99afa3850a9927f24eba18453abe6768.ppt
- Количество слайдов: 131
 Joann L. Data, MD, Ph. D Senior VP, Regulatory Affairs & Quality Assurance Amylin Pharmaceuticals, Inc. Overview P 1
Joann L. Data, MD, Ph. D Senior VP, Regulatory Affairs & Quality Assurance Amylin Pharmaceuticals, Inc. Overview P 1
 SYMLIN™ (Pramlintide Acetate) Amylin Pharmaceuticals, Inc. P 2
SYMLIN™ (Pramlintide Acetate) Amylin Pharmaceuticals, Inc. P 2
 SYMLIN Injection • Indication: Adjunctive therapy to insulin, to improve glycemic and metabolic control in people with type 1 or type 2 diabetes • Administration: Injected subcutaneously approximately 15 minutes prior to a meal • Presentations: Vials and cartridges P 3
SYMLIN Injection • Indication: Adjunctive therapy to insulin, to improve glycemic and metabolic control in people with type 1 or type 2 diabetes • Administration: Injected subcutaneously approximately 15 minutes prior to a meal • Presentations: Vials and cartridges P 3
 Amylin’s Presentation • Unmet Medical Need Kenneth Polonsky, MD Chairman, Department of Medicine Washington University • Pramlintide Pharmacology Andrew Young, MD, Ph. D VP Research Amylin Pharmaceuticals • Clinical Program Orville Kolterman, MD Senior VP Clinical Affairs Amylin Pharmaceuticals • Risk/Benefit Summary Alain Baron, MD VP Clinical Research Amylin Pharmaceuticals P 4
Amylin’s Presentation • Unmet Medical Need Kenneth Polonsky, MD Chairman, Department of Medicine Washington University • Pramlintide Pharmacology Andrew Young, MD, Ph. D VP Research Amylin Pharmaceuticals • Clinical Program Orville Kolterman, MD Senior VP Clinical Affairs Amylin Pharmaceuticals • Risk/Benefit Summary Alain Baron, MD VP Clinical Research Amylin Pharmaceuticals P 4
 Consultants • Hugh E. Black, DVM, Ph. D Toxicology Consultant Hugh E. Black and Associates, Inc. • Wayne Colburn, Ph. D Pharmacokinetics Consultant MDS Pharma Services • Kerry Hafner, Ph. D Statistical Consultant PRA International • Kenneth Polonsky, MD Clinical Consultant Washington University • Richard Dickey, MD Clinical Consultant Private Practice, Hickory, NC P 5
Consultants • Hugh E. Black, DVM, Ph. D Toxicology Consultant Hugh E. Black and Associates, Inc. • Wayne Colburn, Ph. D Pharmacokinetics Consultant MDS Pharma Services • Kerry Hafner, Ph. D Statistical Consultant PRA International • Kenneth Polonsky, MD Clinical Consultant Washington University • Richard Dickey, MD Clinical Consultant Private Practice, Hickory, NC P 5
 Kenneth Polonsky, MD Adolphus Busch Professor of Medicine Chairman, Department of Medicine Washington University School of Medicine Unmet Medical Need P 6
Kenneth Polonsky, MD Adolphus Busch Professor of Medicine Chairman, Department of Medicine Washington University School of Medicine Unmet Medical Need P 6
 A Century of Diabetes Care Type 1 H NP in Insulin therapy 1900 su lin s in og ul al s er in an th n n i a p ul m m s Hu In Pu y ap 1950 1920 2000 1950 2000 Type 2 Oral Agents Insulin therapy 1900 1920 P 7
A Century of Diabetes Care Type 1 H NP in Insulin therapy 1900 su lin s in og ul al s er in an th n n i a p ul m m s Hu In Pu y ap 1950 1920 2000 1950 2000 Type 2 Oral Agents Insulin therapy 1900 1920 P 7
 Insulin Therapy Necessary When -Cell Fails • Type 1 diabetes - -cell failure at outset – Insulin dependent • Type 2 diabetes - Gradual -cell deterioration – Diet and oral agents early stages of disease – Late-stage disease, insulin therapy necessary P 8
Insulin Therapy Necessary When -Cell Fails • Type 1 diabetes - -cell failure at outset – Insulin dependent • Type 2 diabetes - Gradual -cell deterioration – Diet and oral agents early stages of disease – Late-stage disease, insulin therapy necessary P 8
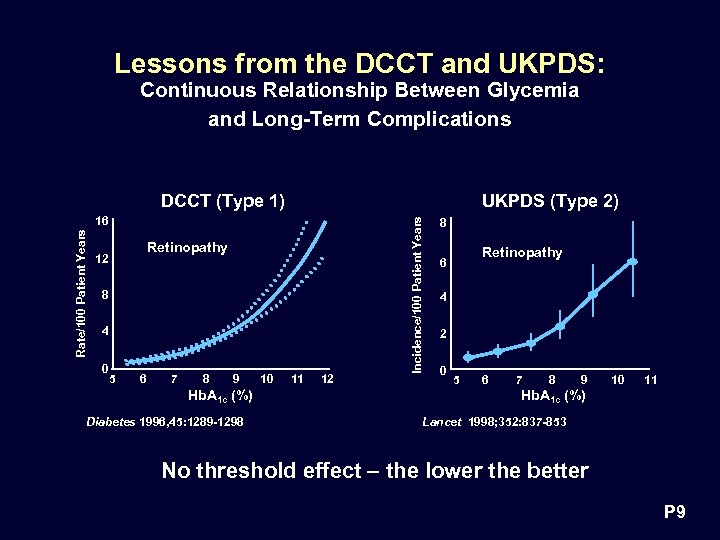 Lessons from the DCCT and UKPDS: Continuous Relationship Between Glycemia and Long-Term Complications DCCT (Type 1) Rate/100 Patient Years 16 Retinopathy 12 8 4 0 5 6 7 8 9 Hb. A 1 c (%) Diabetes 1996, 45: 1289 -1298 10 11 12 Incidence/100 Patient Years UKPDS (Type 2) 8 Retinopathy 6 4 2 0 5 6 7 8 9 10 11 Hb. A 1 c (%) Lancet 1998; 352: 837 -853 No threshold effect – the lower the better P 9
Lessons from the DCCT and UKPDS: Continuous Relationship Between Glycemia and Long-Term Complications DCCT (Type 1) Rate/100 Patient Years 16 Retinopathy 12 8 4 0 5 6 7 8 9 Hb. A 1 c (%) Diabetes 1996, 45: 1289 -1298 10 11 12 Incidence/100 Patient Years UKPDS (Type 2) 8 Retinopathy 6 4 2 0 5 6 7 8 9 10 11 Hb. A 1 c (%) Lancet 1998; 352: 837 -853 No threshold effect – the lower the better P 9
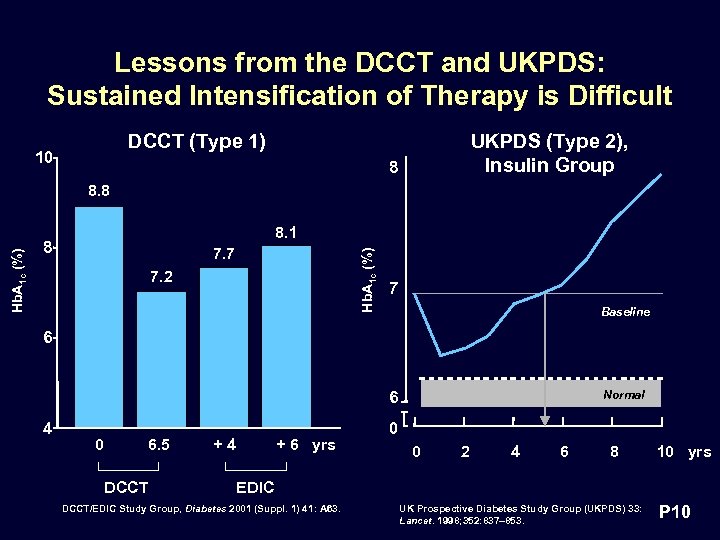 Lessons from the DCCT and UKPDS: Sustained Intensification of Therapy is Difficult DCCT (Type 1) 10 UKPDS (Type 2), Insulin Group 8 8. 1 8 7. 7 Hb. A 1 c (%) 8. 8 7. 2 7 Baseline 6 6 4 0 6. 5 DCCT +4 + 6 yrs Normal 0 0 2 4 6 8 10 yrs EDIC DCCT/EDIC Study Group, Diabetes 2001 (Suppl. 1) 41: A 63. UK Prospective Diabetes Study Group (UKPDS) 33: Lancet. 1998; 352: 837– 853. P 10
Lessons from the DCCT and UKPDS: Sustained Intensification of Therapy is Difficult DCCT (Type 1) 10 UKPDS (Type 2), Insulin Group 8 8. 1 8 7. 7 Hb. A 1 c (%) 8. 8 7. 2 7 Baseline 6 6 4 0 6. 5 DCCT +4 + 6 yrs Normal 0 0 2 4 6 8 10 yrs EDIC DCCT/EDIC Study Group, Diabetes 2001 (Suppl. 1) 41: A 63. UK Prospective Diabetes Study Group (UKPDS) 33: Lancet. 1998; 352: 837– 853. P 10
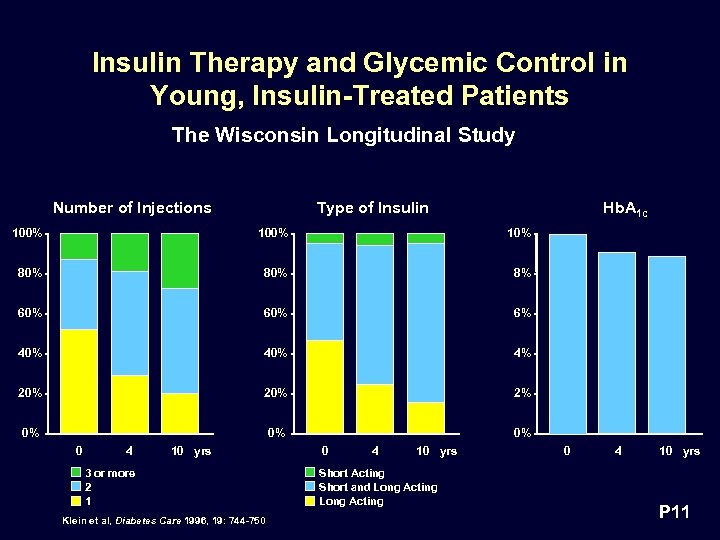 Insulin Therapy and Glycemic Control in Young, Insulin-Treated Patients The Wisconsin Longitudinal Study Number of Injections Type of Insulin Hb. A 1 c 100% 10% 80% 8% 60% 6% 40% 4% 20% 2% 0% 0 4 10 yrs 3 or more 2 1 Klein et al, Diabetes Care 1996, 19: 744 -750 0 4 10 yrs Short Acting Short and Long Acting 0 4 10 yrs P 11
Insulin Therapy and Glycemic Control in Young, Insulin-Treated Patients The Wisconsin Longitudinal Study Number of Injections Type of Insulin Hb. A 1 c 100% 10% 80% 8% 60% 6% 40% 4% 20% 2% 0% 0 4 10 yrs 3 or more 2 1 Klein et al, Diabetes Care 1996, 19: 744 -750 0 4 10 yrs Short Acting Short and Long Acting 0 4 10 yrs P 11
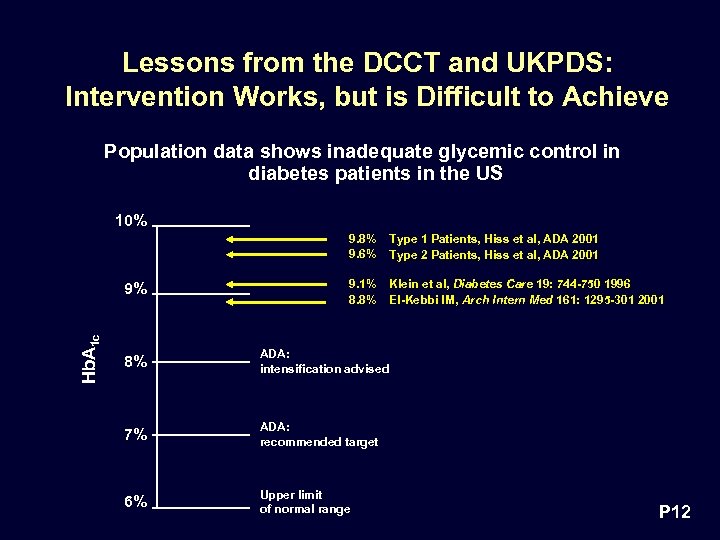 Lessons from the DCCT and UKPDS: Intervention Works, but is Difficult to Achieve Population data shows inadequate glycemic control in diabetes patients in the US 10% 9. 8% 9. 6% Hb. A 1 c 9% Type 1 Patients, Hiss et al, ADA 2001 Type 2 Patients, Hiss et al, ADA 2001 9. 1% 8. 8% Klein et al, Diabetes Care 19: 744 -750 1996 El-Kebbi IM, Arch Intern Med 161: 1295 -301 2001 8% ADA: intensification advised 7% ADA: recommended target 6% Upper limit of normal range P 12
Lessons from the DCCT and UKPDS: Intervention Works, but is Difficult to Achieve Population data shows inadequate glycemic control in diabetes patients in the US 10% 9. 8% 9. 6% Hb. A 1 c 9% Type 1 Patients, Hiss et al, ADA 2001 Type 2 Patients, Hiss et al, ADA 2001 9. 1% 8. 8% Klein et al, Diabetes Care 19: 744 -750 1996 El-Kebbi IM, Arch Intern Med 161: 1295 -301 2001 8% ADA: intensification advised 7% ADA: recommended target 6% Upper limit of normal range P 12
 Our Ability to Achieve Tight Glycemic Control with Insulin Therapy is Limited by: • Hypoglycemia • Weight Gain • Postprandial hyperglycemia P 13
Our Ability to Achieve Tight Glycemic Control with Insulin Therapy is Limited by: • Hypoglycemia • Weight Gain • Postprandial hyperglycemia P 13
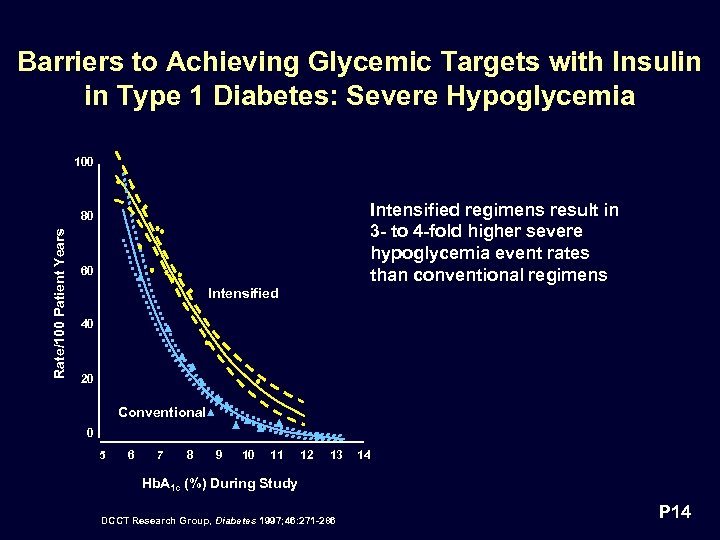 Barriers to Achieving Glycemic Targets with Insulin in Type 1 Diabetes: Severe Hypoglycemia 100 Intensified regimens result in 3 - to 4 -fold higher severe hypoglycemia event rates than conventional regimens Rate/100 Patient Years 80 60 Intensified 40 20 Conventional 0 5 6 7 8 9 10 11 12 13 14 Hb. A 1 c (%) During Study DCCT Research Group, Diabetes 1997; 46: 271 -286 P 14
Barriers to Achieving Glycemic Targets with Insulin in Type 1 Diabetes: Severe Hypoglycemia 100 Intensified regimens result in 3 - to 4 -fold higher severe hypoglycemia event rates than conventional regimens Rate/100 Patient Years 80 60 Intensified 40 20 Conventional 0 5 6 7 8 9 10 11 12 13 14 Hb. A 1 c (%) During Study DCCT Research Group, Diabetes 1997; 46: 271 -286 P 14
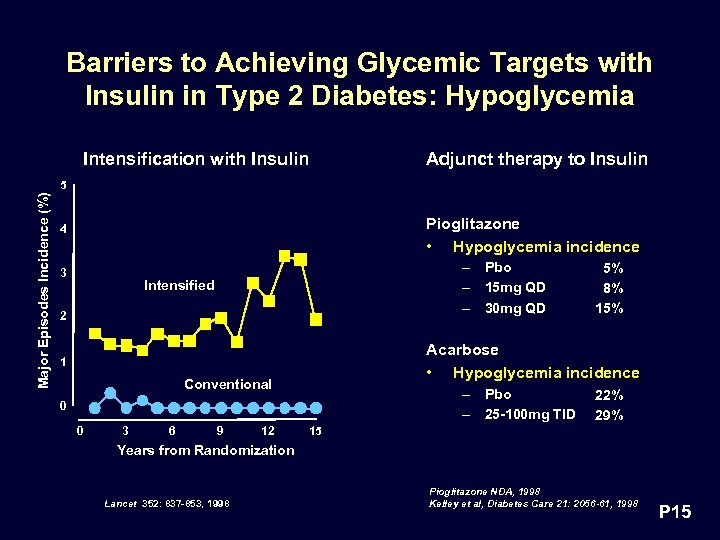 Barriers to Achieving Glycemic Targets with Insulin in Type 2 Diabetes: Hypoglycemia Intensification with Insulin Adjunct therapy to Insulin Major Episodes Incidence (%) 5 Pioglitazone • Hypoglycemia incidence 4 3 – Pbo – 15 mg QD – 30 mg QD Intensified 2 Acarbose • Hypoglycemia incidence 1 Conventional – Pbo – 25 -100 mg TID 0 0 3 6 9 12 5% 8% 15% 22% 29% 15 Years from Randomization Lancet 352: 837 -853, 1998 Pioglitazone NDA, 1998 Kelley et al, Diabetes Care 21: 2056 -61, 1998 P 15
Barriers to Achieving Glycemic Targets with Insulin in Type 2 Diabetes: Hypoglycemia Intensification with Insulin Adjunct therapy to Insulin Major Episodes Incidence (%) 5 Pioglitazone • Hypoglycemia incidence 4 3 – Pbo – 15 mg QD – 30 mg QD Intensified 2 Acarbose • Hypoglycemia incidence 1 Conventional – Pbo – 25 -100 mg TID 0 0 3 6 9 12 5% 8% 15% 22% 29% 15 Years from Randomization Lancet 352: 837 -853, 1998 Pioglitazone NDA, 1998 Kelley et al, Diabetes Care 21: 2056 -61, 1998 P 15
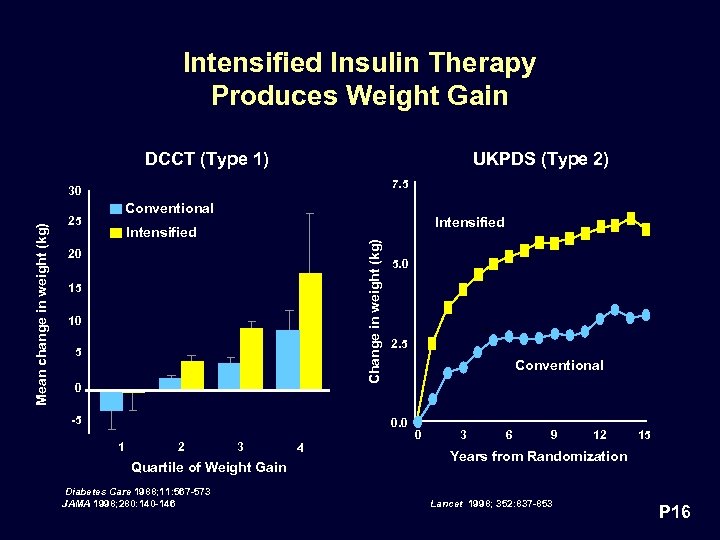 Intensified Insulin Therapy Produces Weight Gain DCCT (Type 1) UKPDS (Type 2) 7. 5 Conventional 25 Intensified Change in weight (kg) Mean change in weight (kg) 30 20 15 10 5 0 -5 5. 0 2. 5 Conventional 0. 0 1 2 3 Quartile of Weight Gain Diabetes Care 1988; 11: 567 -573 JAMA 1998; 280: 140 -146 4 0 3 6 9 12 15 Years from Randomization Lancet 1998; 352: 837 -853 P 16
Intensified Insulin Therapy Produces Weight Gain DCCT (Type 1) UKPDS (Type 2) 7. 5 Conventional 25 Intensified Change in weight (kg) Mean change in weight (kg) 30 20 15 10 5 0 -5 5. 0 2. 5 Conventional 0. 0 1 2 3 Quartile of Weight Gain Diabetes Care 1988; 11: 567 -573 JAMA 1998; 280: 140 -146 4 0 3 6 9 12 15 Years from Randomization Lancet 1998; 352: 837 -853 P 16
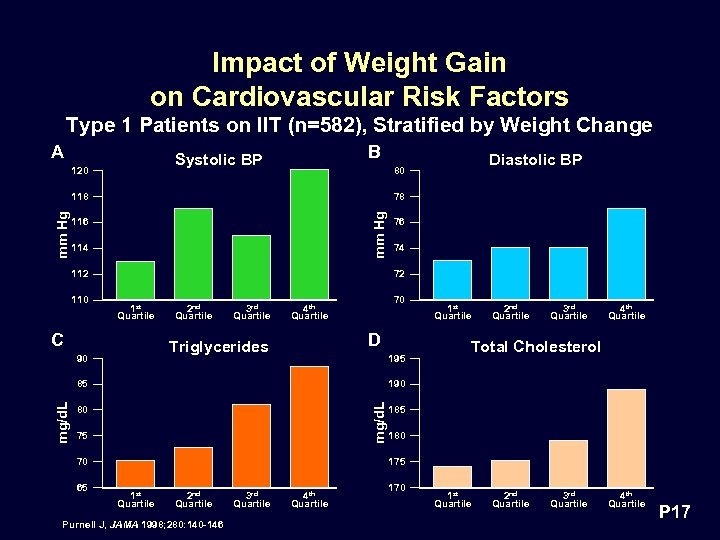 Impact of Weight Gain on Cardiovascular Risk Factors Type 1 Patients on IIT (n=582), Stratified by Weight Change A B Systolic BP 120 78 mm Hg 118 116 114 112 110 1 st Quartile 2 nd Quartile 3 rd Quartile 74 70 4 th Quartile D Triglycerides 90 195 1 st Quartile 2 nd Quartile 3 rd Quartile 4 th Quartile Total Cholesterol 190 mg/d. L 85 mg/d. L 76 72 C 80 75 70 65 Diastolic BP 80 185 180 175 1 st Quartile 2 nd Quartile Purnell J, JAMA 1998; 280: 140 -146 3 rd Quartile 4 th Quartile 170 1 st Quartile 2 nd Quartile 3 rd Quartile 4 th Quartile P 17
Impact of Weight Gain on Cardiovascular Risk Factors Type 1 Patients on IIT (n=582), Stratified by Weight Change A B Systolic BP 120 78 mm Hg 118 116 114 112 110 1 st Quartile 2 nd Quartile 3 rd Quartile 74 70 4 th Quartile D Triglycerides 90 195 1 st Quartile 2 nd Quartile 3 rd Quartile 4 th Quartile Total Cholesterol 190 mg/d. L 85 mg/d. L 76 72 C 80 75 70 65 Diastolic BP 80 185 180 175 1 st Quartile 2 nd Quartile Purnell J, JAMA 1998; 280: 140 -146 3 rd Quartile 4 th Quartile 170 1 st Quartile 2 nd Quartile 3 rd Quartile 4 th Quartile P 17
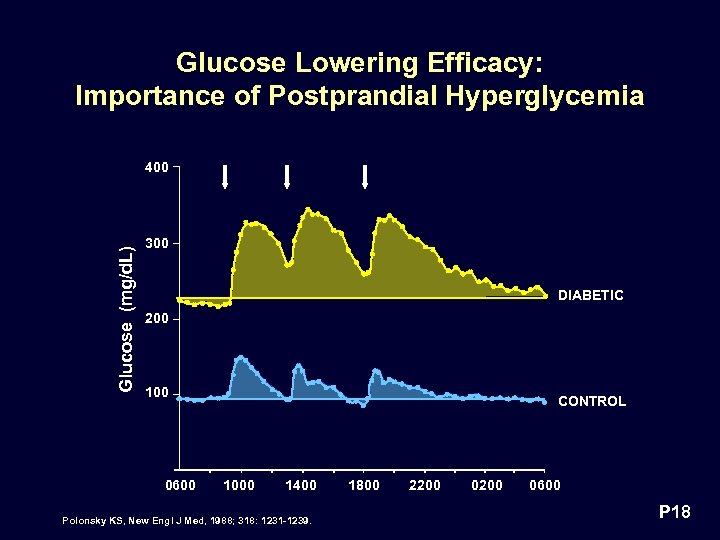 Glucose Lowering Efficacy: Importance of Postprandial Hyperglycemia Glucose (mg/d. L) 400 300 DIABETIC 200 100 0600 CONTROL 1000 1400 Polonsky KS, New Engl J Med, 1988; 318: 1231 -1239. 1800 2200 0600 P 18
Glucose Lowering Efficacy: Importance of Postprandial Hyperglycemia Glucose (mg/d. L) 400 300 DIABETIC 200 100 0600 CONTROL 1000 1400 Polonsky KS, New Engl J Med, 1988; 318: 1231 -1239. 1800 2200 0600 P 18
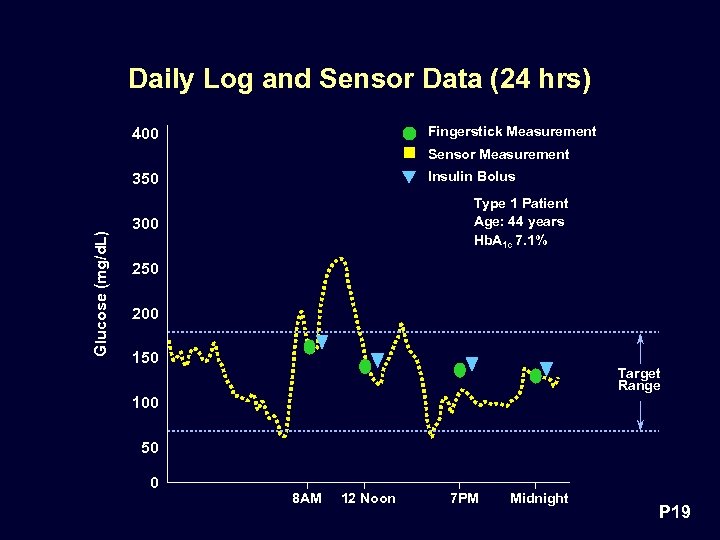 Daily Log and Sensor Data (24 hrs) Fingerstick Measurement 400 Sensor Measurement Insulin Bolus Glucose (mg/d. L) 350 Type 1 Patient Age: 44 years Hb. A 1 c 7. 1% 300 250 200 150 Target Range 100 50 0 8 AM 12 Noon 7 PM Midnight P 19
Daily Log and Sensor Data (24 hrs) Fingerstick Measurement 400 Sensor Measurement Insulin Bolus Glucose (mg/d. L) 350 Type 1 Patient Age: 44 years Hb. A 1 c 7. 1% 300 250 200 150 Target Range 100 50 0 8 AM 12 Noon 7 PM Midnight P 19
 Current Opportunity to Achieve Glycemic Goals • Control Postprandial Glucose • Without Weight Gain • Without Increasing Hypoglycemia P 20
Current Opportunity to Achieve Glycemic Goals • Control Postprandial Glucose • Without Weight Gain • Without Increasing Hypoglycemia P 20
 Andrew Young, MD Ph. D Vice President, Research Amylin Pharmaceuticals, Inc. Pramlintide Pharmacology P 21
Andrew Young, MD Ph. D Vice President, Research Amylin Pharmaceuticals, Inc. Pramlintide Pharmacology P 21
 Pramlintide Pharmacology • Comparison of amylin and pramlintide molecules • Insulin, glucagon and amylin abnormalities in diabetes • Glucose fluxes controlled by amylin/pramlintide • “Glucose-dependence” of amylin/pramlintide action ~ 1700 scientific communications P 22
Pramlintide Pharmacology • Comparison of amylin and pramlintide molecules • Insulin, glucagon and amylin abnormalities in diabetes • Glucose fluxes controlled by amylin/pramlintide • “Glucose-dependence” of amylin/pramlintide action ~ 1700 scientific communications P 22
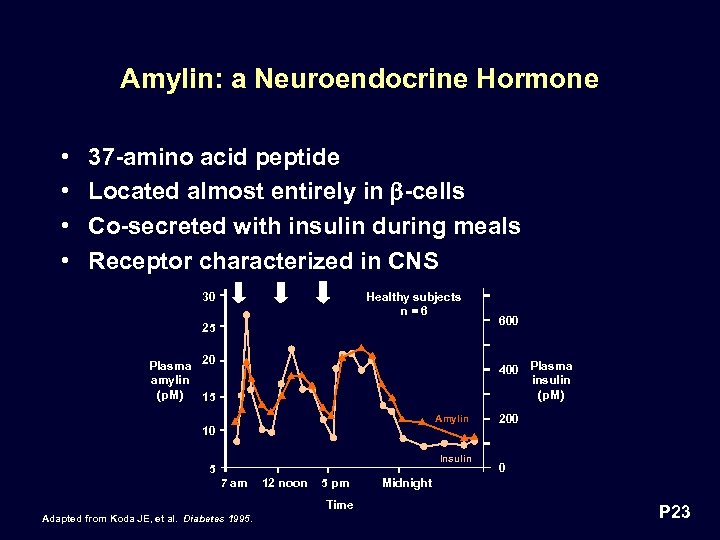 Amylin: a Neuroendocrine Hormone • • 37 -amino acid peptide Located almost entirely in -cells Co-secreted with insulin during meals Receptor characterized in CNS 30 Healthy subjects n=6 25 Plasma 20 amylin (p. M) 15 600 400 Plasma insulin (p. M) Amylin 10 Insulin 5 7 am 12 noon 5 pm Time Adapted from Koda JE, et al. Diabetes 1995. 200 0 Midnight P 23
Amylin: a Neuroendocrine Hormone • • 37 -amino acid peptide Located almost entirely in -cells Co-secreted with insulin during meals Receptor characterized in CNS 30 Healthy subjects n=6 25 Plasma 20 amylin (p. M) 15 600 400 Plasma insulin (p. M) Amylin 10 Insulin 5 7 am 12 noon 5 pm Time Adapted from Koda JE, et al. Diabetes 1995. 200 0 Midnight P 23
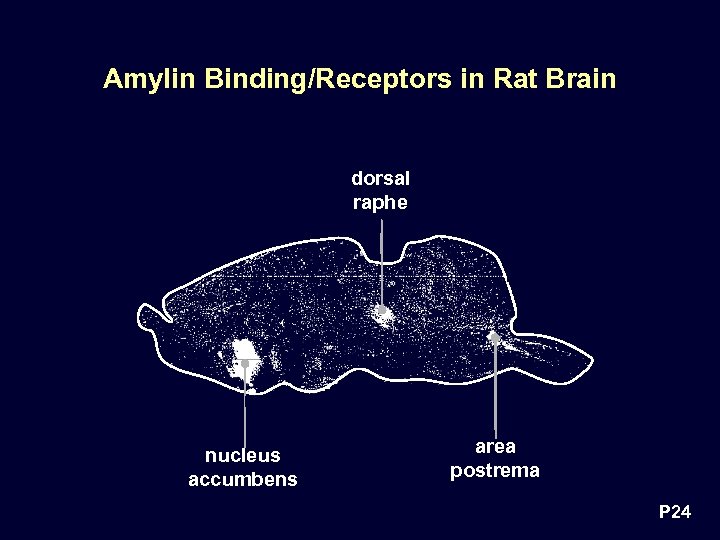 Amylin Binding/Receptors in Rat Brain dorsal raphe nucleus accumbens area postrema P 24
Amylin Binding/Receptors in Rat Brain dorsal raphe nucleus accumbens area postrema P 24
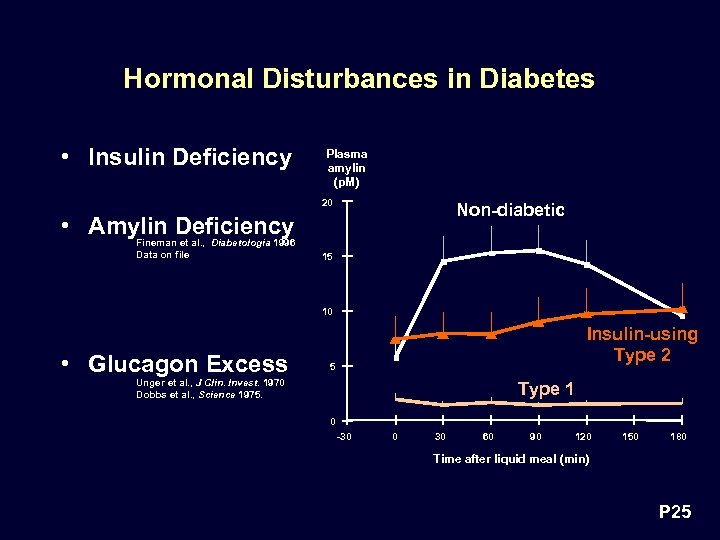 Hormonal Disturbances in Diabetes • Insulin Deficiency Plasma amylin (p. M) 20 Non-diabetic • Amylin Deficiency Fineman et al. , Diabetologia 1996 Data on file 15 10 • Glucagon Excess Insulin-using Type 2 5 Unger et al. , J Clin. Invest. 1970 Dobbs et al. , Science 1975. Type 1 0 -30 0 30 60 90 120 150 180 Time after liquid meal (min) P 25
Hormonal Disturbances in Diabetes • Insulin Deficiency Plasma amylin (p. M) 20 Non-diabetic • Amylin Deficiency Fineman et al. , Diabetologia 1996 Data on file 15 10 • Glucagon Excess Insulin-using Type 2 5 Unger et al. , J Clin. Invest. 1970 Dobbs et al. , Science 1975. Type 1 0 -30 0 30 60 90 120 150 180 Time after liquid meal (min) P 25
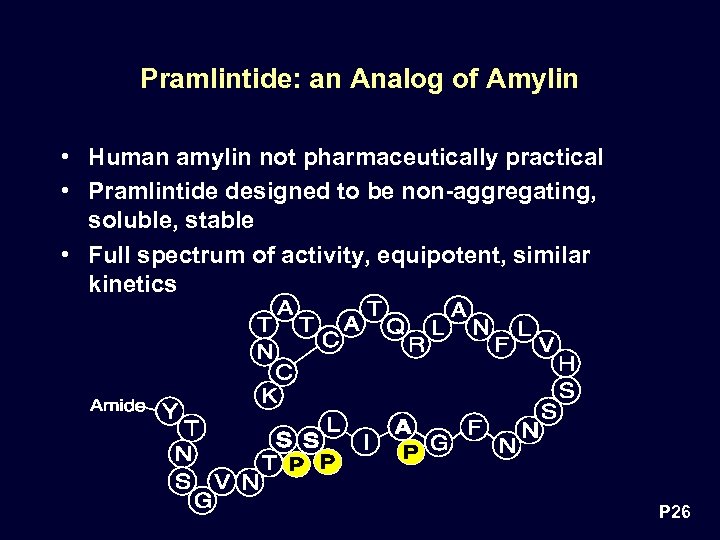 Pramlintide: an Analog of Amylin • Human amylin not pharmaceutically practical • Pramlintide designed to be non-aggregating, soluble, stable • Full spectrum of activity, equipotent, similar kinetics P 26
Pramlintide: an Analog of Amylin • Human amylin not pharmaceutically practical • Pramlintide designed to be non-aggregating, soluble, stable • Full spectrum of activity, equipotent, similar kinetics P 26
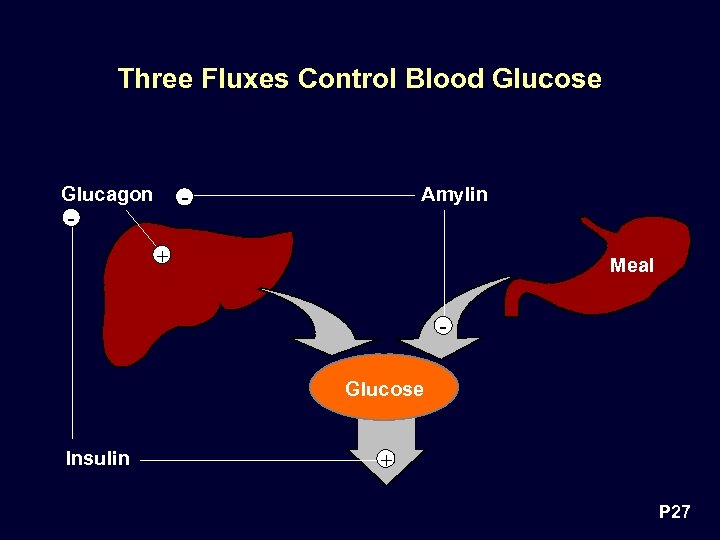 Three Fluxes Control Blood Glucose Glucagon Amylin - - + Meal Glucose Insulin + P 27
Three Fluxes Control Blood Glucose Glucagon Amylin - - + Meal Glucose Insulin + P 27
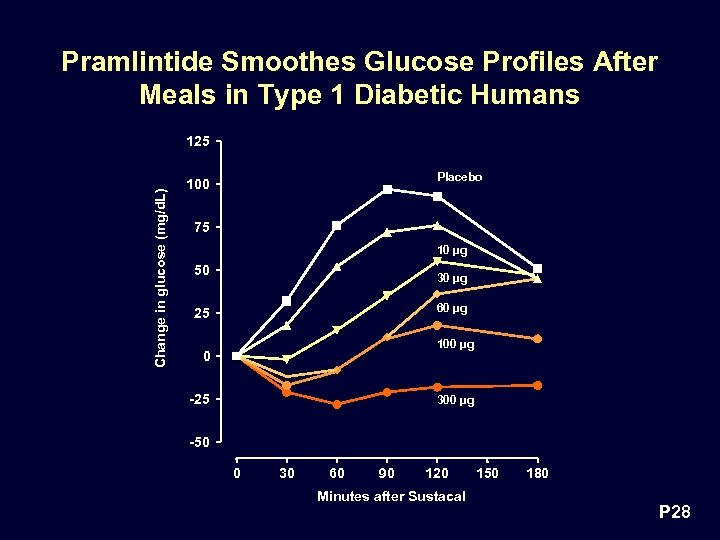 Pramlintide Smoothes Glucose Profiles After Meals in Type 1 Diabetic Humans Change in glucose (mg/d. L) 125 Placebo 100 75 10 µg 50 30 µg 60 µg 25 100 µg 0 -25 300 µg -50 0 30 60 90 120 Minutes after Sustacal 150 180 P 28
Pramlintide Smoothes Glucose Profiles After Meals in Type 1 Diabetic Humans Change in glucose (mg/d. L) 125 Placebo 100 75 10 µg 50 30 µg 60 µg 25 100 µg 0 -25 300 µg -50 0 30 60 90 120 Minutes after Sustacal 150 180 P 28
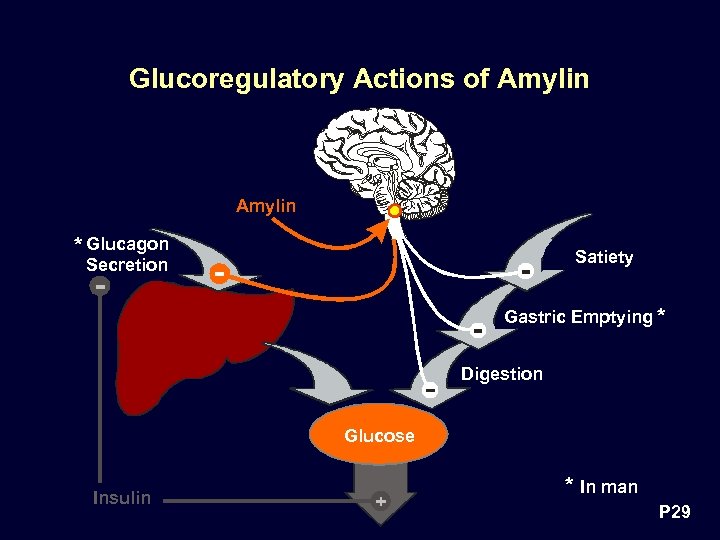 Glucoregulatory Actions of Amylin * Glucagon Satiety Secretion Gastric Emptying * Digestion Glucose Insulin * In man P 29
Glucoregulatory Actions of Amylin * Glucagon Satiety Secretion Gastric Emptying * Digestion Glucose Insulin * In man P 29
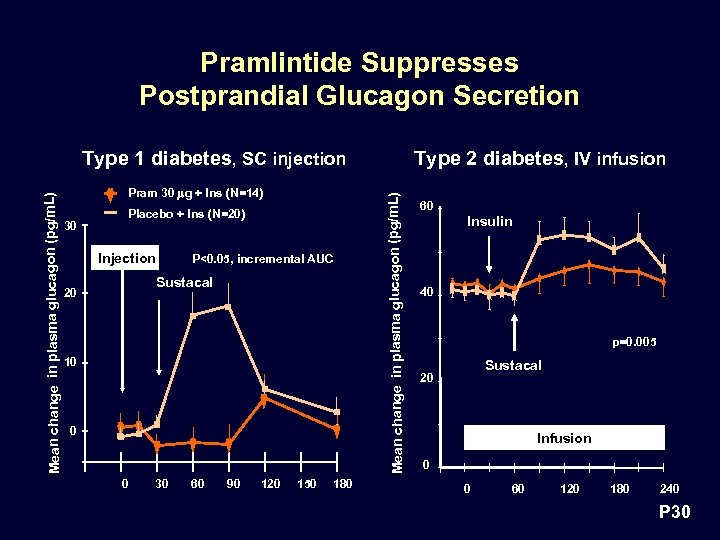 Pramlintide Suppresses Postprandial Glucagon Secretion Pram 30 mg + Ins (N=14) 30 Placebo + Ins (N=20) Injection P<0. 05, incremental AUC Sustacal 20 10 0 0 30 60 90 120 150 180 Type 2 diabetes, IV infusion Mean change in plasma glucagon (pg/m. L) Type 1 diabetes, SC injection 60 Insulin 40 p=0. 005 Sustacal 20 Infusion 0 0 60 120 180 240 P 30
Pramlintide Suppresses Postprandial Glucagon Secretion Pram 30 mg + Ins (N=14) 30 Placebo + Ins (N=20) Injection P<0. 05, incremental AUC Sustacal 20 10 0 0 30 60 90 120 150 180 Type 2 diabetes, IV infusion Mean change in plasma glucagon (pg/m. L) Type 1 diabetes, SC injection 60 Insulin 40 p=0. 005 Sustacal 20 Infusion 0 0 60 120 180 240 P 30
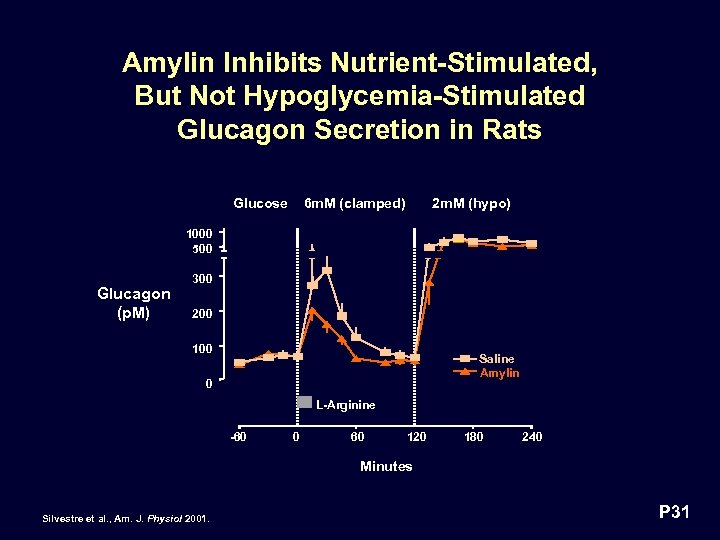 Amylin Inhibits Nutrient-Stimulated, But Not Hypoglycemia-Stimulated Glucagon Secretion in Rats Glucose 6 m. M (clamped) 2 m. M (hypo) 1000 500 Glucagon (p. M) 300 200 100 Saline Amylin 0 L-Arginine -60 0 60 120 180 240 Minutes Silvestre et al. , Am. J. Physiol 2001. P 31
Amylin Inhibits Nutrient-Stimulated, But Not Hypoglycemia-Stimulated Glucagon Secretion in Rats Glucose 6 m. M (clamped) 2 m. M (hypo) 1000 500 Glucagon (p. M) 300 200 100 Saline Amylin 0 L-Arginine -60 0 60 120 180 240 Minutes Silvestre et al. , Am. J. Physiol 2001. P 31
 Pramlintide Does Not Affect Defenses Against Hypoglycemia in Humans • Pramlintide does not suppress secretion of: – – – Glucagon Growth Hormone Cortisol Epinephrine Norepinephrine • Pramlintide does not impede glucose response to glucagon intervention Study AP 93 -04, AP 93 -08 P 32
Pramlintide Does Not Affect Defenses Against Hypoglycemia in Humans • Pramlintide does not suppress secretion of: – – – Glucagon Growth Hormone Cortisol Epinephrine Norepinephrine • Pramlintide does not impede glucose response to glucagon intervention Study AP 93 -04, AP 93 -08 P 32
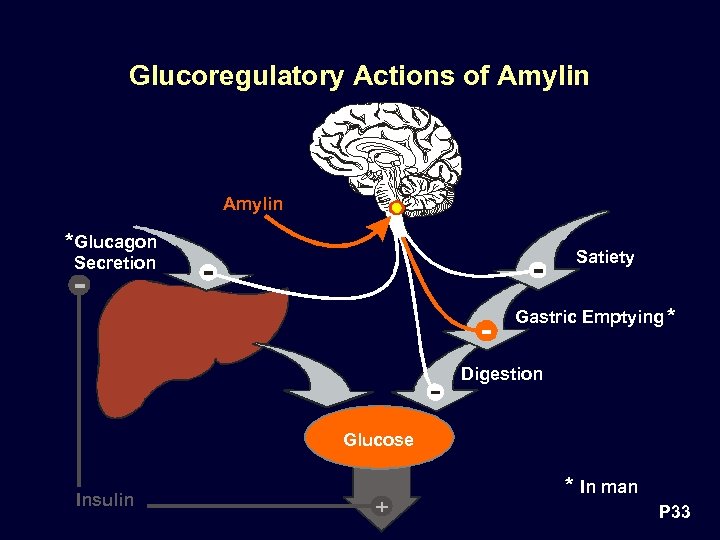 Glucoregulatory Actions of Amylin * Glucagon Satiety Secretion Gastric Emptying * Digestion Glucose Insulin * In man P 33
Glucoregulatory Actions of Amylin * Glucagon Satiety Secretion Gastric Emptying * Digestion Glucose Insulin * In man P 33
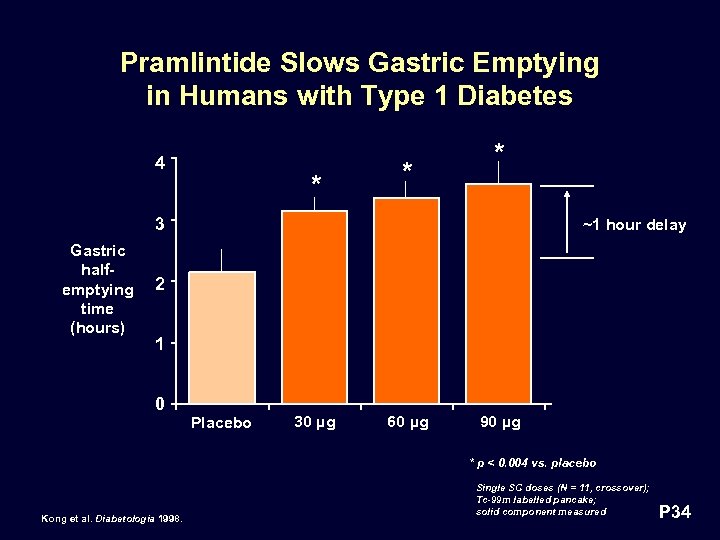 Pramlintide Slows Gastric Emptying in Humans with Type 1 Diabetes 4 * * * 3 Gastric halfemptying time (hours) ~1 hour delay 2 1 0 Placebo 30 µg 60 µg 90 µg * p < 0. 004 vs. placebo Kong et al. Diabetologia 1998. Single SC doses (N = 11, crossover); Tc-99 m labelled pancake; solid component measured P 34
Pramlintide Slows Gastric Emptying in Humans with Type 1 Diabetes 4 * * * 3 Gastric halfemptying time (hours) ~1 hour delay 2 1 0 Placebo 30 µg 60 µg 90 µg * p < 0. 004 vs. placebo Kong et al. Diabetologia 1998. Single SC doses (N = 11, crossover); Tc-99 m labelled pancake; solid component measured P 34
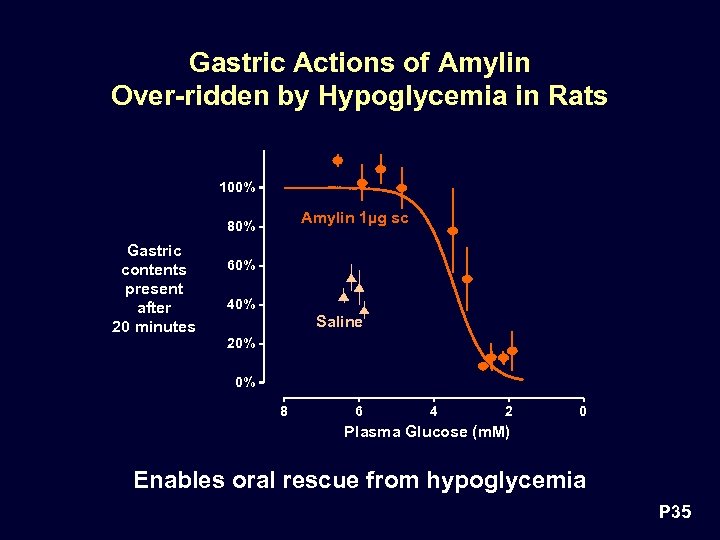 Gastric Actions of Amylin Over-ridden by Hypoglycemia in Rats 100% Amylin 1µg sc 80% Gastric contents present after 20 minutes 60% 40% Saline 20% 0% 8 6 4 2 0 Plasma Glucose (m. M) Enables oral rescue from hypoglycemia P 35
Gastric Actions of Amylin Over-ridden by Hypoglycemia in Rats 100% Amylin 1µg sc 80% Gastric contents present after 20 minutes 60% 40% Saline 20% 0% 8 6 4 2 0 Plasma Glucose (m. M) Enables oral rescue from hypoglycemia P 35
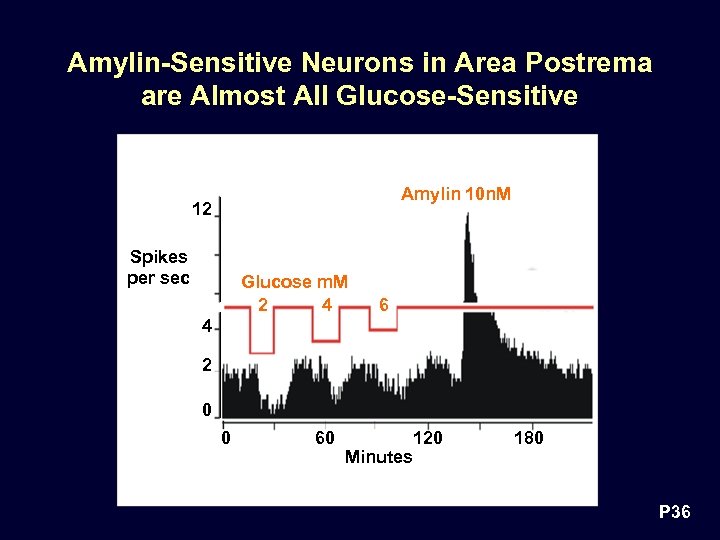 Amylin-Sensitive Neurons in Area Postrema are Almost All Glucose-Sensitive Amylin 10 n. M 12 Spikes per sec Glucose m. M 2 4 6 4 2 0 0 60 Minutes 120 180 P 36
Amylin-Sensitive Neurons in Area Postrema are Almost All Glucose-Sensitive Amylin 10 n. M 12 Spikes per sec Glucose m. M 2 4 6 4 2 0 0 60 Minutes 120 180 P 36
 Summary: Glucoregulatory Actions of Amylin/Pramlintide • Inhibits nutrient stimulated glucagon secretion • Regulates nutrient uptake from the meal • Glucose-lowering actions over-ridden during hypoglycemia P 37
Summary: Glucoregulatory Actions of Amylin/Pramlintide • Inhibits nutrient stimulated glucagon secretion • Regulates nutrient uptake from the meal • Glucose-lowering actions over-ridden during hypoglycemia P 37
 Summary: Rationale for Pramlintide • Pramlintide replaces absent amylin • Pramlintide restores control of glucose influx Complements insulin control of glucose efflux P 38
Summary: Rationale for Pramlintide • Pramlintide replaces absent amylin • Pramlintide restores control of glucose influx Complements insulin control of glucose efflux P 38
 Orville G. Kolterman, MD Senior Vice President, Clinical Affairs, Amylin Pharmaceuticals, Inc. Adjunct Professor of Medicine University of California, San Diego Clinical Program P 39
Orville G. Kolterman, MD Senior Vice President, Clinical Affairs, Amylin Pharmaceuticals, Inc. Adjunct Professor of Medicine University of California, San Diego Clinical Program P 39
 Pramlintide Indication Adjunctive therapy to insulin, to improve glycemic and metabolic control in people with type 1 or type 2 diabetes P 40
Pramlintide Indication Adjunctive therapy to insulin, to improve glycemic and metabolic control in people with type 1 or type 2 diabetes P 40
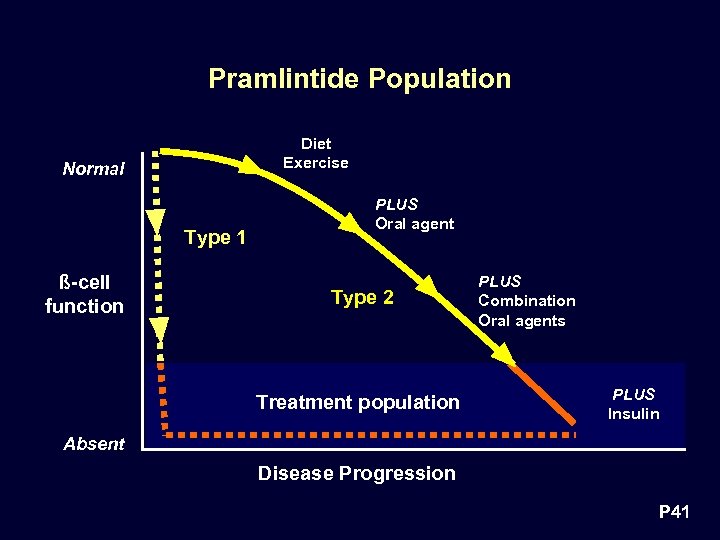 Pramlintide Population Diet Exercise Normal Type 1 ß-cell function PLUS Oral agent Type 2 Treatment population PLUS Combination Oral agents PLUS Insulin Absent Disease Progression P 41
Pramlintide Population Diet Exercise Normal Type 1 ß-cell function PLUS Oral agent Type 2 Treatment population PLUS Combination Oral agents PLUS Insulin Absent Disease Progression P 41
 Pramlintide Therapy à Program Overview • Pharmacodynamic Review • Type 2 Diabetes • Efficacy • Safety • Type 1 Diabetes • Efficacy • Safety P 42
Pramlintide Therapy à Program Overview • Pharmacodynamic Review • Type 2 Diabetes • Efficacy • Safety • Type 1 Diabetes • Efficacy • Safety P 42
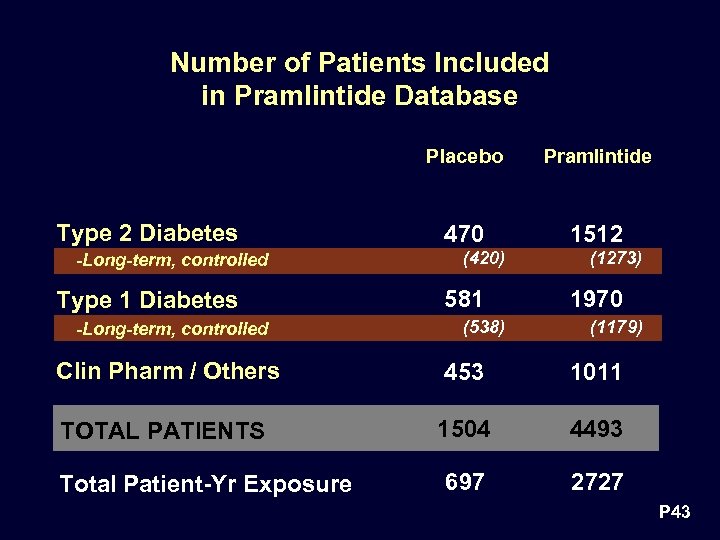 Number of Patients Included in Pramlintide Database Placebo Type 2 Diabetes -Long-term, controlled Type 1 Diabetes -Long-term, controlled Pramlintide 470 1512 (420) 581 (538) (1273) 1970 (1179) Clin Pharm / Others 453 1011 TOTAL PATIENTS 1504 4493 697 2727 Total Patient-Yr Exposure P 43
Number of Patients Included in Pramlintide Database Placebo Type 2 Diabetes -Long-term, controlled Type 1 Diabetes -Long-term, controlled Pramlintide 470 1512 (420) 581 (538) (1273) 1970 (1179) Clin Pharm / Others 453 1011 TOTAL PATIENTS 1504 4493 697 2727 Total Patient-Yr Exposure P 43
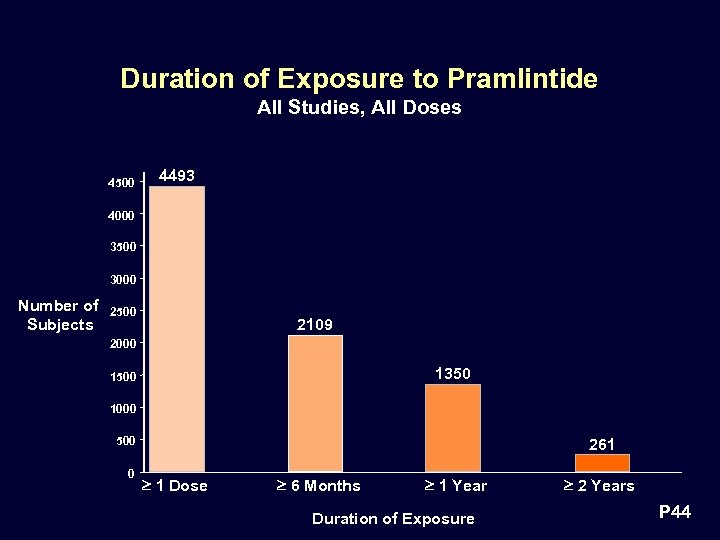 Duration of Exposure to Pramlintide All Studies, All Doses 4500 4493 4000 3500 3000 Number of Subjects 2500 2109 2000 1350 1500 1000 500 0 261 1 Dose 6 Months 1 Year Duration of Exposure 2 Years P 44
Duration of Exposure to Pramlintide All Studies, All Doses 4500 4493 4000 3500 3000 Number of Subjects 2500 2109 2000 1350 1500 1000 500 0 261 1 Dose 6 Months 1 Year Duration of Exposure 2 Years P 44
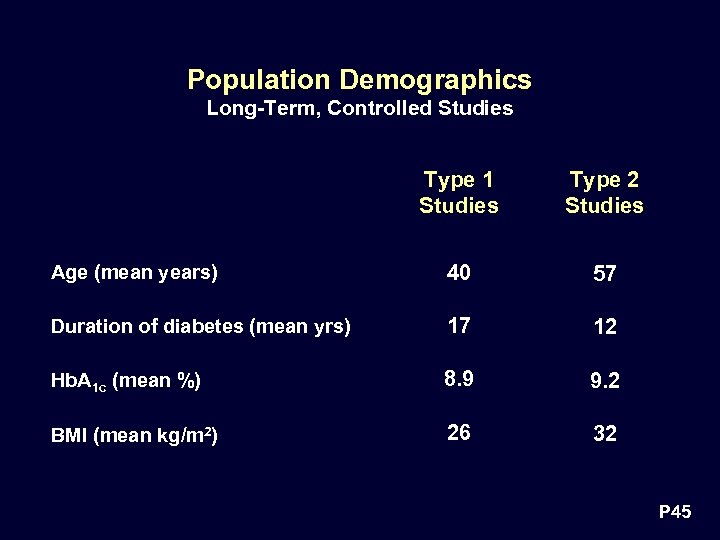 Population Demographics Long-Term, Controlled Studies Type 1 Studies Type 2 Studies Age (mean years) 40 57 Duration of diabetes (mean yrs) 17 12 Hb. A 1 c (mean %) 8. 9 9. 2 BMI (mean kg/m 2) 26 32 P 45
Population Demographics Long-Term, Controlled Studies Type 1 Studies Type 2 Studies Age (mean years) 40 57 Duration of diabetes (mean yrs) 17 12 Hb. A 1 c (mean %) 8. 9 9. 2 BMI (mean kg/m 2) 26 32 P 45
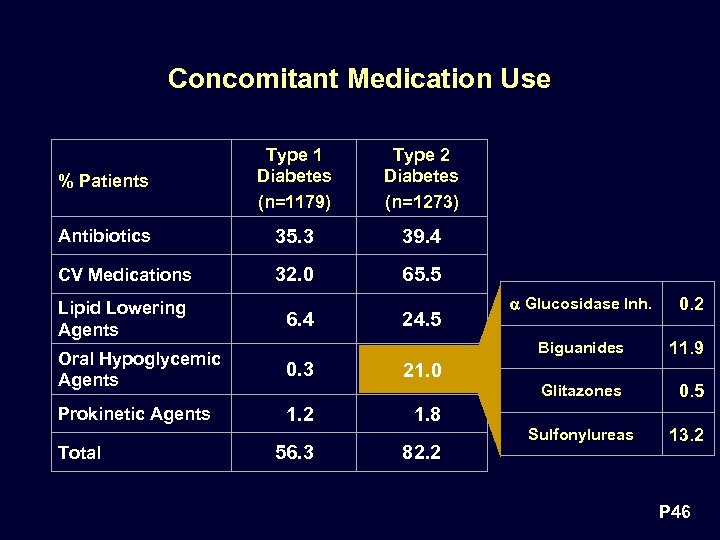 Concomitant Medication Use % Patients Type 1 Diabetes (n=1179) Type 2 Diabetes (n=1273) Antibiotics 35. 3 39. 4 CV Medications 32. 0 65. 5 Lipid Lowering Agents 6. 4 Oral Hypoglycemic Agents 0. 3 Prokinetic Agents 1. 2 Total 24. 5 Glucosidase Inh. 0. 2 Biguanides Glitazones 56. 3 11. 9 0. 5 21. 0 1. 8 82. 2 Sulfonylureas 13. 2 P 46
Concomitant Medication Use % Patients Type 1 Diabetes (n=1179) Type 2 Diabetes (n=1273) Antibiotics 35. 3 39. 4 CV Medications 32. 0 65. 5 Lipid Lowering Agents 6. 4 Oral Hypoglycemic Agents 0. 3 Prokinetic Agents 1. 2 Total 24. 5 Glucosidase Inh. 0. 2 Biguanides Glitazones 56. 3 11. 9 0. 5 21. 0 1. 8 82. 2 Sulfonylureas 13. 2 P 46
 Pramlintide as Adjunctive Therapy to Insulin in Type 1 and Type 2 Diabetes Results in: • Further improvement in glycemic control – Postprandial glucose – Hb. A 1 c • No increase in insulin use • Weight loss P 47
Pramlintide as Adjunctive Therapy to Insulin in Type 1 and Type 2 Diabetes Results in: • Further improvement in glycemic control – Postprandial glucose – Hb. A 1 c • No increase in insulin use • Weight loss P 47
 Pramlintide Therapy • Program Overview à Pharmacodynamic Review • Type 2 Diabetes – Efficacy – Safety • Type 1 Diabetes – Efficacy – Safety P 48
Pramlintide Therapy • Program Overview à Pharmacodynamic Review • Type 2 Diabetes – Efficacy – Safety • Type 1 Diabetes – Efficacy – Safety P 48
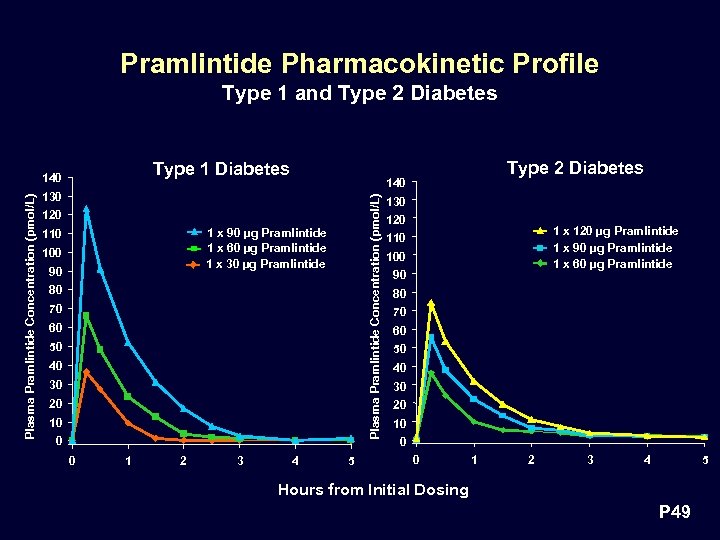 Pramlintide Pharmacokinetic Profile Type 1 and Type 2 Diabetes Type 1 Diabetes 130 120 1 x 90 µg Pramlintide 1 x 60 µg Pramlintide 1 x 30 µg Pramlintide 110 100 90 80 70 60 50 40 30 20 10 0 0 1 2 3 Type 2 Diabetes 140 Plasma Pramlintide Concentration (pmol/L) 140 4 5 130 120 1 x 120 µg Pramlintide 1 x 90 µg Pramlintide 1 x 60 µg Pramlintide 110 100 90 80 70 60 50 40 30 20 10 0 0 1 2 3 4 5 Hours from Initial Dosing P 49
Pramlintide Pharmacokinetic Profile Type 1 and Type 2 Diabetes Type 1 Diabetes 130 120 1 x 90 µg Pramlintide 1 x 60 µg Pramlintide 1 x 30 µg Pramlintide 110 100 90 80 70 60 50 40 30 20 10 0 0 1 2 3 Type 2 Diabetes 140 Plasma Pramlintide Concentration (pmol/L) 140 4 5 130 120 1 x 120 µg Pramlintide 1 x 90 µg Pramlintide 1 x 60 µg Pramlintide 110 100 90 80 70 60 50 40 30 20 10 0 0 1 2 3 4 5 Hours from Initial Dosing P 49
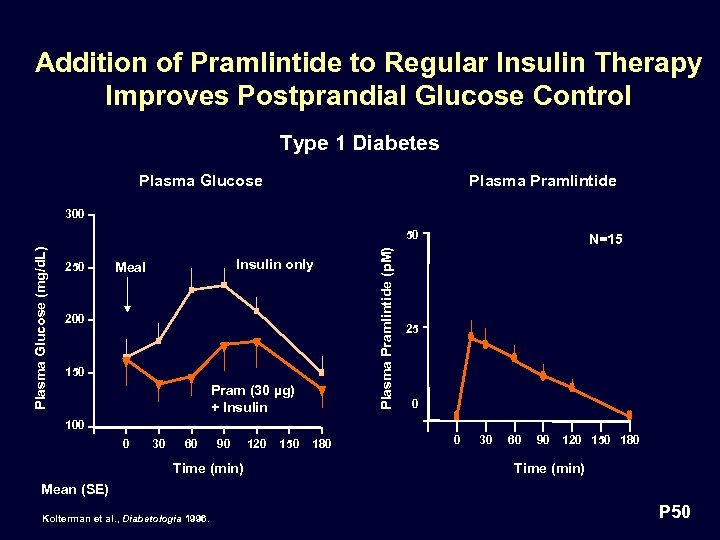 Addition of Pramlintide to Regular Insulin Therapy Improves Postprandial Glucose Control Type 1 Diabetes Plasma Glucose Plasma Pramlintide 300 250 Insulin only Meal 200 150 Pram (30 µg) + Insulin Plasma Pramlintide (p. M) Plasma Glucose (mg/d. L) 50 N=15 25 0 100 0 30 60 90 Time (min) 120 150 180 0 30 60 90 120 150 180 Time (min) Mean (SE) Kolterman et al. , Diabetologia 1996. P 50
Addition of Pramlintide to Regular Insulin Therapy Improves Postprandial Glucose Control Type 1 Diabetes Plasma Glucose Plasma Pramlintide 300 250 Insulin only Meal 200 150 Pram (30 µg) + Insulin Plasma Pramlintide (p. M) Plasma Glucose (mg/d. L) 50 N=15 25 0 100 0 30 60 90 Time (min) 120 150 180 0 30 60 90 120 150 180 Time (min) Mean (SE) Kolterman et al. , Diabetologia 1996. P 50
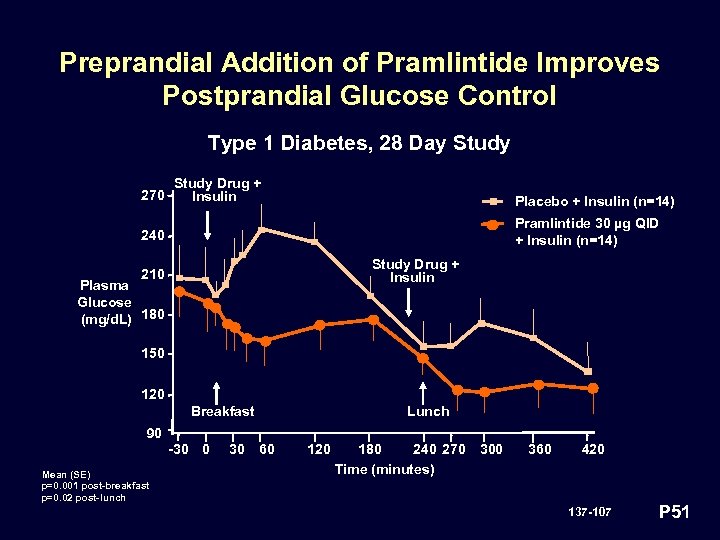 Preprandial Addition of Pramlintide Improves Postprandial Glucose Control Type 1 Diabetes, 28 Day Study 270 Study Drug + Insulin Placebo + Insulin (n=14) Pramlintide 30 µg QID + Insulin (n=14) 240 Study Drug + Insulin 210 Plasma Glucose (mg/d. L) 180 150 120 Breakfast Lunch 90 -30 0 Mean (SE) p=0. 001 post-breakfast p=0. 02 post-lunch 30 60 120 180 240 270 Time (minutes) 300 360 420 137 -107 P 51
Preprandial Addition of Pramlintide Improves Postprandial Glucose Control Type 1 Diabetes, 28 Day Study 270 Study Drug + Insulin Placebo + Insulin (n=14) Pramlintide 30 µg QID + Insulin (n=14) 240 Study Drug + Insulin 210 Plasma Glucose (mg/d. L) 180 150 120 Breakfast Lunch 90 -30 0 Mean (SE) p=0. 001 post-breakfast p=0. 02 post-lunch 30 60 120 180 240 270 Time (minutes) 300 360 420 137 -107 P 51
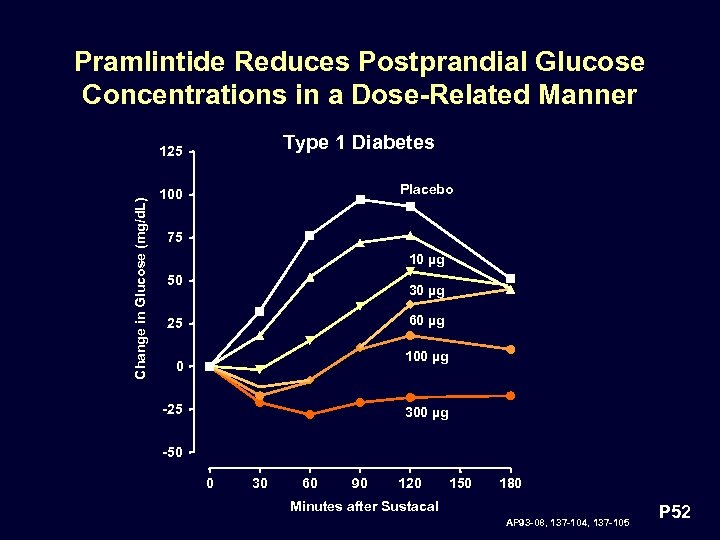 Pramlintide Reduces Postprandial Glucose Concentrations in a Dose-Related Manner Type 1 Diabetes Change in Glucose (mg/d. L) 125 Placebo 100 75 10 µg 50 30 µg 60 µg 25 100 µg 0 -25 300 µg -50 0 30 60 90 120 150 180 Minutes after Sustacal AP 93 -08, 137 -104, 137 -105 P 52
Pramlintide Reduces Postprandial Glucose Concentrations in a Dose-Related Manner Type 1 Diabetes Change in Glucose (mg/d. L) 125 Placebo 100 75 10 µg 50 30 µg 60 µg 25 100 µg 0 -25 300 µg -50 0 30 60 90 120 150 180 Minutes after Sustacal AP 93 -08, 137 -104, 137 -105 P 52
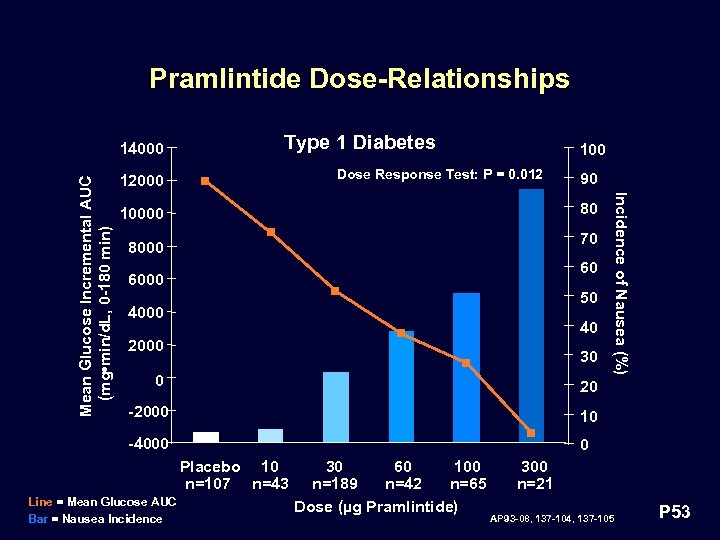 Pramlintide Dose-Relationships Type 1 Diabetes 100 Dose Response Test: P = 0. 012 12000 90 80 10000 70 8000 60 6000 50 4000 40 2000 30 0 Incidence of Nausea (%) Mean Glucose Incremental AUC (mg • min/d. L, 0 -180 min) 14000 20 -2000 10 -4000 0 Placebo 10 n=107 n=43 Line = Mean Glucose AUC Bar = Nausea Incidence 30 60 100 n=189 n=42 n=65 Dose (µg Pramlintide) 300 n=21 AP 93 -08, 137 -104, 137 -105 P 53
Pramlintide Dose-Relationships Type 1 Diabetes 100 Dose Response Test: P = 0. 012 12000 90 80 10000 70 8000 60 6000 50 4000 40 2000 30 0 Incidence of Nausea (%) Mean Glucose Incremental AUC (mg • min/d. L, 0 -180 min) 14000 20 -2000 10 -4000 0 Placebo 10 n=107 n=43 Line = Mean Glucose AUC Bar = Nausea Incidence 30 60 100 n=189 n=42 n=65 Dose (µg Pramlintide) 300 n=21 AP 93 -08, 137 -104, 137 -105 P 53
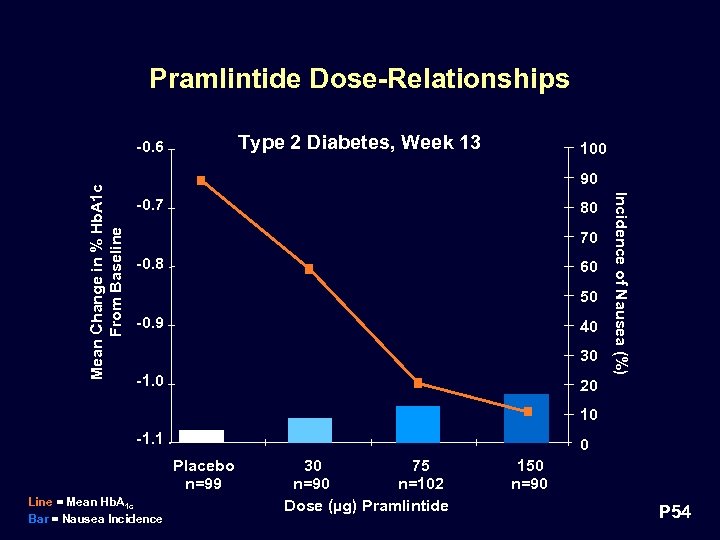 Pramlintide Dose-Relationships Type 2 Diabetes, Week 13 100 90 -0. 7 80 70 -0. 8 60 50 -0. 9 40 30 -1. 0 Incidence of Nausea (%) Mean Change in % Hb. A 1 c From Baseline -0. 6 20 10 -1. 1 0 Placebo n=99 Line = Mean Hb. A 1 c Bar = Nausea Incidence 30 75 n=90 n=102 Dose (µg) Pramlintide 150 n=90 P 54
Pramlintide Dose-Relationships Type 2 Diabetes, Week 13 100 90 -0. 7 80 70 -0. 8 60 50 -0. 9 40 30 -1. 0 Incidence of Nausea (%) Mean Change in % Hb. A 1 c From Baseline -0. 6 20 10 -1. 1 0 Placebo n=99 Line = Mean Hb. A 1 c Bar = Nausea Incidence 30 75 n=90 n=102 Dose (µg) Pramlintide 150 n=90 P 54
 Doses Selected for Phase 3 Studies • Type 2 diabetes range: 30 to 150 µg • Type 1 diabetes range: 30 to 90 µg P 55
Doses Selected for Phase 3 Studies • Type 2 diabetes range: 30 to 150 µg • Type 1 diabetes range: 30 to 90 µg P 55
 Phase 3 Clinical Trials • Demonstration of efficacy • Assessment of safety • Guidance for clinical use P 56
Phase 3 Clinical Trials • Demonstration of efficacy • Assessment of safety • Guidance for clinical use P 56
 Study Design Considerations • No precedent for efficacy studies in insulin-treated subjects • DCCT established Hb. A 1 c as surrogate endpoint for glycemic control • On-going debate regarding “threshold effect” • Ancillary metabolic effects (weight, insulin use, lipid profile) not fully appreciated P 57
Study Design Considerations • No precedent for efficacy studies in insulin-treated subjects • DCCT established Hb. A 1 c as surrogate endpoint for glycemic control • On-going debate regarding “threshold effect” • Ancillary metabolic effects (weight, insulin use, lipid profile) not fully appreciated P 57
 General Approach to Pramlintide Phase 3 Clinical Studies • All subjects were treated with insulin • All studies employed an “add-on” design – Pramlintide or placebo was added to existing therapies – Oral hypoglycemic agents were to be unchanged • Sulfonylurea • Metformin P 58
General Approach to Pramlintide Phase 3 Clinical Studies • All subjects were treated with insulin • All studies employed an “add-on” design – Pramlintide or placebo was added to existing therapies – Oral hypoglycemic agents were to be unchanged • Sulfonylurea • Metformin P 58
 Approaches to Insulin Management Clinical Trial Setting • Insulin should ideally remain constant to isolate effect of “add-on” drug • Changes in insulin use during the study period confound data interpretation Clinical Practice Setting • Involves frequent changes in insulin regimens – Patient safety (hypoglycemia) – Pursuit of glycemic targets P 59
Approaches to Insulin Management Clinical Trial Setting • Insulin should ideally remain constant to isolate effect of “add-on” drug • Changes in insulin use during the study period confound data interpretation Clinical Practice Setting • Involves frequent changes in insulin regimens – Patient safety (hypoglycemia) – Pursuit of glycemic targets P 59
 Insulin Use in Pramlintide Phase 3 Clinical Studies • Four studies Consistent insulin dosing encouraged • Two studies No constraints on insulin dosing • Allowed changes for patient safety • Patients were not discontinued due to changes in insulin regimen • Analysis plan pre-defined “stable insulin” subgroup – Total daily dose at baseline ± 10% – Isolates “true” drug effect P 60
Insulin Use in Pramlintide Phase 3 Clinical Studies • Four studies Consistent insulin dosing encouraged • Two studies No constraints on insulin dosing • Allowed changes for patient safety • Patients were not discontinued due to changes in insulin regimen • Analysis plan pre-defined “stable insulin” subgroup – Total daily dose at baseline ± 10% – Isolates “true” drug effect P 60
 Phase 3 Study Design Type 2 and Type 1 Diabetes • Multicenter, randomized, placebo-controlled • Primary endpoint – Hb. A 1 c, week 26 or 52 • Secondary endpoints – Weight – Insulin use – Safety parameters P 61
Phase 3 Study Design Type 2 and Type 1 Diabetes • Multicenter, randomized, placebo-controlled • Primary endpoint – Hb. A 1 c, week 26 or 52 • Secondary endpoints – Weight – Insulin use – Safety parameters P 61
 Pramlintide Therapy • Program Overview • Pharmacodynamic Review à Type 2 Diabetes à Efficacy • Safety • Type 1 Diabetes • Efficacy • Safety P 62
Pramlintide Therapy • Program Overview • Pharmacodynamic Review à Type 2 Diabetes à Efficacy • Safety • Type 1 Diabetes • Efficacy • Safety P 62
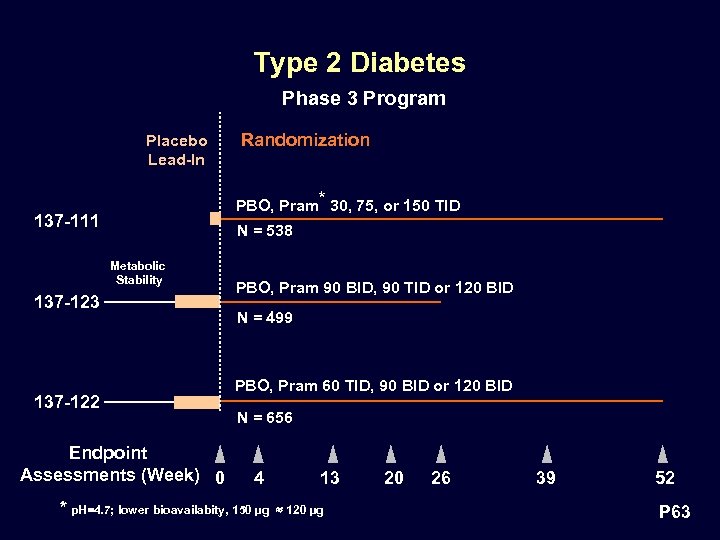 Type 2 Diabetes Phase 3 Program Placebo Lead-In Randomization PBO, Pram* 30, 75, or 150 TID 137 -111 N = 538 Metabolic Stability 137 -123 137 -122 Endpoint Assessments (Week) 0 PBO, Pram 90 BID, 90 TID or 120 BID N = 499 PBO, Pram 60 TID, 90 BID or 120 BID N = 656 4 * p. H=4. 7; lower bioavailabity, 150 µg 13 120 µg 20 26 39 52 P 63
Type 2 Diabetes Phase 3 Program Placebo Lead-In Randomization PBO, Pram* 30, 75, or 150 TID 137 -111 N = 538 Metabolic Stability 137 -123 137 -122 Endpoint Assessments (Week) 0 PBO, Pram 90 BID, 90 TID or 120 BID N = 499 PBO, Pram 60 TID, 90 BID or 120 BID N = 656 4 * p. H=4. 7; lower bioavailabity, 150 µg 13 120 µg 20 26 39 52 P 63
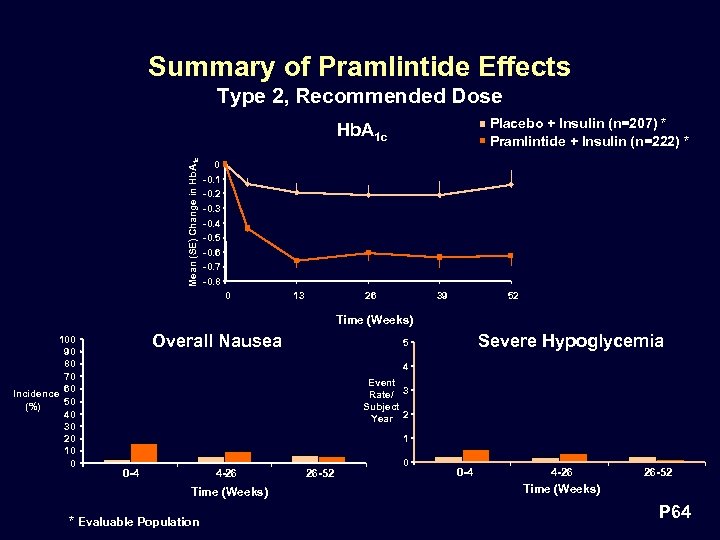 Summary of Pramlintide Effects Type 2, Recommended Dose Placebo + Insulin (n=207) * Pramlintide + Insulin (n=222) * Mean (SE) Change in Hb. A 1 c 0 -0. 1 -0. 2 -0. 3 -0. 4 -0. 5 -0. 6 -0. 7 -0. 8 0 13 26 39 52 Time (Weeks) 100 90 80 70 Incidence 60 50 (%) 40 30 20 10 0 Overall Nausea Severe Hypoglycemia 5 4 Event Rate/ 3 Subject Year 2 1 0 -4 4 -26 Time (Weeks) * Evaluable Population 26 -52 0 0 -4 4 -26 26 -52 Time (Weeks) P 64
Summary of Pramlintide Effects Type 2, Recommended Dose Placebo + Insulin (n=207) * Pramlintide + Insulin (n=222) * Mean (SE) Change in Hb. A 1 c 0 -0. 1 -0. 2 -0. 3 -0. 4 -0. 5 -0. 6 -0. 7 -0. 8 0 13 26 39 52 Time (Weeks) 100 90 80 70 Incidence 60 50 (%) 40 30 20 10 0 Overall Nausea Severe Hypoglycemia 5 4 Event Rate/ 3 Subject Year 2 1 0 -4 4 -26 Time (Weeks) * Evaluable Population 26 -52 0 0 -4 4 -26 26 -52 Time (Weeks) P 64
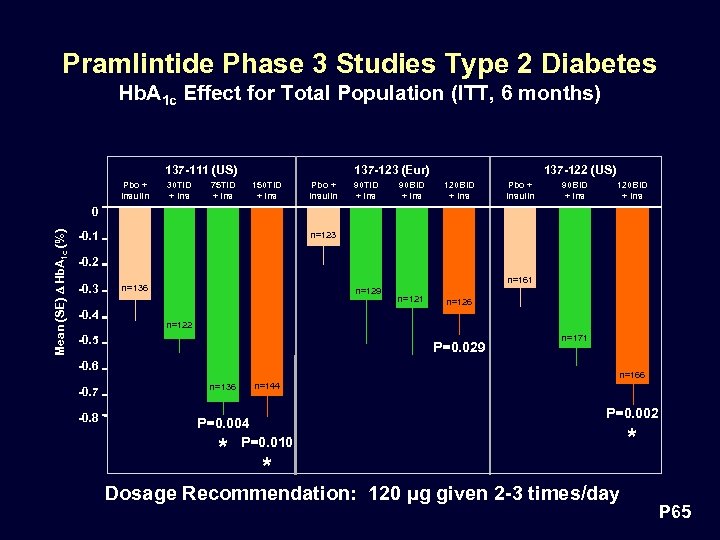 Pramlintide Phase 3 Studies Type 2 Diabetes Hb. A 1 c Effect for Total Population (ITT, 6 months) 137 -123 (Eur) 137 -111 (US) Pbo + Insulin 30 TID + Ins 75 TID + Ins 150 TID + Ins Pbo + Insulin 90 TID + Ins 90 BID + Ins 137 -122 (US) 120 BID + Ins Pbo + Insulin 90 BID + Ins 120 BID + Ins Mean (SE) D Hb. A 1 c (%) 0 -0. 1 n=123 -0. 2 -0. 3 -0. 4 n=161 n=136 n=129 P=0. 029 -0. 6 -0. 8 n=126 n=122 -0. 5 -0. 7 n=121 n=171 n=166 n=136 n=144 P=0. 004 P=0. 010 * P=0. 002 * Dosage Recommendation: 120 µg given 2 -3 times/day * P 65
Pramlintide Phase 3 Studies Type 2 Diabetes Hb. A 1 c Effect for Total Population (ITT, 6 months) 137 -123 (Eur) 137 -111 (US) Pbo + Insulin 30 TID + Ins 75 TID + Ins 150 TID + Ins Pbo + Insulin 90 TID + Ins 90 BID + Ins 137 -122 (US) 120 BID + Ins Pbo + Insulin 90 BID + Ins 120 BID + Ins Mean (SE) D Hb. A 1 c (%) 0 -0. 1 n=123 -0. 2 -0. 3 -0. 4 n=161 n=136 n=129 P=0. 029 -0. 6 -0. 8 n=126 n=122 -0. 5 -0. 7 n=121 n=171 n=166 n=136 n=144 P=0. 004 P=0. 010 * P=0. 002 * Dosage Recommendation: 120 µg given 2 -3 times/day * P 65
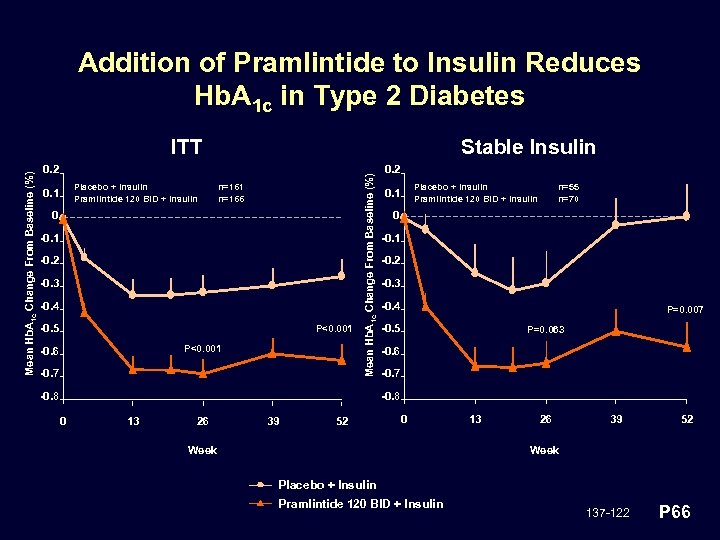 Addition of Pramlintide to Insulin Reduces Hb. A 1 c in Type 2 Diabetes Stable Insulin 0. 2 Placebo + Insulin Pramlintide 120 BID + Insulin 0. 1 n=166 0 -0. 1 -0. 2 -0. 3 -0. 4 -0. 5 P<0. 001 -0. 6 -0. 7 Mean Hb. A 1 c Change From Baseline (%) ITT -0. 8 0. 2 Placebo + Insulin Pramlintide 120 BID + Insulin 0. 1 n=55 n=70 0 -0. 1 -0. 2 -0. 3 -0. 4 P=0. 007 -0. 5 P=0. 063 -0. 6 -0. 7 -0. 8 0 13 26 39 52 0 Week 13 26 39 52 Week Placebo + Insulin Pramlintide 120 BID + Insulin 137 -122 P 66
Addition of Pramlintide to Insulin Reduces Hb. A 1 c in Type 2 Diabetes Stable Insulin 0. 2 Placebo + Insulin Pramlintide 120 BID + Insulin 0. 1 n=166 0 -0. 1 -0. 2 -0. 3 -0. 4 -0. 5 P<0. 001 -0. 6 -0. 7 Mean Hb. A 1 c Change From Baseline (%) ITT -0. 8 0. 2 Placebo + Insulin Pramlintide 120 BID + Insulin 0. 1 n=55 n=70 0 -0. 1 -0. 2 -0. 3 -0. 4 P=0. 007 -0. 5 P=0. 063 -0. 6 -0. 7 -0. 8 0 13 26 39 52 0 Week 13 26 39 52 Week Placebo + Insulin Pramlintide 120 BID + Insulin 137 -122 P 66
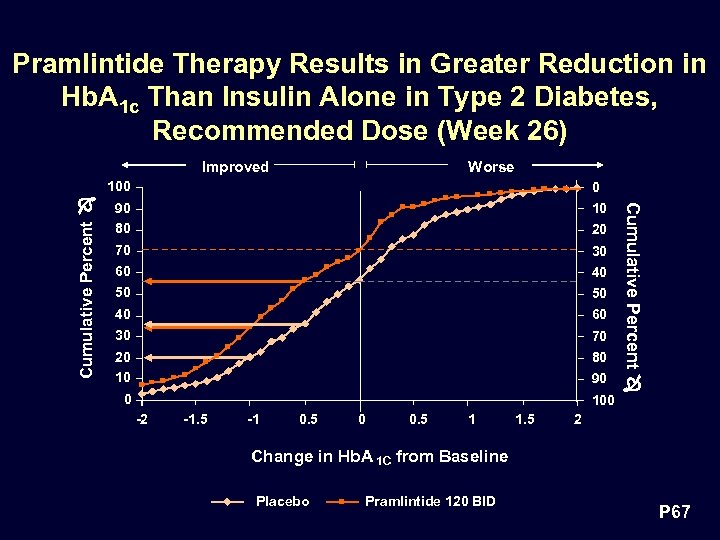 Pramlintide Therapy Results in Greater Reduction in Hb. A 1 c Than Insulin Alone in Type 2 Diabetes, Recommended Dose (Week 26) Improved Worse Cumulative Percent 0 90 80 10 70 60 30 50 50 40 30 60 20 10 80 20 40 70 90 0 100 -2 -1. 5 -1 0. 5 0 0. 5 1 1. 5 Cumulative Percent 100 2 Change in Hb. A 1 C from Baseline Placebo Pramlintide 120 BID P 67
Pramlintide Therapy Results in Greater Reduction in Hb. A 1 c Than Insulin Alone in Type 2 Diabetes, Recommended Dose (Week 26) Improved Worse Cumulative Percent 0 90 80 10 70 60 30 50 50 40 30 60 20 10 80 20 40 70 90 0 100 -2 -1. 5 -1 0. 5 0 0. 5 1 1. 5 Cumulative Percent 100 2 Change in Hb. A 1 C from Baseline Placebo Pramlintide 120 BID P 67
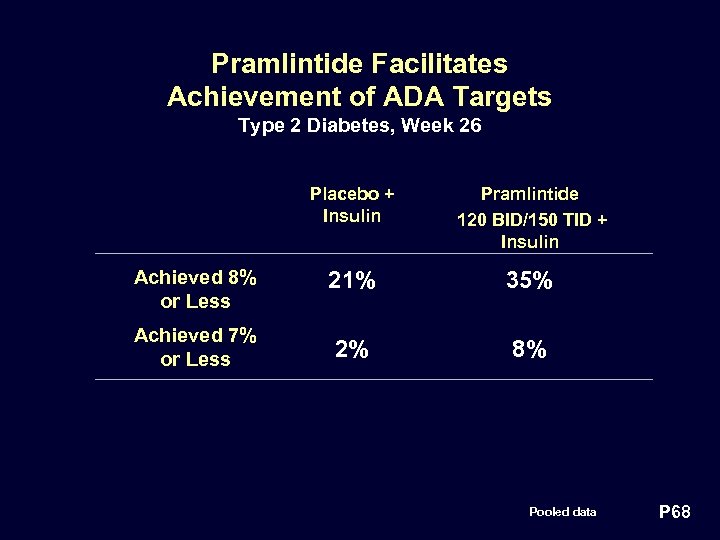 Pramlintide Facilitates Achievement of ADA Targets Type 2 Diabetes, Week 26 Placebo + Insulin Pramlintide 120 BID/150 TID + Insulin Achieved 8% or Less 21% 35% Achieved 7% or Less 2% 8% Pooled data P 68
Pramlintide Facilitates Achievement of ADA Targets Type 2 Diabetes, Week 26 Placebo + Insulin Pramlintide 120 BID/150 TID + Insulin Achieved 8% or Less 21% 35% Achieved 7% or Less 2% 8% Pooled data P 68
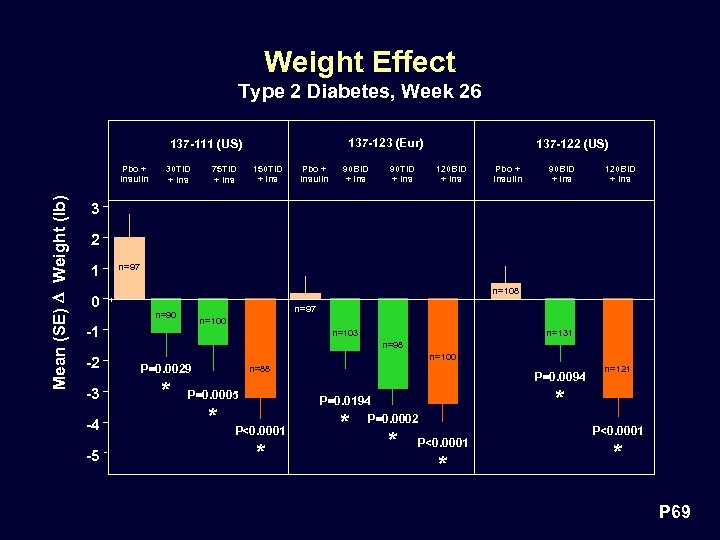 Weight Effect Type 2 Diabetes, Week 26 137 -123 (Eur) 137 -111 (US) Mean (SE) D Weight (lb) Pbo + Insulin 30 TID + Ins 75 TID + Ins 150 TID + Ins Pbo + Insulin 90 BID + Ins 90 TID + Ins 137 -122 (US) 120 BID + Ins Pbo + Insulin 90 BID + Ins 120 BID + Ins 3 2 1 n=97 n=108 0 n=97 n=90 n=100 -1 n=98 n=100 -2 P=0. 0029 -3 * -4 -5 n=131 n=103 n=88 P=0. 0005 * P<0. 0001 * P=0. 0094 * P=0. 0194 P=0. 0002 * * n=121 P<0. 0001 * P 69
Weight Effect Type 2 Diabetes, Week 26 137 -123 (Eur) 137 -111 (US) Mean (SE) D Weight (lb) Pbo + Insulin 30 TID + Ins 75 TID + Ins 150 TID + Ins Pbo + Insulin 90 BID + Ins 90 TID + Ins 137 -122 (US) 120 BID + Ins Pbo + Insulin 90 BID + Ins 120 BID + Ins 3 2 1 n=97 n=108 0 n=97 n=90 n=100 -1 n=98 n=100 -2 P=0. 0029 -3 * -4 -5 n=131 n=103 n=88 P=0. 0005 * P<0. 0001 * P=0. 0094 * P=0. 0194 P=0. 0002 * * n=121 P<0. 0001 * P 69
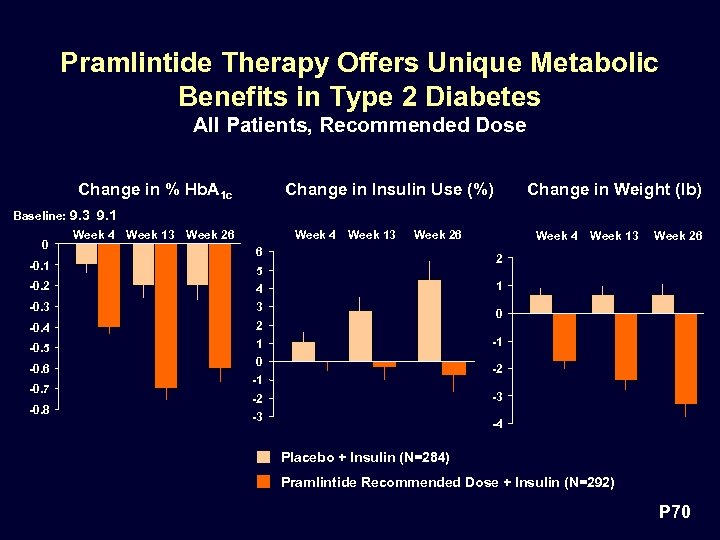 Pramlintide Therapy Offers Unique Metabolic Benefits in Type 2 Diabetes All Patients, Recommended Dose Change in % Hb. A 1 c Change in Insulin Use (%) Change in Weight (lb) Baseline: 9. 3 9. 1 0 -0. 1 Week 4 Week 13 Week 26 6 4 -0. 3 3 -0. 4 2 -0. 5 1 -0. 6 -0. 7 -0. 8 Week 13 Week 26 2 5 -0. 2 Week 4 1 0 -2 -1 -3 -2 -3 -4 Placebo + Insulin (N=284) Pramlintide Recommended Dose + Insulin (N=292) P 70
Pramlintide Therapy Offers Unique Metabolic Benefits in Type 2 Diabetes All Patients, Recommended Dose Change in % Hb. A 1 c Change in Insulin Use (%) Change in Weight (lb) Baseline: 9. 3 9. 1 0 -0. 1 Week 4 Week 13 Week 26 6 4 -0. 3 3 -0. 4 2 -0. 5 1 -0. 6 -0. 7 -0. 8 Week 13 Week 26 2 5 -0. 2 Week 4 1 0 -2 -1 -3 -2 -3 -4 Placebo + Insulin (N=284) Pramlintide Recommended Dose + Insulin (N=292) P 70
 Pramlintide Therapy • Program Overview • Pharmacodynamic Review • Type 2 Diabetes • Efficacy àSafety • Type 1 Diabetes • Efficacy • Safety P 71
Pramlintide Therapy • Program Overview • Pharmacodynamic Review • Type 2 Diabetes • Efficacy àSafety • Type 1 Diabetes • Efficacy • Safety P 71
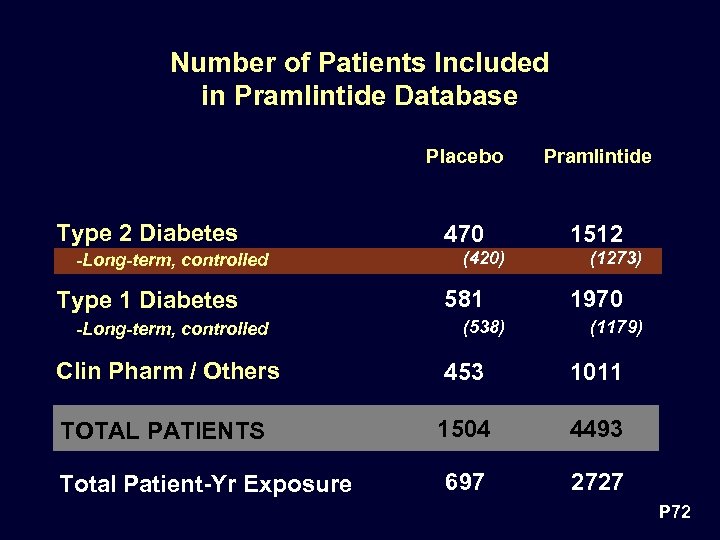 Number of Patients Included in Pramlintide Database Placebo Type 2 Diabetes -Long-term, controlled Type 1 Diabetes -Long-term, controlled Pramlintide 470 1512 (420) 581 (538) (1273) 1970 (1179) Clin Pharm / Others 453 1011 TOTAL PATIENTS 1504 4493 697 2727 Total Patient-Yr Exposure P 72
Number of Patients Included in Pramlintide Database Placebo Type 2 Diabetes -Long-term, controlled Type 1 Diabetes -Long-term, controlled Pramlintide 470 1512 (420) 581 (538) (1273) 1970 (1179) Clin Pharm / Others 453 1011 TOTAL PATIENTS 1504 4493 697 2727 Total Patient-Yr Exposure P 72
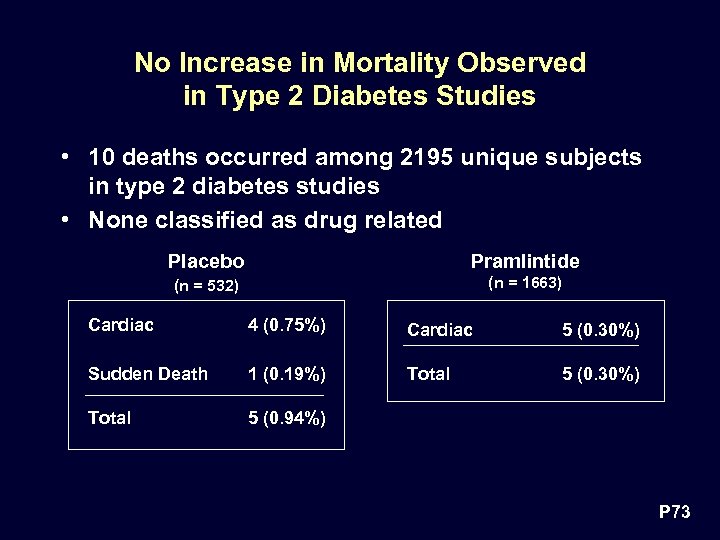 No Increase in Mortality Observed in Type 2 Diabetes Studies • 10 deaths occurred among 2195 unique subjects in type 2 diabetes studies • None classified as drug related Placebo Pramlintide (n = 532) (n = 1663) Cardiac 4 (0. 75%) Cardiac 5 (0. 30%) Sudden Death 1 (0. 19%) Total 5 (0. 30%) Total 5 (0. 94%) P 73
No Increase in Mortality Observed in Type 2 Diabetes Studies • 10 deaths occurred among 2195 unique subjects in type 2 diabetes studies • None classified as drug related Placebo Pramlintide (n = 532) (n = 1663) Cardiac 4 (0. 75%) Cardiac 5 (0. 30%) Sudden Death 1 (0. 19%) Total 5 (0. 30%) Total 5 (0. 94%) P 73
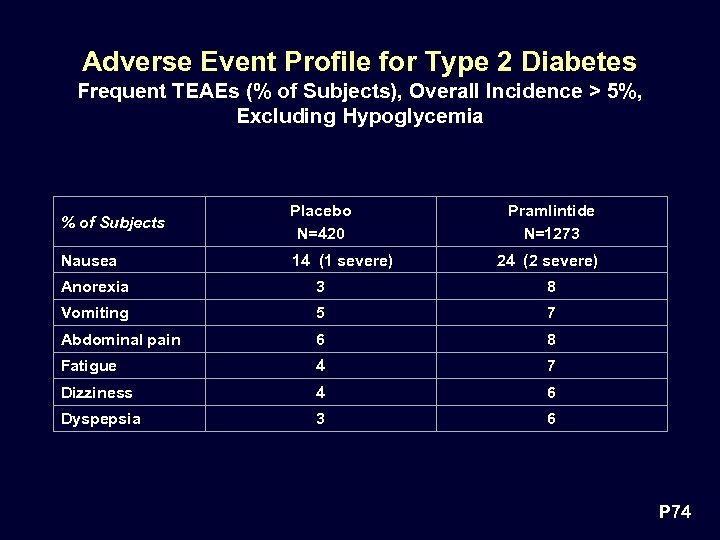 Adverse Event Profile for Type 2 Diabetes Frequent TEAEs (% of Subjects), Overall Incidence > 5%, Excluding Hypoglycemia % of Subjects Placebo N=420 Nausea 14 (1 severe) Pramlintide N=1273 24 (2 severe) Anorexia 3 8 Vomiting 5 7 Abdominal pain 6 8 Fatigue 4 7 Dizziness 4 6 Dyspepsia 3 6 P 74
Adverse Event Profile for Type 2 Diabetes Frequent TEAEs (% of Subjects), Overall Incidence > 5%, Excluding Hypoglycemia % of Subjects Placebo N=420 Nausea 14 (1 severe) Pramlintide N=1273 24 (2 severe) Anorexia 3 8 Vomiting 5 7 Abdominal pain 6 8 Fatigue 4 7 Dizziness 4 6 Dyspepsia 3 6 P 74
 Vision/Retinal Disorder Adverse Events • One study (137 -111) had an apparent increase in incidence of retinal disorder in the 150 µg treatment arm compared with placebo • No apparent pramlintide-related increase in incidence of retinal disorder or other vision disorders at doses of 75 µg TID or 120 µg BID • No safety concern P 75
Vision/Retinal Disorder Adverse Events • One study (137 -111) had an apparent increase in incidence of retinal disorder in the 150 µg treatment arm compared with placebo • No apparent pramlintide-related increase in incidence of retinal disorder or other vision disorders at doses of 75 µg TID or 120 µg BID • No safety concern P 75
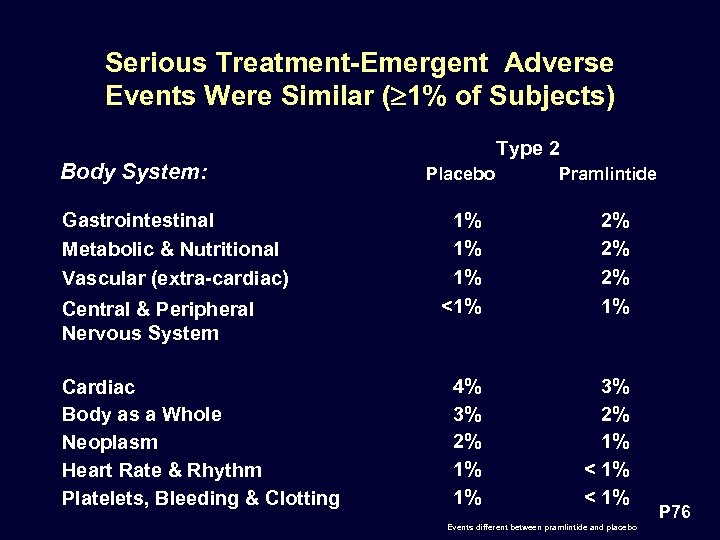 Serious Treatment-Emergent Adverse Events Were Similar ( 1% of Subjects) Type 2 Body System: Gastrointestinal Metabolic & Nutritional Vascular (extra-cardiac) Central & Peripheral Nervous System Cardiac Body as a Whole Neoplasm Heart Rate & Rhythm Platelets, Bleeding & Clotting Placebo Pramlintide 1% 1% 1% <1% 2% 2% 2% 1% 4% 3% 2% 1% 1% 3% 2% 1% < 1% Events different between pramlintide and placebo P 76
Serious Treatment-Emergent Adverse Events Were Similar ( 1% of Subjects) Type 2 Body System: Gastrointestinal Metabolic & Nutritional Vascular (extra-cardiac) Central & Peripheral Nervous System Cardiac Body as a Whole Neoplasm Heart Rate & Rhythm Platelets, Bleeding & Clotting Placebo Pramlintide 1% 1% 1% <1% 2% 2% 2% 1% 4% 3% 2% 1% 1% 3% 2% 1% < 1% Events different between pramlintide and placebo P 76
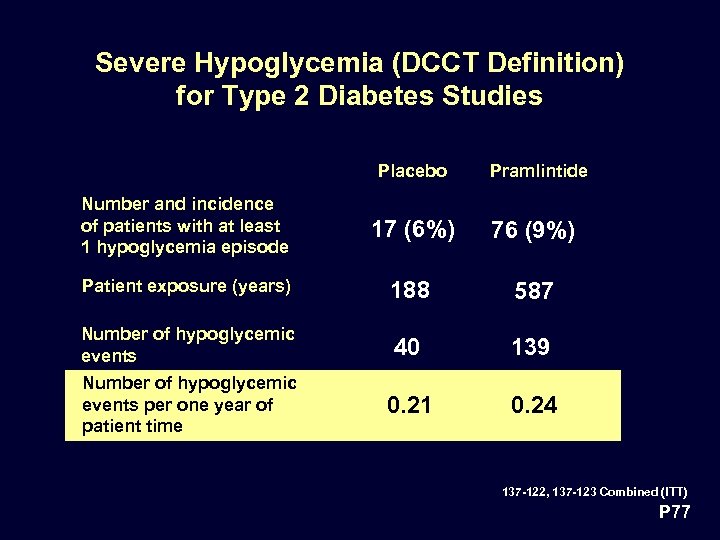 Severe Hypoglycemia (DCCT Definition) for Type 2 Diabetes Studies Placebo Pramlintide Number and incidence of patients with at least 1 hypoglycemia episode 17 (6%) 76 (9%) Patient exposure (years) 188 587 Number of hypoglycemic events 40 139 Number of hypoglycemic events per one year of patient time 0. 21 0. 24 137 -122, 137 -123 Combined (ITT) P 77
Severe Hypoglycemia (DCCT Definition) for Type 2 Diabetes Studies Placebo Pramlintide Number and incidence of patients with at least 1 hypoglycemia episode 17 (6%) 76 (9%) Patient exposure (years) 188 587 Number of hypoglycemic events 40 139 Number of hypoglycemic events per one year of patient time 0. 21 0. 24 137 -122, 137 -123 Combined (ITT) P 77
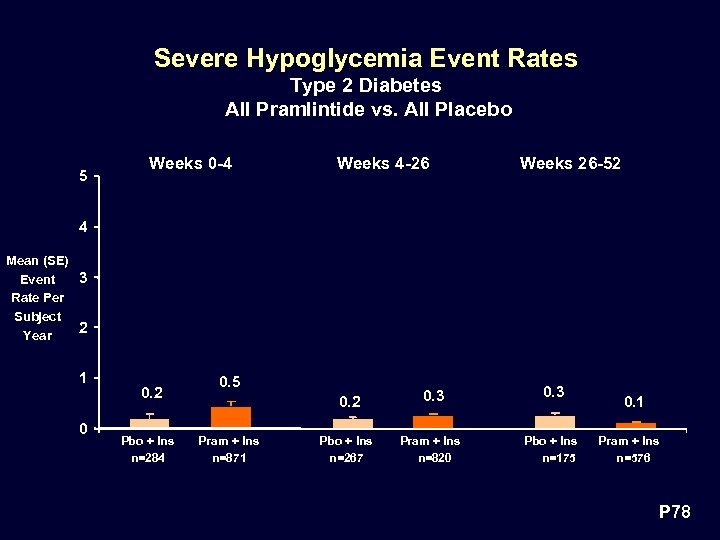 Severe Hypoglycemia Event Rates Type 2 Diabetes All Pramlintide vs. All Placebo 5 Weeks 0 -4 Weeks 4 -26 Weeks 26 -52 4 Mean (SE) 3 Event Rate Per Subject 2 Year 1 0 0. 2 Pbo + Ins n=284 0. 5 0. 2 Pram + Ins n=871 0. 3 Pbo + Ins n=267 Pram + Ins n=820 Pbo + Ins n=175 0. 1 Pram + Ins n=576 P 78
Severe Hypoglycemia Event Rates Type 2 Diabetes All Pramlintide vs. All Placebo 5 Weeks 0 -4 Weeks 4 -26 Weeks 26 -52 4 Mean (SE) 3 Event Rate Per Subject 2 Year 1 0 0. 2 Pbo + Ins n=284 0. 5 0. 2 Pram + Ins n=871 0. 3 Pbo + Ins n=267 Pram + Ins n=820 Pbo + Ins n=175 0. 1 Pram + Ins n=576 P 78
 Other Safety Observations Type 2 Diabetes • No evidence of: – Serious events that are unusual in the absence of drug therapy – Cardiac toxicity – Hepatic toxicity – Renal toxicity • No increase in frequency of clinically significant: – Lipid abnormalities – ECG changes – Changes in vital signs • Systolic blood pressure • Diastolic blood pressure – Laboratory abnormalities P 79
Other Safety Observations Type 2 Diabetes • No evidence of: – Serious events that are unusual in the absence of drug therapy – Cardiac toxicity – Hepatic toxicity – Renal toxicity • No increase in frequency of clinically significant: – Lipid abnormalities – ECG changes – Changes in vital signs • Systolic blood pressure • Diastolic blood pressure – Laboratory abnormalities P 79
 Pramlintide is Efficacious and Safe in Type 2 Diabetes • Improves glycemic control • No increase in insulin use • Weight loss • No safety issues • Dosage recommendation: 120 µg given 2 -3 times/day before meals P 80
Pramlintide is Efficacious and Safe in Type 2 Diabetes • Improves glycemic control • No increase in insulin use • Weight loss • No safety issues • Dosage recommendation: 120 µg given 2 -3 times/day before meals P 80
 Pramlintide Therapy • Program Overview • Pharmacodynamic Review • Type 2 Diabetes • Efficacy • Safety • Type 1 Diabetes àEfficacy • Safety P 81
Pramlintide Therapy • Program Overview • Pharmacodynamic Review • Type 2 Diabetes • Efficacy • Safety • Type 1 Diabetes àEfficacy • Safety P 81
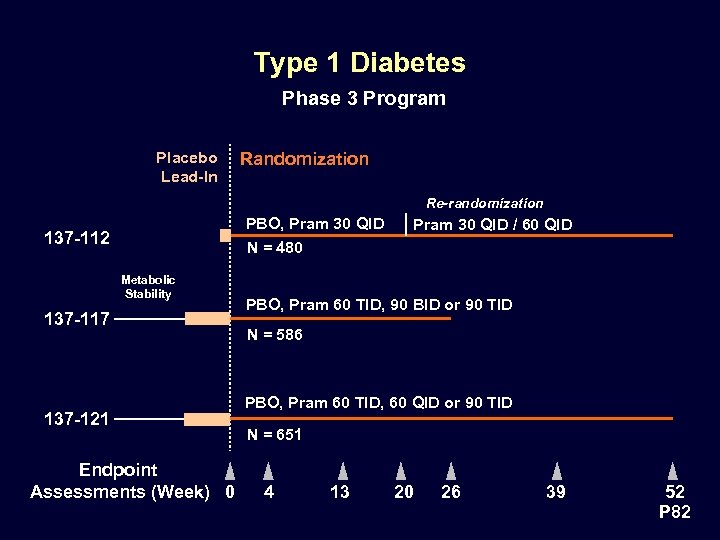 Type 1 Diabetes Phase 3 Program Placebo Lead-In Randomization Re-randomization PBO, Pram 30 QID 137 -112 Pram 30 QID / 60 QID N = 480 Metabolic Stability 137 -117 137 -121 Endpoint Assessments (Week) 0 PBO, Pram 60 TID, 90 BID or 90 TID N = 586 PBO, Pram 60 TID, 60 QID or 90 TID N = 651 4 13 20 26 39 52 P 82
Type 1 Diabetes Phase 3 Program Placebo Lead-In Randomization Re-randomization PBO, Pram 30 QID 137 -112 Pram 30 QID / 60 QID N = 480 Metabolic Stability 137 -117 137 -121 Endpoint Assessments (Week) 0 PBO, Pram 60 TID, 90 BID or 90 TID N = 586 PBO, Pram 60 TID, 60 QID or 90 TID N = 651 4 13 20 26 39 52 P 82
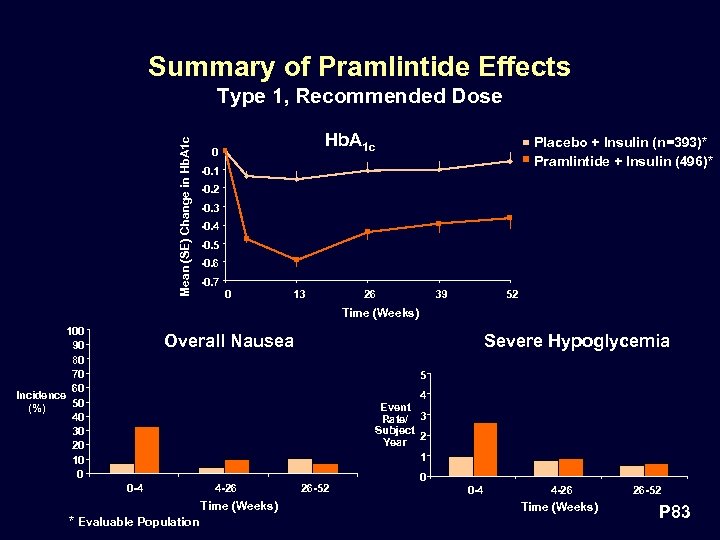 Summary of Pramlintide Effects Mean (SE) Change in Hb. A 1 c Type 1, Recommended Dose Hb. A 1 c 0 Placebo + Insulin (n=393)* Pramlintide + Insulin (496)* -0. 1 -0. 2 -0. 3 -0. 4 -0. 5 -0. 6 -0. 7 0 13 26 39 52 Time (Weeks) 100 90 80 70 60 Incidence 50 (%) 40 30 20 10 0 Overall Nausea Severe Hypoglycemia 5 4 Event Rate/ 3 Subject Year 2 1 0 -4 4 -26 Time (Weeks) * Evaluable Population 26 -52 0 0 -4 4 -26 Time (Weeks) 26 -52 P 83
Summary of Pramlintide Effects Mean (SE) Change in Hb. A 1 c Type 1, Recommended Dose Hb. A 1 c 0 Placebo + Insulin (n=393)* Pramlintide + Insulin (496)* -0. 1 -0. 2 -0. 3 -0. 4 -0. 5 -0. 6 -0. 7 0 13 26 39 52 Time (Weeks) 100 90 80 70 60 Incidence 50 (%) 40 30 20 10 0 Overall Nausea Severe Hypoglycemia 5 4 Event Rate/ 3 Subject Year 2 1 0 -4 4 -26 Time (Weeks) * Evaluable Population 26 -52 0 0 -4 4 -26 Time (Weeks) 26 -52 P 83
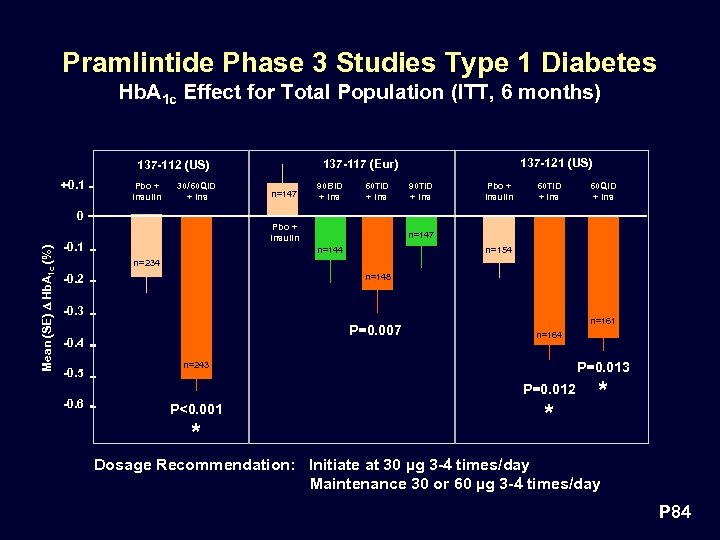 Pramlintide Phase 3 Studies Type 1 Diabetes Hb. A 1 c Effect for Total Population (ITT, 6 months) +0. 1 Pbo + Insulin 30/60 QID + Ins 137 -121 (US) 137 -117 (Eur) 137 -112 (US) n=147 90 BID + Ins 60 TID + Ins 90 TID + Ins Pbo + Insulin 60 TID + Ins 60 QID + Ins Mean (SE) D Hb. A 1 c (%) 0 Pbo + Insulin -0. 1 n=147 n=144 n=154 n=234 -0. 2 n=148 -0. 3 P=0. 007 -0. 4 -0. 5 n=161 n=164 P=0. 013 n=243 P=0. 012 -0. 6 P<0. 001 * * * Dosage Recommendation: Initiate at 30 µg 3 -4 times/day Maintenance 30 or 60 µg 3 -4 times/day P 84
Pramlintide Phase 3 Studies Type 1 Diabetes Hb. A 1 c Effect for Total Population (ITT, 6 months) +0. 1 Pbo + Insulin 30/60 QID + Ins 137 -121 (US) 137 -117 (Eur) 137 -112 (US) n=147 90 BID + Ins 60 TID + Ins 90 TID + Ins Pbo + Insulin 60 TID + Ins 60 QID + Ins Mean (SE) D Hb. A 1 c (%) 0 Pbo + Insulin -0. 1 n=147 n=144 n=154 n=234 -0. 2 n=148 -0. 3 P=0. 007 -0. 4 -0. 5 n=161 n=164 P=0. 013 n=243 P=0. 012 -0. 6 P<0. 001 * * * Dosage Recommendation: Initiate at 30 µg 3 -4 times/day Maintenance 30 or 60 µg 3 -4 times/day P 84
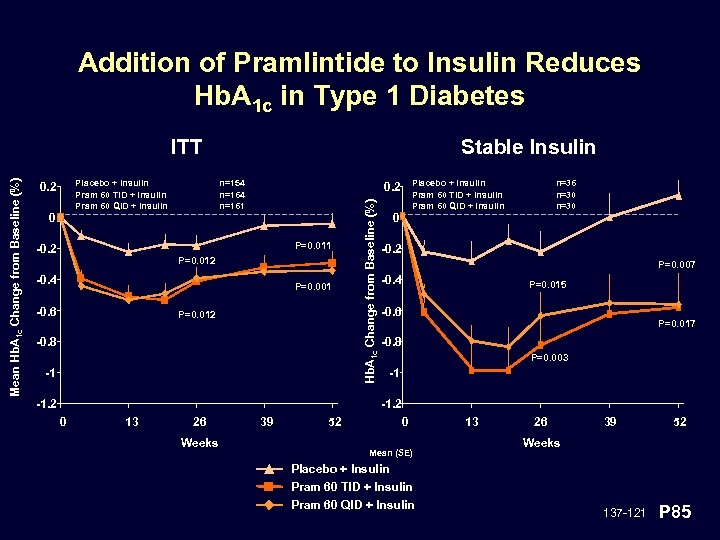 Addition of Pramlintide to Insulin Reduces Hb. A 1 c in Type 1 Diabetes Placebo + Insulin Pram 60 TID + Insulin Pram 60 QID + Insulin 0. 2 0 Stable Insulin n=154 n=161 P=0. 011 -0. 2 P=0. 012 -0. 4 P=0. 001 -0. 6 Placebo + Insulin Pram 60 TID + Insulin Pram 60 QID + Insulin 0. 2 P=0. 012 -0. 8 -1 -1. 2 Hb. A 1 c Change from Baseline (%) Mean Hb. A 1 c Change from Baseline (%) ITT 0 n=36 n=30 -0. 2 P=0. 007 -0. 4 P=0. 015 -0. 6 P=0. 017 -0. 8 P=0. 003 -1 -1. 2 0 13 26 Weeks 39 52 0 Mean (SE) 13 26 39 52 Weeks Placebo + Insulin Pram 60 TID + Insulin Pram 60 QID + Insulin 137 -121 P 85
Addition of Pramlintide to Insulin Reduces Hb. A 1 c in Type 1 Diabetes Placebo + Insulin Pram 60 TID + Insulin Pram 60 QID + Insulin 0. 2 0 Stable Insulin n=154 n=161 P=0. 011 -0. 2 P=0. 012 -0. 4 P=0. 001 -0. 6 Placebo + Insulin Pram 60 TID + Insulin Pram 60 QID + Insulin 0. 2 P=0. 012 -0. 8 -1 -1. 2 Hb. A 1 c Change from Baseline (%) Mean Hb. A 1 c Change from Baseline (%) ITT 0 n=36 n=30 -0. 2 P=0. 007 -0. 4 P=0. 015 -0. 6 P=0. 017 -0. 8 P=0. 003 -1 -1. 2 0 13 26 Weeks 39 52 0 Mean (SE) 13 26 39 52 Weeks Placebo + Insulin Pram 60 TID + Insulin Pram 60 QID + Insulin 137 -121 P 85
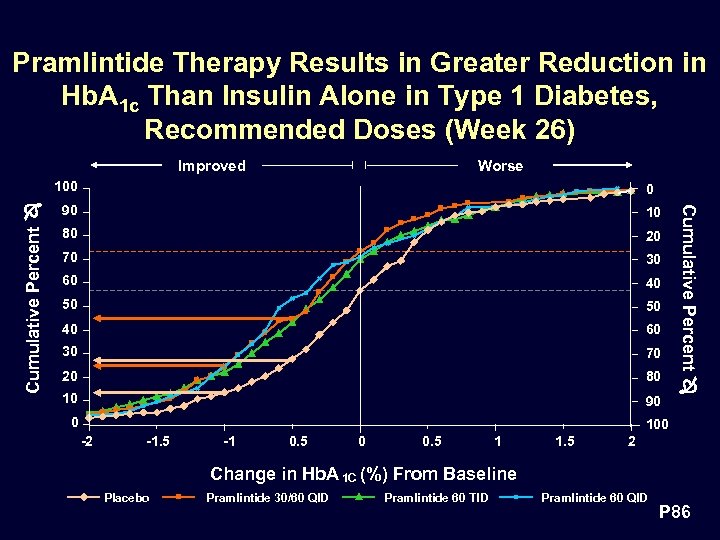 Pramlintide Therapy Results in Greater Reduction in Hb. A 1 c Than Insulin Alone in Type 1 Diabetes, Recommended Doses (Week 26) Improved Worse Cumulative Percent 0 90 10 80 20 70 30 60 40 50 50 40 60 30 70 20 80 10 90 0 Cumulative Percent 100 -2 -1. 5 -1 0. 5 0 0. 5 1 1. 5 2 Change in Hb. A 1 C (%) From Baseline Placebo Pramlintide 30/60 QID Pramlintide 60 TID Pramlintide 60 QID P 86
Pramlintide Therapy Results in Greater Reduction in Hb. A 1 c Than Insulin Alone in Type 1 Diabetes, Recommended Doses (Week 26) Improved Worse Cumulative Percent 0 90 10 80 20 70 30 60 40 50 50 40 60 30 70 20 80 10 90 0 Cumulative Percent 100 -2 -1. 5 -1 0. 5 0 0. 5 1 1. 5 2 Change in Hb. A 1 C (%) From Baseline Placebo Pramlintide 30/60 QID Pramlintide 60 TID Pramlintide 60 QID P 86
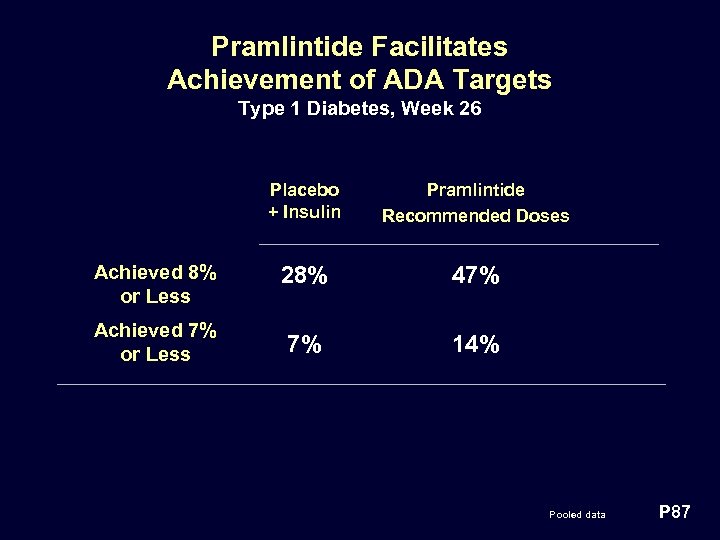 Pramlintide Facilitates Achievement of ADA Targets Type 1 Diabetes, Week 26 Placebo + Insulin Pramlintide Recommended Doses Achieved 8% or Less 28% 47% Achieved 7% or Less 7% 14% Pooled data P 87
Pramlintide Facilitates Achievement of ADA Targets Type 1 Diabetes, Week 26 Placebo + Insulin Pramlintide Recommended Doses Achieved 8% or Less 28% 47% Achieved 7% or Less 7% 14% Pooled data P 87
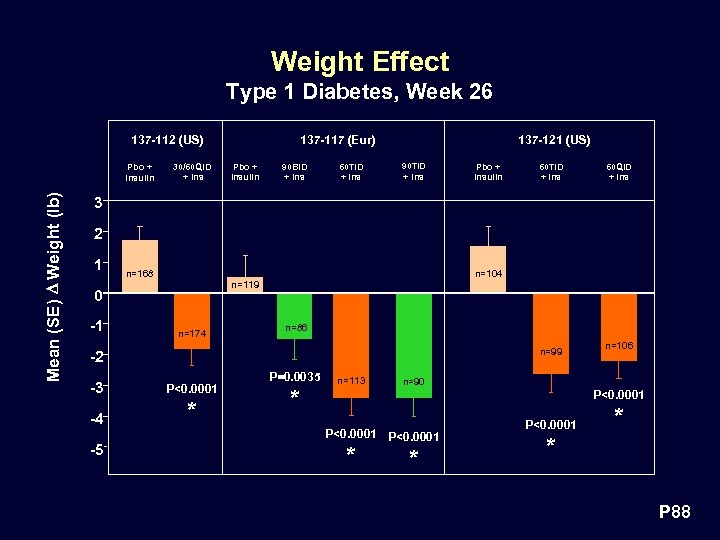 Weight Effect Type 1 Diabetes, Week 26 137 -112 (US) Mean (SE) D Weight (lb) Pbo + Insulin 30/60 QID + Ins 137 -117 (Eur) Pbo + Insulin 90 BID + Ins 60 TID + Ins 137 -121 (US) 90 TID + Ins Pbo + Insulin 60 TID + Ins 60 QID + Ins 3 2 1 n=168 0 -1 n=104 n=119 n=174 n=86 n=99 -2 -3 P<0. 0001 -4 * -5 P=0. 0035 * n=113 n=106 n=90 P<0. 0001 * * P 88
Weight Effect Type 1 Diabetes, Week 26 137 -112 (US) Mean (SE) D Weight (lb) Pbo + Insulin 30/60 QID + Ins 137 -117 (Eur) Pbo + Insulin 90 BID + Ins 60 TID + Ins 137 -121 (US) 90 TID + Ins Pbo + Insulin 60 TID + Ins 60 QID + Ins 3 2 1 n=168 0 -1 n=104 n=119 n=174 n=86 n=99 -2 -3 P<0. 0001 -4 * -5 P=0. 0035 * n=113 n=106 n=90 P<0. 0001 * * P 88
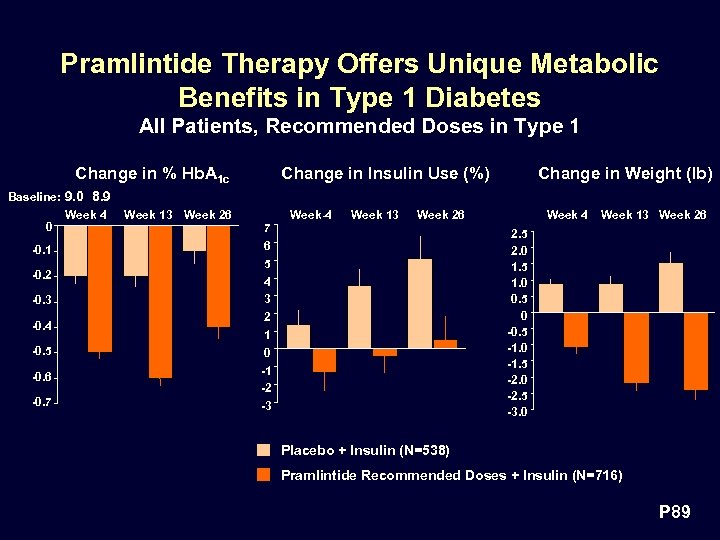 Pramlintide Therapy Offers Unique Metabolic Benefits in Type 1 Diabetes All Patients, Recommended Doses in Type 1 Change in % Hb. A 1 c Change in Insulin Use (%) Change in Weight (lb) Baseline: 9. 0 8. 9 0 -0. 1 -0. 2 -0. 3 -0. 4 -0. 5 -0. 6 -0. 7 Week 4 Week 13 Week 26 7 6 5 4 3 2 1 0 -1 -2 -3 Week 4 Week 13 Week 26 2. 5 2. 0 1. 5 1. 0 0. 5 0 -0. 5 -1. 0 -1. 5 -2. 0 -2. 5 -3. 0 Placebo + Insulin (N=538) Pramlintide Recommended Doses + Insulin (N=716) P 89
Pramlintide Therapy Offers Unique Metabolic Benefits in Type 1 Diabetes All Patients, Recommended Doses in Type 1 Change in % Hb. A 1 c Change in Insulin Use (%) Change in Weight (lb) Baseline: 9. 0 8. 9 0 -0. 1 -0. 2 -0. 3 -0. 4 -0. 5 -0. 6 -0. 7 Week 4 Week 13 Week 26 7 6 5 4 3 2 1 0 -1 -2 -3 Week 4 Week 13 Week 26 2. 5 2. 0 1. 5 1. 0 0. 5 0 -0. 5 -1. 0 -1. 5 -2. 0 -2. 5 -3. 0 Placebo + Insulin (N=538) Pramlintide Recommended Doses + Insulin (N=716) P 89
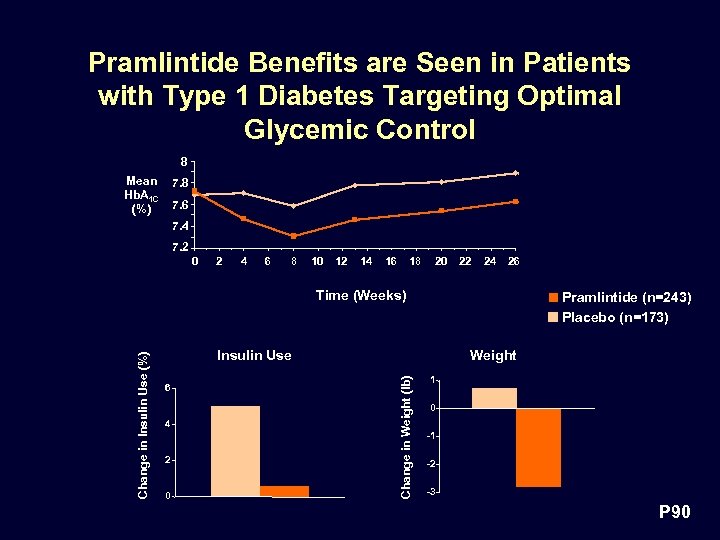 Pramlintide Benefits are Seen in Patients with Type 1 Diabetes Targeting Optimal Glycemic Control 8 Mean Hb. A 1 C (%) 7. 8 7. 6 7. 4 7. 2 0 2 4 6 8 10 12 14 16 18 20 22 24 26 Pramlintide (n=243) Placebo (n=173) Insulin Use 6 4 2 0 Weight Change in Weight (lb) Change in Insulin Use (%) Time (Weeks) 1 0 -1 -2 -3 P 90
Pramlintide Benefits are Seen in Patients with Type 1 Diabetes Targeting Optimal Glycemic Control 8 Mean Hb. A 1 C (%) 7. 8 7. 6 7. 4 7. 2 0 2 4 6 8 10 12 14 16 18 20 22 24 26 Pramlintide (n=243) Placebo (n=173) Insulin Use 6 4 2 0 Weight Change in Weight (lb) Change in Insulin Use (%) Time (Weeks) 1 0 -1 -2 -3 P 90
 Pramlintide Therapy • Program Overview • Pharmacodynamic Review • Type 2 Diabetes • Efficacy • Safety • Type 1 Diabetes • Efficacy àSafety P 91
Pramlintide Therapy • Program Overview • Pharmacodynamic Review • Type 2 Diabetes • Efficacy • Safety • Type 1 Diabetes • Efficacy àSafety P 91
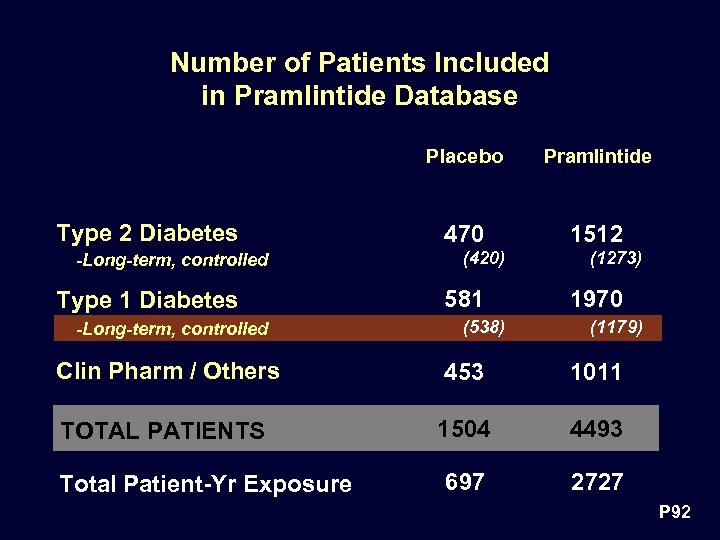 Number of Patients Included in Pramlintide Database Placebo Type 2 Diabetes -Long-term, controlled Type 1 Diabetes -Long-term, controlled Pramlintide 470 1512 (420) 581 (538) (1273) 1970 (1179) Clin Pharm / Others 453 1011 TOTAL PATIENTS 1504 4493 697 2727 Total Patient-Yr Exposure P 92
Number of Patients Included in Pramlintide Database Placebo Type 2 Diabetes -Long-term, controlled Type 1 Diabetes -Long-term, controlled Pramlintide 470 1512 (420) 581 (538) (1273) 1970 (1179) Clin Pharm / Others 453 1011 TOTAL PATIENTS 1504 4493 697 2727 Total Patient-Yr Exposure P 92
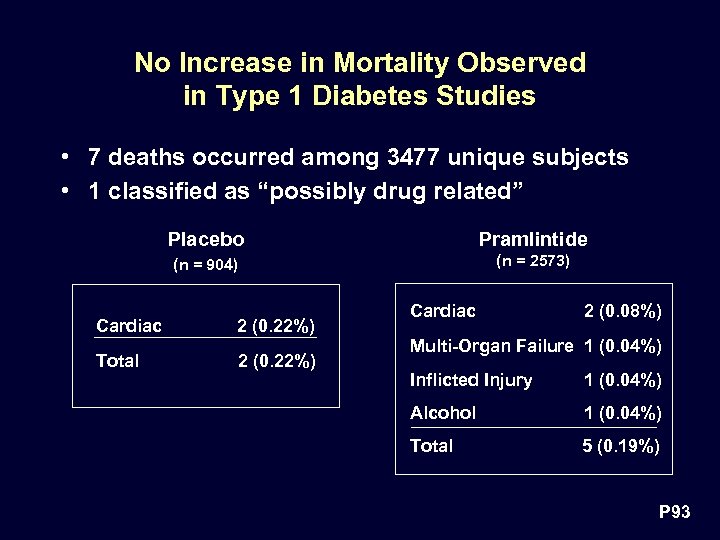 No Increase in Mortality Observed in Type 1 Diabetes Studies • 7 deaths occurred among 3477 unique subjects • 1 classified as “possibly drug related” Placebo Pramlintide (n = 904) (n = 2573) Cardiac 2 (0. 22%) Total 2 (0. 22%) Cardiac 2 (0. 08%) Multi-Organ Failure 1 (0. 04%) Inflicted Injury 1 (0. 04%) Alcohol 1 (0. 04%) Total 5 (0. 19%) P 93
No Increase in Mortality Observed in Type 1 Diabetes Studies • 7 deaths occurred among 3477 unique subjects • 1 classified as “possibly drug related” Placebo Pramlintide (n = 904) (n = 2573) Cardiac 2 (0. 22%) Total 2 (0. 22%) Cardiac 2 (0. 08%) Multi-Organ Failure 1 (0. 04%) Inflicted Injury 1 (0. 04%) Alcohol 1 (0. 04%) Total 5 (0. 19%) P 93
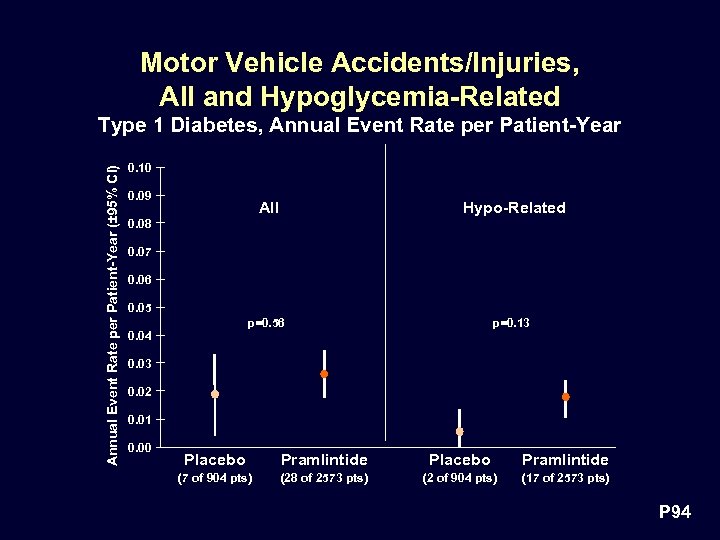 Motor Vehicle Accidents/Injuries, All and Hypoglycemia-Related Annual Event Rate per Patient-Year (± 95% CI) Type 1 Diabetes, Annual Event Rate per Patient-Year 0. 10 0. 09 All Hypo-Related p=0. 56 p=0. 13 0. 08 0. 07 0. 06 0. 05 0. 04 0. 03 0. 02 0. 01 0. 00 Placebo Pramlintide (7 of 904 pts) (28 of 2573 pts) (2 of 904 pts) (17 of 2573 pts) P 94
Motor Vehicle Accidents/Injuries, All and Hypoglycemia-Related Annual Event Rate per Patient-Year (± 95% CI) Type 1 Diabetes, Annual Event Rate per Patient-Year 0. 10 0. 09 All Hypo-Related p=0. 56 p=0. 13 0. 08 0. 07 0. 06 0. 05 0. 04 0. 03 0. 02 0. 01 0. 00 Placebo Pramlintide (7 of 904 pts) (28 of 2573 pts) (2 of 904 pts) (17 of 2573 pts) P 94
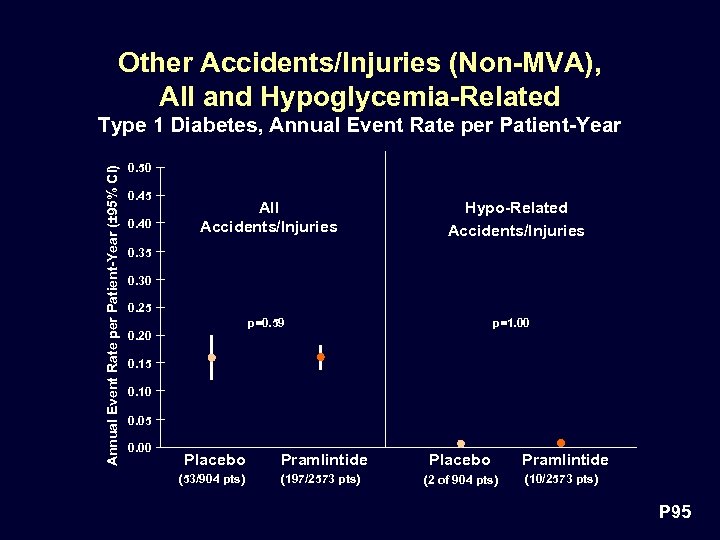 Other Accidents/Injuries (Non-MVA), All and Hypoglycemia-Related Annual Event Rate per Patient-Year (± 95% CI) Type 1 Diabetes, Annual Event Rate per Patient-Year 0. 50 0. 45 0. 40 All Accidents/Injuries Hypo-Related Accidents/Injuries 0. 35 0. 30 0. 25 p=0. 59 p=1. 00 0. 20 0. 15 0. 10 0. 05 0. 00 Placebo Pramlintide Placebo (53/904 pts) (197/2573 pts) (2 of 904 pts) Pramlintide (10/2573 pts) P 95
Other Accidents/Injuries (Non-MVA), All and Hypoglycemia-Related Annual Event Rate per Patient-Year (± 95% CI) Type 1 Diabetes, Annual Event Rate per Patient-Year 0. 50 0. 45 0. 40 All Accidents/Injuries Hypo-Related Accidents/Injuries 0. 35 0. 30 0. 25 p=0. 59 p=1. 00 0. 20 0. 15 0. 10 0. 05 0. 00 Placebo Pramlintide Placebo (53/904 pts) (197/2573 pts) (2 of 904 pts) Pramlintide (10/2573 pts) P 95
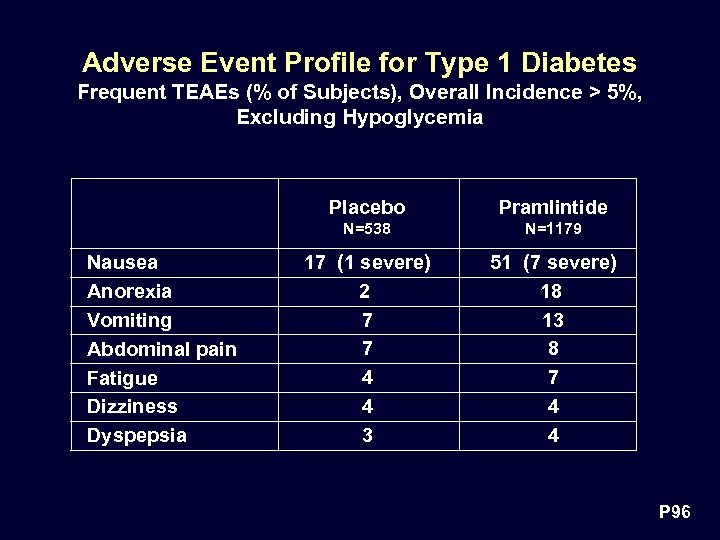 Adverse Event Profile for Type 1 Diabetes Frequent TEAEs (% of Subjects), Overall Incidence > 5%, Excluding Hypoglycemia Placebo N=538 Nausea Anorexia Vomiting Abdominal pain Fatigue Dizziness Dyspepsia Pramlintide N=1179 17 (1 severe) 2 7 7 4 4 3 51 (7 severe) 18 13 8 7 4 4 P 96
Adverse Event Profile for Type 1 Diabetes Frequent TEAEs (% of Subjects), Overall Incidence > 5%, Excluding Hypoglycemia Placebo N=538 Nausea Anorexia Vomiting Abdominal pain Fatigue Dizziness Dyspepsia Pramlintide N=1179 17 (1 severe) 2 7 7 4 4 3 51 (7 severe) 18 13 8 7 4 4 P 96
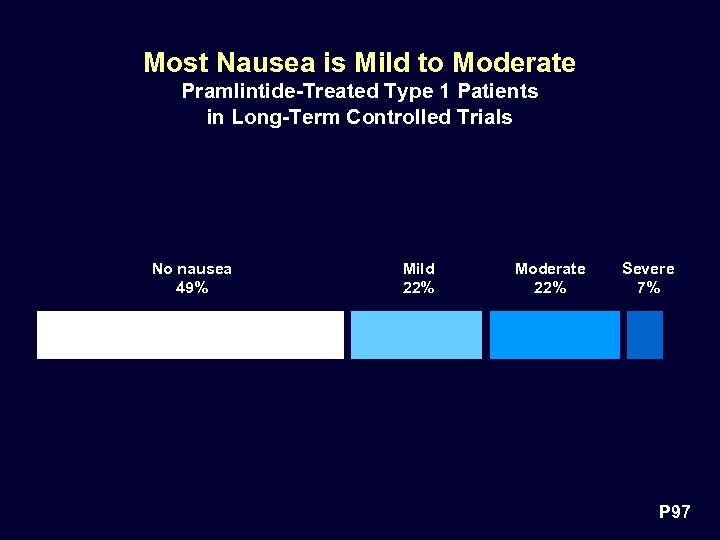 Most Nausea is Mild to Moderate Pramlintide-Treated Type 1 Patients in Long-Term Controlled Trials No nausea 49% Mild 22% Moderate 22% Severe 7% P 97
Most Nausea is Mild to Moderate Pramlintide-Treated Type 1 Patients in Long-Term Controlled Trials No nausea 49% Mild 22% Moderate 22% Severe 7% P 97
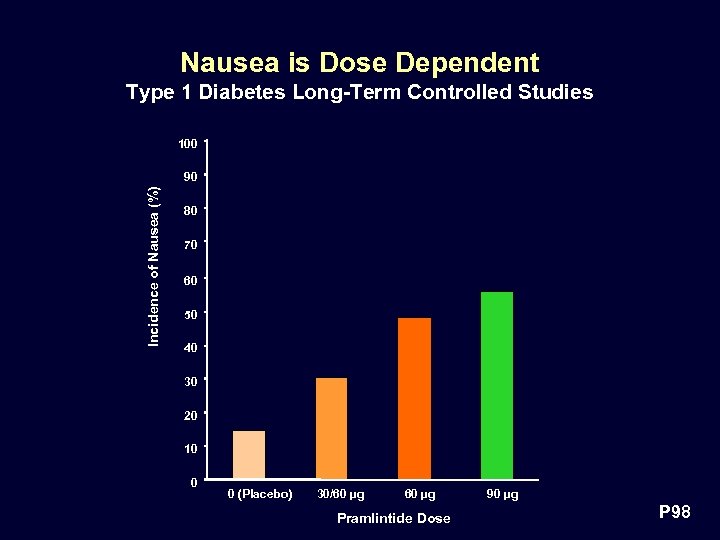 Nausea is Dose Dependent Type 1 Diabetes Long-Term Controlled Studies 100 Incidence of Nausea (%) 90 80 70 60 50 40 30 20 10 0 0 (Placebo) 30/60 µg Pramlintide Dose 90 µg P 98
Nausea is Dose Dependent Type 1 Diabetes Long-Term Controlled Studies 100 Incidence of Nausea (%) 90 80 70 60 50 40 30 20 10 0 0 (Placebo) 30/60 µg Pramlintide Dose 90 µg P 98
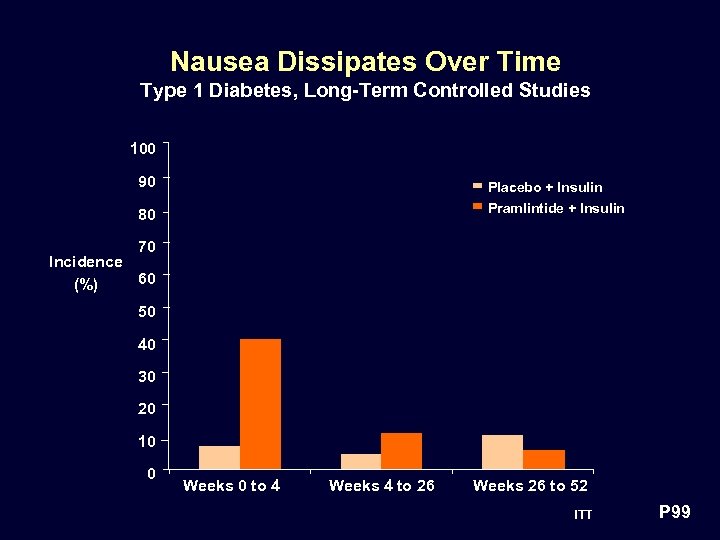 Nausea Dissipates Over Time Type 1 Diabetes, Long-Term Controlled Studies 100 90 Placebo + Insulin Pramlintide + Insulin 80 70 Incidence 60 (%) 50 40 30 20 10 0 Weeks 0 to 4 Weeks 4 to 26 Weeks 26 to 52 ITT P 99
Nausea Dissipates Over Time Type 1 Diabetes, Long-Term Controlled Studies 100 90 Placebo + Insulin Pramlintide + Insulin 80 70 Incidence 60 (%) 50 40 30 20 10 0 Weeks 0 to 4 Weeks 4 to 26 Weeks 26 to 52 ITT P 99
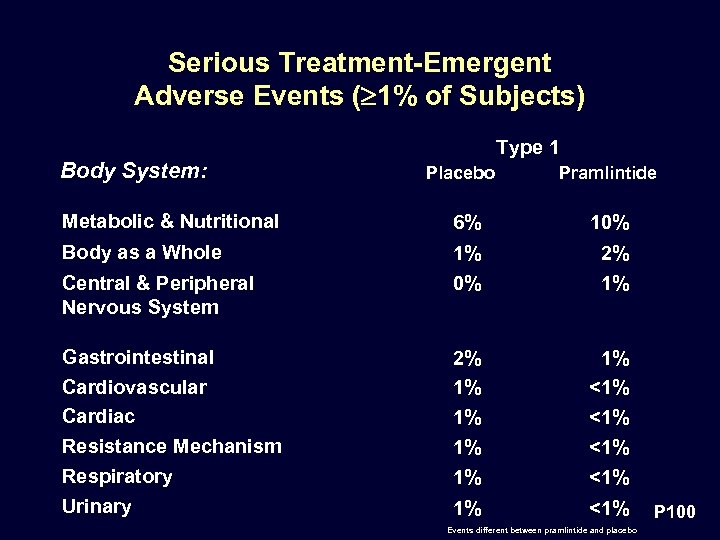 Serious Treatment-Emergent Adverse Events ( 1% of Subjects) Type 1 Body System: Placebo Pramlintide Metabolic & Nutritional 6% 10% Body as a Whole 1% 0% 2% 1% Gastrointestinal Cardiovascular Cardiac Resistance Mechanism 2% 1% 1% <1% <1% Respiratory 1% <1% Urinary 1% <1% Central & Peripheral Nervous System Events different between pramlintide and placebo P 100
Serious Treatment-Emergent Adverse Events ( 1% of Subjects) Type 1 Body System: Placebo Pramlintide Metabolic & Nutritional 6% 10% Body as a Whole 1% 0% 2% 1% Gastrointestinal Cardiovascular Cardiac Resistance Mechanism 2% 1% 1% <1% <1% Respiratory 1% <1% Urinary 1% <1% Central & Peripheral Nervous System Events different between pramlintide and placebo P 100
 Assessment of Severe Hypoglycemia in Long-Term Controlled Trials • Objective endpoints employed (DCCT) – Requiring the assistance of another individual (including aid in ingestion of oral carbohydrate) -or– Requiring the administration of glucagon injection or intravenous glucose • Sponsor’s intent was to have severe hypoglycemia reported as Serious Adverse Events P 101
Assessment of Severe Hypoglycemia in Long-Term Controlled Trials • Objective endpoints employed (DCCT) – Requiring the assistance of another individual (including aid in ingestion of oral carbohydrate) -or– Requiring the administration of glucagon injection or intravenous glucose • Sponsor’s intent was to have severe hypoglycemia reported as Serious Adverse Events P 101
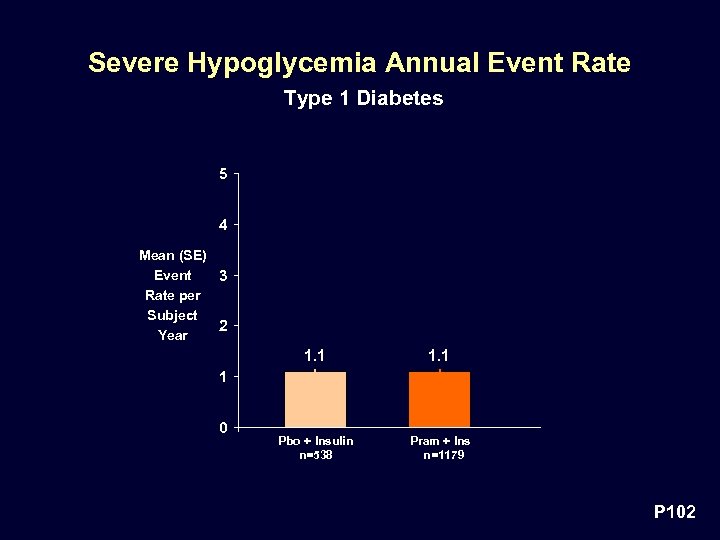 Severe Hypoglycemia Annual Event Rate Type 1 Diabetes 5 4 Mean (SE) Event 3 Rate per Subject 2 Year 1. 1 Pbo + Insulin n=538 Pram + Ins n=1179 1 0 P 102
Severe Hypoglycemia Annual Event Rate Type 1 Diabetes 5 4 Mean (SE) Event 3 Rate per Subject 2 Year 1. 1 Pbo + Insulin n=538 Pram + Ins n=1179 1 0 P 102
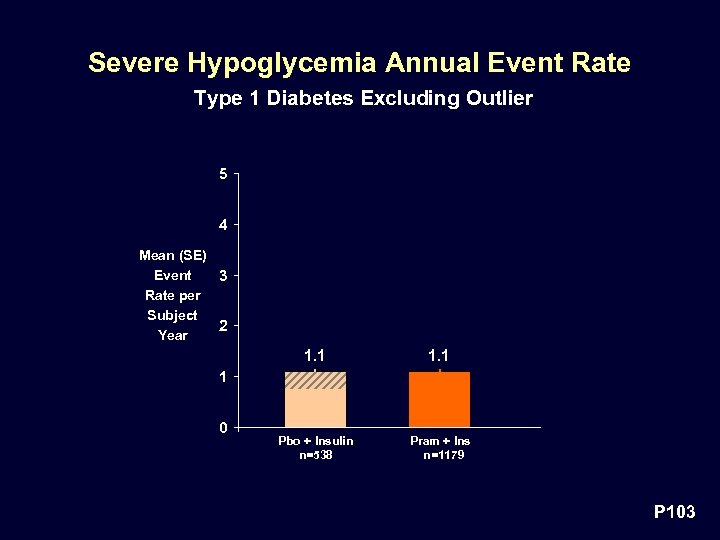 Severe Hypoglycemia Annual Event Rate Type 1 Diabetes Excluding Outlier 5 4 Mean (SE) Event 3 Rate per Subject 2 Year 1. 1 Pbo + Insulin n=538 Pram + Ins n=1179 1 0 P 103
Severe Hypoglycemia Annual Event Rate Type 1 Diabetes Excluding Outlier 5 4 Mean (SE) Event 3 Rate per Subject 2 Year 1. 1 Pbo + Insulin n=538 Pram + Ins n=1179 1 0 P 103
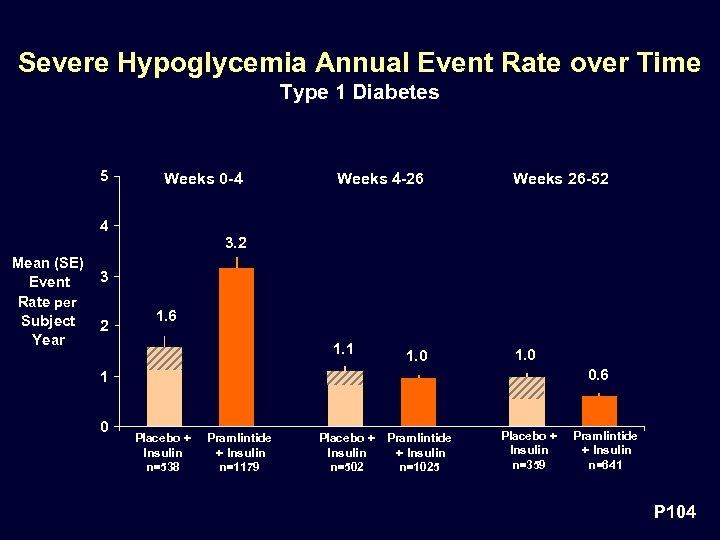 Severe Hypoglycemia Annual Event Rate over Time Type 1 Diabetes 5 Weeks 0 -4 Weeks 4 -26 Weeks 26 -52 1. 1 1. 0 4 3. 2 Mean (SE) Event Rate per Subject Year 3 2 1. 6 1. 0 0. 6 1 0 Placebo + Insulin n=538 Pramlintide + Insulin n=1179 Placebo + Pramlintide Insulin + Insulin n=502 n=1025 Placebo + Insulin n=359 Pramlintide + Insulin n=641 P 104
Severe Hypoglycemia Annual Event Rate over Time Type 1 Diabetes 5 Weeks 0 -4 Weeks 4 -26 Weeks 26 -52 1. 1 1. 0 4 3. 2 Mean (SE) Event Rate per Subject Year 3 2 1. 6 1. 0 0. 6 1 0 Placebo + Insulin n=538 Pramlintide + Insulin n=1179 Placebo + Pramlintide Insulin + Insulin n=502 n=1025 Placebo + Insulin n=359 Pramlintide + Insulin n=641 P 104
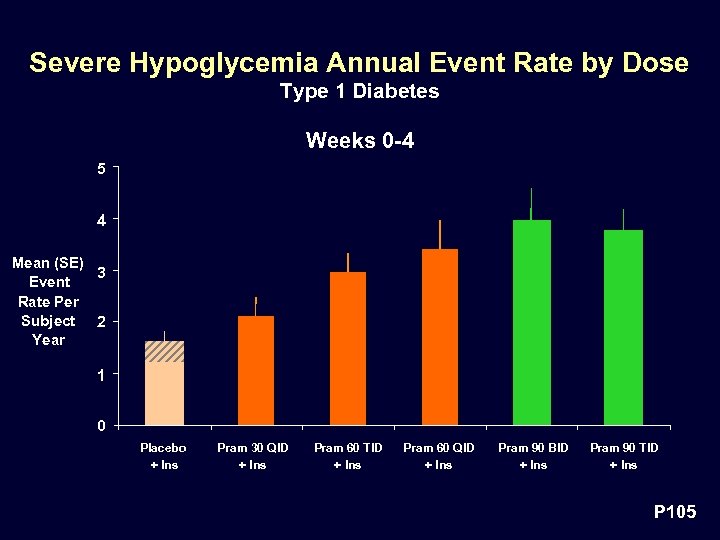 Severe Hypoglycemia Annual Event Rate by Dose Type 1 Diabetes Weeks 0 -4 5 4 Mean (SE) 3 Event Rate Per Subject 2 Year 1 0 Placebo + Ins Pram 30 QID + Ins Pram 60 TID + Ins Pram 60 QID + Ins Pram 90 BID + Ins Pram 90 TID + Ins P 105
Severe Hypoglycemia Annual Event Rate by Dose Type 1 Diabetes Weeks 0 -4 5 4 Mean (SE) 3 Event Rate Per Subject 2 Year 1 0 Placebo + Ins Pram 30 QID + Ins Pram 60 TID + Ins Pram 60 QID + Ins Pram 90 BID + Ins Pram 90 TID + Ins P 105
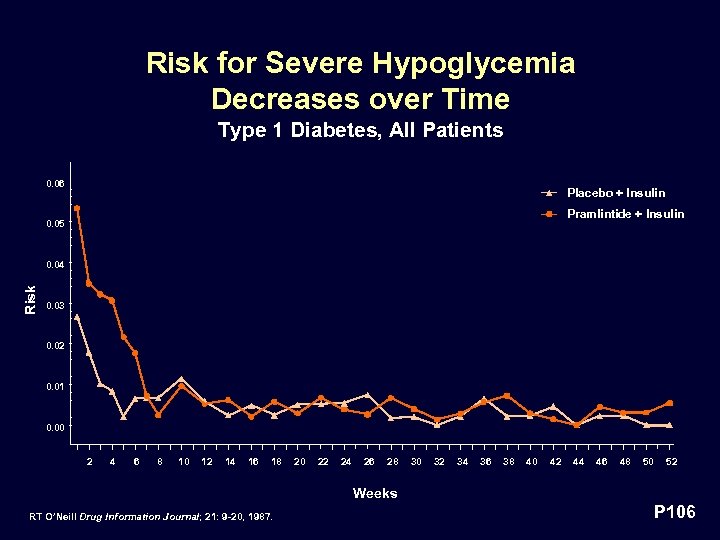 Risk for Severe Hypoglycemia Decreases over Time Type 1 Diabetes, All Patients 0. 06 Placebo + Insulin Pramlintide + Insulin 0. 05 Risk 0. 04 0. 03 0. 02 0. 01 0. 00 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46 48 50 52 Weeks RT O’Neill Drug Information Journal; 21: 9 -20, 1987. P 106
Risk for Severe Hypoglycemia Decreases over Time Type 1 Diabetes, All Patients 0. 06 Placebo + Insulin Pramlintide + Insulin 0. 05 Risk 0. 04 0. 03 0. 02 0. 01 0. 00 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46 48 50 52 Weeks RT O’Neill Drug Information Journal; 21: 9 -20, 1987. P 106
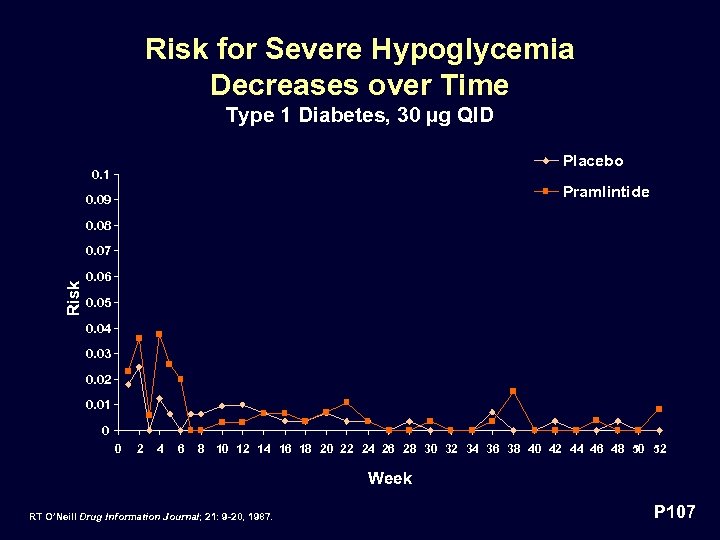 Risk for Severe Hypoglycemia Decreases over Time Type 1 Diabetes, 30 µg QID Placebo 0. 1 Pramlintide 0. 09 0. 08 Risk 0. 07 0. 06 0. 05 0. 04 0. 03 0. 02 0. 01 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46 48 50 52 Week RT O’Neill Drug Information Journal; 21: 9 -20, 1987. P 107
Risk for Severe Hypoglycemia Decreases over Time Type 1 Diabetes, 30 µg QID Placebo 0. 1 Pramlintide 0. 09 0. 08 Risk 0. 07 0. 06 0. 05 0. 04 0. 03 0. 02 0. 01 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46 48 50 52 Week RT O’Neill Drug Information Journal; 21: 9 -20, 1987. P 107
 Pramlintide Alone Does Not Cause Hypoglycemia Normal volunteers did not become hypoglycemic following 10, 000 µg dose 80 x maximum recommended dose P 108
Pramlintide Alone Does Not Cause Hypoglycemia Normal volunteers did not become hypoglycemic following 10, 000 µg dose 80 x maximum recommended dose P 108
 Pramlintide Does Not Alter the Response to Hypoglycemia In Type 1 Diabetes • Pramlintide did not inhibit the counter-regulatory response to hypoglycemia – Time to counter-regulatory hormone release and time to glucose recovery unaffected • No impact on hypoglycemia awareness – Catecholamine responses preserved – Perception of symptoms not diminished P 109
Pramlintide Does Not Alter the Response to Hypoglycemia In Type 1 Diabetes • Pramlintide did not inhibit the counter-regulatory response to hypoglycemia – Time to counter-regulatory hormone release and time to glucose recovery unaffected • No impact on hypoglycemia awareness – Catecholamine responses preserved – Perception of symptoms not diminished P 109
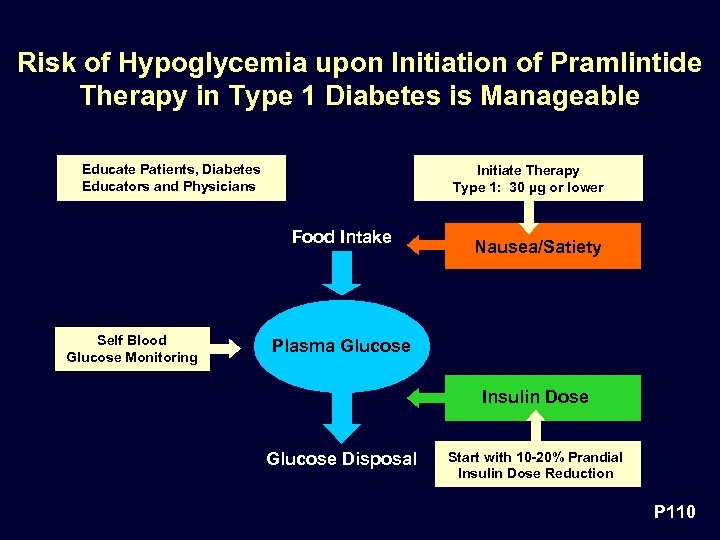 Risk of Hypoglycemia upon Initiation of Pramlintide Therapy in Type 1 Diabetes is Manageable Educate Patients, Diabetes Educators and Physicians Initiate Therapy Type 1: 30 µg or lower Food Intake Self Blood Glucose Monitoring Nausea/Satiety Plasma Glucose Insulin Dose Glucose Disposal Start with 10 -20% Prandial Insulin Dose Reduction P 110
Risk of Hypoglycemia upon Initiation of Pramlintide Therapy in Type 1 Diabetes is Manageable Educate Patients, Diabetes Educators and Physicians Initiate Therapy Type 1: 30 µg or lower Food Intake Self Blood Glucose Monitoring Nausea/Satiety Plasma Glucose Insulin Dose Glucose Disposal Start with 10 -20% Prandial Insulin Dose Reduction P 110
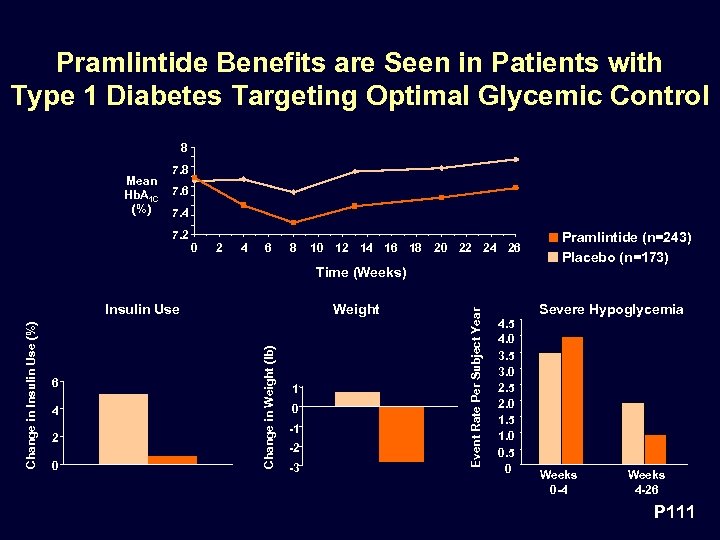 Pramlintide Benefits are Seen in Patients with Type 1 Diabetes Targeting Optimal Glycemic Control 8 Mean Hb. A 1 C (%) 7. 8 7. 6 7. 4 7. 2 0 2 4 6 8 10 12 14 16 18 20 22 24 26 6 4 2 0 Weight Change in Weight (lb) Change in Insulin Use (%) Insulin Use 1 0 -1 -2 -3 Event Rate Per Subject Year Time (Weeks) 4. 5 4. 0 3. 5 3. 0 2. 5 2. 0 1. 5 1. 0 0. 5 0 Pramlintide (n=243) Placebo (n=173) Severe Hypoglycemia Weeks 0 -4 Weeks 4 -26 P 111
Pramlintide Benefits are Seen in Patients with Type 1 Diabetes Targeting Optimal Glycemic Control 8 Mean Hb. A 1 C (%) 7. 8 7. 6 7. 4 7. 2 0 2 4 6 8 10 12 14 16 18 20 22 24 26 6 4 2 0 Weight Change in Weight (lb) Change in Insulin Use (%) Insulin Use 1 0 -1 -2 -3 Event Rate Per Subject Year Time (Weeks) 4. 5 4. 0 3. 5 3. 0 2. 5 2. 0 1. 5 1. 0 0. 5 0 Pramlintide (n=243) Placebo (n=173) Severe Hypoglycemia Weeks 0 -4 Weeks 4 -26 P 111
 Other Safety Observations Type 1 Diabetes • No evidence of: – Serious events that are unusual in the absence of drug therapy – Cardiac toxicity – Hepatic toxicity – Renal toxicity • No increase in frequency of clinically significant: – Lipid abnormalities – ECG changes – Changes in vital signs • Systolic blood pressure • Diastolic blood pressure – Laboratory abnormalities P 112
Other Safety Observations Type 1 Diabetes • No evidence of: – Serious events that are unusual in the absence of drug therapy – Cardiac toxicity – Hepatic toxicity – Renal toxicity • No increase in frequency of clinically significant: – Lipid abnormalities – ECG changes – Changes in vital signs • Systolic blood pressure • Diastolic blood pressure – Laboratory abnormalities P 112
 Pramlintide is Efficacious and Safe in Type 1 Diabetes • Improves glycemic control • Weight loss • Increased insulin-induced hypoglycemia only during initiation of therapy – No increase in insulin-induced hypoglycemia after initiation of therapy • No other safety issues • Dosage recommendation: – Initiate at 30 µg 3 -4 times/day before meals – Maintenance 30 or 60 µg 3 -4 times/day before meals P 113
Pramlintide is Efficacious and Safe in Type 1 Diabetes • Improves glycemic control • Weight loss • Increased insulin-induced hypoglycemia only during initiation of therapy – No increase in insulin-induced hypoglycemia after initiation of therapy • No other safety issues • Dosage recommendation: – Initiate at 30 µg 3 -4 times/day before meals – Maintenance 30 or 60 µg 3 -4 times/day before meals P 113
 Guidelines for Initiation of Therapy • Initial Dose – Type 2: 120 µg – Type 1: 30 µg or lower • Dose Frequency – Determined by meal pattern – Administered within 15 minutes before a meal • Insulin Reduction – 10%-20% of preprandial, short-acting insulin dose P 114
Guidelines for Initiation of Therapy • Initial Dose – Type 2: 120 µg – Type 1: 30 µg or lower • Dose Frequency – Determined by meal pattern – Administered within 15 minutes before a meal • Insulin Reduction – 10%-20% of preprandial, short-acting insulin dose P 114
 Guidelines for Chronic Therapy • Pramlintide Dose – Type 2: 120 µg – Type 1: 30 or 60 µg • Insulin Dose – Adjusted according to standard clinical practice – Guided by self blood glucose monitoring P 115
Guidelines for Chronic Therapy • Pramlintide Dose – Type 2: 120 µg – Type 1: 30 or 60 µg • Insulin Dose – Adjusted according to standard clinical practice – Guided by self blood glucose monitoring P 115
 Pramlintide as Adjunctive Therapy to Insulin Type 2 Diabetes ü Efficacious ü Safe ü Dosage recommendation: 120 µg given 2 -3 times/day before meals Type 1 Diabetes ü Efficacious ü Safe ü Dosage recommendation: Initiate at 30 µg 3 -4 times/day before meals Maintenance 30 or 60 µg 3 -4 times/day before meals P 116
Pramlintide as Adjunctive Therapy to Insulin Type 2 Diabetes ü Efficacious ü Safe ü Dosage recommendation: 120 µg given 2 -3 times/day before meals Type 1 Diabetes ü Efficacious ü Safe ü Dosage recommendation: Initiate at 30 µg 3 -4 times/day before meals Maintenance 30 or 60 µg 3 -4 times/day before meals P 116
 Alain Baron, MD Vice President, Clinical Research, Amylin Pharmaceuticals, Inc. Professor of Medicine Indiana University, School of Medicine Risk/Benefit Summary P 117
Alain Baron, MD Vice President, Clinical Research, Amylin Pharmaceuticals, Inc. Professor of Medicine Indiana University, School of Medicine Risk/Benefit Summary P 117
 Risk and Barriers to Current Insulin Therapy • Hypoglycemia • Inadequate postprandial control – associated glycemic swings • Weight gain • Novel delivery and monitoring devices and insulin analogs are valuable therapeutic advances but still fall short of overcoming these barriers WE NEED NEW TOOLS P 118
Risk and Barriers to Current Insulin Therapy • Hypoglycemia • Inadequate postprandial control – associated glycemic swings • Weight gain • Novel delivery and monitoring devices and insulin analogs are valuable therapeutic advances but still fall short of overcoming these barriers WE NEED NEW TOOLS P 118
 % Hb. A 1 c Reduction Risk of Current Insulin Therapy Insulin Alone • Insulin Dose • Hypoglycemia • Weight Gain P 119
% Hb. A 1 c Reduction Risk of Current Insulin Therapy Insulin Alone • Insulin Dose • Hypoglycemia • Weight Gain P 119
 Type 2 Diabetes Pramlintide offers clear benefits outweighing expected, well recognized and manageable risks P 120
Type 2 Diabetes Pramlintide offers clear benefits outweighing expected, well recognized and manageable risks P 120
 Type 2 Diabetes — Adjunctive to Insulin Therapy Pramlintide is safe Risk Nausea – Mild, infrequent and transient Severe Hypoglycemia – No overall increased risk Management – Good clinical care – Adjustment of insulin dose P 121
Type 2 Diabetes — Adjunctive to Insulin Therapy Pramlintide is safe Risk Nausea – Mild, infrequent and transient Severe Hypoglycemia – No overall increased risk Management – Good clinical care – Adjustment of insulin dose P 121
 Pramlintide Overcomes Barriers and Challenges to Insulin Therapy in Type 2 Diabetes Barriers Benefits Postprandial hyperglycemia – Reduced postprandial glucose excursions Weight gain – Weight loss Hypoglycemia – No overall increased risk Hyperinsulinemia – Permits reduction of insulin dose P 122
Pramlintide Overcomes Barriers and Challenges to Insulin Therapy in Type 2 Diabetes Barriers Benefits Postprandial hyperglycemia – Reduced postprandial glucose excursions Weight gain – Weight loss Hypoglycemia – No overall increased risk Hyperinsulinemia – Permits reduction of insulin dose P 122
 Type 1 Diabetes Pramlintide offers clear benefits outweighing expected, well recognized and manageable risks P 123
Type 1 Diabetes Pramlintide offers clear benefits outweighing expected, well recognized and manageable risks P 123
 Type 1 Diabetes — Adjunctive to Insulin Therapy Pramlintide can be used safely Risk Management Nausea – Mild-moderate-severe – Dose-dependent and transient – Start therapy at 30 µg or less Severe Hypoglycemia – Increased risk upon initiation – Increased nausea/satiety – Dose-dependent – Start therapy at 30 µg or less – Adjustment of insulin dose P 124
Type 1 Diabetes — Adjunctive to Insulin Therapy Pramlintide can be used safely Risk Management Nausea – Mild-moderate-severe – Dose-dependent and transient – Start therapy at 30 µg or less Severe Hypoglycemia – Increased risk upon initiation – Increased nausea/satiety – Dose-dependent – Start therapy at 30 µg or less – Adjustment of insulin dose P 124
 Pramlintide Overcomes Barriers and Challenges to Insulin Therapy in Type 1 Diabetes Barriers Benefits Hypoglycemia – No overall increased risk – Possible reduction of risk post-initiation Weight gain – Weight loss particularly in overweight patients Postprandial hyperglycemia and glycemic swings – Reduces postprandial glucose excursions P 125
Pramlintide Overcomes Barriers and Challenges to Insulin Therapy in Type 1 Diabetes Barriers Benefits Hypoglycemia – No overall increased risk – Possible reduction of risk post-initiation Weight gain – Weight loss particularly in overweight patients Postprandial hyperglycemia and glycemic swings – Reduces postprandial glucose excursions P 125
 Is the Reduction in Hb. A 1 c Obtained with Pramlintide Worthwhile? • Average reductions in Hb. A 1 c of 0. 3 – 0. 7% vs. insulin alone and 0. 5 – 1. 0% vs. baseline are worthwhile • According to DCCT data a 0. 5% reduction in Hb. A 1 c leads to ~ 30% decrease in risk of retinopathy P 126
Is the Reduction in Hb. A 1 c Obtained with Pramlintide Worthwhile? • Average reductions in Hb. A 1 c of 0. 3 – 0. 7% vs. insulin alone and 0. 5 – 1. 0% vs. baseline are worthwhile • According to DCCT data a 0. 5% reduction in Hb. A 1 c leads to ~ 30% decrease in risk of retinopathy P 126
 Benefit of Pramlintide Therapy in Addition to Insulin • To further reduce Hb. A 1 c and attain glycemic goals • To control postprandial hyperglycemia and associated glycemic swings • Minimize weight gain P 127
Benefit of Pramlintide Therapy in Addition to Insulin • To further reduce Hb. A 1 c and attain glycemic goals • To control postprandial hyperglycemia and associated glycemic swings • Minimize weight gain P 127
 Benefits of Pramlintide Therapy in Addition to Insulin Unique Mode of Action • Limits postprandial glycemic excursions by: – Suppressing postprandial glucagon secretion (not achievable by exogenous insulin therapy), and – Modulating the rate of nutrient delivery • Both effects are complementary and additive to the actions of insulin to limit postprandial glycemic excursions P 128
Benefits of Pramlintide Therapy in Addition to Insulin Unique Mode of Action • Limits postprandial glycemic excursions by: – Suppressing postprandial glucagon secretion (not achievable by exogenous insulin therapy), and – Modulating the rate of nutrient delivery • Both effects are complementary and additive to the actions of insulin to limit postprandial glycemic excursions P 128
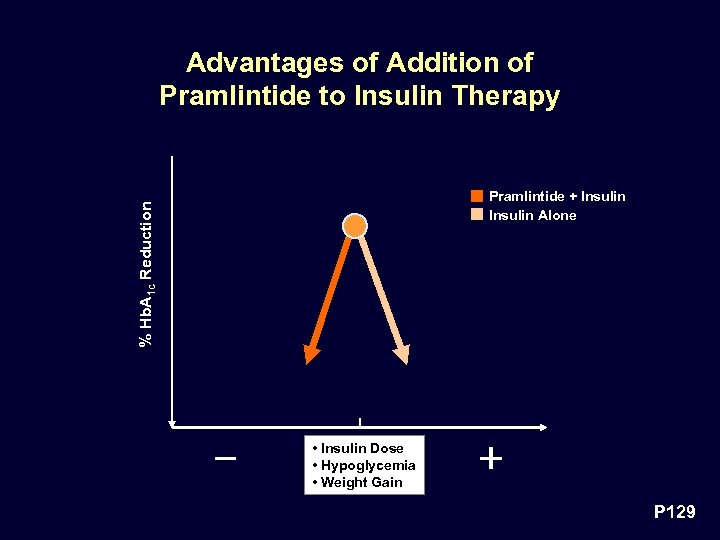 Advantages of Addition of Pramlintide to Insulin Therapy % Hb. A 1 c Reduction Pramlintide + Insulin Alone • Insulin Dose • Hypoglycemia • Weight Gain P 129
Advantages of Addition of Pramlintide to Insulin Therapy % Hb. A 1 c Reduction Pramlintide + Insulin Alone • Insulin Dose • Hypoglycemia • Weight Gain P 129
 The complementary actions of insulin and pramlintide form a potent binary therapeutic tool to lower postprandial plasma glucose P 130
The complementary actions of insulin and pramlintide form a potent binary therapeutic tool to lower postprandial plasma glucose P 130
 Conclusion Amylin replacement with pramlintide represents a novel and unique therapeutic advance that fulfills a need for patients with diabetes treated with insulin P 131
Conclusion Amylin replacement with pramlintide represents a novel and unique therapeutic advance that fulfills a need for patients with diabetes treated with insulin P 131


