fa3e00e32ac3bf85a54b666e151a48a8.ppt
- Количество слайдов: 21
 ISO 15189 in Histopathology Louise O’Callaghan MSc FAMLS Histology Department Bon Secours Hospital, Cork
ISO 15189 in Histopathology Louise O’Callaghan MSc FAMLS Histology Department Bon Secours Hospital, Cork
 Bon Secours Hospital, Cork
Bon Secours Hospital, Cork
 Bon Secours Hospital, Cork Private hospital 340 Bed hospital 18, 000 in patients; 29, 000 outpatients /year Endoscopy Theatres Breast Unit Dermatology Oncology
Bon Secours Hospital, Cork Private hospital 340 Bed hospital 18, 000 in patients; 29, 000 outpatients /year Endoscopy Theatres Breast Unit Dermatology Oncology
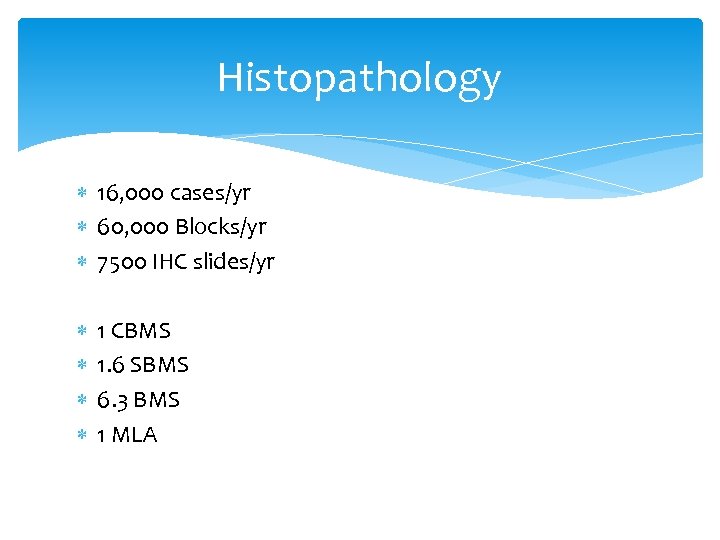 Histopathology 16, 000 cases/yr 60, 000 Blocks/yr 7500 IHC slides/yr 1 CBMS 1. 6 SBMS 6. 3 BMS 1 MLA
Histopathology 16, 000 cases/yr 60, 000 Blocks/yr 7500 IHC slides/yr 1 CBMS 1. 6 SBMS 6. 3 BMS 1 MLA
 Histopathology First accredited ISO 15189 – 2004 April 2015 – ISO 15189: 2012 Cross discipline audits - annually Quality manager + Document Controller + Validation Technician Currently paper based Quality System
Histopathology First accredited ISO 15189 – 2004 April 2015 – ISO 15189: 2012 Cross discipline audits - annually Quality manager + Document Controller + Validation Technician Currently paper based Quality System
 ISO 15189 - Where to start? ? ? The standard: Read / review/ interpret Section 4 – Management requirements Section 5 – Technical requirements Identify road blocks/ obstacles gap analysis Create a plan
ISO 15189 - Where to start? ? ? The standard: Read / review/ interpret Section 4 – Management requirements Section 5 – Technical requirements Identify road blocks/ obstacles gap analysis Create a plan
 Assessments Accrediting body – INAB / UKAS Pre assessment Accreditation for 5 years, with annual surveillance visits – allow extension to scope ‘Surprise’ visits ‘Only answer what you are asked’
Assessments Accrediting body – INAB / UKAS Pre assessment Accreditation for 5 years, with annual surveillance visits – allow extension to scope ‘Surprise’ visits ‘Only answer what you are asked’
 4 Management Requirements 4. 1 Organization and management responsibility Covers: legal entity / ethical conduct/ Lab director / Quality policy / needs of users/ communication/ quality manager 4. 2 Quality Management System Covers: general requirements/ documentation requirements/ quality manual 4. 3 Document control
4 Management Requirements 4. 1 Organization and management responsibility Covers: legal entity / ethical conduct/ Lab director / Quality policy / needs of users/ communication/ quality manager 4. 2 Quality Management System Covers: general requirements/ documentation requirements/ quality manual 4. 3 Document control
 4 Management Requirements 4. 4 service agreements Establish & review service agreements 4. 5 Examination by referral laboratories Select/ review/ maintain list of referral labs & expert opinions Keep record of periodic reviews
4 Management Requirements 4. 4 service agreements Establish & review service agreements 4. 5 Examination by referral laboratories Select/ review/ maintain list of referral labs & expert opinions Keep record of periodic reviews
 4 Management Requirements 4. 6 External services and supplies Procedure for selecting and purchasing equipment/ external services/ reagents and consumable supplies Select and approve suppliers – may need to collaborate with other organizational departments List of selected and approved suppliers maintained Monitor performance of suppliers
4 Management Requirements 4. 6 External services and supplies Procedure for selecting and purchasing equipment/ external services/ reagents and consumable supplies Select and approve suppliers – may need to collaborate with other organizational departments List of selected and approved suppliers maintained Monitor performance of suppliers
 4 Management Requirements 4. 7 Advisory services Arrangements for communicating with users- sample requirements/ advice on clinical cases/ effective utilisation of lab services / logistical issues 4. 8 Resolution of complaints 4. 9 Identification and control of non conformities
4 Management Requirements 4. 7 Advisory services Arrangements for communicating with users- sample requirements/ advice on clinical cases/ effective utilisation of lab services / logistical issues 4. 8 Resolution of complaints 4. 9 Identification and control of non conformities
 4 Management Requirements 4. 10 Corrective Action 4. 11 Preventative Action 4. 12 Continual Improvement 4. 13 Control of records
4 Management Requirements 4. 10 Corrective Action 4. 11 Preventative Action 4. 12 Continual Improvement 4. 13 Control of records
 4 Management Requirements 4. 14 Evaluation and audits Review of requests/ user feedback/ staff suggestions/ internal audit/ risk management/ quality indicators 4. 15 Management review
4 Management Requirements 4. 14 Evaluation and audits Review of requests/ user feedback/ staff suggestions/ internal audit/ risk management/ quality indicators 4. 15 Management review
 5 Technical Requirements 5. 1 Personnel qualifications/ job descriptions/ introduction to organization/ training/ competence/ review of performance/ continuing education and professional development/ personnel records 5. 2 Accommodation and environmental conditions Lab and office facilities/ staff facilities/ storage/ sample collection facilities/ facility maintenance and environmental conditions
5 Technical Requirements 5. 1 Personnel qualifications/ job descriptions/ introduction to organization/ training/ competence/ review of performance/ continuing education and professional development/ personnel records 5. 2 Accommodation and environmental conditions Lab and office facilities/ staff facilities/ storage/ sample collection facilities/ facility maintenance and environmental conditions
 5 Technical Requirements 5. 3 Lab equipment reagents and consumables Equipment acceptance testing/ instructions/ calibration and metrological traceability/ maintenance and repair/ adverse incident reporting/ records Reagent and consumables reception and storage/ acceptance testing/ inventory management/ instructions for use/ adverse incident reporting/ records
5 Technical Requirements 5. 3 Lab equipment reagents and consumables Equipment acceptance testing/ instructions/ calibration and metrological traceability/ maintenance and repair/ adverse incident reporting/ records Reagent and consumables reception and storage/ acceptance testing/ inventory management/ instructions for use/ adverse incident reporting/ records
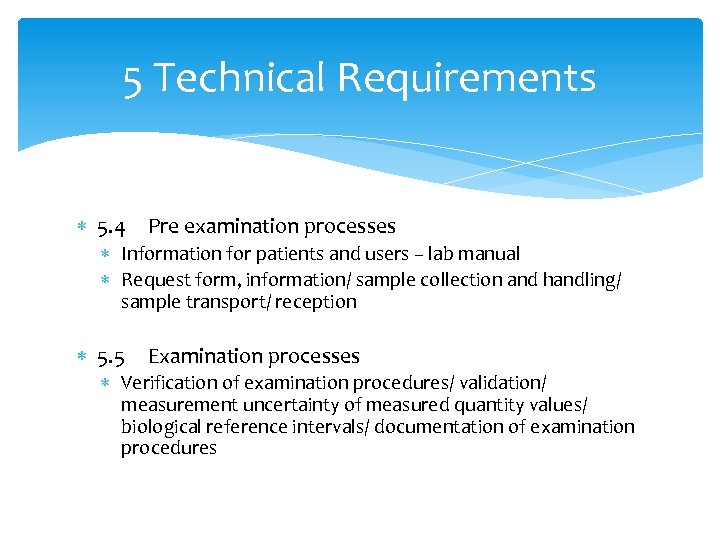 5 Technical Requirements 5. 4 Pre examination processes Information for patients and users – lab manual Request form, information/ sample collection and handling/ sample transport/ reception 5. 5 Examination processes Verification of examination procedures/ validation/ measurement uncertainty of measured quantity values/ biological reference intervals/ documentation of examination procedures
5 Technical Requirements 5. 4 Pre examination processes Information for patients and users – lab manual Request form, information/ sample collection and handling/ sample transport/ reception 5. 5 Examination processes Verification of examination procedures/ validation/ measurement uncertainty of measured quantity values/ biological reference intervals/ documentation of examination procedures
 5 Technical Requirements 5. 6 Ensuring quality of examination results Quality control materials/ data Inter lab comparisons – Participation/ alternative approach 5. 7 Post examination processes Review of results/ storage retention of samples
5 Technical Requirements 5. 6 Ensuring quality of examination results Quality control materials/ data Inter lab comparisons – Participation/ alternative approach 5. 7 Post examination processes Review of results/ storage retention of samples
 5 Technical Requirements 5. 8 Reporting results Report attributes/ content 5. 9 Release of results Critical values Automated selection and reporting of results
5 Technical Requirements 5. 8 Reporting results Report attributes/ content 5. 9 Release of results Critical values Automated selection and reporting of results
 5 Technical Requirements 5. 10 Laboratory Information Management Confidentiality of patient information Define authorities and responsibilities of all personnel System should be validated/ protected/ safeguarded
5 Technical Requirements 5. 10 Laboratory Information Management Confidentiality of patient information Define authorities and responsibilities of all personnel System should be validated/ protected/ safeguarded
 Information Lab manual http: //www. bonsecours. ie/content. Files/files//Cork/path ology/New%20 Folder/BSCPATHGDE 001%20 Ver. %2013. 5. p df
Information Lab manual http: //www. bonsecours. ie/content. Files/files//Cork/path ology/New%20 Folder/BSCPATHGDE 001%20 Ver. %2013. 5. p df
 Thank you! Questions?
Thank you! Questions?


