763559a76ab624bc2d4d253f0c203112.ppt
- Количество слайдов: 15
 June 13, 2007 Performance of Chiron Quantitative and Qualitative HBV PCR Assay and Confirmation of HBV Yield Cases Yiu-Lian Fong, Ph. D, Associate Director
June 13, 2007 Performance of Chiron Quantitative and Qualitative HBV PCR Assay and Confirmation of HBV Yield Cases Yiu-Lian Fong, Ph. D, Associate Director
 Agenda A qualitative and quantitative HBV DNA assay has been developed and validated in house for sensitive detection and accurate quantification of HBV DNA for confirmatory purposes. § Assay Performance § Confirming HBV Yield Cases § Issues raised for standardized calibration of various genotypes 2 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)
Agenda A qualitative and quantitative HBV DNA assay has been developed and validated in house for sensitive detection and accurate quantification of HBV DNA for confirmatory purposes. § Assay Performance § Confirming HBV Yield Cases § Issues raised for standardized calibration of various genotypes 2 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)
 Assay Performance § Analytical Sensitivity and LOD for WHO HBV Standard § Analytical Sensitivities for DDL Genotypes A-G § Linearity, LLOQ, reproducibility and precision § Clinical Sensitivity § Analytical and Clinical Specificities 3 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)
Assay Performance § Analytical Sensitivity and LOD for WHO HBV Standard § Analytical Sensitivities for DDL Genotypes A-G § Linearity, LLOQ, reproducibility and precision § Clinical Sensitivity § Analytical and Clinical Specificities 3 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)
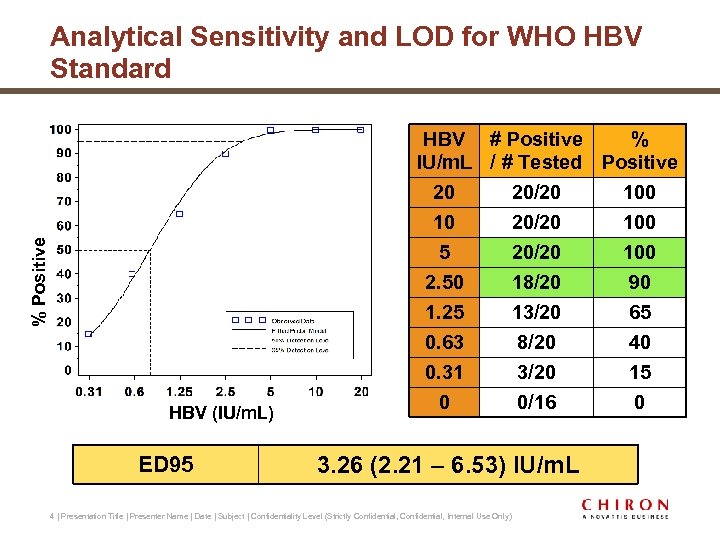 % Positive Analytical Sensitivity and LOD for WHO HBV Standard HBV (IU/m. L) ED 95 HBV # Positive % IU/m. L / # Tested Positive 20 20/20 100 5 20/20 100 2. 50 18/20 90 1. 25 13/20 65 0. 63 8/20 40 0. 31 3/20 15 0 0/16 0 3. 26 (2. 21 – 6. 53) IU/m. L 4 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)
% Positive Analytical Sensitivity and LOD for WHO HBV Standard HBV (IU/m. L) ED 95 HBV # Positive % IU/m. L / # Tested Positive 20 20/20 100 5 20/20 100 2. 50 18/20 90 1. 25 13/20 65 0. 63 8/20 40 0. 31 3/20 15 0 0/16 0 3. 26 (2. 21 – 6. 53) IU/m. L 4 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)
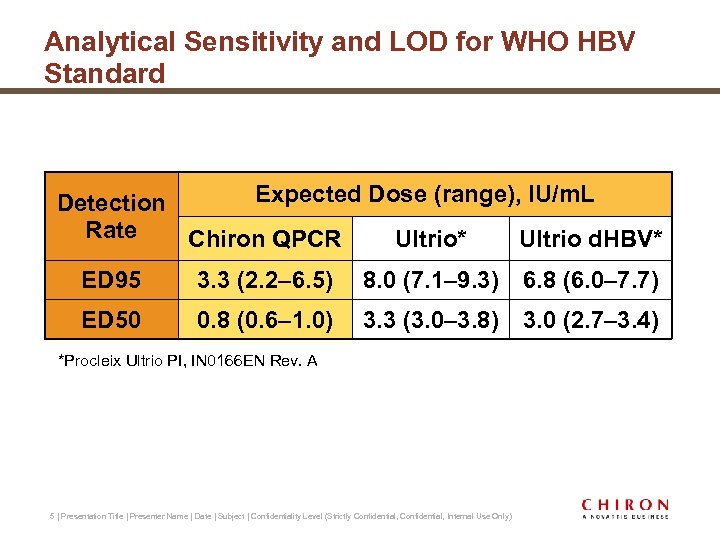 Analytical Sensitivity and LOD for WHO HBV Standard Expected Dose (range), IU/m. L Detection Rate Chiron QPCR Ultrio* Ultrio d. HBV* ED 95 3. 3 (2. 2– 6. 5) 8. 0 (7. 1– 9. 3) 6. 8 (6. 0– 7. 7) ED 50 0. 8 (0. 6– 1. 0) 3. 3 (3. 0– 3. 8) 3. 0 (2. 7– 3. 4) *Procleix Ultrio PI, IN 0166 EN Rev. A 5 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)
Analytical Sensitivity and LOD for WHO HBV Standard Expected Dose (range), IU/m. L Detection Rate Chiron QPCR Ultrio* Ultrio d. HBV* ED 95 3. 3 (2. 2– 6. 5) 8. 0 (7. 1– 9. 3) 6. 8 (6. 0– 7. 7) ED 50 0. 8 (0. 6– 1. 0) 3. 3 (3. 0– 3. 8) 3. 0 (2. 7– 3. 4) *Procleix Ultrio PI, IN 0166 EN Rev. A 5 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)
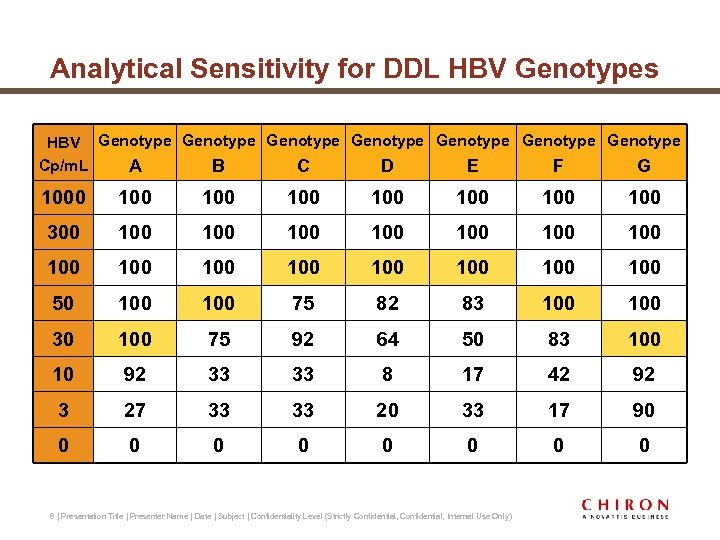 Analytical Sensitivity for DDL HBV Genotypes HBV Genotype Genotype Cp/m. L A B C D E F G 1000 100 100 300 100 100 100 100 50 100 75 82 83 100 30 100 75 92 64 50 83 100 10 92 33 33 8 17 42 92 3 27 33 33 20 33 17 90 0 0 0 0 6 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)
Analytical Sensitivity for DDL HBV Genotypes HBV Genotype Genotype Cp/m. L A B C D E F G 1000 100 100 300 100 100 100 100 50 100 75 82 83 100 30 100 75 92 64 50 83 100 10 92 33 33 8 17 42 92 3 27 33 33 20 33 17 90 0 0 0 0 6 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)
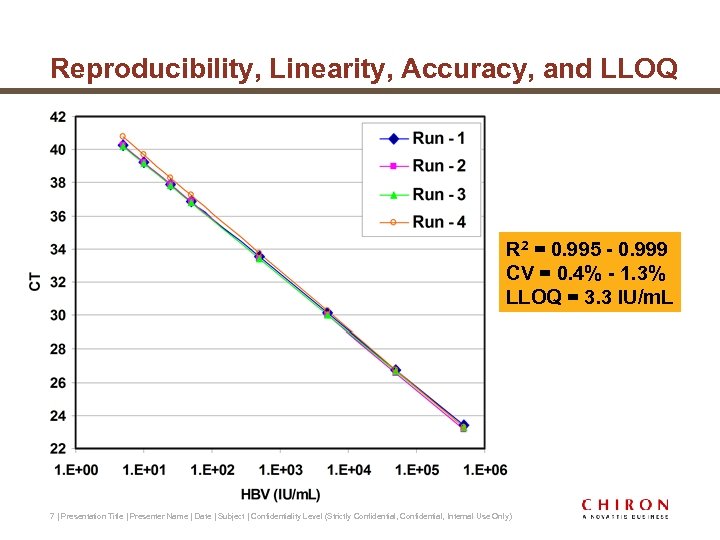 Reproducibility, Linearity, Accuracy, and LLOQ R 2 = 0. 995 - 0. 999 CV = 0. 4% - 1. 3% LLOQ = 3. 3 IU/m. L 7 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)
Reproducibility, Linearity, Accuracy, and LLOQ R 2 = 0. 995 - 0. 999 CV = 0. 4% - 1. 3% LLOQ = 3. 3 IU/m. L 7 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)
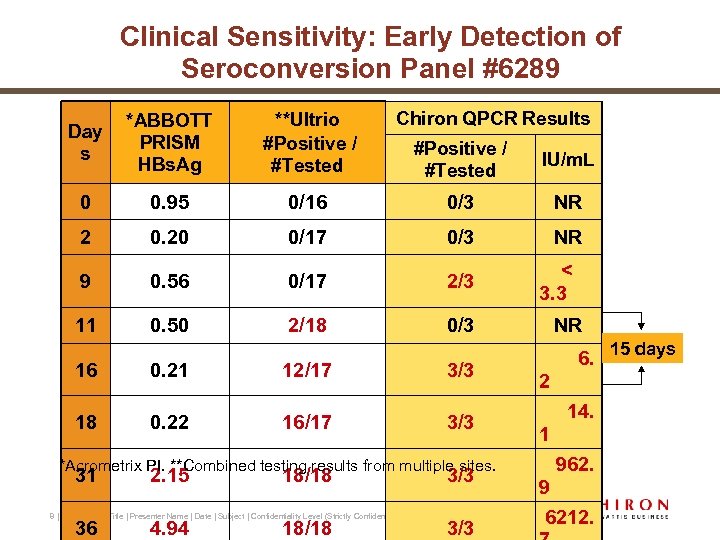 Clinical Sensitivity: Early Detection of Seroconversion Panel #6289 Day s *ABBOTT PRISM HBs. Ag **Ultrio #Positive / #Tested 0 0. 95 2 Chiron QPCR Results #Positive / #Tested IU/m. L 0/16 0/3 NR 0. 20 0/17 0/3 NR 9 0. 56 0/17 2/3 11 0. 50 2/18 0/3 16 0. 21 12/17 3/3 18 0. 22 16/17 3/3 *Acrometrix PI. **Combined testing results from multiple sites. 31 2. 15 18/18 3/3 8 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only) 36 4. 94 18/18 3/3 < 3. 3 NR 6. 2 14. 1 962. 9 6212. 15 days
Clinical Sensitivity: Early Detection of Seroconversion Panel #6289 Day s *ABBOTT PRISM HBs. Ag **Ultrio #Positive / #Tested 0 0. 95 2 Chiron QPCR Results #Positive / #Tested IU/m. L 0/16 0/3 NR 0. 20 0/17 0/3 NR 9 0. 56 0/17 2/3 11 0. 50 2/18 0/3 16 0. 21 12/17 3/3 18 0. 22 16/17 3/3 *Acrometrix PI. **Combined testing results from multiple sites. 31 2. 15 18/18 3/3 8 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only) 36 4. 94 18/18 3/3 < 3. 3 NR 6. 2 14. 1 962. 9 6212. 15 days
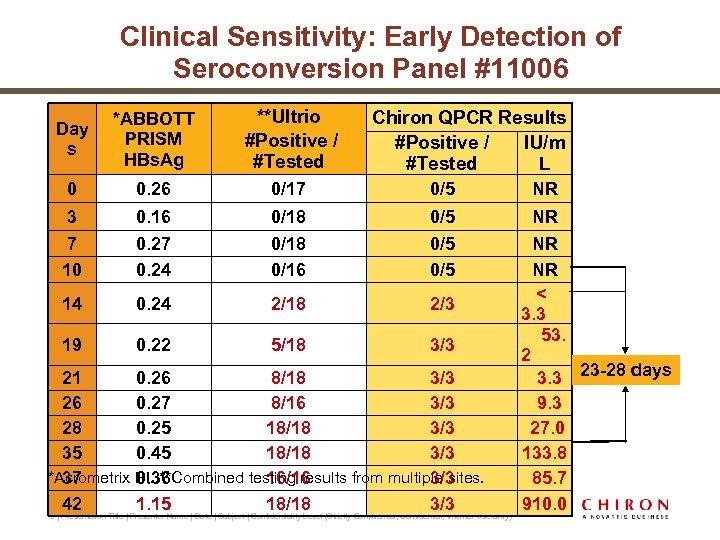 Clinical Sensitivity: Early Detection of Seroconversion Panel #11006 Day s *ABBOTT PRISM HBs. Ag **Ultrio #Positive / #Tested 0 0. 26 0/17 0/5 3 7 10 0. 16 0. 27 0. 24 0/18 0/16 0/5 0/5 14 0. 24 2/18 2/3 19 0. 22 5/18 3/3 Chiron QPCR Results #Positive / IU/m #Tested L 21 0. 26 8/18 3/3 26 0. 27 8/16 3/3 28 0. 25 18/18 3/3 35 0. 45 18/18 3/3 *Acrometrix PI. **Combined testing results from multiple sites. 37 0. 36 16/16 3/3 42 1. 15 18/18 3/3 9 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only) NR NR < 3. 3 53. 2 3. 3 23 -28 days 9. 3 27. 0 133. 8 85. 7 910. 0
Clinical Sensitivity: Early Detection of Seroconversion Panel #11006 Day s *ABBOTT PRISM HBs. Ag **Ultrio #Positive / #Tested 0 0. 26 0/17 0/5 3 7 10 0. 16 0. 27 0. 24 0/18 0/16 0/5 0/5 14 0. 24 2/18 2/3 19 0. 22 5/18 3/3 Chiron QPCR Results #Positive / IU/m #Tested L 21 0. 26 8/18 3/3 26 0. 27 8/16 3/3 28 0. 25 18/18 3/3 35 0. 45 18/18 3/3 *Acrometrix PI. **Combined testing results from multiple sites. 37 0. 36 16/16 3/3 42 1. 15 18/18 3/3 9 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only) NR NR < 3. 3 53. 2 3. 3 23 -28 days 9. 3 27. 0 133. 8 85. 7 910. 0
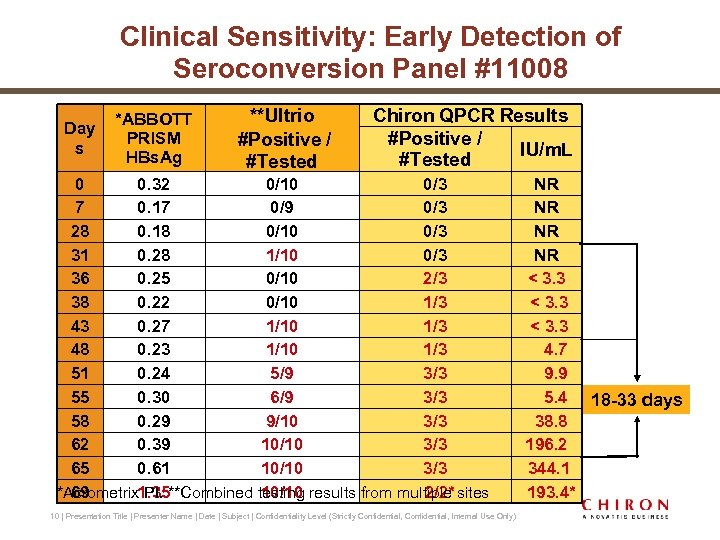 Clinical Sensitivity: Early Detection of Seroconversion Panel #11008 Day s *ABBOTT PRISM HBs. Ag **Ultrio #Positive / #Tested Chiron QPCR Results #Positive / IU/m. L #Tested 0 0. 32 0/10 0/3 7 0. 17 0/9 0/3 28 0. 18 0/10 0/3 31 0. 28 1/10 0/3 36 0. 25 0/10 2/3 38 0. 22 0/10 1/3 43 0. 27 1/10 1/3 48 0. 23 1/10 1/3 51 0. 24 5/9 3/3 55 0. 30 6/9 3/3 58 0. 29 9/10 3/3 62 0. 39 10/10 3/3 65 0. 61 10/10 3/3 69 10/10 2/2* *Acrometrix 1. 35**Combined testing results from multiple sites PI. 10 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only) NR NR < 3. 3 4. 7 9. 9 5. 4 18 -33 days 38. 8 196. 2 344. 1 193. 4*
Clinical Sensitivity: Early Detection of Seroconversion Panel #11008 Day s *ABBOTT PRISM HBs. Ag **Ultrio #Positive / #Tested Chiron QPCR Results #Positive / IU/m. L #Tested 0 0. 32 0/10 0/3 7 0. 17 0/9 0/3 28 0. 18 0/10 0/3 31 0. 28 1/10 0/3 36 0. 25 0/10 2/3 38 0. 22 0/10 1/3 43 0. 27 1/10 1/3 48 0. 23 1/10 1/3 51 0. 24 5/9 3/3 55 0. 30 6/9 3/3 58 0. 29 9/10 3/3 62 0. 39 10/10 3/3 65 0. 61 10/10 3/3 69 10/10 2/2* *Acrometrix 1. 35**Combined testing results from multiple sites PI. 10 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only) NR NR < 3. 3 4. 7 9. 9 5. 4 18 -33 days 38. 8 196. 2 344. 1 193. 4*
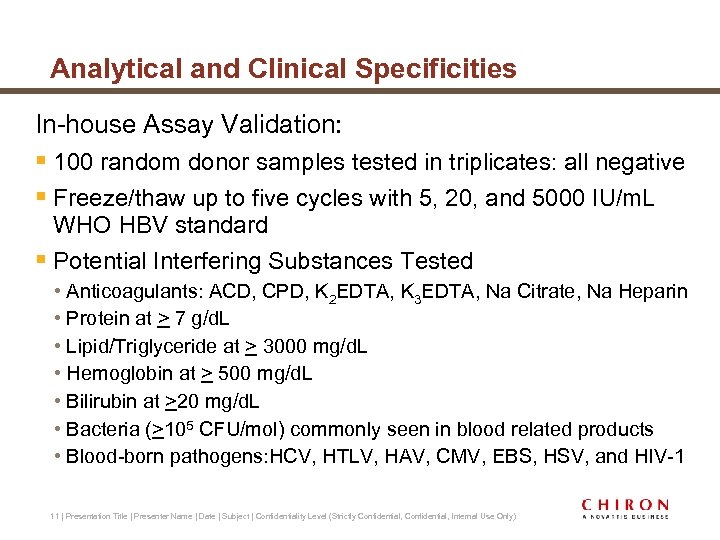 Analytical and Clinical Specificities In-house Assay Validation: § 100 random donor samples tested in triplicates: all negative § Freeze/thaw up to five cycles with 5, 20, and 5000 IU/m. L WHO HBV standard § Potential Interfering Substances Tested • Anticoagulants: ACD, CPD, K 2 EDTA, K 3 EDTA, Na Citrate, Na Heparin • Protein at > 7 g/d. L • Lipid/Triglyceride at > 3000 mg/d. L • Hemoglobin at > 500 mg/d. L • Bilirubin at >20 mg/d. L • Bacteria (>105 CFU/mol) commonly seen in blood related products • Blood-born pathogens: HCV, HTLV, HAV, CMV, EBS, HSV, and HIV-1 11 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)
Analytical and Clinical Specificities In-house Assay Validation: § 100 random donor samples tested in triplicates: all negative § Freeze/thaw up to five cycles with 5, 20, and 5000 IU/m. L WHO HBV standard § Potential Interfering Substances Tested • Anticoagulants: ACD, CPD, K 2 EDTA, K 3 EDTA, Na Citrate, Na Heparin • Protein at > 7 g/d. L • Lipid/Triglyceride at > 3000 mg/d. L • Hemoglobin at > 500 mg/d. L • Bilirubin at >20 mg/d. L • Bacteria (>105 CFU/mol) commonly seen in blood related products • Blood-born pathogens: HCV, HTLV, HAV, CMV, EBS, HSV, and HIV-1 11 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)
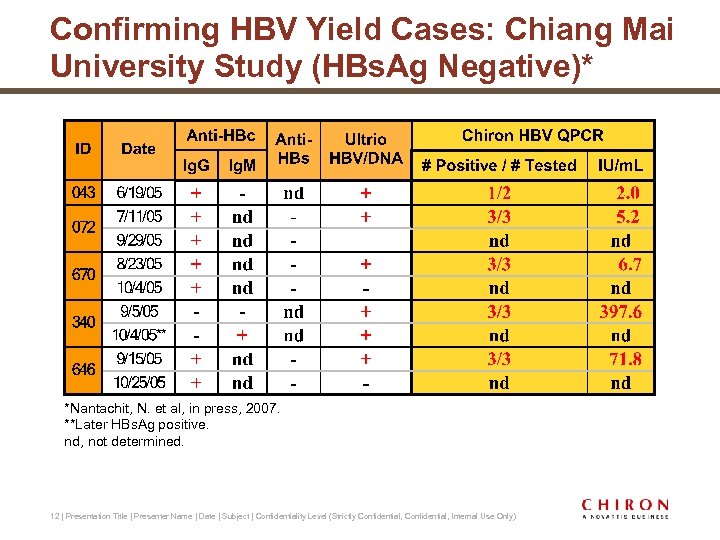 Confirming HBV Yield Cases: Chiang Mai University Study (HBs. Ag Negative)* *Nantachit, N. et al, in press, 2007. **Later HBs. Ag positive. nd, not determined. 12 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)
Confirming HBV Yield Cases: Chiang Mai University Study (HBs. Ag Negative)* *Nantachit, N. et al, in press, 2007. **Later HBs. Ag positive. nd, not determined. 12 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)
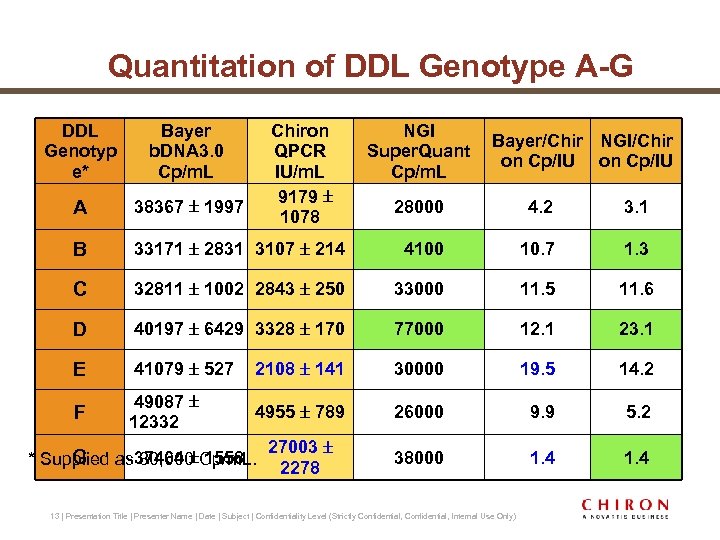 Quantitation of DDL Genotype A-G DDL Genotyp e* Bayer b. DNA 3. 0 Cp/m. L A 38367 1997 B Chiron QPCR IU/m. L 9179 1078 NGI Super. Quant Cp/m. L Bayer/Chir NGI/Chir on Cp/IU 28000 4. 2 3. 1 33171 2831 3107 214 4100 10. 7 1. 3 C 32811 1002 2843 250 33000 11. 5 11. 6 D 40197 6429 3328 170 77000 12. 1 23. 1 E 41079 527 2108 141 30000 19. 5 14. 2 F 49087 12332 4955 789 26000 9. 9 5. 2 27003 2278 38000 1. 4 G * Supplied as 37464 Cp/m. L. 30, 000 1558 13 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)
Quantitation of DDL Genotype A-G DDL Genotyp e* Bayer b. DNA 3. 0 Cp/m. L A 38367 1997 B Chiron QPCR IU/m. L 9179 1078 NGI Super. Quant Cp/m. L Bayer/Chir NGI/Chir on Cp/IU 28000 4. 2 3. 1 33171 2831 3107 214 4100 10. 7 1. 3 C 32811 1002 2843 250 33000 11. 5 11. 6 D 40197 6429 3328 170 77000 12. 1 23. 1 E 41079 527 2108 141 30000 19. 5 14. 2 F 49087 12332 4955 789 26000 9. 9 5. 2 27003 2278 38000 1. 4 G * Supplied as 37464 Cp/m. L. 30, 000 1558 13 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)
 Summary § A sensitive qualitative and quantitative HBV DNA assay has been developed and validated in house • LOD at 3. 3 IU/m. L for WHO HBV standard • Detects various genotypes • Detects window phase low viremia samples • Confirms yield cases § Conclusion: This highly sensitive and reliable assay is suitable for confirmatory purposes § Remaining Issues: • How to standardize? - How to calibrate various genotypes? - Which test method to use? 14 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)
Summary § A sensitive qualitative and quantitative HBV DNA assay has been developed and validated in house • LOD at 3. 3 IU/m. L for WHO HBV standard • Detects various genotypes • Detects window phase low viremia samples • Confirms yield cases § Conclusion: This highly sensitive and reliable assay is suitable for confirmatory purposes § Remaining Issues: • How to standardize? - How to calibrate various genotypes? - Which test method to use? 14 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)
 Acknowledgement § Dennis Madriaga § Baohe Shen § Barney Krebs 15 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)
Acknowledgement § Dennis Madriaga § Baohe Shen § Barney Krebs 15 | Presentation Title | Presenter Name | Date | Subject | Confidentiality Level (Strictly Confidential, Internal Use Only)


