a2d45f0d7e756391d894711f78521ce7.ppt
- Количество слайдов: 36

ISO/IEEE 11073 IEEE, EMB, S 2 E Standards Opportunities for Medical Devices and Healthcare Informatics Presentation to IEEE S 2 E Ex. Com, 31 July 2008: The 21 st Century Healthcare Informatics Industry & IEEE’s leadership roles and opportunities Elliot B. Sloane Villanova University EMBS Ad. Com, IEEE 11073 Sponsor Chair, IEEE’s Healthcare Industry Segment Initiative (’ 03 -’ 05) Presentation developed with input and permission from Todd Cooper, President of Breakthrough Solutions, IEEE 11073 Chair, and ISO TC 215 Chair 1

ISO/IEEE 11073 Bio. Brief: Elliot Sloane, Ph. D, CCE Dual Clinical Engineering and Information Technology “Citizenship!” • 32+ Years of CE and IT/IS Industry and Academic Expertise – Initially, Vice President, ECRI Institute – 15 years, CIO & COO • World’s largest medical device research, testing, standards, and education agency – Then, Vice President, MEDIQ/PRN – 10 Years, COO & CTO • Medical device & drug distribution, service, and manufacturing – Since 2000, Information Systems faculty at Villanova University School of Business, near of Philadelphia. • Teaching, research and publishing in health informatics. • #12 undergraduate Business School in US (Business Week) • Member and Officer in CE/IT/MIS Societies since 1980 – IEEE EMBS Board of Directors since 2003; Member of IEEE SA • Senior Member IEEE, member since 1974. – Board of Directors ANSI HITSP committee – Co-chair International IHE Board of directors representing HIMSS, and co-chair of the IHE Patient Care Device Domain. 2 – Past-President, American College of Clinical Engineering

ISO/IEEE 11073 What is a medical device? • According to FDA, it is “any product (or portion of a product) that affects a patient’s diagnosis or therapy of” 3

ISO/IEEE 11073 Important Update for IEEE standards activities/opportunities In a recent 2008 FDA proposed ruling data communication or storage devices or networks that merely transmit or store patient data will become “medical devices” • e. g. , This might be an opportunity to introduce IEEE S 2 E standards into life-critical healthcare applications, because formal 4 validation is required.

ISO/IEEE 11073 What standards govern medical devices in the US? • Unlike Europe the FDA has NO written federal/state standards for medical devices! – FDA chooses to regulate quality and safety by pre-market screening and post-market surveillance. – The furthest FDA goes is to provide a few “guidance documents” for manufacturers. 5

ISO/IEEE 11073 What standards really govern medical devices in the US? • AAMI, an industry association, develops consensus clinical, technical, and safety standards for specific medical devices like IV Pumps. – Now “importing” European standards from IEC and other sources. • IEC 60601 and some ISO standards cover European medical devices – Some of those standards include “smart” functions such as intelligent alarms, but not the transmission of data. 6

ISO/IEEE 11073 What standards really govern medical devices? • IEEE 11073 committees develop most of the medical information standards(a. k. a. medical informatics standards) related to nomenclature, structure, and transmission of data between medical devices and/lr computer systems used for medical care. – Medical image data standards are handled by DICOM, but realtime waveform data like heart rhythms are not yet addressed there. • Following approval, IEEE 11073 standards are presented for ISO balloting and approval, to make them visible for widespread global adoption. 7

ISO/IEEE 11073 What else is a “medical device? ” • Strong movement now occurring inside/outside government to include “consumer health/medical devices” such as heart monitors used with treadmills as non-regulated, but still partially valid, sources of medical data. – Low cost products suit Medicare plans to reduce costs • Allows people to purchase many products for their medical care at Costco, BJs, and Wal-Mart for own “basic” medical care at home. • HHS plans use “telemedicine” to leverage these low cost devices. – IEEE 802. x (Zigbee, Bluetooth, Wi-Fi, Wi-Max), USB devices, laptops, disk drives, PDAs, etc, will acquire new liabilities when used in such applications. 8

ISO/IEEE 11073 “Industry” (e. g. , Intel, Microsoft, etc) is leading this effort to create standards for this new class of “consumer health/medical devices” and associated communication, storage, and computing accessories. Continua. Alliance. org is the group leading that effort. – Continua Alliance is beginning to build out their standards as a subcommittee within IEEE 11073. – Intel HAS achieved FDA 510 K approval for one consumer product this July! (http: //www. healthnewsdirect. com/? p=372) 9

ISO/IEEE 11073 • How big is the healthcare market in the US? – Roughly $2 trillion/year in 2006 – Projected to reach $4 trillion, or 25% GDP, by 2015. – IOM/National Academies of Engineering report in 2005 gave these facts: • Waste is estimated by government at 30 -40% • Errors are running at 2 -3 Sigma levels • Medical errors are killing 70 -100, 000 patients each year 10

ISO/IEEE 11073 The global solution perceived for spiraling medical costs? Medical Informatics Since the current trends are unsustainable; US and worldwide governments are “changing the game” by building National Healthcare Information Networks (NHINs), essentially bring e. Commerce and automated manufacturing tools and techniques to healthcare. – The US NHIN Program launched in 2004 – Second pilot projects under way in 2008 – Funding to physicians and hospitals in tax incentives and payment bonuses will begin in late 2008 – Participation will remain voluntary, BUT, all payments for non-NHIN -participants will be cut off in 4 -5 years AND data-mining will be used to cull out (i. e. , fire) underperformers 11

ISO/IEEE 11073 • How is the US NHIN being created? – The ANSI Healthcare Technology Standards Panel (HITSP) is given a portfolio of annual informatics projects at the beginning of each year. • HITSP has over 350 associations now, spanning government, vendor, SDO, and other stakeholders. • The HITSP Board has about 15 elected members. – Todd Cooper (an IEEE SA delegate) was elected to the Board by the SDOs because of his medical device and informatics expertise. – Because of my own medical device, informatics, and patient safety background, and the fact that I co-chair the International Integrating the Healthcare Enterprise (IHE) organization, I was elected to the Board by the SDOs as well. 12
Chairman of the ANSI/HITSP Board: Dr. John Halamka, CMIO of Harvard 13

ISO/IEEE 11073 How many IEEE standards have been incorporated in the US NHIN so far? • The first are being added to this year’s drafts – Medical device-related tasks were deferred by the HHS until 2008, which delayed IEEE 11073, and – Despite the fact that the meetings are open, free, and are mostly conducted by teleconference, and despite repeated requests from Todd Cooper and myself, nobody from any IEEE standards committees except 11073 has participated since 2004 14

ISO/IEEE 11073 Will IEEE’s involvement change? • Yes, for the 11073 standards • Maybe for the huge battery of relevant IEEE standards that SHOULD be considered. – S 2 E has finally stepped forward to at least consider bringing the library of software engineering and verification and validation standards into the ANSI/HITSP discussion – We are trying to mobilize IEEE 802. x or various quality, genomics, security/encryption, and other IEEE standards leaders… 15

ISO/IEEE 11073 What’s involved? • HITSP panels do not write primary standards. • HITSP committees do their best to identify all relevant potential standards • HITSP committees do their best to interpret the advantages and disadvantages of all available standards – They post their findings and recommendations for internal and then public review • The bottom line: whoever shows up or speaks up gets to vote, and the earlier, the better! – Committee and subcommittee leaders are volunteers who are voted in mostly due to their willingness to do the work. 16

HITSP TC Existing Constructs Security Privacy & Infrastructure C 19 - Entity Identity Assertion C 25 - Anonymize Care Management & Health Records Administrative & Finance C 35 - Lab Result Terminology C 47 - Resource Utilization C 36 - Lab Result Message C 37 - Lab Report Document T 40 - Patient Eligibility Verification T 17 - Secured Communication Channel T 18 - View Lab Result From Web App TP 46 - Medication Formulary and Benefits Information T 24 - Pseudonymize C 39 - Encounter Message TP 20 - Access Control C 41 - Radiology Result Message TP 30 - Manage Consent Directives TP 49 - Sharing Radiology Results C 26 - Nonrepudiation of Origin T 15 - Collect and Comm Security Audit Trail T 16 - Consistent Time C 44 - Secure Web Connection T 29 - Notification of Document Availability T 31 - Document Reliable Interchange T 23 - Patient Demographics Query T 33 - Transfer of Documents on Media TP 13 - Manage Sharing of Documents TP 14 - Send Lab Result Message TP 21 - Query for Existing Data TP 22 - Patient ID Cross-Referencing TP 50 - Retrieve Form for Data Capture C 48 - Encounter Document C 32 - Summary Docs Using CCD C 28 - Emergency Care Summary Document C 34 - Patient Level Quality Data Message C 38 - Patient Level Quality Data Document T 42 - Medication Dispensing Status TP 43 - Medication Orders 17
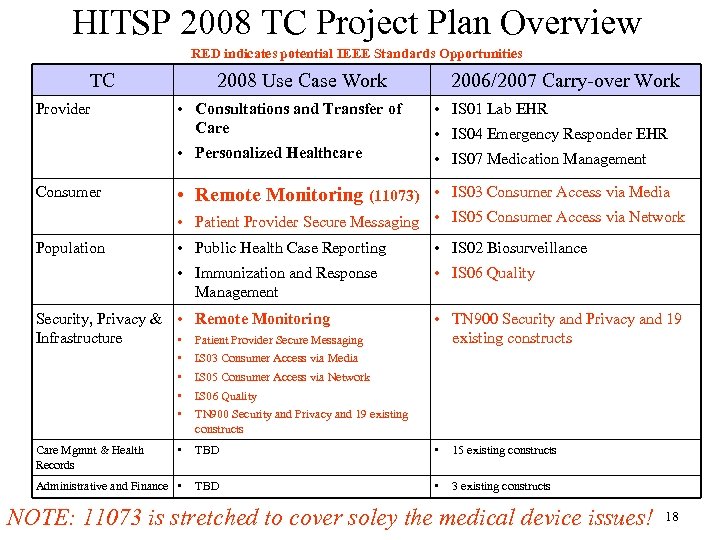
HITSP 2008 TC Project Plan Overview RED indicates potential IEEE Standards Opportunities TC 2008 Use Case Work 2006/2007 Carry-over Work Consumer • Consultations and Transfer of Care • IS 01 Lab EHR • Personalized Healthcare Provider • IS 07 Medication Management • IS 04 Emergency Responder EHR • Remote Monitoring (11073) • IS 03 Consumer Access via Media • Patient Provider Secure Messaging • IS 05 Consumer Access via Network • Public Health Case Reporting • IS 02 Biosurveillance • Immunization and Response Management Population • IS 06 Quality Security, Privacy & • Remote Monitoring Infrastructure • Patient Provider Secure Messaging • TN 900 Security and Privacy and 19 existing constructs • • IS 03 Consumer Access via Media • TBD • 15 existing constructs Administrative and Finance • TBD • 3 existing constructs Care Mgmnt & Health Records IS 05 Consumer Access via Network IS 06 Quality TN 900 Security and Privacy and 19 existing constructs NOTE: 11073 is stretched to cover soley the medical device issues! 18

ISO/IEEE 11073 Tutorial for IEEE EMBS - 2003 -09 -17 ISO/IEEE 11073 The 11073 standards are pretty simple, and constitute a Medical Device Data Language (MDDL): i. e. , the Semantics needed to communicate a device’s application status and control information. Consists of three main components: Ø Nomenclature (1073. 1. 1. 1) Ø Domain Information Model (DIM) (1073. 1. 2. 1) Ø Device Specializations (1073. 1. 3. x) 19
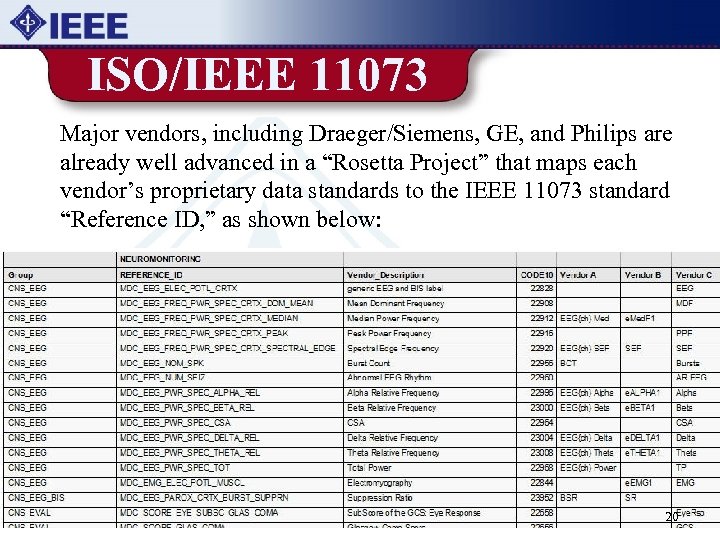
ISO/IEEE 11073 Major vendors, including Draeger/Siemens, GE, and Philips are already well advanced in a “Rosetta Project” that maps each vendor’s proprietary data standards to the IEEE 11073 standard “Reference ID, ” as shown below: 20

21
22
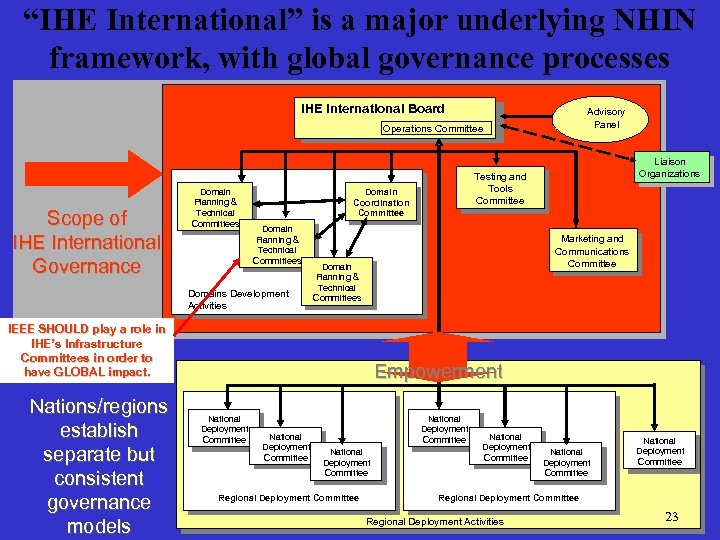
“IHE International” is a major underlying NHIN framework, with global governance processes IHE International Board Advisory Panel Operations Committee Scope of IHE International Governance Domain Planning && Planning Technical Committees Domain Coordination Committee Domain Planning && Planning Technical Committees Domains Development Activities Nations/regions establish separate but consistent governance models Marketing and Communications Committee Domain Planning && Planning Technical Committees IEEE SHOULD play a role in IHE’s Infrastructure Committees in order to have GLOBAL impact. Liaison Organizations Testing and Tools Committee Empowerment National Deployment Committee National Deployment Committee Regional Deployment Committee National National Deployment Deployment Committee Committee Regional Deployment Activities 23
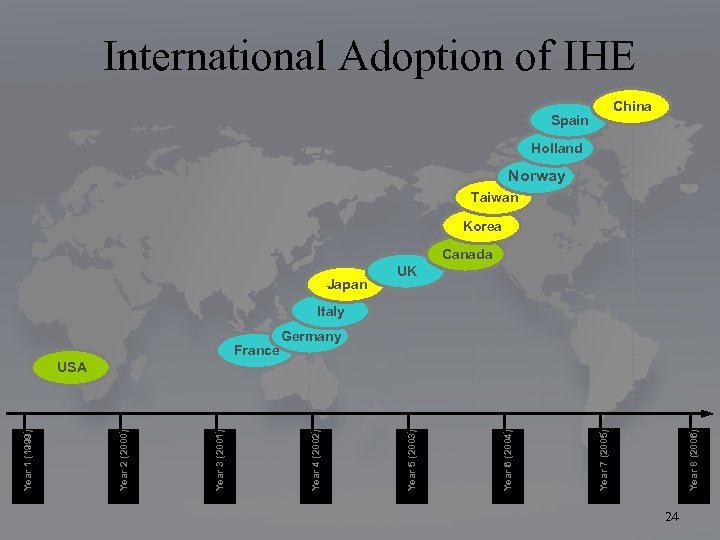
Spain Year 8 (2006) Year 7 (2005) Year 6 (2004) Japan Year 5 (2003) France Year 4 (2002) Year 3 (2001) Year 2 (2000) Year 1 (1999) International Adoption of IHE China Holland Norway Taiwan Korea Canada UK Italy Germany USA 24
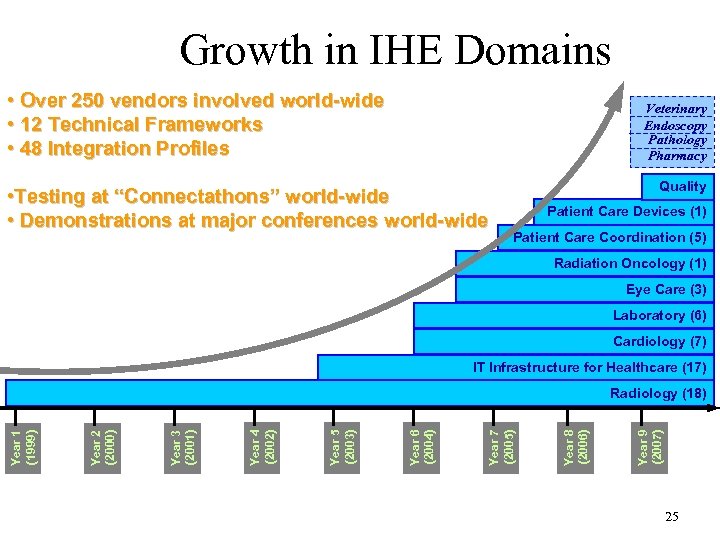
Growth in IHE Domains • Over 250 vendors involved world-wide • 12 Technical Frameworks • 48 Integration Profiles Veterinary Endoscopy Pathology Pharmacy • Testing at “Connectathons” world-wide • Demonstrations at major conferences world-wide Quality Patient Care Devices (1) Patient Care Coordination (5) Radiation Oncology (1) Eye Care (3) Laboratory (6) Cardiology (7) IT Infrastructure for Healthcare (17) Year 9 (2007) Year 8 (2006) Year 7 (2005) Year 6 (2004) Year 5 (2003) Year 4 (2002) Year 3 (2001) Year 2 (2000) Year 1 (1999) Radiology (18) 25

IHE Interoperability Showcases demonstrate working systems by leading COMPETING companies: 26

IHE PCD in the HIMSS 2007 and 2008 Interoperability Showcase 27
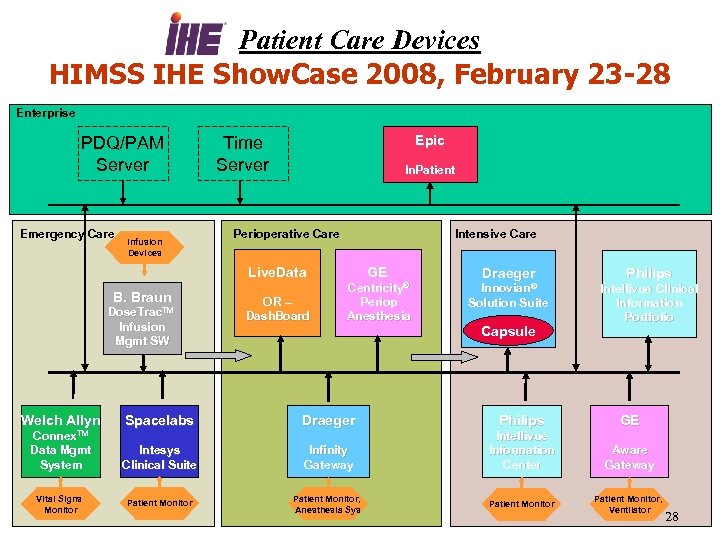
Patient Care Devices HIMSS IHE Show. Case 2008, February 23 -28 Enterprise PDQ/PAM Server Emergency Care Infusion Devices Epic Time Server In. Patient Perioperative Care Intensive Care Live. Data B. Braun Dose. Trac. TM Infusion Mgmt SW GE OR – Dash. Board Centricity® Periop Anesthesia Draeger Philips Innovian® Intellivue Clinical Information Portfolio Solution Suite Capsule Welch Allyn Spacelabs Draeger Philips GE Connex. TM Data Mgmt System Intesys Clinical Suite Infinity Gateway Intellivue Information Center Aware Gateway Patient Monitor, Anesthesia Sys Vital Signs Monitor Patient Monitor, Ventilator 28

ISO/IEEE 11073 Is IEEE represented in IHE International? • I am co-chair of the International Board of Directors • IEEE 11073 members Todd Cooper, Jack Harrington, and have all been co-chairs of the IHE Patient Care Device Domain since its founding in 2002. – The Technical and Planning Committees are also full of IEEE 11073 subcommittee members 29

ISO/IEEE 11073 • Progress? – Worldwide, IEEE 11073 standards are becoming integral to all IHE International Patient Care Device “integration profiles” • The entire IHE family of integration profiles are the largest single resource selected globally by “Ministry of Health” task forces who have been building national electronic healthcare information networks and systems since 2002 or so. • In the US, since 2005, most of the ANSI HITSP panel members and leaders are drawn from the same pool of volunteers who have been drafting the IHE integration profiles since 1998. – Virtually all HITSP standards recommendations since 2005 have therefore been based on the IHE integration profiles. 30

ISO/IEEE 11073 Where is this headed in the US? • All of the State and Federal Medicare agencies, and then all private insurers, will use the next generation of communicating, interoperable medical devices to install telemedicine as the standard of care for chronic disease and wellness care and to automate data capture for electronic health records. – Eventually, most homes will have multiple products that we, today, categorize as “medical devices” – Unless we really mess this up, all state, federal, and home medical devices, systems, and accessories will interoperable with the NHIN via IEEE 11073 standards. • FDA-regulated devices for hospitals and acute will follow suit because, frankly, that makes more economic sense than inventing a separate system than the IEEE 11073 that the vendors, themselves, created! 31

ISO/IEEE 11073 HUGE GAPS exist? • None of the State or Federal healthcare informatics initiatives in the US related to bootstrapping our National Healthcare Information Network have formal S 2 E concepts incorporated (e. g. , no V&V, no formal system development processes, etc. ) – Medical devices themselves have general constraints, I do not believe that NO IEEE Standards are identified as exemplars, nor are courses offered to mfrs to expedite the adoption. 32

ISO/IEEE 11073 Three basic questions we have in front of us: How large does IEEE want its role to be in 21 st Century Healthcare? How, or is, IEEE going to make that happen? Can IEEE even afford to consider sitting on the sidelines in healthcare any longer (e. g. , what if IEEE 802. x is displaced in healthcare, or IEEE Software and Systems Engineering Standards are 33 simply overlooked? )

ISO/IEEE 11073 One proposal: • Get the 11073 and S 2 E experts together to consider formulating a presentation to ANSI/HITSP and IHE leadership. – This will have to be a high-level “gloss” that excites and invites them to welcome the S 2 E expertise quickly in order to create a “fast path” to infuse the concepts into their cultures. – Fortunately, there is a lot of overlap between ANSI/HITSP and IHE membership, though by no means complete… 34

ISO/IEEE 11073 They said it couldn’t be done…. “The young do not know enough to be prudent, and therefore they attempt the impossible -- and achieve it, generation after generation. ” - Pearl S. Buck 35

ISO/IEEE 11073 THANK YOU! ? ? ? QUESTIONS? Elliot B. Sloane, Ph. D, CCE ebsloane@villanova. edu, & @ieee. org, @gmail. com, @aol. com, @yahoo. com, @hotmail. com, etc. www. homepage. villanova. edu/ebsloane or just Google™ me! 36
a2d45f0d7e756391d894711f78521ce7.ppt