e5ea687d496c545e56b3d7a54bbf0d89.ppt
- Количество слайдов: 17
Journal Club Plasma Renin Activity by Liquid Chromatography– Tandem Mass Spectrometry (LC-MS/MS): Development of a Prototypical Clinical Assay Reveals a Subpopulation of Human Plasma Samples with Substantial Peptidase Activity C. E. Bystrom, W. Salameh, R. Reitz, and N. J. Clarke October 2010 http: //www. clinchem. org/cgi/reprint/56/10/1561 © Copyright 2010 by the American Association for Clinical Chemistry © Copyright 2009 by the American Association for Clinical Chemistry
Introduction ØPlasma renin activity (PRA) is useful in diagnosis of secondary hypertension Traditionally determined via measurement of angiotensin (Ang) I using a broadly accepted RIA Accurate measurement of low activity is essential for diagnostic utility Narrow dynamic range of RIA is overcome via extended incubation of patient samples with low activity. Low activity samples are incubated for 18 h rather than 3 h © Copyright 2009 by the American Association for Clinical Chemistry
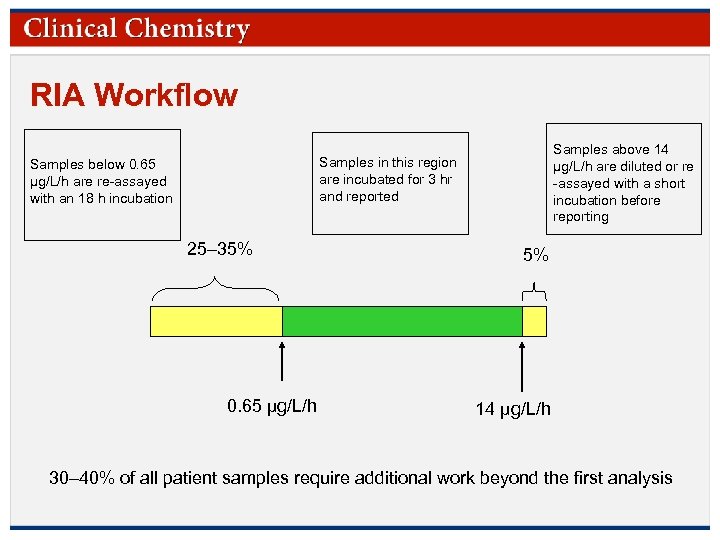
RIA Workflow Samples above 14 µg/L/h are diluted or re -assayed with a short incubation before reporting Samples in this region are incubated for 3 hr and reported Samples below 0. 65 µg/L/h are re-assayed with an 18 h incubation 25– 35% 0. 65 µg/L/h 5% 14 µg/L/h 30– 40% of all patient samples require additional work beyond the first analysis © Copyright 2009 by the American Association for Clinical Chemistry
Introduction (cont) ØAng I can be measured using mass spectrometry Extended incubation is not required due to the high sensitivity and wide dynamic range of the mass spectrometer Benefits in sample preparation improve throughput Improvements in precision may improve diagnostic utility © Copyright 2009 by the American Association for Clinical Chemistry
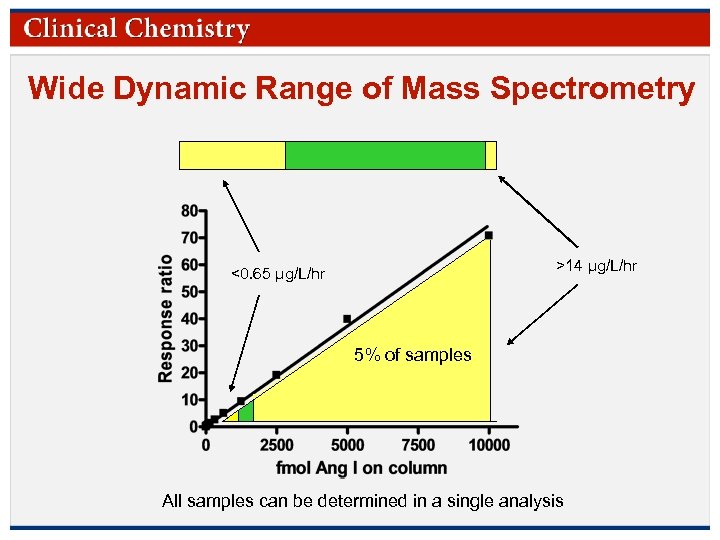
Wide Dynamic Range of Mass Spectrometry >14 µg/L/hr <0. 65 µg/L/hr 5% of samples All samples can be determined in a single analysis © Copyright 2009 by the American Association for Clinical Chemistry
Introduction (cont) ØA method for determination of PRA by mass spectrometry was validated Discordant results between 3 h LC-MS/MS and 18 h RIA results were observed with low frequency and suggested strong degradation activity Use of multiple, isotope labeled internal standards was used to identify samples with strong propensity for Ang I degradation. Mass spectrometry was used to characterize degradation products © Copyright 2009 by the American Association for Clinical Chemistry
Question Ø Why is Ang I degradation difficult to assess by RIA alone? © Copyright 2009 by the American Association for Clinical Chemistry
Materials and Methods Ø Thermo Quantum Ultra triple quadrupole Ø Cohesive automated HPLC system Ø Waters HLB online extraction cartridges Ø Waters X-bridge C 18 analytical columns Ø De-identified residual clinical samples Ø Freshly collected non-hypertensive control samples Ø Heavy isotope labeled peptides © Copyright 2009 by the American Association for Clinical Chemistry
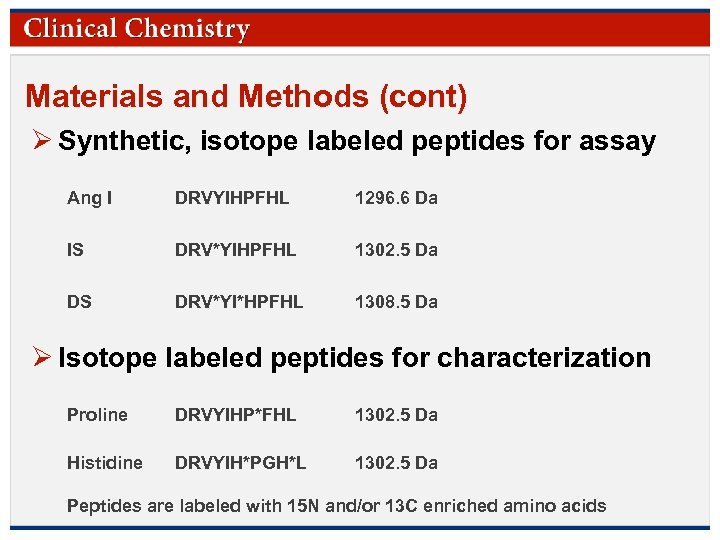
Materials and Methods (cont) Ø Synthetic, isotope labeled peptides for assay Ang I DRVYIHPFHL 1296. 6 Da IS DRV*YIHPFHL 1302. 5 Da DS DRV*YI*HPFHL 1308. 5 Da Ø Isotope labeled peptides for characterization Proline DRVYIHP*FHL 1302. 5 Da Histidine DRVYIH*PGH*L 1302. 5 Da Peptides are labeled with 15 N and/or 13 C enriched amino acids. Clinical Chemistry © Copyright 2009 by the American Association for
Question Ø What are the advantages of using isotopelabeled peptides in these types of experiments? © Copyright 2009 by the American Association for Clinical Chemistry
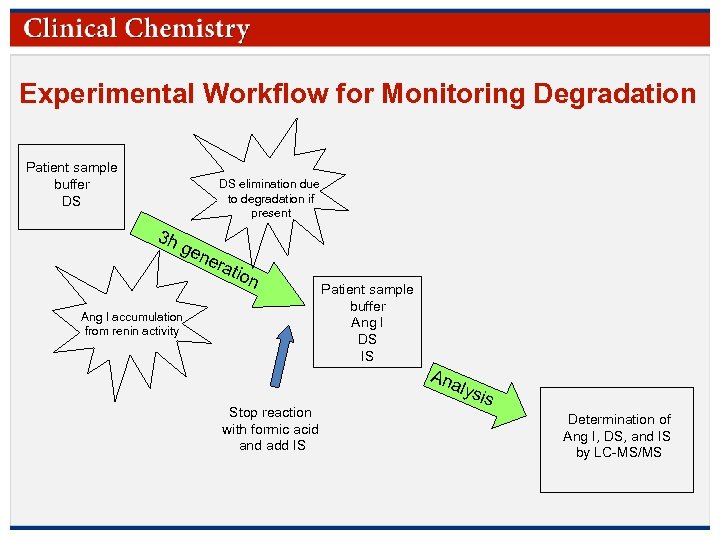
Experimental Workflow for Monitoring Degradation Patient sample buffer DS DS elimination due to degradation if present 3 h gen era tion Ang I accumulation from renin activity Patient sample buffer Ang I DS IS Ana Stop reaction with formic acid and add IS lysi s Determination of Ang I, DS, and IS by LC-MS/MS © Copyright 2009 by the American Association for Clinical Chemistry
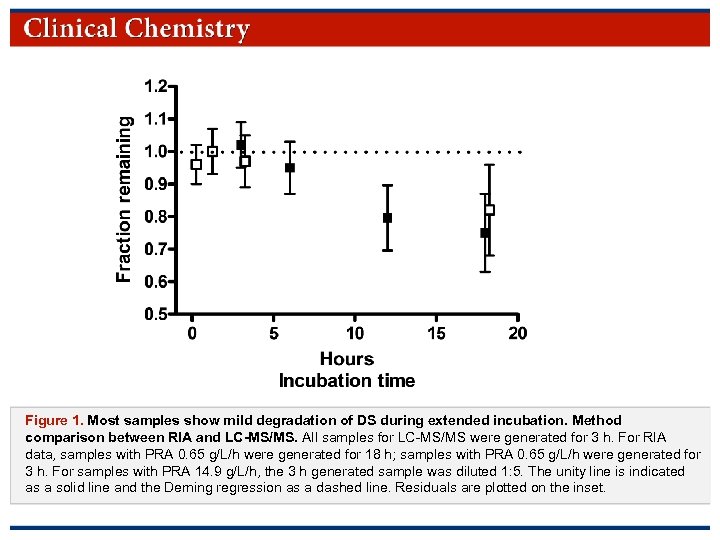
Figure 1. Most samples show mild degradation of DS during extended incubation. Method comparison between RIA and LC-MS/MS. All samples for LC-MS/MS were generated for 3 h. For RIA data, samples with PRA 0. 65 g/L/h were generated for 18 h; samples with PRA 0. 65 g/L/h were generated for 3 h. For samples with PRA 14. 9 g/L/h, the 3 h generated sample was diluted 1: 5. The unity line is indicated as a solid line and the Deming regression as a dashed line. Residuals are plotted on the inset. © Copyright 2009 by the American Association for Clinical Chemistry
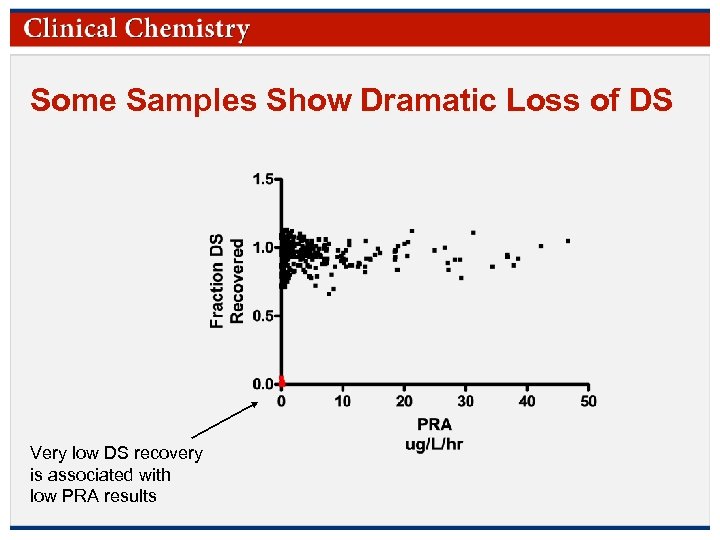
Some Samples Show Dramatic Loss of DS Very low DS recovery is associated with low PRA results © Copyright 2009 by the American Association for Clinical Chemistry
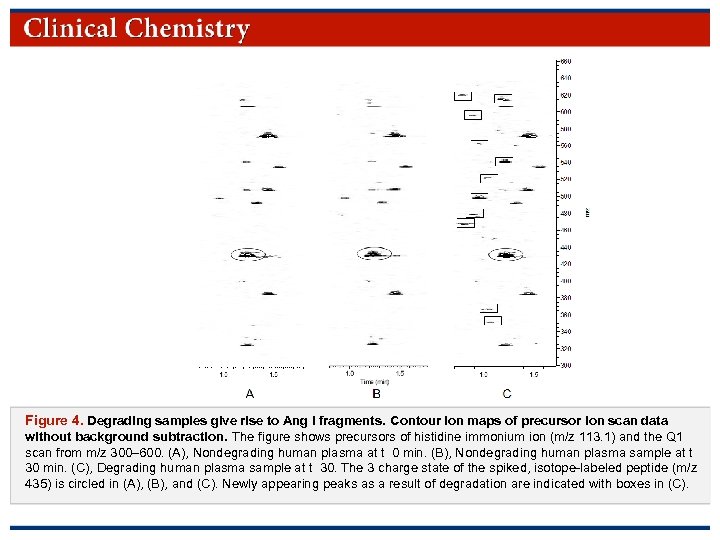
Figure 4. Degrading samples give rise to Ang I fragments. Contour ion maps of precursor ion scan data without background subtraction. The figure shows precursors of histidine immonium ion (m/z 113. 1) and the Q 1 scan from m/z 300– 600. (A), Nondegrading human plasma at t 0 min. (B), Nondegrading human plasma sample at t 30 min. (C), Degrading human plasma sample at t 30. The 3 charge state of the spiked, isotope-labeled peptide (m/z 435) is circled in (A), (B), and (C). Newly appearing peaks as a result of degradation are indicated with boxes in (C). © Copyright 2009 by the American Association for Clinical Chemistry
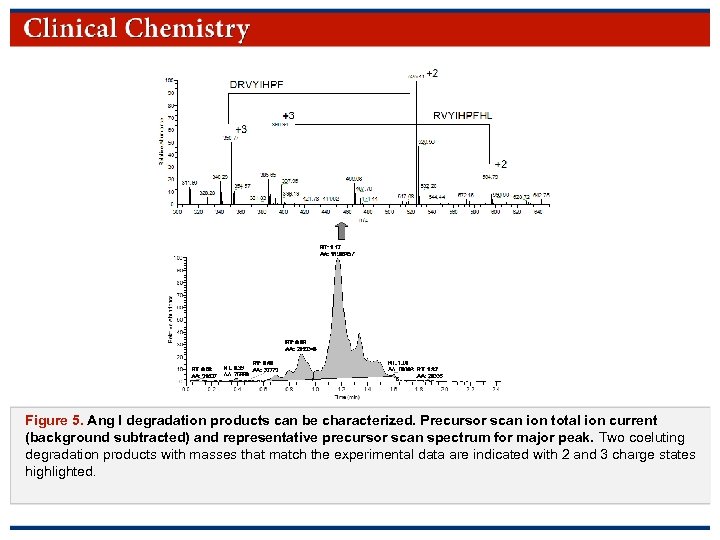
Figure 5. Ang I degradation products can be characterized. Precursor scan ion total ion current (background subtracted) and representative precursor scan spectrum for major peak. Two coeluting degradation products with masses that match the experimental data are indicated with 2 and 3 charge states highlighted. © Copyright 2009 by the American Association for Clinical Chemistry
Conclusions Ø Profound degradation of Ang I is observed in some patient samples Ø Degradation of Ang I may be a confounding factor in the interpretation of some PRA results Ø Use of multiple isotope-labeled peptides may be employed to monitor degradation concomitant with Ang I generation © Copyright 2009 by the American Association for Clinical Chemistry
Questions Ø What results would you expect if direct renin measurements were made in degrading samples? © Copyright 2009 by the American Association for Clinical Chemistry
e5ea687d496c545e56b3d7a54bbf0d89.ppt