Recommendations for Personal CGM “Good” Candidates Other Candidates • A 1 C levels >7% and able to use • the device near-continuously • Type 1 diabetes with hypoglycemia unawareness or • frequent hypoglycemia • Hyperglycemia over target or with excessive glycemic variability • Requiring A 1 C lowering without excessive hypoglycemia (e. g. , potentially disabling or life-threatening) • Pregnancy Youth who frequently monitor their BG levels children (<8 years of age), especially if there are problems with hypoglycemia 2 - to 4 -week trial recommended

AACE Recommendations for Professional CGM for Youth • May be useful if there are major changes in diabetes regimen • Nocturnal hypoglycemia/dawn phenomenon • Hypoglycemia unawareness • Postprandial hyperglycemia
Blood glucose monitors (glucometers)

Predictors of Poor Glycemic Control • • • Younger age Longer diabetes duration Weight < 85 th percentile Not living in a 2 -parent household Type of diabetes care provider Nonwhite race/ethnicity Female gender Lower parental education Poor early glycemic control (2 nd year after diagnosis; predictive of poor glycemic control later)

EDUCATION • Educate child and care givers about: q Diabetes q Insulin q Life-saving skills q Recognition of Hypoglycemia and DKA q Meal plan q regular glycemic control

DIET REGULATION • Regular meal plans with calorie exchange options are encouraged. • 50 -60% of required energy to be obtained from complex carbohydrates. • Distribute carbohydrate load evenly during the day preferably 3 meals and 2 snacks with avoidance of simple sugars. • Encouraged low salt, low saturated fats and high fiber diet.

EXERCISE • Decreases insulin requirement in diabetic subjects by increasing both sensitivity of muscle cells to insulin and glucose utilization. • It can precipitate hypoglycemia in the unprepared diabetic patient. • It may worsen pre-existing diabetic retinopathy.

INSULIN • A polypeptide made of 2 b-chains. • Discovered by Bants and Best in 1921. • Animal types (porcine and bovine) were used before the introduction of human-like insulin (DNA-recombinant types). • Recently more potent insulin analogs are produced by changing aminoacid sequence.

FUNCTION OF INSULIN § § Insulin being an anabolic hormone stimulates protein and fatty acids synthesis. Insulin decreases blood sugar 1. By inhibiting hepatic glycogenolysis and gluconeogenesis. 2. By stimulating glucose uptake, utilization and storage by the liver, muscles and adipose tissue.

TYPES OF INSULIN • Short acting (neutral, soluble, regular) üPeak 2 -3 hours and duration up to 8 hours • Intermediate acting üIsophane (peak 6 -8 h and duration 16 -24 h) üBiphasic (peak 4 -6 h and duration 12 -20 h) üSemilente (peak 5 -7 h and duration 12 -18 h) • Long acting (lente, ultralente , insulin protamine zinc) üPeak 8 -14 h and duration 20 -36 h

INSULIN ANALOGS • Ultra short acting q Insulin Lispro q Insulin Aspart • Long acting without peak action to simulate normal basal insulin q Glargine
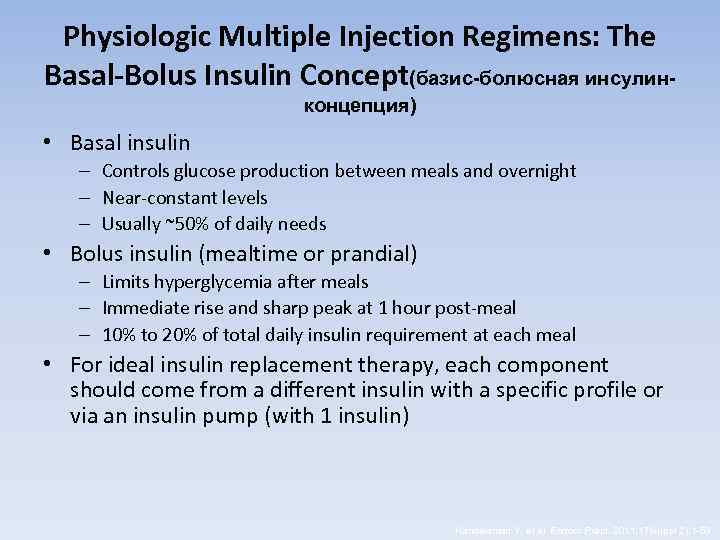
Physiologic Multiple Injection Regimens: The Basal-Bolus Insulin Concept(базис-болюсная инсулинконцепция) • Basal insulin – Controls glucose production between meals and overnight – Near-constant levels – Usually ~50% of daily needs • Bolus insulin (mealtime or prandial) – Limits hyperglycemia after meals – Immediate rise and sharp peak at 1 hour post-meal – 10% to 20% of total daily insulin requirement at each meal • For ideal insulin replacement therapy, each component should come from a different insulin with a specific profile or via an insulin pump (with 1 insulin) Handelsman Y, et al. Endocr Pract. 2011; 17(suppl 2): 1 -53.
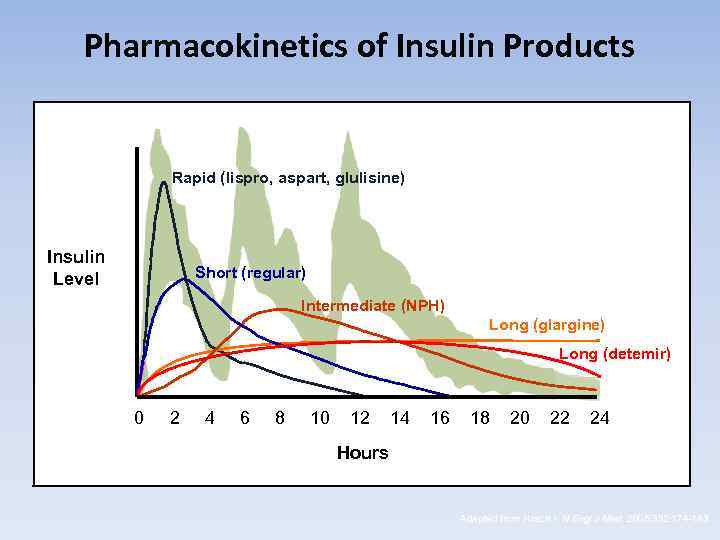
Pharmacokinetics of Insulin Products Rapid (lispro, aspart, glulisine) Insulin Level Short (regular) Intermediate (NPH) Long (glargine) Long (detemir) 0 2 4 6 8 10 12 14 16 18 20 22 24 Hours Adapted from Hirsch I. N Engl J Med. 2005; 352: 174 -183.
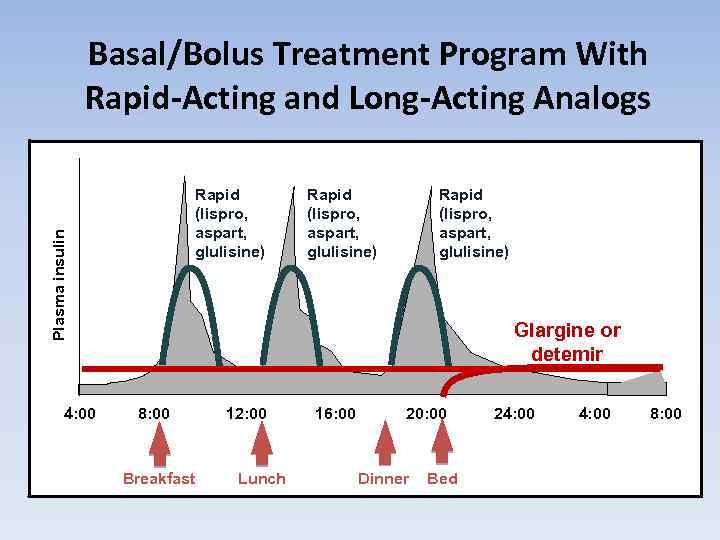
Basal/Bolus Treatment Program With Rapid-Acting and Long-Acting Analogs Plasma insulin Rapid (lispro, aspart, glulisine) 4: 00 Rapid (lispro, aspart, glulisine) Glargine or detemir 8: 00 Breakfast 12: 00 Lunch 16: 00 20: 00 Dinner Bed 24: 00 8: 00

INSULIN CONCENTRATIONS • Insulin is available in different concentrations 40, 80 and 100 Unit/ml. • WHO now recommends U 100 to be the only used insulin to prevent confusion. • Special preparation for infusion pumps is soluble insulin 500 U/ml.
Insulin pumps

Insulin Pump Use in Children Advantages • Improved blood sugar control • Insulin availability and convenience • Use of multiple basal rates, temporary basal rates • Ease of administering multiple boluses • Reduction of hypoglycemia • Flexibility and freedom • Control of post-meal blood sugar/CGM values • Ease of adjusting insulin doses with exercise and travel Disadvantages • Remembering to give insulin boluses with food intake • Ketonuria or ketoacidosis • Psychological factors • Expense • Weight gain • Skin infections • Insulin unavailability and instability • Infusion site locations and set changes • Physical/logistical considerations Maahs DM, et al. Diabetes Technol Ther. 2010; 12(S 1): S-59 -S-65.

Injection Pens

INSULIN REGIMENS • Twice daily: either NPH alone or NPH+SI. • Thrice daily: SI before each meal and NPH only before dinner. • Intensive 4 times/day: SI before meals + NPH or Glargine at bed time. • Continuous s/c infusion using pumps loaded with SI.

ADVERSE EFFECTS OF INSULIN • • Hypoglycemia Lipoatrophy Lipohypertrophy Obesity Insulin allergy Insulin antibodies Insulin induced edema
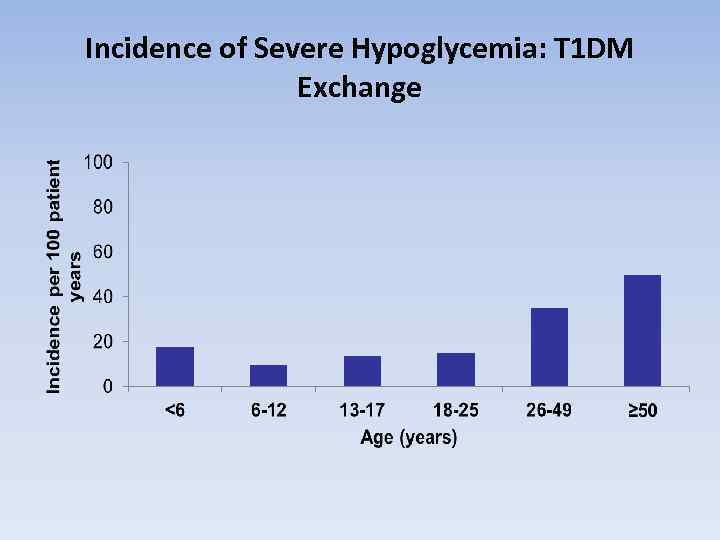
Incidence of Severe Hypoglycemia: T 1 DM Exchange
Cognitive Effects of Hypoglycemia in Children • Repeated severe hypoglycemia has been reported to reduce long-term spatial memory in children with type 1 diabetes • Early exposure to hypoglycemia may be more damaging to cognitive function than later exposure • High frequency of and early evolvement severe hypoglycemia during development negatively affects spatial long-term memory performance Hershey T, et al. Diabetes Care. 2005; 28: 2372 -2377.
Causes of Hypoglycemia in Toddlers and Preschoolers • Unpredictable food intake and physical activity • Imprecise administration of low doses of insulin • Frequent viral infections • Inability to convey the symptoms of low blood sugar Litton J, et al. J Pediatr. 2002; 141: 490 -495.

PREVENTION OF DIABETES q. Primary prevention • Identification of diabetes gene • Tampering with the immune system • Elimination of environmental factor q. Secondary prevention • Immunosuppressive therapy q. Tertiary prevention • Tight metabolic control and good monitoring

Happy new year!!!
diabetes mellitus.ppt