3ac1b99e98782a55eaa45f7e54f2aa4a.ppt
- Количество слайдов: 33
 IONIC COMPOUNDS Naming and Formula Writing
IONIC COMPOUNDS Naming and Formula Writing
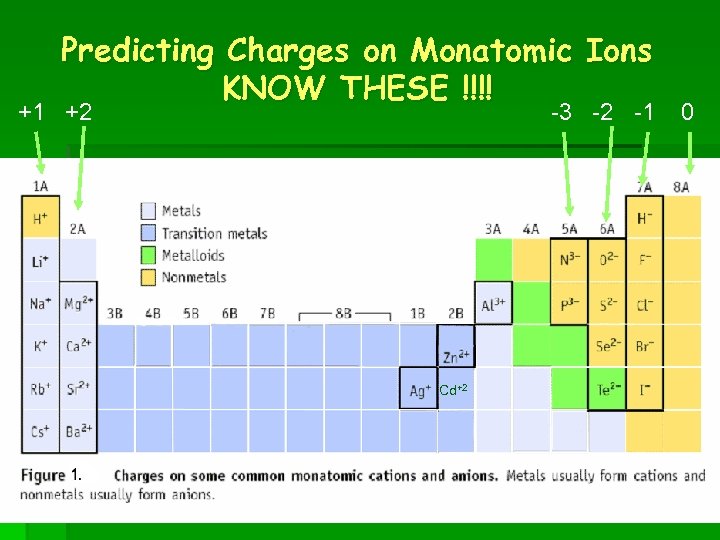 Predicting Charges on Monatomic Ions KNOW THESE !!!! +1 +2 -3 -2 -1 Cd+2 0
Predicting Charges on Monatomic Ions KNOW THESE !!!! +1 +2 -3 -2 -1 Cd+2 0
 Naming Positive Ions § Before you name an Ion you have to know the charge. § Group 1 = always +1 § Group 2 = always +2 § Aluminum = always +3 § Zinc and Cadmium = always +2 § Silver = always +1
Naming Positive Ions § Before you name an Ion you have to know the charge. § Group 1 = always +1 § Group 2 = always +2 § Aluminum = always +3 § Zinc and Cadmium = always +2 § Silver = always +1
 The Other Metals § All other metals can have various positive charges from +1 to +7 § Roman Numerals are used to tell the charge
The Other Metals § All other metals can have various positive charges from +1 to +7 § Roman Numerals are used to tell the charge
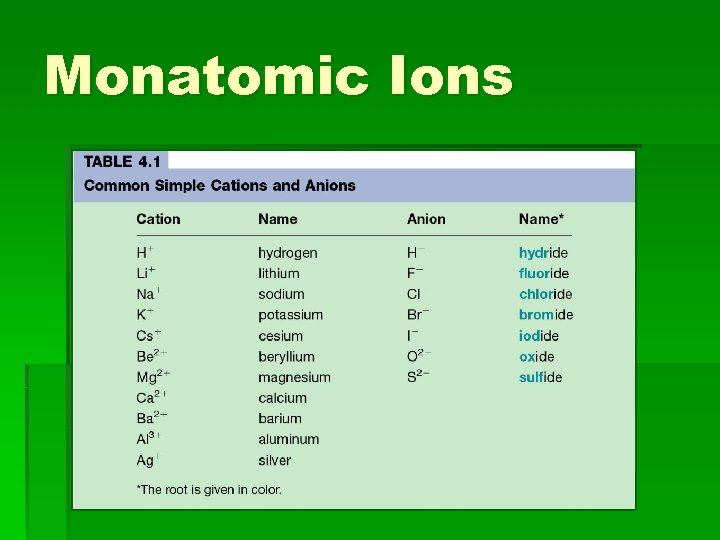 Monatomic Ions
Monatomic Ions
 Naming Postive Ions § With group 1, 2, Al, Zn, Cd, and Ag you just give the name of the atom § Na+ is Sodium § Mg+2 is Magnesium § Ag+ is Silver § Al+3 is Aluminum
Naming Postive Ions § With group 1, 2, Al, Zn, Cd, and Ag you just give the name of the atom § Na+ is Sodium § Mg+2 is Magnesium § Ag+ is Silver § Al+3 is Aluminum
 All the other metals § With all other metals you give the name Metal followed by a roman numerial § Cu+ - Copper (I) § Cu+2 – Copper (II) § Fe+2 – Iron (II) § Fe+3 – Iron (III) § U+6 – Uranium (VI)
All the other metals § With all other metals you give the name Metal followed by a roman numerial § Cu+ - Copper (I) § Cu+2 – Copper (II) § Fe+2 – Iron (II) § Fe+3 – Iron (III) § U+6 – Uranium (VI)
 Naming negative ions § All negative ions have their endings changed to –ide § Oxygen becomes oxide § Fluorine becomes Fluoride § Nitrogen becomes Nitride § Chlorine becomes Chloride
Naming negative ions § All negative ions have their endings changed to –ide § Oxygen becomes oxide § Fluorine becomes Fluoride § Nitrogen becomes Nitride § Chlorine becomes Chloride
 Names of other Negative Ions § Carbide = what you want your parents to do for you § Boride = what happened at the rodeo § Silicide = what you are seeing right now § Fluoride = a state in the union § Iodide = Very sad, I guess we will have to Barium.
Names of other Negative Ions § Carbide = what you want your parents to do for you § Boride = what happened at the rodeo § Silicide = what you are seeing right now § Fluoride = a state in the union § Iodide = Very sad, I guess we will have to Barium.
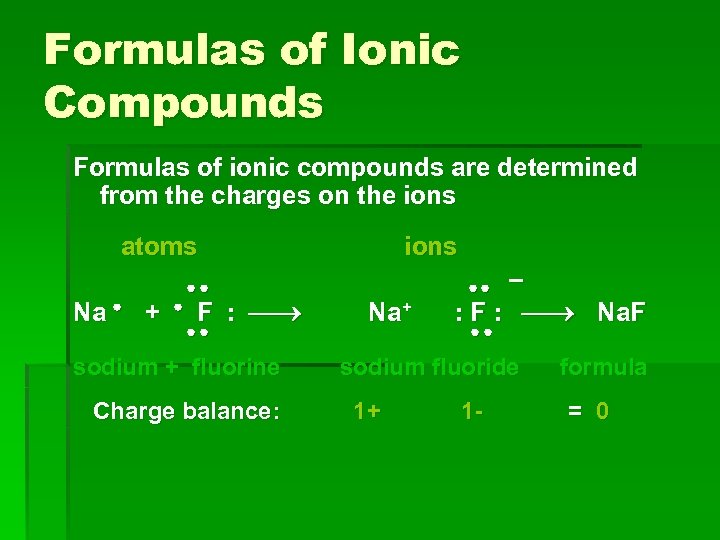 Formulas of Ionic Compounds Formulas of ionic compounds are determined from the charges on the ions atoms Na + F : sodium + fluorine Charge balance: ions Na+ – : F : Na. F sodium fluoride 1+ 1 - formula = 0
Formulas of Ionic Compounds Formulas of ionic compounds are determined from the charges on the ions atoms Na + F : sodium + fluorine Charge balance: ions Na+ – : F : Na. F sodium fluoride 1+ 1 - formula = 0
 Writing a Formula Write the formula for the ionic compound that will form between Ba 2+ and Cl. To cancel out Ba’s +2, then two -1 Cl’s are needed. Solution: 1. Balance charge with + and – ions 2. Write the positive ion of metal first, and the negative ion Ba 2+ Cl Cl 3. Write the number of ions needed as subscripts Ba. Cl 2
Writing a Formula Write the formula for the ionic compound that will form between Ba 2+ and Cl. To cancel out Ba’s +2, then two -1 Cl’s are needed. Solution: 1. Balance charge with + and – ions 2. Write the positive ion of metal first, and the negative ion Ba 2+ Cl Cl 3. Write the number of ions needed as subscripts Ba. Cl 2
 Balancing a formula by math § Every ionic compound should have a formula that has a charge that equals zero. § Barium Fluoride § Ba+2 F§ How many F’s are needed to balance Ba+2 ? § Two § Formula is Ba. F 2
Balancing a formula by math § Every ionic compound should have a formula that has a charge that equals zero. § Barium Fluoride § Ba+2 F§ How many F’s are needed to balance Ba+2 ? § Two § Formula is Ba. F 2
 Another way – Drop, Swap, Reduce § § § § Aluminum Oxide Al is always +3 Oxide is always -2 Al+3, O-2 DROP: Al 3 O 2 SWAP: Al 2 O 3 REDUCE: 2 and 3 are lowest integers, so leave alone
Another way – Drop, Swap, Reduce § § § § Aluminum Oxide Al is always +3 Oxide is always -2 Al+3, O-2 DROP: Al 3 O 2 SWAP: Al 2 O 3 REDUCE: 2 and 3 are lowest integers, so leave alone
 Another example § § § § Magnesium Oxide Mg is +2 Oxide is -2 Mg+2, O-2 DROP: Mg 2 O 2 SWAP: Mg 2 O 2 REDUCE: Mg. O, 2 and 2 divide each other out.
Another example § § § § Magnesium Oxide Mg is +2 Oxide is -2 Mg+2, O-2 DROP: Mg 2 O 2 SWAP: Mg 2 O 2 REDUCE: Mg. O, 2 and 2 divide each other out.
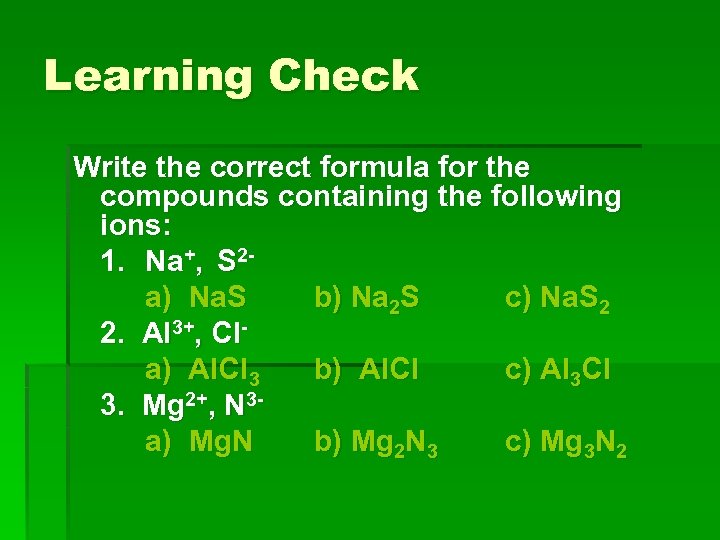 Learning Check Write the correct formula for the compounds containing the following ions: 1. Na+, S 2 a) Na. S b) Na 2 S c) Na. S 2 2. Al 3+, Cla) Al. Cl 3 b) Al. Cl c) Al 3 Cl 3. Mg 2+, N 3 a) Mg. N b) Mg 2 N 3 c) Mg 3 N 2
Learning Check Write the correct formula for the compounds containing the following ions: 1. Na+, S 2 a) Na. S b) Na 2 S c) Na. S 2 2. Al 3+, Cla) Al. Cl 3 b) Al. Cl c) Al 3 Cl 3. Mg 2+, N 3 a) Mg. N b) Mg 2 N 3 c) Mg 3 N 2
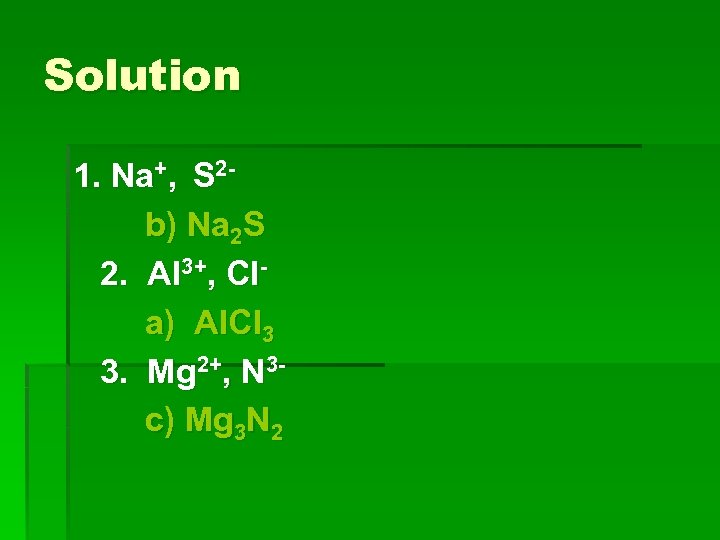 Solution 1. Na+, S 2 b) Na 2 S 2. Al 3+, Cla) Al. Cl 3 3. Mg 2+, N 3 c) Mg 3 N 2
Solution 1. Na+, S 2 b) Na 2 S 2. Al 3+, Cla) Al. Cl 3 3. Mg 2+, N 3 c) Mg 3 N 2
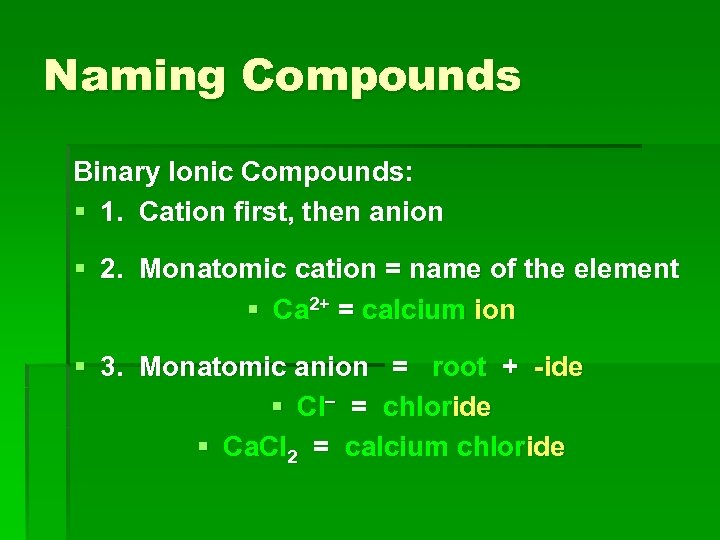 Naming Compounds Binary Ionic Compounds: § 1. Cation first, then anion § 2. Monatomic cation = name of the element § Ca 2+ = calcium ion § 3. Monatomic anion = root + -ide § Cl = chloride § Ca. Cl 2 = calcium chloride
Naming Compounds Binary Ionic Compounds: § 1. Cation first, then anion § 2. Monatomic cation = name of the element § Ca 2+ = calcium ion § 3. Monatomic anion = root + -ide § Cl = chloride § Ca. Cl 2 = calcium chloride
 Naming Binary Ionic Compounds l Examples: Na. Cl § sodium chloride Zn. I 2 § zinc iodide Al 2 O 3 § aluminum oxide
Naming Binary Ionic Compounds l Examples: Na. Cl § sodium chloride Zn. I 2 § zinc iodide Al 2 O 3 § aluminum oxide
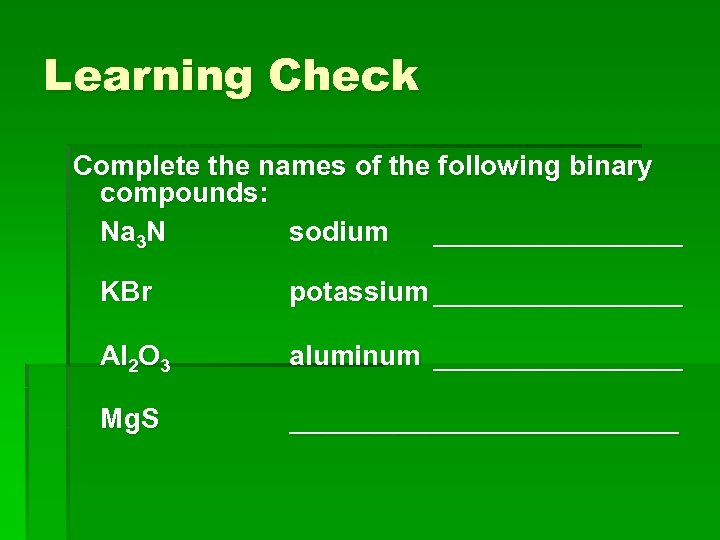 Learning Check Complete the names of the following binary compounds: Na 3 N sodium ________ KBr potassium ________ Al 2 O 3 aluminum ________ Mg. S _____________
Learning Check Complete the names of the following binary compounds: Na 3 N sodium ________ KBr potassium ________ Al 2 O 3 aluminum ________ Mg. S _____________
 Transition Metals Elements that can have more than one possible charge MUST have a Roman Numeral to indicate the charge on the individual ion. 1+ or 2+ 2+ or 3+ Cu+, Cu 2+ copper(I) ion copper (II) ion Fe 2+, Fe 3+ iron(II) ion iron(III) ion
Transition Metals Elements that can have more than one possible charge MUST have a Roman Numeral to indicate the charge on the individual ion. 1+ or 2+ 2+ or 3+ Cu+, Cu 2+ copper(I) ion copper (II) ion Fe 2+, Fe 3+ iron(II) ion iron(III) ion
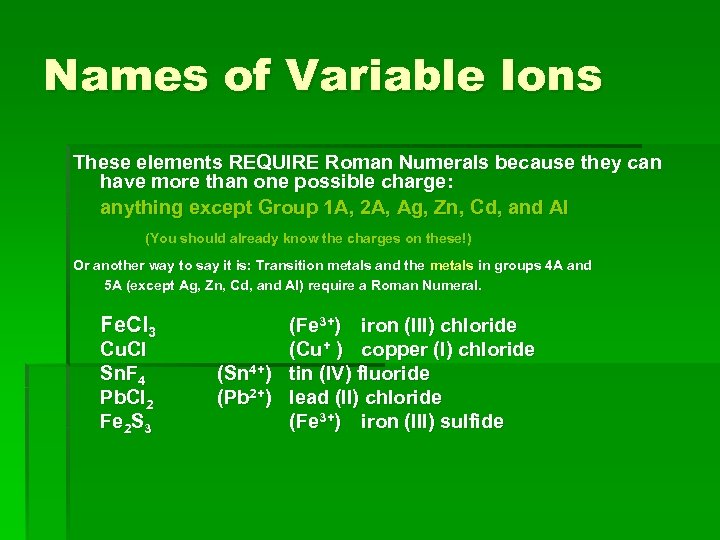 Names of Variable Ions These elements REQUIRE Roman Numerals because they can have more than one possible charge: anything except Group 1 A, 2 A, Ag, Zn, Cd, and Al (You should already know the charges on these!) Or another way to say it is: Transition metals and the metals in groups 4 A and 5 A (except Ag, Zn, Cd, and Al) require a Roman Numeral. Fe. Cl 3 Cu. Cl Sn. F 4 Pb. Cl 2 Fe 2 S 3 (Fe 3+) iron (III) chloride (Cu+ ) copper (I) chloride (Sn 4+) tin (IV) fluoride (Pb 2+) lead (II) chloride (Fe 3+) iron (III) sulfide
Names of Variable Ions These elements REQUIRE Roman Numerals because they can have more than one possible charge: anything except Group 1 A, 2 A, Ag, Zn, Cd, and Al (You should already know the charges on these!) Or another way to say it is: Transition metals and the metals in groups 4 A and 5 A (except Ag, Zn, Cd, and Al) require a Roman Numeral. Fe. Cl 3 Cu. Cl Sn. F 4 Pb. Cl 2 Fe 2 S 3 (Fe 3+) iron (III) chloride (Cu+ ) copper (I) chloride (Sn 4+) tin (IV) fluoride (Pb 2+) lead (II) chloride (Fe 3+) iron (III) sulfide
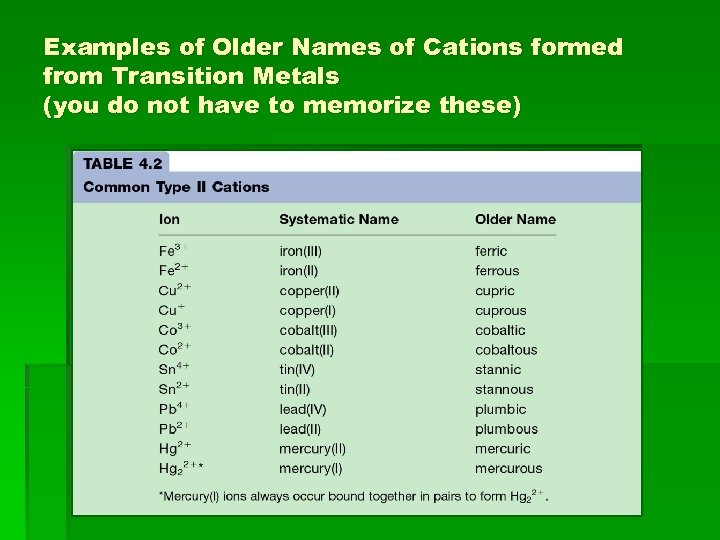 Examples of Older Names of Cations formed from Transition Metals (you do not have to memorize these)
Examples of Older Names of Cations formed from Transition Metals (you do not have to memorize these)
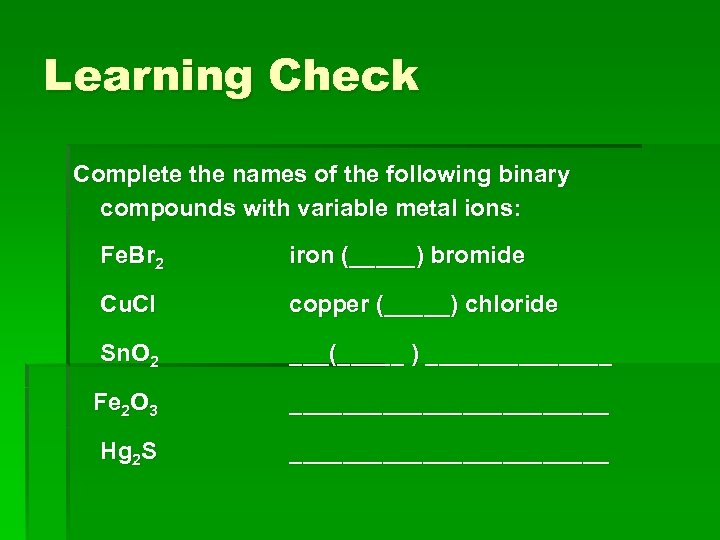 Learning Check Complete the names of the following binary compounds with variable metal ions: Fe. Br 2 iron (_____) bromide Cu. Cl copper (_____) chloride Sn. O 2 ___(_____ ) _______ Fe 2 O 3 ____________ Hg 2 S ____________
Learning Check Complete the names of the following binary compounds with variable metal ions: Fe. Br 2 iron (_____) bromide Cu. Cl copper (_____) chloride Sn. O 2 ___(_____ ) _______ Fe 2 O 3 ____________ Hg 2 S ____________
 Polyatomic Ions § Some ions are composed of more then one atom. § These are called polyatomic ions § Poly = more
Polyatomic Ions § Some ions are composed of more then one atom. § These are called polyatomic ions § Poly = more
 Formulas and names § § § Nitrate = NO 3 Sulfate = SO 4 -2 Silver nitrate § Ag+ NO 3§ DSR = Ag. NO 3 § Copper (I) Sulfate § Cu+ SO 4 -2 § Cu 2 SO 4
Formulas and names § § § Nitrate = NO 3 Sulfate = SO 4 -2 Silver nitrate § Ag+ NO 3§ DSR = Ag. NO 3 § Copper (I) Sulfate § Cu+ SO 4 -2 § Cu 2 SO 4
 More Polyatomics § Lead (IV) Phosphate § Pb+4 PO 4 -3 § Pb 3(PO 4)4 § Notice: When more then one Polyatomic is present you surround it with () § Big: The subscripts on Polyatomic ions are NEVER changed.
More Polyatomics § Lead (IV) Phosphate § Pb+4 PO 4 -3 § Pb 3(PO 4)4 § Notice: When more then one Polyatomic is present you surround it with () § Big: The subscripts on Polyatomic ions are NEVER changed.
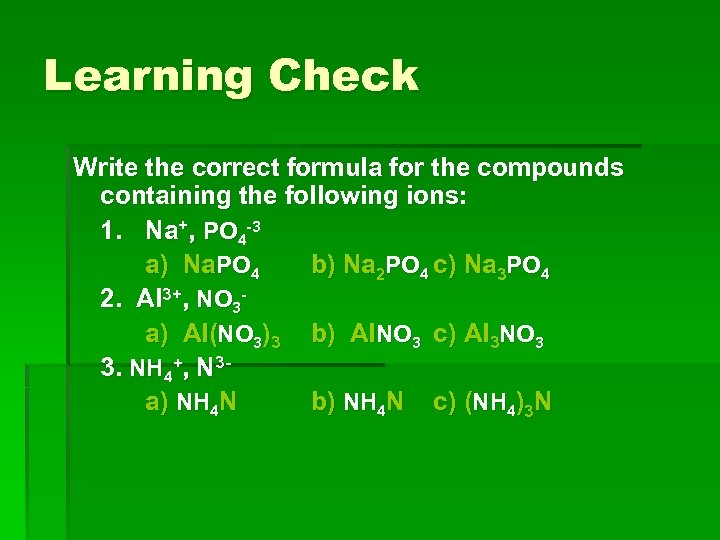 Learning Check Write the correct formula for the compounds containing the following ions: 1. Na+, PO 4 -3 a) Na. PO 4 b) Na 2 PO 4 c) Na 3 PO 4 2. Al 3+, NO 3 a) Al(NO 3)3 b) Al. NO 3 c) Al 3 NO 3 3. NH 4+, N 3 a) NH 4 N b) NH 4 N c) (NH 4)3 N
Learning Check Write the correct formula for the compounds containing the following ions: 1. Na+, PO 4 -3 a) Na. PO 4 b) Na 2 PO 4 c) Na 3 PO 4 2. Al 3+, NO 3 a) Al(NO 3)3 b) Al. NO 3 c) Al 3 NO 3 3. NH 4+, N 3 a) NH 4 N b) NH 4 N c) (NH 4)3 N
 Answers § 1. Na+, PO 4 -3 c) Na 3 PO 4 § 2. Al 3+, NO 3§ a) Al(NO 3)3 § 3. NH 4+, N 3§ c) (NH 4)3 N
Answers § 1. Na+, PO 4 -3 c) Na 3 PO 4 § 2. Al 3+, NO 3§ a) Al(NO 3)3 § 3. NH 4+, N 3§ c) (NH 4)3 N
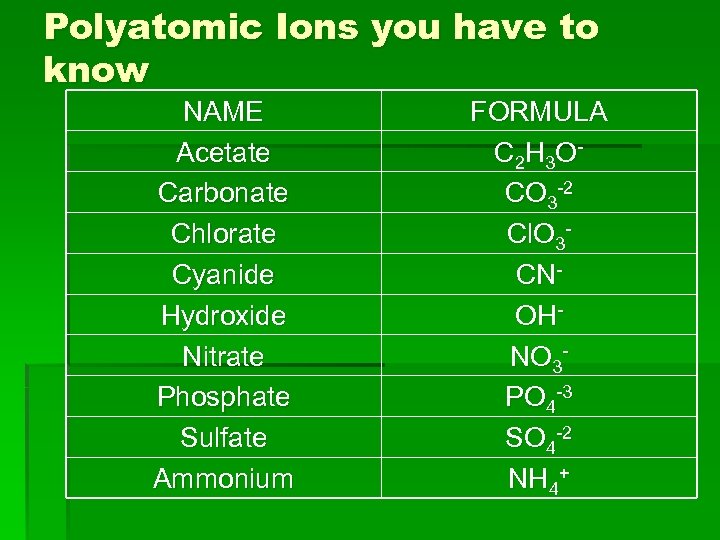 Polyatomic Ions you have to know NAME Acetate Carbonate Chlorate Cyanide Hydroxide Nitrate Phosphate Sulfate Ammonium FORMULA C 2 H 3 O CO 3 -2 Cl. O 3 CNOHNO 3 PO 4 -3 SO 4 -2 NH 4+
Polyatomic Ions you have to know NAME Acetate Carbonate Chlorate Cyanide Hydroxide Nitrate Phosphate Sulfate Ammonium FORMULA C 2 H 3 O CO 3 -2 Cl. O 3 CNOHNO 3 PO 4 -3 SO 4 -2 NH 4+
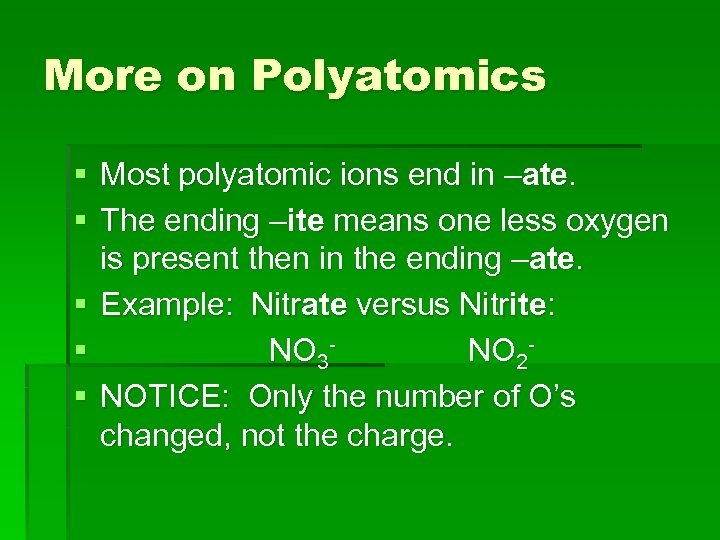 More on Polyatomics § Most polyatomic ions end in –ate. § The ending –ite means one less oxygen is present then in the ending –ate. § Example: Nitrate versus Nitrite: § NO 3 NO 2§ NOTICE: Only the number of O’s changed, not the charge.
More on Polyatomics § Most polyatomic ions end in –ate. § The ending –ite means one less oxygen is present then in the ending –ate. § Example: Nitrate versus Nitrite: § NO 3 NO 2§ NOTICE: Only the number of O’s changed, not the charge.
 Even More § The Prefix Hypo means two less oxygen’s are present then in –ate. § Example: Hyposulfite: SO 2 -2 § The Prefix Per- means one extra oxygen is present then in –ate. § Examples: § Perchlorate Cl. O 4§ Chlorate Cl. O 3§ Chlorite Cl. O 2§ Hypo. Chlorite Cl. O-
Even More § The Prefix Hypo means two less oxygen’s are present then in –ate. § Example: Hyposulfite: SO 2 -2 § The Prefix Per- means one extra oxygen is present then in –ate. § Examples: § Perchlorate Cl. O 4§ Chlorate Cl. O 3§ Chlorite Cl. O 2§ Hypo. Chlorite Cl. O-
 What if Hydrogen is in it? § The Prefix Bi means that a Hydrogen is added, and the charge is reduced by one. § Example: § Bi. Carbonate HCO 3 -
What if Hydrogen is in it? § The Prefix Bi means that a Hydrogen is added, and the charge is reduced by one. § Example: § Bi. Carbonate HCO 3 -
 Properties of Ionic Compounds § Ionic compounds are: § also known as salts § They are usually hard and brittle § Conduct electricity when molten or dissolved § Have very high melting and boiling points § Most are soluble in water § Normally composed of at least one metal and one nonmetal
Properties of Ionic Compounds § Ionic compounds are: § also known as salts § They are usually hard and brittle § Conduct electricity when molten or dissolved § Have very high melting and boiling points § Most are soluble in water § Normally composed of at least one metal and one nonmetal


