4eb50fc9e07cd7f71577316eed3d87ca.ppt
- Количество слайдов: 35
 Is Your Document Control… Out of Control? Donna M. Wolk, Ph. D. , D(ABMM) Southern Arizona VA Health Care System / Tucson & University of Arizona 4/6/2006
Is Your Document Control… Out of Control? Donna M. Wolk, Ph. D. , D(ABMM) Southern Arizona VA Health Care System / Tucson & University of Arizona 4/6/2006
 Overview n n Background, beginning 2001 Needs Assessment Software Assessment & Implementation Future Applications for Quality System Recommendations
Overview n n Background, beginning 2001 Needs Assessment Software Assessment & Implementation Future Applications for Quality System Recommendations
 BACKGROUND: Laboratory Medicine and Pathology provides support for: CLINICAL SERVICES VA Hospital plus Comprehensive Health Care Service to 8 Counties (Community-Based Outreach Clinics) RESEARCH VA Research Service Line and Biomedical Research Foundation of Southern Arizona
BACKGROUND: Laboratory Medicine and Pathology provides support for: CLINICAL SERVICES VA Hospital plus Comprehensive Health Care Service to 8 Counties (Community-Based Outreach Clinics) RESEARCH VA Research Service Line and Biomedical Research Foundation of Southern Arizona
 Clinical Laboratory & Research Core
Clinical Laboratory & Research Core
 OUR DOCUMENTS, Sept. , 2001 n n n > 800 documents in 35 manuals Hundreds of gov’t policies & memos, paper and web-based 6 research protocols Educational modules Records
OUR DOCUMENTS, Sept. , 2001 n n n > 800 documents in 35 manuals Hundreds of gov’t policies & memos, paper and web-based 6 research protocols Educational modules Records
 Types of Documents Found n n Personnel: Training, competency, qualifications, job/position descriptions Organization: Organizational charts, definitions, responsibilities & relationships, inspection and accreditation records, Provision of Service Plan, Quality Plan Safety: Accidents reports, Chemical Hygiene Plan, Biohazardous Waste Disposal Plan, Shipping and Handling of Biologicals, Infection Control Plan Audits: Internal and external n n n Performance: Quality assurance records, performance improvement records, faults, reporting errors and accidents, root cause analysis records and corrective action plans Supplies and Equipment: Identification, inventory list, validation records, operation/maintenance checks, and quality control records Manuals: Policies, processes and standard operating procedures Research Protocols Misc. Records
Types of Documents Found n n Personnel: Training, competency, qualifications, job/position descriptions Organization: Organizational charts, definitions, responsibilities & relationships, inspection and accreditation records, Provision of Service Plan, Quality Plan Safety: Accidents reports, Chemical Hygiene Plan, Biohazardous Waste Disposal Plan, Shipping and Handling of Biologicals, Infection Control Plan Audits: Internal and external n n n Performance: Quality assurance records, performance improvement records, faults, reporting errors and accidents, root cause analysis records and corrective action plans Supplies and Equipment: Identification, inventory list, validation records, operation/maintenance checks, and quality control records Manuals: Policies, processes and standard operating procedures Research Protocols Misc. Records
 Internal Assessment Concerns n Development of the new Molecular Lab Program and Clinical Research Core « Rapid changes in the lab and increased training requirements « Future compliance to quality systems regulations, especially document control « Research issues (GLP)
Internal Assessment Concerns n Development of the new Molecular Lab Program and Clinical Research Core « Rapid changes in the lab and increased training requirements « Future compliance to quality systems regulations, especially document control « Research issues (GLP)
 Main Trigger for Concerns: CAP Checklist Item, GEN 13806 n Does the laboratory quality improvement program follow a documented operational plan? NOTE: Plan may be based on: u u u n NCCLS GP-26 ISO JCAHO AABB Lab’s own design Quality Plan Info. has strong emphasis on document control, our # 1 CAP deficiency (1996 -2001)
Main Trigger for Concerns: CAP Checklist Item, GEN 13806 n Does the laboratory quality improvement program follow a documented operational plan? NOTE: Plan may be based on: u u u n NCCLS GP-26 ISO JCAHO AABB Lab’s own design Quality Plan Info. has strong emphasis on document control, our # 1 CAP deficiency (1996 -2001)
 Benchmarking: National Regulatory Compliance by Review of Inspection Deficiencies n n Region 6, VA Regional Commissioner’s Office CAP, JCAHO, VA
Benchmarking: National Regulatory Compliance by Review of Inspection Deficiencies n n Region 6, VA Regional Commissioner’s Office CAP, JCAHO, VA
 Benchmark 2001: We are not alone…. . NATIONALLY: Most Frequent CAP Deficiencies in Clinical Laboratory Inspections Continually Relate to Document Control Issues
Benchmark 2001: We are not alone…. . NATIONALLY: Most Frequent CAP Deficiencies in Clinical Laboratory Inspections Continually Relate to Document Control Issues
 Among top five CAP deficiencies, 1996. n n Is a complete procedure manual written substantially in compliance with NCCLS GP 2 A 2 available at the workbench or in the work area? Is there documentation of at least annual review of all procedures in the
Among top five CAP deficiencies, 1996. n n Is a complete procedure manual written substantially in compliance with NCCLS GP 2 A 2 available at the workbench or in the work area? Is there documentation of at least annual review of all procedures in the
 Most Common CAP Deficiencies 1998 -2001 # 1: Procedure Manuals: Annual review and date/sign each procedure. # 2: Procedure Manual Format: in NCCLS format; personalize manufacturer’s manuals/inserts. Reported by Albert Rabinovitch, MD, Ph. D, CAP in 1998 and Reported again by Francis Sharkey, MD, CAP in 2001
Most Common CAP Deficiencies 1998 -2001 # 1: Procedure Manuals: Annual review and date/sign each procedure. # 2: Procedure Manual Format: in NCCLS format; personalize manufacturer’s manuals/inserts. Reported by Albert Rabinovitch, MD, Ph. D, CAP in 1998 and Reported again by Francis Sharkey, MD, CAP in 2001
 JCAHO Deficiencies Document control in top 10 of all deficiencies, nationally IM. 7. 10 The laboratory has current descriptions and instructions for all analytical methods and procedures. For entire VA (Also among most common deficiency, mainly in CBOCs) (Most frequently cited standards in laboratory inspections of Jan-Dec. 1997, as published by JCAHO)
JCAHO Deficiencies Document control in top 10 of all deficiencies, nationally IM. 7. 10 The laboratory has current descriptions and instructions for all analytical methods and procedures. For entire VA (Also among most common deficiency, mainly in CBOCs) (Most frequently cited standards in laboratory inspections of Jan-Dec. 1997, as published by JCAHO)
 Search for Information, Helpful Resources
Search for Information, Helpful Resources
 NCCLS, now CLSI n GP 26 A: A Quality System Model for Health Care, Approved Guidelines (October 1999) u n Part 4. 6, Quality System Essentials, Documents and Records GP 2 -A 4 Clinical Laboratory Technical Procedure Manuals; 4 th Edition
NCCLS, now CLSI n GP 26 A: A Quality System Model for Health Care, Approved Guidelines (October 1999) u n Part 4. 6, Quality System Essentials, Documents and Records GP 2 -A 4 Clinical Laboratory Technical Procedure Manuals; 4 th Edition
 International Organization for Standardization (ISO) Guidelines n ISO 9001 (Third edition 12 -15 -2000) u Section 4. Quality Management System 4. 2 Document requirements « 4. 2. 1 General; documented procedures to control all documents and data « 4. 2. 2 Quality manual « 4. 2. 3 Control of documents « 4. 2. 4 Control of records «
International Organization for Standardization (ISO) Guidelines n ISO 9001 (Third edition 12 -15 -2000) u Section 4. Quality Management System 4. 2 Document requirements « 4. 2. 1 General; documented procedures to control all documents and data « 4. 2. 2 Quality manual « 4. 2. 3 Control of documents « 4. 2. 4 Control of records «
 Regulations and Regulatory Agency Requirements n n n College of American Pathologist Standards Joint Commission on Accreditation of Healthcare Organizations Standards U. S. Federal Laws, Code of Federal Regulations, Federal Register, CLIA, FDA, GLP, etc. n Veterans Health Administration Directive and Handbook n American Association of Blood Banks Standards
Regulations and Regulatory Agency Requirements n n n College of American Pathologist Standards Joint Commission on Accreditation of Healthcare Organizations Standards U. S. Federal Laws, Code of Federal Regulations, Federal Register, CLIA, FDA, GLP, etc. n Veterans Health Administration Directive and Handbook n American Association of Blood Banks Standards
 Summary: The Document Control Needs are: n n Documents (policies, processes and procedures) must be identified, reviewed, approved, and retained Records must be created, stored and archived
Summary: The Document Control Needs are: n n Documents (policies, processes and procedures) must be identified, reviewed, approved, and retained Records must be created, stored and archived
 Historical Document Control System Did Not Meet Our Needs n n n Paper copies in duplicate locations Retired copies in manager’s file cabinets Electronic documents saved in various locations u u n C drives Common S drive on network server Floppy disks Not at all Reviews performed yearly typically en masse
Historical Document Control System Did Not Meet Our Needs n n n Paper copies in duplicate locations Retired copies in manager’s file cabinets Electronic documents saved in various locations u u n C drives Common S drive on network server Floppy disks Not at all Reviews performed yearly typically en masse
 Software Assessment Help for controlling documents
Software Assessment Help for controlling documents
 Software Resources Review www. qualitydigest. com/feb 01/html/docbg. html www. documantmanagement. org. uk/pages/vendors. htm www. pdmic. com/vendors/docimage 1. html Examples of Software Solutions MS Office Suite & Front Page Adobe Acrobat Integrum Share. Point, Microsoft Visio Proquis/All Clear Documentum
Software Resources Review www. qualitydigest. com/feb 01/html/docbg. html www. documantmanagement. org. uk/pages/vendors. htm www. pdmic. com/vendors/docimage 1. html Examples of Software Solutions MS Office Suite & Front Page Adobe Acrobat Integrum Share. Point, Microsoft Visio Proquis/All Clear Documentum
 Document Control with Proquis n n n Keeps all the details of the documents, including the documents (. doc, . xls. , . pdf, etc) themselves. Maintains the controls and security levels (authorship, authorization, etc. ) to be applied to each document. Details distribution and access of each document Records change requests Records approvals and confirmations of changes Archives documents and keeps history
Document Control with Proquis n n n Keeps all the details of the documents, including the documents (. doc, . xls. , . pdf, etc) themselves. Maintains the controls and security levels (authorship, authorization, etc. ) to be applied to each document. Details distribution and access of each document Records change requests Records approvals and confirmations of changes Archives documents and keeps history
 Proquis DEMO!!!!!
Proquis DEMO!!!!!
 Access on PC & Thin Client Desktop
Access on PC & Thin Client Desktop
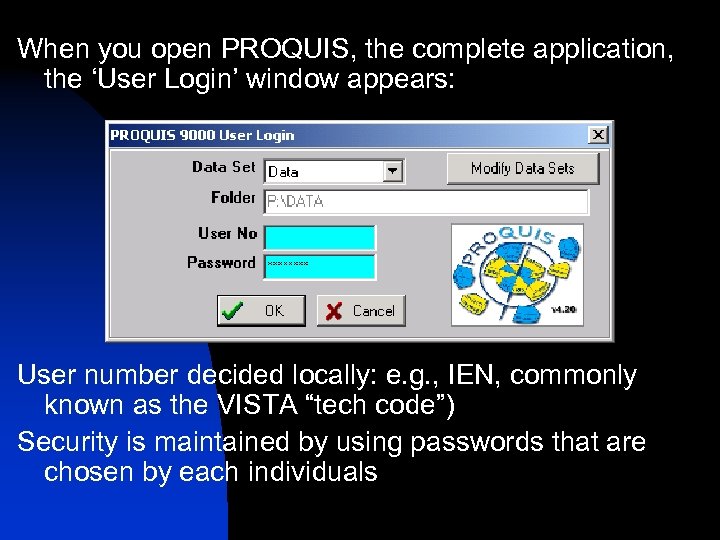 When you open PROQUIS, the complete application, the ‘User Login’ window appears: User number decided locally: e. g. , IEN, commonly known as the VISTA “tech code”) Security is maintained by using passwords that are chosen by each individuals
When you open PROQUIS, the complete application, the ‘User Login’ window appears: User number decided locally: e. g. , IEN, commonly known as the VISTA “tech code”) Security is maintained by using passwords that are chosen by each individuals
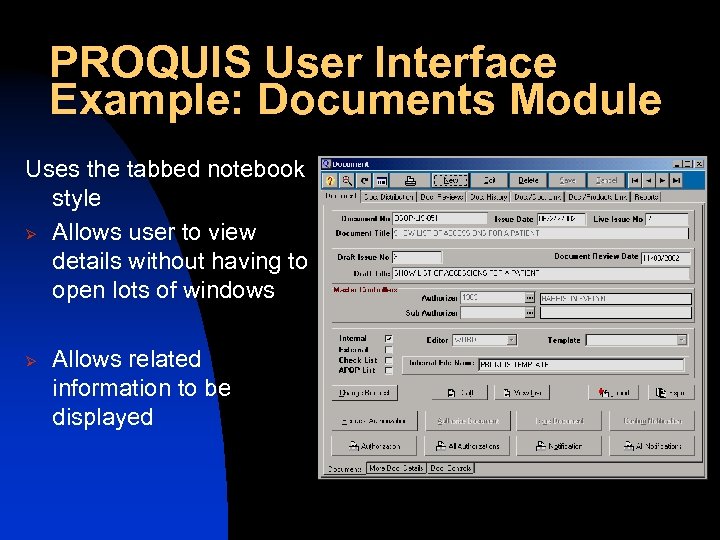 PROQUIS User Interface Example: Documents Module Uses the tabbed notebook style Ø Allows user to view details without having to open lots of windows Ø Allows related information to be displayed
PROQUIS User Interface Example: Documents Module Uses the tabbed notebook style Ø Allows user to view details without having to open lots of windows Ø Allows related information to be displayed
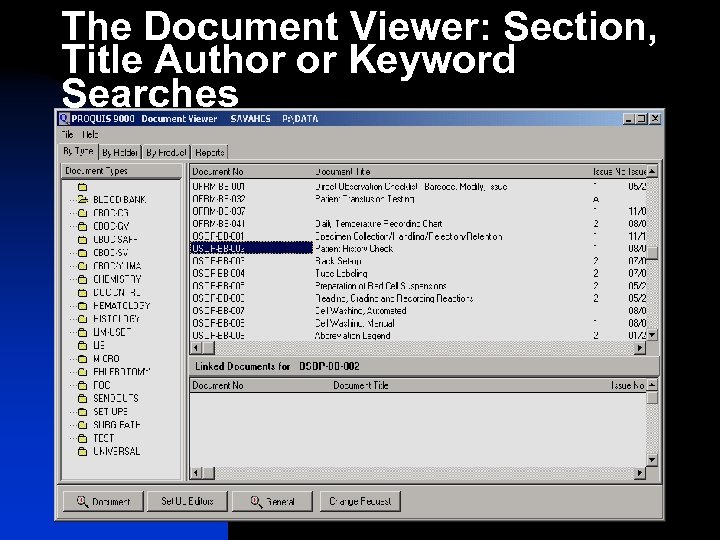 The Document Viewer: Section, Title Author or Keyword Searches
The Document Viewer: Section, Title Author or Keyword Searches
 Document Control, Current Status n Control u u u Access Distribution Changes Authorizations Confirmations n n Maintains u History of changes u Superseded documents Improves u Security of controlled documents u Communication u Retrieval of archived documents
Document Control, Current Status n Control u u u Access Distribution Changes Authorizations Confirmations n n Maintains u History of changes u Superseded documents Improves u Security of controlled documents u Communication u Retrieval of archived documents
 Automated warnings Document review date Unauthorized change request Change notification unconfirmed Documents can be linked or crossreferenced Facilitates ease of access to related documents Microsoft Access Based Integrated database system: changes in one section trigger any required updates of other areas of the system automatically
Automated warnings Document review date Unauthorized change request Change notification unconfirmed Documents can be linked or crossreferenced Facilitates ease of access to related documents Microsoft Access Based Integrated database system: changes in one section trigger any required updates of other areas of the system automatically
 Software Facilitates Compliance to NCCLS Guidelines n n n n n Standardized formats for all documents Document identification including version Change Control for documents Distribution Lists Master file of all documents with current and historical versions Master index of documents Documentation of approval and review Use of only current documents Identification, archiving and retreival
Software Facilitates Compliance to NCCLS Guidelines n n n n n Standardized formats for all documents Document identification including version Change Control for documents Distribution Lists Master file of all documents with current and historical versions Master index of documents Documentation of approval and review Use of only current documents Identification, archiving and retreival
 Other Software Modules at SAVAHCS n n n Customer Care Document Control FMEA/Design Control Vendor Control Equipment Control Customized Reports n n n Testing House Fault Log Audit & Management Review Health & Safety Personnel and Training Flow Charting
Other Software Modules at SAVAHCS n n n Customer Care Document Control FMEA/Design Control Vendor Control Equipment Control Customized Reports n n n Testing House Fault Log Audit & Management Review Health & Safety Personnel and Training Flow Charting
 Software Links to QSEs Assessing and grouping each policy or procedure by the key elements of the Quality System Essentials
Software Links to QSEs Assessing and grouping each policy or procedure by the key elements of the Quality System Essentials
 Expansion Processes Ø Ø Ø Fault Logs Equipment Maintenance Logs (limited) Personnel Training Records Assessments in Progress Ø Ø Ø Staff’s Feedback after Training and Hands-on Supervisor and Manager’s Review as SOP Review Dates are Automatically Generated Expectation/Preparation for CAP Inspection Web-links in process Encryption for signatures/GLP in process Outlook e-mail is easist, not compatible with VISTA
Expansion Processes Ø Ø Ø Fault Logs Equipment Maintenance Logs (limited) Personnel Training Records Assessments in Progress Ø Ø Ø Staff’s Feedback after Training and Hands-on Supervisor and Manager’s Review as SOP Review Dates are Automatically Generated Expectation/Preparation for CAP Inspection Web-links in process Encryption for signatures/GLP in process Outlook e-mail is easist, not compatible with VISTA
 Summary: Why must we control documents? To ensure. . . n n n regulatory requirements are satisfied. provision of adequate personnel to perform, verify, and manage all activities. performance of calibration, maintenance, and monitoring of equipment. provision of consistent high quality critical materials and services from contracted suppliers. provision of safe and adequate environmental conditions in the work place. control of processes.
Summary: Why must we control documents? To ensure. . . n n n regulatory requirements are satisfied. provision of adequate personnel to perform, verify, and manage all activities. performance of calibration, maintenance, and monitoring of equipment. provision of consistent high quality critical materials and services from contracted suppliers. provision of safe and adequate environmental conditions in the work place. control of processes.
 Thank you
Thank you


