0e46d9f8d0da441eb5dd929f89afdd27.ppt
- Количество слайдов: 56
 Jeopardy
Jeopardy
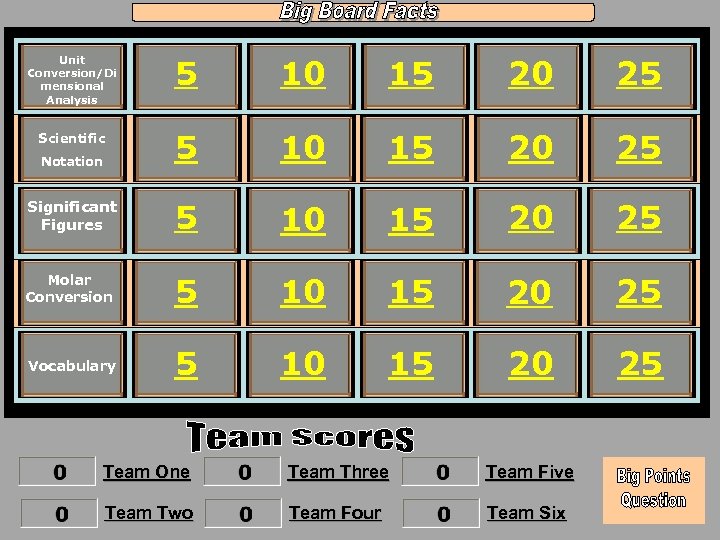 Unit Conversion/Di mensional Analysis 5 10 15 20 25 Scientific Notation 5 10 15 20 25 Significant Figures 5 10 15 20 25 Molar Conversion 5 10 15 20 25 Vocabulary 5 10 15 20 25 Team One Team Three Team Five Team Two Team Four Team Six
Unit Conversion/Di mensional Analysis 5 10 15 20 25 Scientific Notation 5 10 15 20 25 Significant Figures 5 10 15 20 25 Molar Conversion 5 10 15 20 25 Vocabulary 5 10 15 20 25 Team One Team Three Team Five Team Two Team Four Team Six
 Unit Conversion/Dimensional Analysis Your brother goes to the store and buys 5 kg of rice. What amount of rice did he buy in grams? HINT: 1 kg= 1000 g Show Answer
Unit Conversion/Dimensional Analysis Your brother goes to the store and buys 5 kg of rice. What amount of rice did he buy in grams? HINT: 1 kg= 1000 g Show Answer
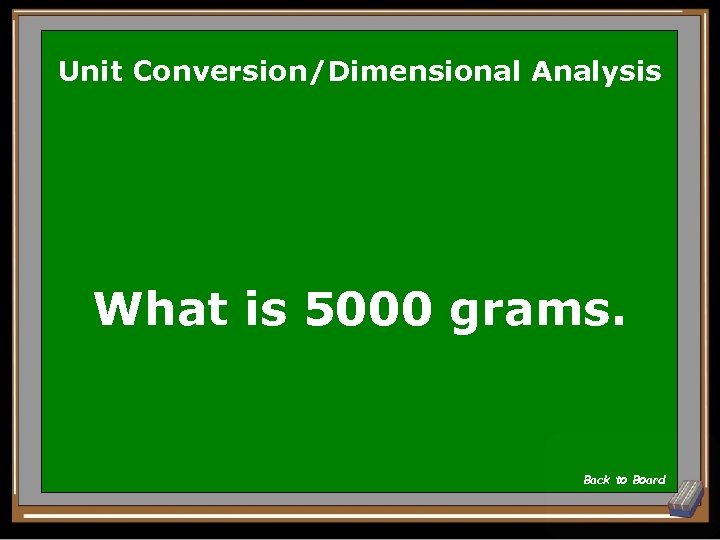 Unit Conversion/Dimensional Analysis What is 5000 grams. Back to Board
Unit Conversion/Dimensional Analysis What is 5000 grams. Back to Board
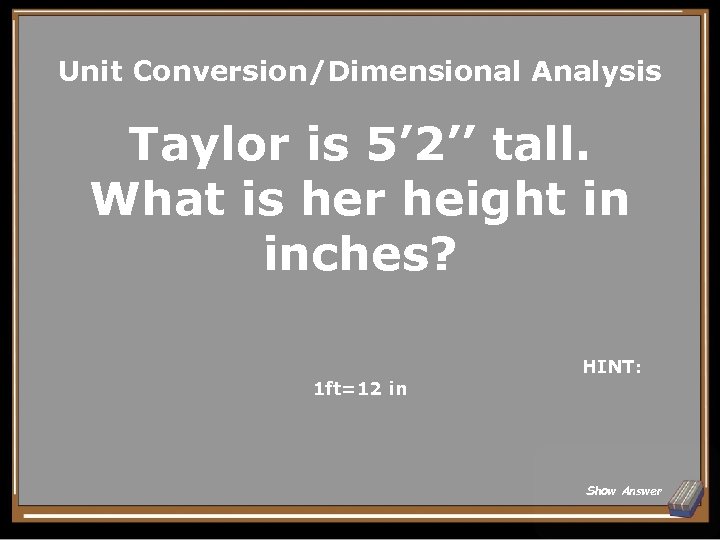 Unit Conversion/Dimensional Analysis Taylor is 5’ 2’’ tall. What is her height in inches? 1 ft=12 in HINT: Show Answer
Unit Conversion/Dimensional Analysis Taylor is 5’ 2’’ tall. What is her height in inches? 1 ft=12 in HINT: Show Answer
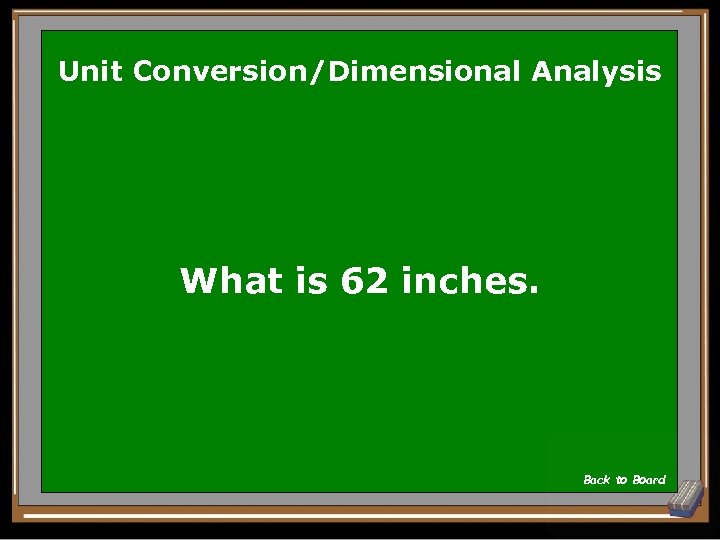 Unit Conversion/Dimensional Analysis What is 62 inches. Back to Board
Unit Conversion/Dimensional Analysis What is 62 inches. Back to Board
 Unit Conversion/Dimensional Analysis A table measures 72. 5 centimeters. How many inches does the table measure? HINT: 1 in=2. 54 cm Show Answer
Unit Conversion/Dimensional Analysis A table measures 72. 5 centimeters. How many inches does the table measure? HINT: 1 in=2. 54 cm Show Answer
 Unit Conversion/Dimensional Analysis What is 28. 5 inches. Back to Board
Unit Conversion/Dimensional Analysis What is 28. 5 inches. Back to Board
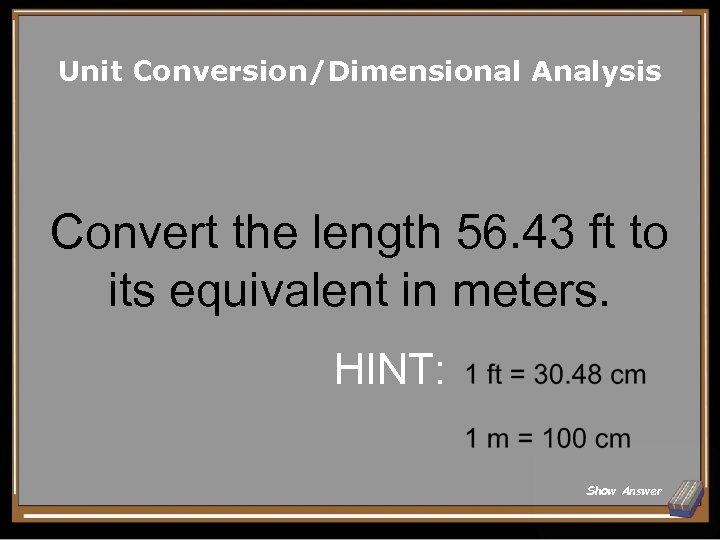 Unit Conversion/Dimensional Analysis Convert the length 56. 43 ft to its equivalent in meters. HINT: Show Answer
Unit Conversion/Dimensional Analysis Convert the length 56. 43 ft to its equivalent in meters. HINT: Show Answer
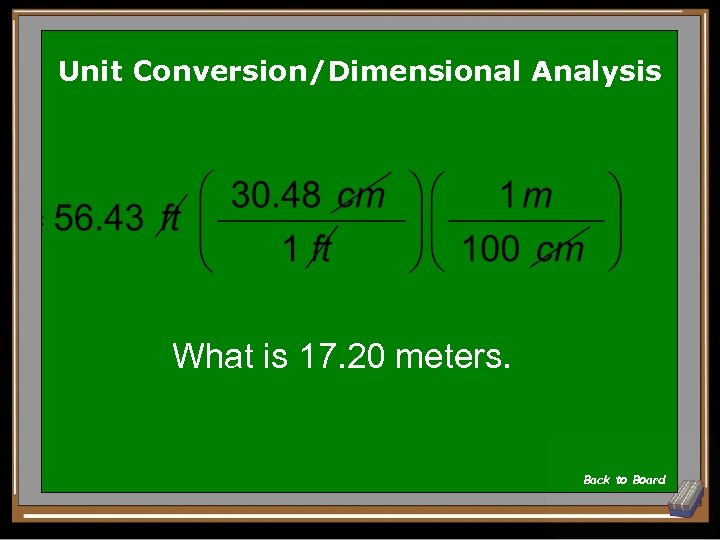 Unit Conversion/Dimensional Analysis What is 17. 20 meters. Back to Board
Unit Conversion/Dimensional Analysis What is 17. 20 meters. Back to Board
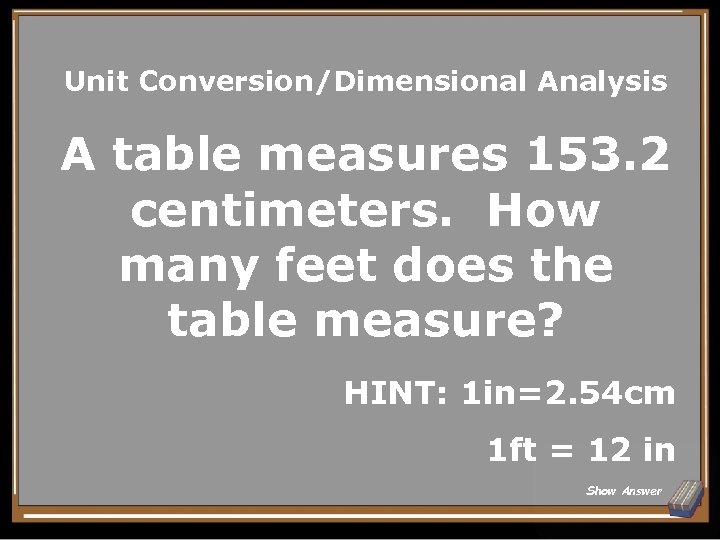 Unit Conversion/Dimensional Analysis A table measures 153. 2 centimeters. How many feet does the table measure? HINT: 1 in=2. 54 cm 1 ft = 12 in Show Answer
Unit Conversion/Dimensional Analysis A table measures 153. 2 centimeters. How many feet does the table measure? HINT: 1 in=2. 54 cm 1 ft = 12 in Show Answer
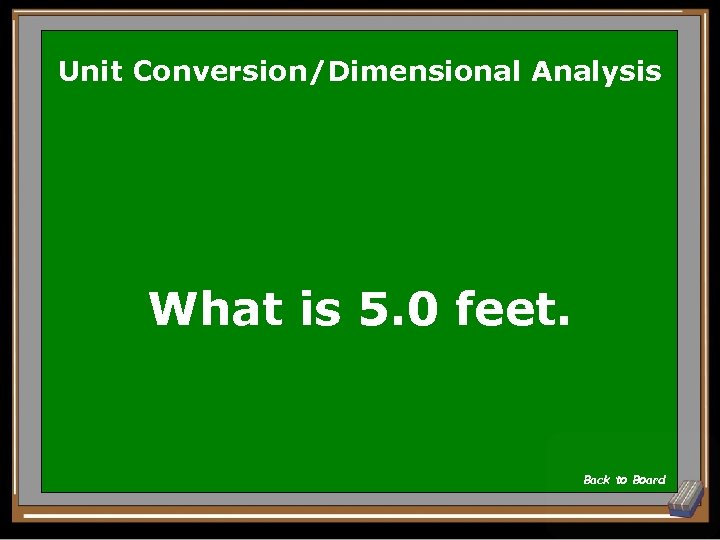 Unit Conversion/Dimensional Analysis What is 5. 0 feet. Back to Board
Unit Conversion/Dimensional Analysis What is 5. 0 feet. Back to Board
 Scientific Notation Write the following in scientific notation: 0. 07882. Show Answer
Scientific Notation Write the following in scientific notation: 0. 07882. Show Answer
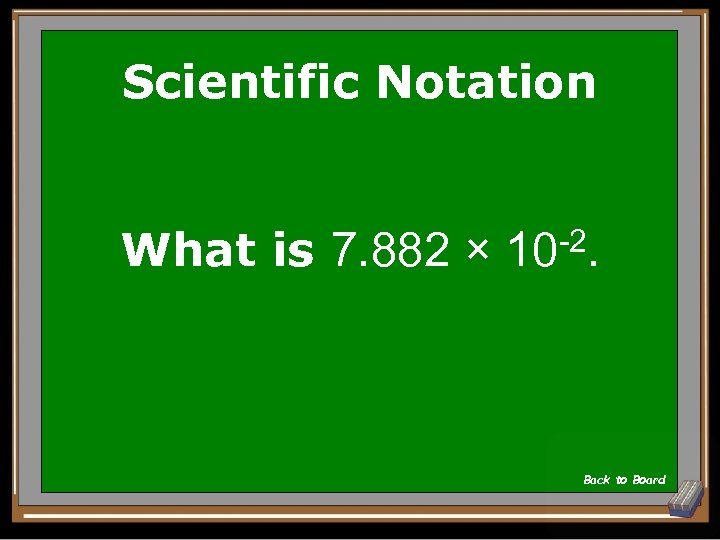 Scientific Notation What is 7. 882 × -2. 10 Back to Board
Scientific Notation What is 7. 882 × -2. 10 Back to Board
 Scientific Notation Write the following in scientific notation: 0. 0000085. Show Answer
Scientific Notation Write the following in scientific notation: 0. 0000085. Show Answer
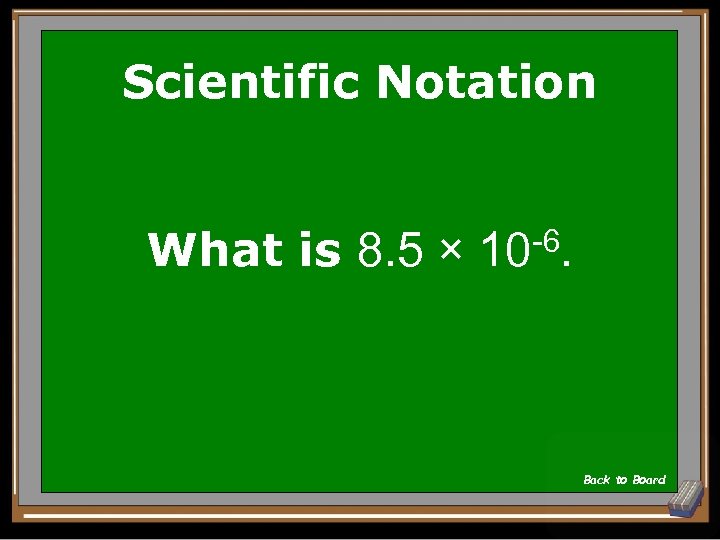 Scientific Notation What is 8. 5 × -6. 10 Back to Board
Scientific Notation What is 8. 5 × -6. 10 Back to Board
 Scientific Notation Write the following number in standard form: 6. 58157 × 107 Show Answer
Scientific Notation Write the following number in standard form: 6. 58157 × 107 Show Answer
 Scientific Notation What is 65815700. Back to Board
Scientific Notation What is 65815700. Back to Board
 Scientific Notation Convert the following into scientific notation. 45. 01 Show Answer
Scientific Notation Convert the following into scientific notation. 45. 01 Show Answer
 Scientific Notation What is 1 4. 501 x 10 Back to Board
Scientific Notation What is 1 4. 501 x 10 Back to Board
 Scientific Notation Express the following in standard form: 3. 6 x 101 Show Answer
Scientific Notation Express the following in standard form: 3. 6 x 101 Show Answer
 Scientific Notation What is 36. Back to Board
Scientific Notation What is 36. Back to Board
 Significant Figures How many significant figures in the following number? 90299 Show Answer
Significant Figures How many significant figures in the following number? 90299 Show Answer
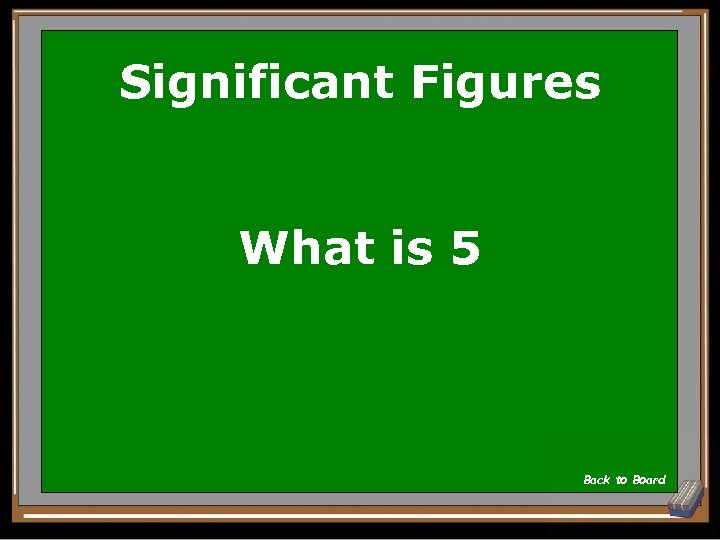 Significant Figures What is 5 Back to Board
Significant Figures What is 5 Back to Board
 Significant Figures How many significant figures in 0. 0253? Show Answer
Significant Figures How many significant figures in 0. 0253? Show Answer
 Significant Figures What is 3. Back to Board
Significant Figures What is 3. Back to Board
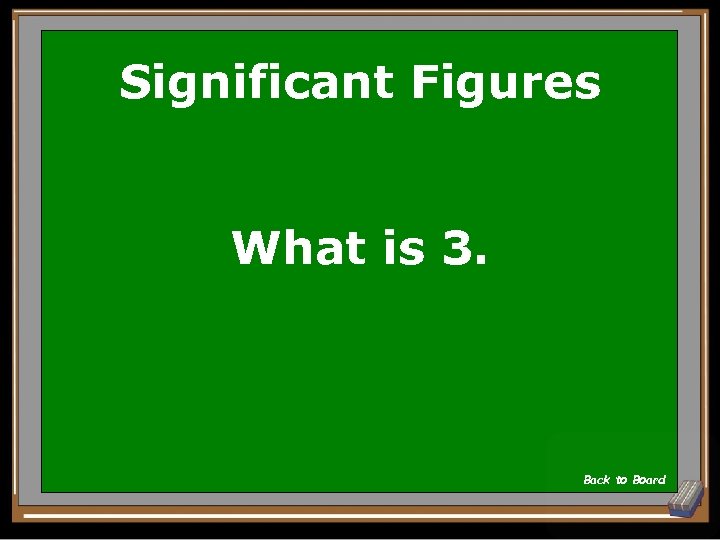
 Significant Figures 0. 00840 has how many significant figures? Show Answer
Significant Figures 0. 00840 has how many significant figures? Show Answer
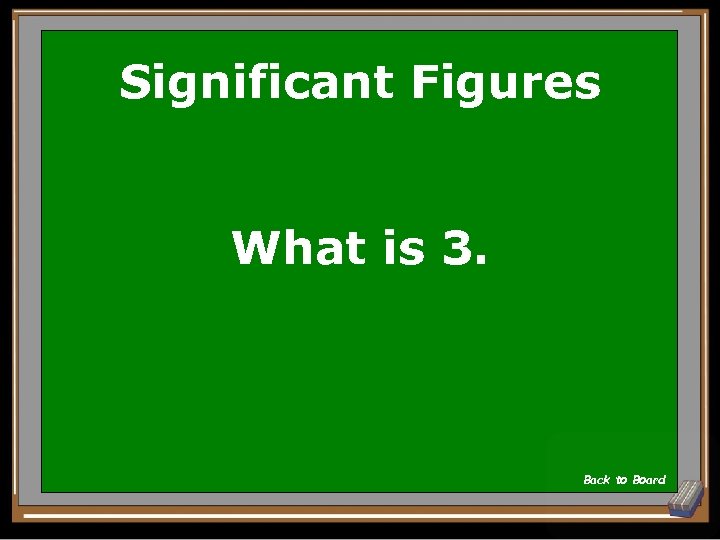 Significant Figures What is 3. Back to Board
Significant Figures What is 3. Back to Board
 Significant Figures What is the area of a rectangle whose sides are 3. 235 cm by 2. 32 cm. Give the answer in the correct number of sig figs. Area = w x l Show Answer
Significant Figures What is the area of a rectangle whose sides are 3. 235 cm by 2. 32 cm. Give the answer in the correct number of sig figs. Area = w x l Show Answer
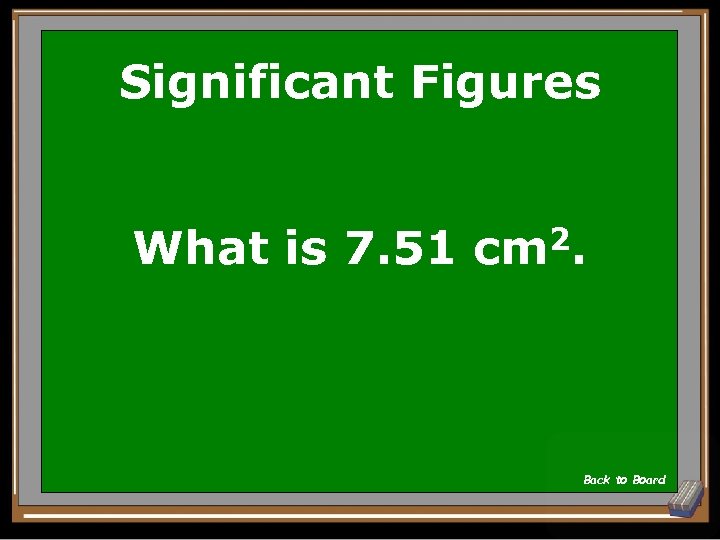 Significant Figures What is 7. 51 cm 2. Back to Board
Significant Figures What is 7. 51 cm 2. Back to Board
 Significant Figures You have $329. 91 and Peter gives you $2. 20. What is the total amount of money you have to the correct number of sig figs? Show Answer
Significant Figures You have $329. 91 and Peter gives you $2. 20. What is the total amount of money you have to the correct number of sig figs? Show Answer
 Significant Figures What is $332. 11. Back to Board
Significant Figures What is $332. 11. Back to Board
 Molar Conversion How many items/particles are in one mole? Show Answer
Molar Conversion How many items/particles are in one mole? Show Answer
 Molar Conversion What is 6. 02 x 10^23 Back to Board
Molar Conversion What is 6. 02 x 10^23 Back to Board
 Molar Conversion How many grams are there in one mole of Pb(NO 3)2 ? Show Answer
Molar Conversion How many grams are there in one mole of Pb(NO 3)2 ? Show Answer
 Molar Conversion What is 331 g Pb(NO 3)2. Back to Board
Molar Conversion What is 331 g Pb(NO 3)2. Back to Board
 Molar Conversion How many mols are there in 428. 6 g of Na. Br? Show Answer
Molar Conversion How many mols are there in 428. 6 g of Na. Br? Show Answer
 Molar Conversion What is 4. 16 mols Na. Br. Back to Board
Molar Conversion What is 4. 16 mols Na. Br. Back to Board
 Molar Conversion How many liters of Hydrogen gas at STP are in 73. 9 mols? Show Answer
Molar Conversion How many liters of Hydrogen gas at STP are in 73. 9 mols? Show Answer
 Molar Conversion What is 1, 655. 4 L of Hydrogen gas. Back to Board
Molar Conversion What is 1, 655. 4 L of Hydrogen gas. Back to Board
 Molar Conversion If Tiaja wants to create 456 g of Re. F 7, how many particles should she begin with? Show Answer
Molar Conversion If Tiaja wants to create 456 g of Re. F 7, how many particles should she begin with? Show Answer
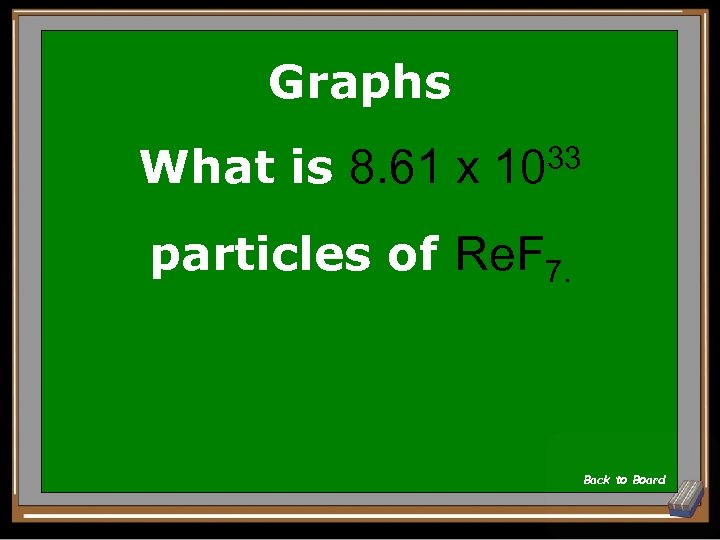 Graphs What is 8. 61 x 1033 particles of Re. F 7. Back to Board
Graphs What is 8. 61 x 1033 particles of Re. F 7. Back to Board
 Vocabulary What is a hypothesis? Show Answer
Vocabulary What is a hypothesis? Show Answer
 Vocabulary What is an educated guess in an “If. . then…” format. Back to Board
Vocabulary What is an educated guess in an “If. . then…” format. Back to Board
 Vocabulary What is density—needs explanation and formula Show Answer
Vocabulary What is density—needs explanation and formula Show Answer
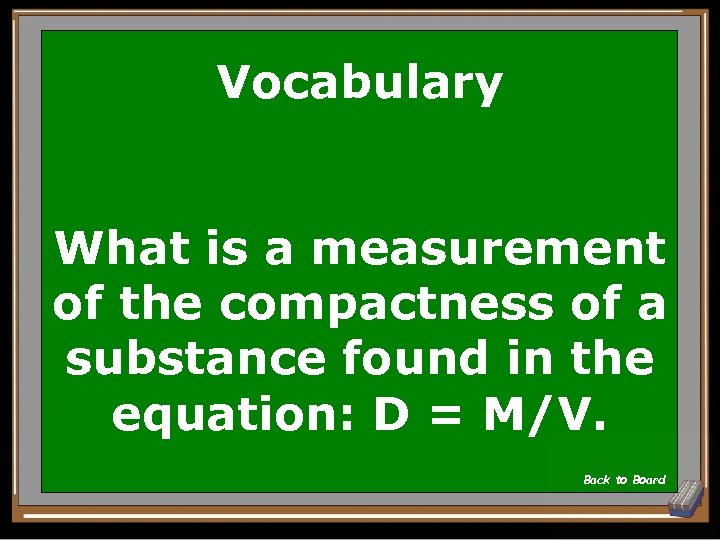 Vocabulary What is a measurement of the compactness of a substance found in the equation: D = M/V. Back to Board
Vocabulary What is a measurement of the compactness of a substance found in the equation: D = M/V. Back to Board
 Vocabulary Describe three examples of a chemical change/property. Show Answer
Vocabulary Describe three examples of a chemical change/property. Show Answer
 Vocabulary Answers will vary. Back to Board
Vocabulary Answers will vary. Back to Board
 Vocabulary Who was Ernest Rutherford? Show Answer
Vocabulary Who was Ernest Rutherford? Show Answer
 Vocabulary Who is the scientist who discovered the nucleus of the atom as well as its positive charge! Back to Board
Vocabulary Who is the scientist who discovered the nucleus of the atom as well as its positive charge! Back to Board
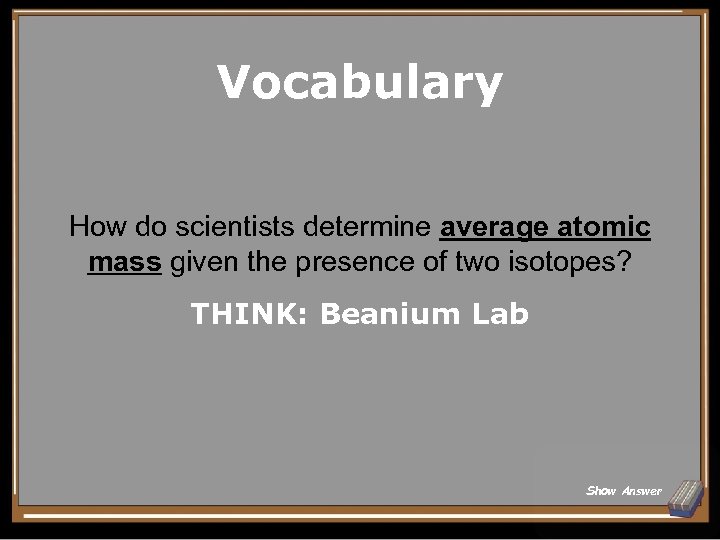 Vocabulary How do scientists determine average atomic mass given the presence of two isotopes? THINK: Beanium Lab Show Answer
Vocabulary How do scientists determine average atomic mass given the presence of two isotopes? THINK: Beanium Lab Show Answer
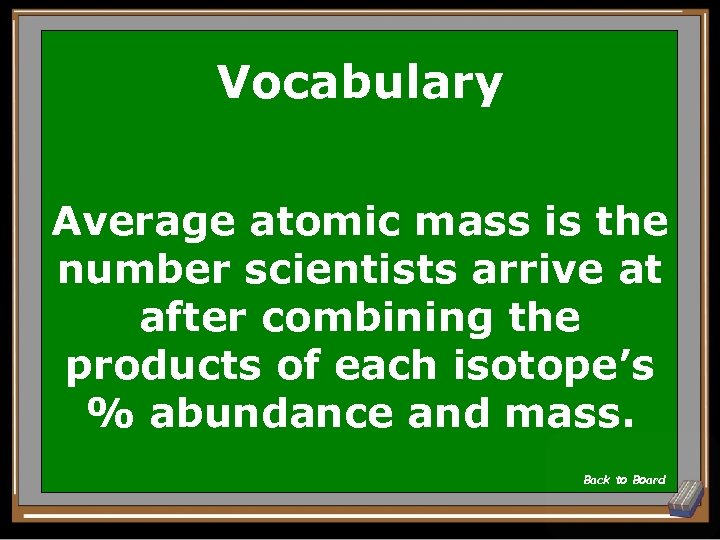 Vocabulary Average atomic mass is the number scientists arrive at after combining the products of each isotope’s % abundance and mass. Back to Board
Vocabulary Average atomic mass is the number scientists arrive at after combining the products of each isotope’s % abundance and mass. Back to Board
 Show Question
Show Question
 25 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 26 27 28 29 30 1 2 3 4 5 6 7 8 9 Where did Uranium come from? NOTE : You must explain the whole story! Show Answer
25 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 26 27 28 29 30 1 2 3 4 5 6 7 8 9 Where did Uranium come from? NOTE : You must explain the whole story! Show Answer
 Big Points Answer Uranium is a product of nuclear fusion that began in the sun’s core with the fusion of Hydrogen and Helium nuclei into bigger and bigger elements. Once the element iron began to form the star (which couldn’t burn iron as fuel) became unstable, collapsed internally, and then exploded. The massive explosion (brighter than millions of our suns) provided the energy necessary to create all elements bigger than iron—including uranium! Back to Board
Big Points Answer Uranium is a product of nuclear fusion that began in the sun’s core with the fusion of Hydrogen and Helium nuclei into bigger and bigger elements. Once the element iron began to form the star (which couldn’t burn iron as fuel) became unstable, collapsed internally, and then exploded. The massive explosion (brighter than millions of our suns) provided the energy necessary to create all elements bigger than iron—including uranium! Back to Board
 Big Board Facts © 2010 Jeff Ertzberger All rights reserved. All Clipart copyright Graphics. Factory. com– All Rights Reserved. Some images have been modified from original version. This presentation may not be sold, or redistributed in any form without written permission of the author. For even more template games and great resources visit: uncw. edu/Ed. Games By using this game you are agreeing to our terms of use. End
Big Board Facts © 2010 Jeff Ertzberger All rights reserved. All Clipart copyright Graphics. Factory. com– All Rights Reserved. Some images have been modified from original version. This presentation may not be sold, or redistributed in any form without written permission of the author. For even more template games and great resources visit: uncw. edu/Ed. Games By using this game you are agreeing to our terms of use. End


