21d7f7ea3400ecd4f1d6578379478f2b.ppt
- Количество слайдов: 85
 Introductory Chemistry, 3 rd Edition Nivaldo Tro Chapter 6 Chemical Composition Roy Kennedy Massachusetts Bay Community College Wellesley Hills, MA 2009, Prentice Hall
Introductory Chemistry, 3 rd Edition Nivaldo Tro Chapter 6 Chemical Composition Roy Kennedy Massachusetts Bay Community College Wellesley Hills, MA 2009, Prentice Hall
 Why Is Knowledge of Composition Important? • All matter is either chemically or physically combined into substances. • Knowing the fraction of material you have can tell you: ü the amount of sodium in sodium chloride for diet. ü the amount of iron in iron ore for steel production. ü the amount of hydrogen in water for hydrogen fuel. ü the amount of chlorine in freon to estimate ozone depletion. Tro's "Introductory Chemistry", Chapter 6 2
Why Is Knowledge of Composition Important? • All matter is either chemically or physically combined into substances. • Knowing the fraction of material you have can tell you: ü the amount of sodium in sodium chloride for diet. ü the amount of iron in iron ore for steel production. ü the amount of hydrogen in water for hydrogen fuel. ü the amount of chlorine in freon to estimate ozone depletion. Tro's "Introductory Chemistry", Chapter 6 2
 How much seed do you plant? • In a garden you count the seeds by hand. How many seeds would you know to plant in a field? 3
How much seed do you plant? • In a garden you count the seeds by hand. How many seeds would you know to plant in a field? 3
 Counting by Weighing • Building a house requires a lot of nails. • If you know that a single nail weighs. 0122 g, than 100 nails weigh 1. 22 g, a 1000 nails weigh 12. 2 g and so on. • Analogy: üYou want to make 100 lbs of Al 2 O 3, how much aluminum do you use Tro's "Introductory Chemistry", Chapter 6 4
Counting by Weighing • Building a house requires a lot of nails. • If you know that a single nail weighs. 0122 g, than 100 nails weigh 1. 22 g, a 1000 nails weigh 12. 2 g and so on. • Analogy: üYou want to make 100 lbs of Al 2 O 3, how much aluminum do you use Tro's "Introductory Chemistry", Chapter 6 4
 Counting Nails by the Pound, Continued A hardware store customer buys 2. 60 pounds of nails. A dozen nails has a mass of 0. 150 pounds. How many nails did the customer buy? 1 dozen nails = 0. 150 lbs. 12 nails = 1 dozen nails Solution map: Tro's "Introductory Chemistry", Chapter 6 5
Counting Nails by the Pound, Continued A hardware store customer buys 2. 60 pounds of nails. A dozen nails has a mass of 0. 150 pounds. How many nails did the customer buy? 1 dozen nails = 0. 150 lbs. 12 nails = 1 dozen nails Solution map: Tro's "Introductory Chemistry", Chapter 6 5
 Counting Nails by the Pound, Continued • The customer bought 2. 60 lbs of nails and received 208 nails. He counted the nails by weighing them! Tro's "Introductory Chemistry", Chapter 6 6
Counting Nails by the Pound, Continued • The customer bought 2. 60 lbs of nails and received 208 nails. He counted the nails by weighing them! Tro's "Introductory Chemistry", Chapter 6 6
 Counting Nails by the Pound, Continued • What if he bought a different size nail? üWould the mass of a dozen be 0. 150 lbs? üWould there still be 12 nails in a dozen? üWould there be 208 nails in 2. 60 lbs? üHow would this effect the conversion factors? Tro's "Introductory Chemistry", Chapter 6 7
Counting Nails by the Pound, Continued • What if he bought a different size nail? üWould the mass of a dozen be 0. 150 lbs? üWould there still be 12 nails in a dozen? üWould there be 208 nails in 2. 60 lbs? üHow would this effect the conversion factors? Tro's "Introductory Chemistry", Chapter 6 7
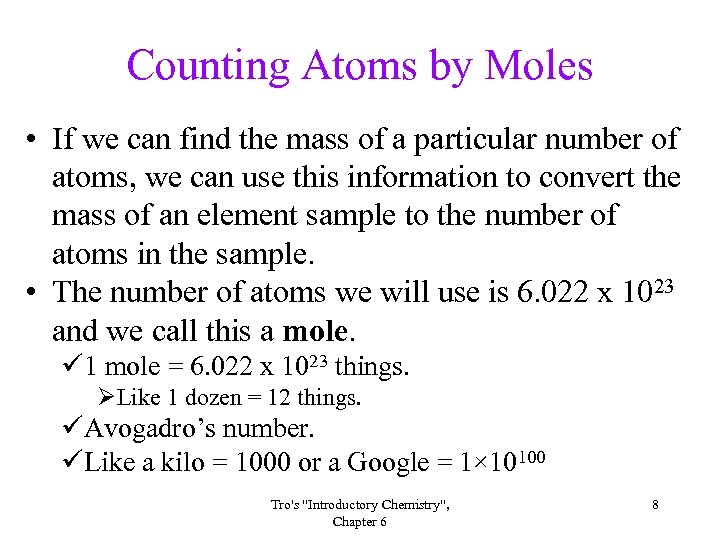 Counting Atoms by Moles • If we can find the mass of a particular number of atoms, we can use this information to convert the mass of an element sample to the number of atoms in the sample. • The number of atoms we will use is 6. 022 x 1023 and we call this a mole. ü 1 mole = 6. 022 x 1023 things. ØLike 1 dozen = 12 things. üAvogadro’s number. üLike a kilo = 1000 or a Google = 1× 10100 Tro's "Introductory Chemistry", Chapter 6 8
Counting Atoms by Moles • If we can find the mass of a particular number of atoms, we can use this information to convert the mass of an element sample to the number of atoms in the sample. • The number of atoms we will use is 6. 022 x 1023 and we call this a mole. ü 1 mole = 6. 022 x 1023 things. ØLike 1 dozen = 12 things. üAvogadro’s number. üLike a kilo = 1000 or a Google = 1× 10100 Tro's "Introductory Chemistry", Chapter 6 8
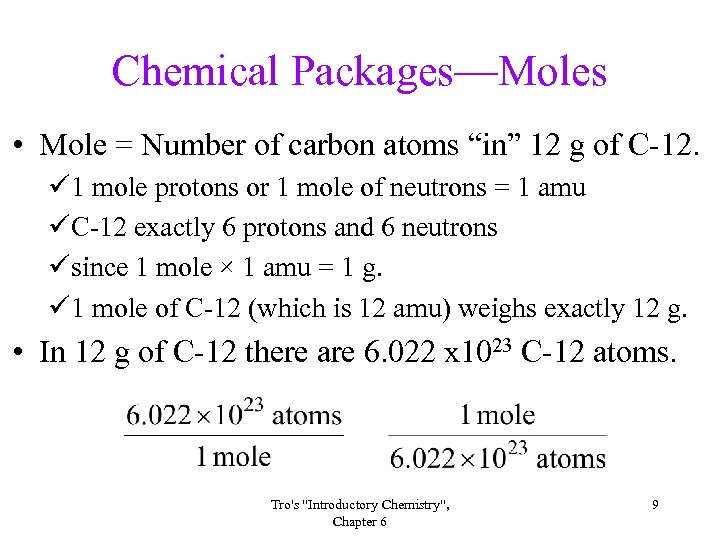 Chemical Packages—Moles • Mole = Number of carbon atoms “in” 12 g of C-12. ü 1 mole protons or 1 mole of neutrons = 1 amu üC-12 exactly 6 protons and 6 neutrons üsince 1 mole × 1 amu = 1 g. ü 1 mole of C-12 (which is 12 amu) weighs exactly 12 g. • In 12 g of C-12 there are 6. 022 x 1023 C-12 atoms. Tro's "Introductory Chemistry", Chapter 6 9
Chemical Packages—Moles • Mole = Number of carbon atoms “in” 12 g of C-12. ü 1 mole protons or 1 mole of neutrons = 1 amu üC-12 exactly 6 protons and 6 neutrons üsince 1 mole × 1 amu = 1 g. ü 1 mole of C-12 (which is 12 amu) weighs exactly 12 g. • In 12 g of C-12 there are 6. 022 x 1023 C-12 atoms. Tro's "Introductory Chemistry", Chapter 6 9
 Example 6. 1: • A silver ring contains 1. 1 x 1022 silver atoms. How many moles of silver are in the ring? Tro's "Introductory Chemistry", Chapter 6 10
Example 6. 1: • A silver ring contains 1. 1 x 1022 silver atoms. How many moles of silver are in the ring? Tro's "Introductory Chemistry", Chapter 6 10
 Example: A silver ring contains 1. 1 x 1022 silver atoms. How many moles of silver are in the ring? • Write down the given quantity and its units. Given: 1. 1 x 1022 Ag atoms Tro's "Introductory Chemistry", Chapter 6 11
Example: A silver ring contains 1. 1 x 1022 silver atoms. How many moles of silver are in the ring? • Write down the given quantity and its units. Given: 1. 1 x 1022 Ag atoms Tro's "Introductory Chemistry", Chapter 6 11
 Example: A silver ring contains 1. 1 x 1022 silver atoms. How many moles of silver are in the ring? Information: Given: 1. 1 x 1022 Ag atoms • Write down the quantity to find and/or its units. Find: ? moles Tro's "Introductory Chemistry", Chapter 6 12
Example: A silver ring contains 1. 1 x 1022 silver atoms. How many moles of silver are in the ring? Information: Given: 1. 1 x 1022 Ag atoms • Write down the quantity to find and/or its units. Find: ? moles Tro's "Introductory Chemistry", Chapter 6 12
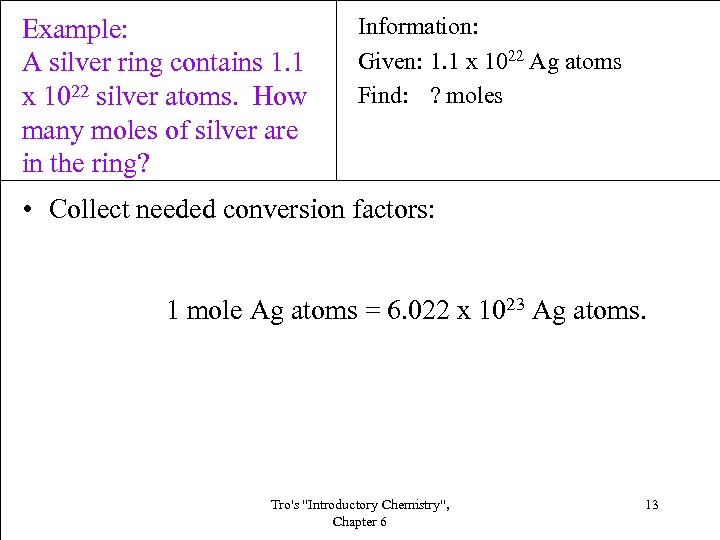 Example: A silver ring contains 1. 1 x 1022 silver atoms. How many moles of silver are in the ring? Information: Given: 1. 1 x 1022 Ag atoms Find: ? moles • Collect needed conversion factors: 1 mole Ag atoms = 6. 022 x 1023 Ag atoms. Tro's "Introductory Chemistry", Chapter 6 13
Example: A silver ring contains 1. 1 x 1022 silver atoms. How many moles of silver are in the ring? Information: Given: 1. 1 x 1022 Ag atoms Find: ? moles • Collect needed conversion factors: 1 mole Ag atoms = 6. 022 x 1023 Ag atoms. Tro's "Introductory Chemistry", Chapter 6 13
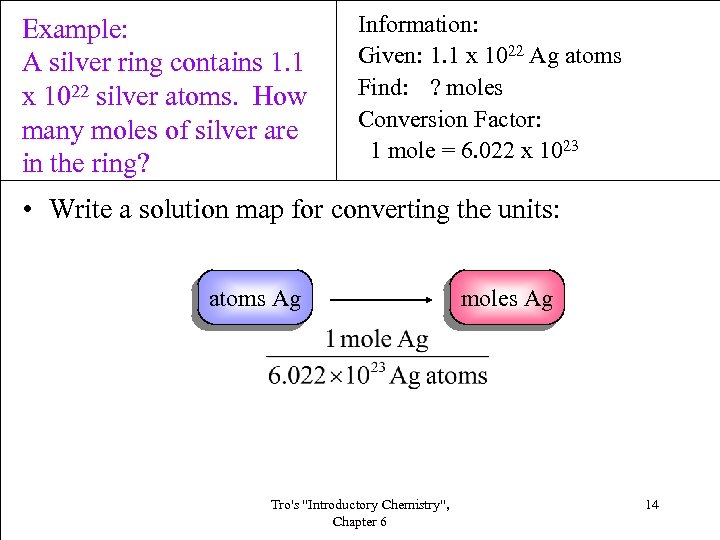 Example: A silver ring contains 1. 1 x 1022 silver atoms. How many moles of silver are in the ring? Information: Given: 1. 1 x 1022 Ag atoms Find: ? moles Conversion Factor: 1 mole = 6. 022 x 1023 • Write a solution map for converting the units: atoms Ag Tro's "Introductory Chemistry", Chapter 6 moles Ag 14
Example: A silver ring contains 1. 1 x 1022 silver atoms. How many moles of silver are in the ring? Information: Given: 1. 1 x 1022 Ag atoms Find: ? moles Conversion Factor: 1 mole = 6. 022 x 1023 • Write a solution map for converting the units: atoms Ag Tro's "Introductory Chemistry", Chapter 6 moles Ag 14
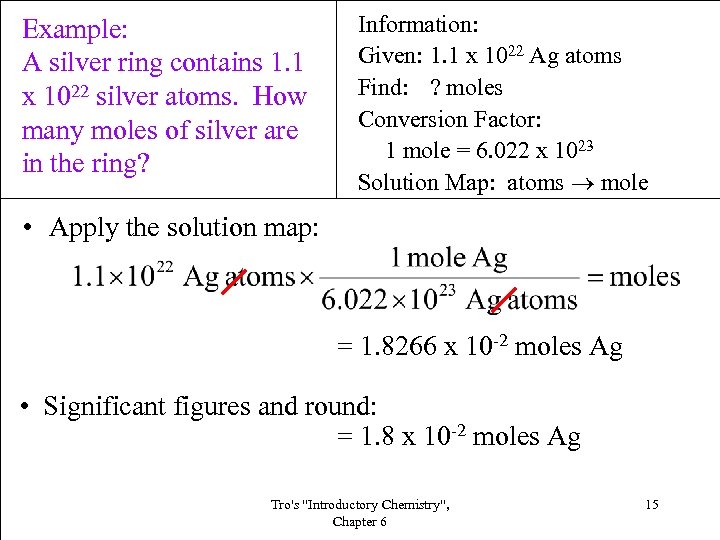 Example: A silver ring contains 1. 1 x 1022 silver atoms. How many moles of silver are in the ring? Information: Given: 1. 1 x 1022 Ag atoms Find: ? moles Conversion Factor: 1 mole = 6. 022 x 1023 Solution Map: atoms mole • Apply the solution map: = 1. 8266 x 10 -2 moles Ag • Significant figures and round: = 1. 8 x 10 -2 moles Ag Tro's "Introductory Chemistry", Chapter 6 15
Example: A silver ring contains 1. 1 x 1022 silver atoms. How many moles of silver are in the ring? Information: Given: 1. 1 x 1022 Ag atoms Find: ? moles Conversion Factor: 1 mole = 6. 022 x 1023 Solution Map: atoms mole • Apply the solution map: = 1. 8266 x 10 -2 moles Ag • Significant figures and round: = 1. 8 x 10 -2 moles Ag Tro's "Introductory Chemistry", Chapter 6 15
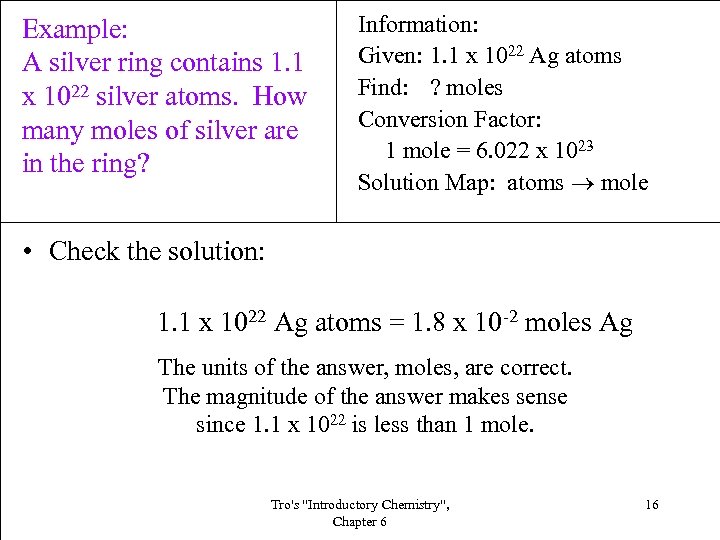 Example: A silver ring contains 1. 1 x 1022 silver atoms. How many moles of silver are in the ring? Information: Given: 1. 1 x 1022 Ag atoms Find: ? moles Conversion Factor: 1 mole = 6. 022 x 1023 Solution Map: atoms mole • Check the solution: 1. 1 x 1022 Ag atoms = 1. 8 x 10 -2 moles Ag The units of the answer, moles, are correct. The magnitude of the answer makes sense since 1. 1 x 1022 is less than 1 mole. Tro's "Introductory Chemistry", Chapter 6 16
Example: A silver ring contains 1. 1 x 1022 silver atoms. How many moles of silver are in the ring? Information: Given: 1. 1 x 1022 Ag atoms Find: ? moles Conversion Factor: 1 mole = 6. 022 x 1023 Solution Map: atoms mole • Check the solution: 1. 1 x 1022 Ag atoms = 1. 8 x 10 -2 moles Ag The units of the answer, moles, are correct. The magnitude of the answer makes sense since 1. 1 x 1022 is less than 1 mole. Tro's "Introductory Chemistry", Chapter 6 16
 Practice—Calculate the Number of Atoms in 2. 45 Mol of Copper. Tro's "Introductory Chemistry", Chapter 6 17
Practice—Calculate the Number of Atoms in 2. 45 Mol of Copper. Tro's "Introductory Chemistry", Chapter 6 17
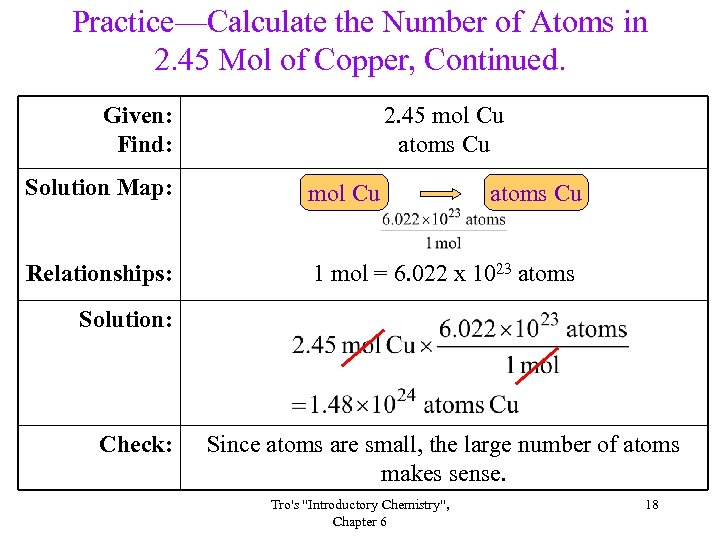 Practice—Calculate the Number of Atoms in 2. 45 Mol of Copper, Continued. Given: Find: 2. 45 mol Cu atoms Cu Solution Map: mol Cu Relationships: 1 mol = 6. 022 x 1023 atoms Cu Solution: Check: Since atoms are small, the large number of atoms makes sense. Tro's "Introductory Chemistry", Chapter 6 18
Practice—Calculate the Number of Atoms in 2. 45 Mol of Copper, Continued. Given: Find: 2. 45 mol Cu atoms Cu Solution Map: mol Cu Relationships: 1 mol = 6. 022 x 1023 atoms Cu Solution: Check: Since atoms are small, the large number of atoms makes sense. Tro's "Introductory Chemistry", Chapter 6 18
 Relationship Between Moles and Mass • The mass of one mole of atoms is called the molar mass. • The molar mass of an element, in grams, is numerically equal to the element’s atomic mass, in amu. • The lighter the atom, the less a mole weighs. • The lighter the atom, the more atoms there are in 1 g. Tro's "Introductory Chemistry", Chapter 6 19
Relationship Between Moles and Mass • The mass of one mole of atoms is called the molar mass. • The molar mass of an element, in grams, is numerically equal to the element’s atomic mass, in amu. • The lighter the atom, the less a mole weighs. • The lighter the atom, the more atoms there are in 1 g. Tro's "Introductory Chemistry", Chapter 6 19
 Mole and Mass Relationships 1 mole sulfur 32. 06 g 1 mole carbon 12. 01 g Tro's "Introductory Chemistry", Chapter 6 20
Mole and Mass Relationships 1 mole sulfur 32. 06 g 1 mole carbon 12. 01 g Tro's "Introductory Chemistry", Chapter 6 20
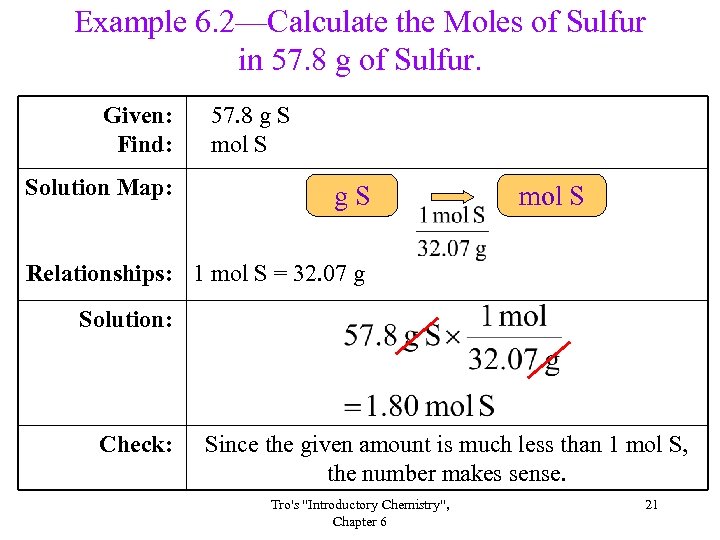 Example 6. 2—Calculate the Moles of Sulfur in 57. 8 g of Sulfur. Given: Find: Solution Map: 57. 8 g S mol S g. S mol S Relationships: 1 mol S = 32. 07 g Solution: Check: Since the given amount is much less than 1 mol S, the number makes sense. Tro's "Introductory Chemistry", Chapter 6 21
Example 6. 2—Calculate the Moles of Sulfur in 57. 8 g of Sulfur. Given: Find: Solution Map: 57. 8 g S mol S g. S mol S Relationships: 1 mol S = 32. 07 g Solution: Check: Since the given amount is much less than 1 mol S, the number makes sense. Tro's "Introductory Chemistry", Chapter 6 21
 Practice—Calculate the Moles of Carbon in 0. 0265 g of Pencil Lead. Tro's "Introductory Chemistry", Chapter 6 22
Practice—Calculate the Moles of Carbon in 0. 0265 g of Pencil Lead. Tro's "Introductory Chemistry", Chapter 6 22
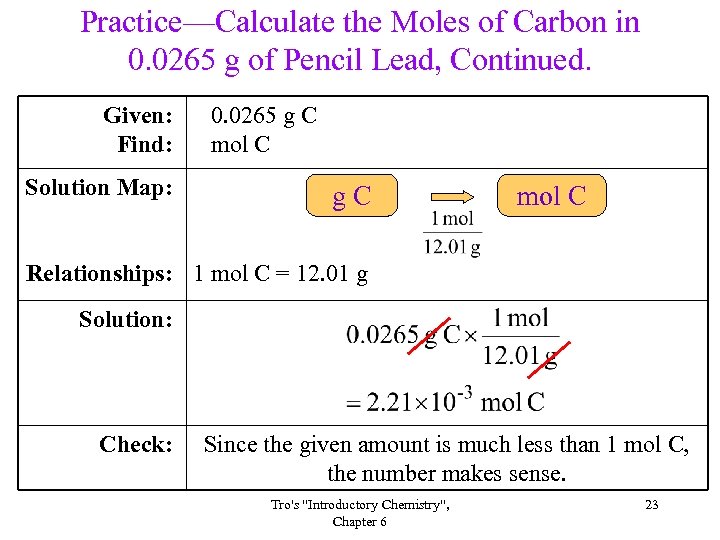 Practice—Calculate the Moles of Carbon in 0. 0265 g of Pencil Lead, Continued. Given: Find: Solution Map: 0. 0265 g C mol C g. C mol C Relationships: 1 mol C = 12. 01 g Solution: Check: Since the given amount is much less than 1 mol C, the number makes sense. Tro's "Introductory Chemistry", Chapter 6 23
Practice—Calculate the Moles of Carbon in 0. 0265 g of Pencil Lead, Continued. Given: Find: Solution Map: 0. 0265 g C mol C g. C mol C Relationships: 1 mol C = 12. 01 g Solution: Check: Since the given amount is much less than 1 mol C, the number makes sense. Tro's "Introductory Chemistry", Chapter 6 23
 Example 6. 3: • How many aluminum atoms are in an aluminum can with a mass of 16. 2 g? Tro's "Introductory Chemistry", Chapter 6 24
Example 6. 3: • How many aluminum atoms are in an aluminum can with a mass of 16. 2 g? Tro's "Introductory Chemistry", Chapter 6 24
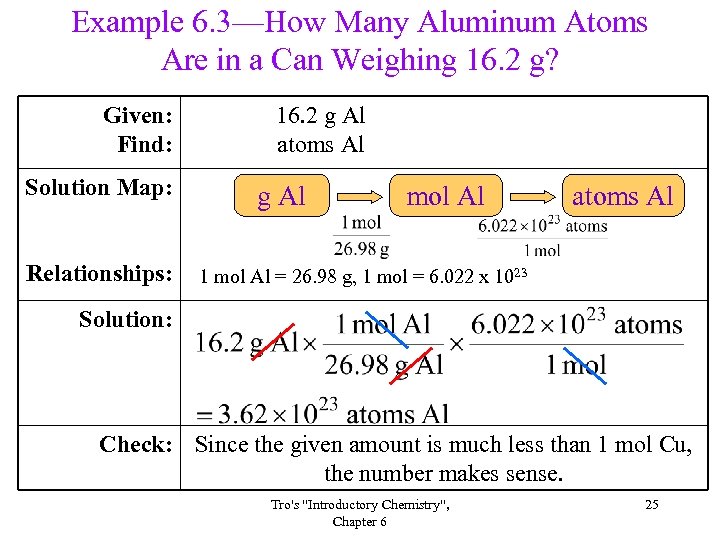 Example 6. 3—How Many Aluminum Atoms Are in a Can Weighing 16. 2 g? Given: Find: Solution Map: Relationships: 16. 2 g Al atoms Al g Al mol Al atoms Al 1 mol Al = 26. 98 g, 1 mol = 6. 022 x 1023 Solution: Check: Since the given amount is much less than 1 mol Cu, the number makes sense. Tro's "Introductory Chemistry", Chapter 6 25
Example 6. 3—How Many Aluminum Atoms Are in a Can Weighing 16. 2 g? Given: Find: Solution Map: Relationships: 16. 2 g Al atoms Al g Al mol Al atoms Al 1 mol Al = 26. 98 g, 1 mol = 6. 022 x 1023 Solution: Check: Since the given amount is much less than 1 mol Cu, the number makes sense. Tro's "Introductory Chemistry", Chapter 6 25
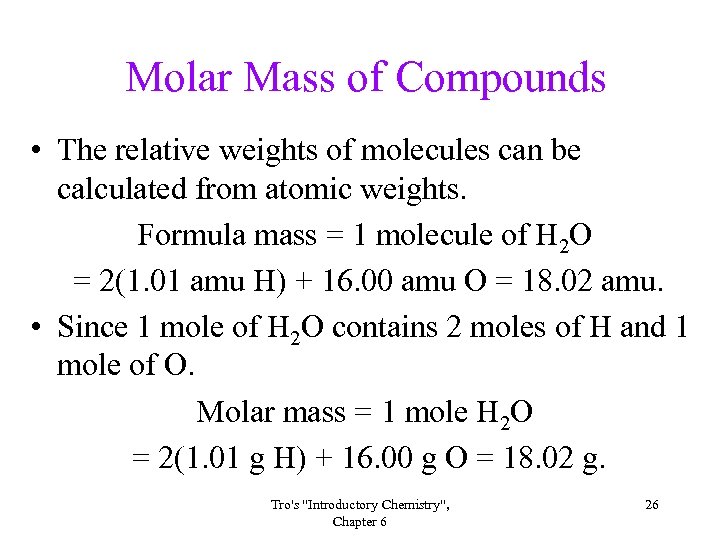 Molar Mass of Compounds • The relative weights of molecules can be calculated from atomic weights. Formula mass = 1 molecule of H 2 O = 2(1. 01 amu H) + 16. 00 amu O = 18. 02 amu. • Since 1 mole of H 2 O contains 2 moles of H and 1 mole of O. Molar mass = 1 mole H 2 O = 2(1. 01 g H) + 16. 00 g O = 18. 02 g. Tro's "Introductory Chemistry", Chapter 6 26
Molar Mass of Compounds • The relative weights of molecules can be calculated from atomic weights. Formula mass = 1 molecule of H 2 O = 2(1. 01 amu H) + 16. 00 amu O = 18. 02 amu. • Since 1 mole of H 2 O contains 2 moles of H and 1 mole of O. Molar mass = 1 mole H 2 O = 2(1. 01 g H) + 16. 00 g O = 18. 02 g. Tro's "Introductory Chemistry", Chapter 6 26
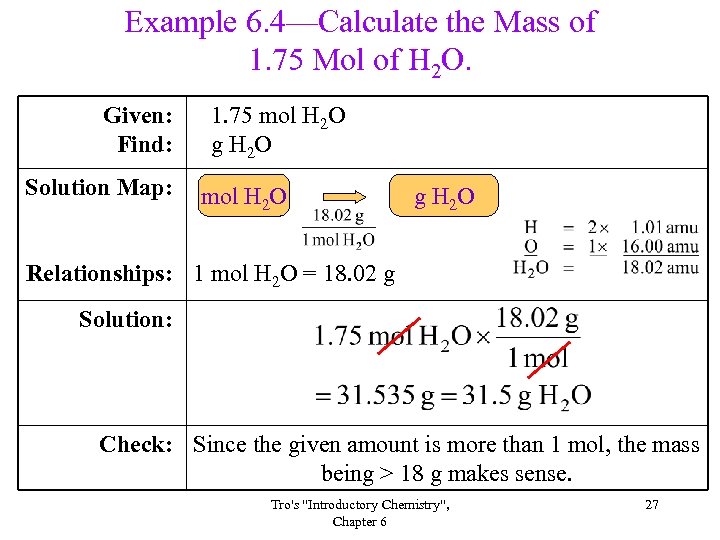 Example 6. 4—Calculate the Mass of 1. 75 Mol of H 2 O. Given: Find: Solution Map: 1. 75 mol H 2 O g H 2 O Relationships: 1 mol H 2 O = 18. 02 g Solution: Check: Since the given amount is more than 1 mol, the mass being > 18 g makes sense. Tro's "Introductory Chemistry", Chapter 6 27
Example 6. 4—Calculate the Mass of 1. 75 Mol of H 2 O. Given: Find: Solution Map: 1. 75 mol H 2 O g H 2 O Relationships: 1 mol H 2 O = 18. 02 g Solution: Check: Since the given amount is more than 1 mol, the mass being > 18 g makes sense. Tro's "Introductory Chemistry", Chapter 6 27
 Practice—How Many Moles Are in 50. 0 g of Pb. O 2? (Pb = 207. 2, O = 16. 00) Tro's "Introductory Chemistry", Chapter 6 28
Practice—How Many Moles Are in 50. 0 g of Pb. O 2? (Pb = 207. 2, O = 16. 00) Tro's "Introductory Chemistry", Chapter 6 28
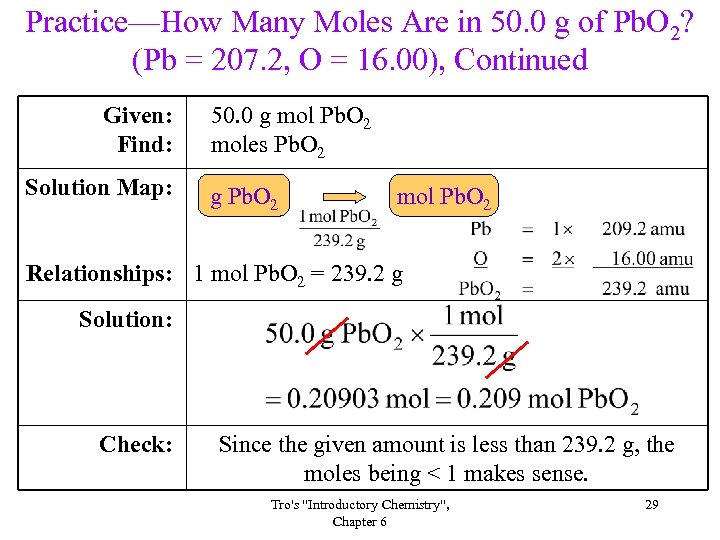 Practice—How Many Moles Are in 50. 0 g of Pb. O 2? (Pb = 207. 2, O = 16. 00), Continued Given: Find: Solution Map: 50. 0 g mol Pb. O 2 moles Pb. O 2 g Pb. O 2 mol Pb. O 2 Relationships: 1 mol Pb. O 2 = 239. 2 g Solution: Check: Since the given amount is less than 239. 2 g, the moles being < 1 makes sense. Tro's "Introductory Chemistry", Chapter 6 29
Practice—How Many Moles Are in 50. 0 g of Pb. O 2? (Pb = 207. 2, O = 16. 00), Continued Given: Find: Solution Map: 50. 0 g mol Pb. O 2 moles Pb. O 2 g Pb. O 2 mol Pb. O 2 Relationships: 1 mol Pb. O 2 = 239. 2 g Solution: Check: Since the given amount is less than 239. 2 g, the moles being < 1 makes sense. Tro's "Introductory Chemistry", Chapter 6 29
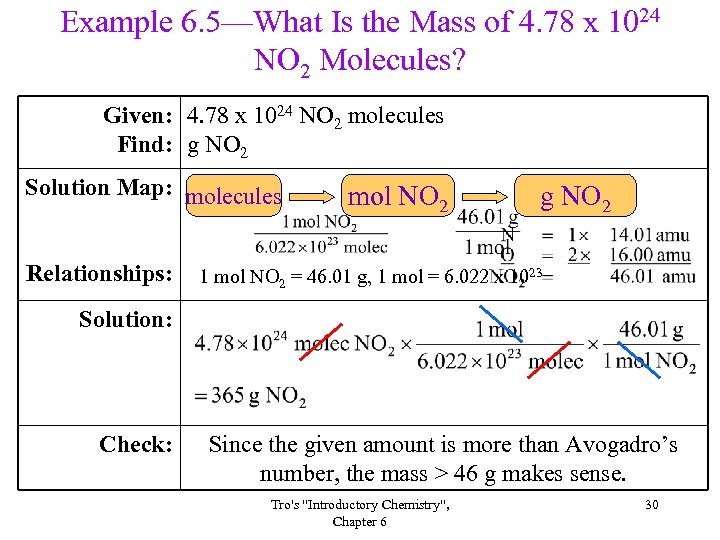 Example 6. 5—What Is the Mass of 4. 78 x 1024 NO 2 Molecules? Given: 4. 78 x 1024 NO 2 molecules Find: g NO 2 Solution Map: molecules Relationships: mol NO 2 g NO 2 1 mol NO 2 = 46. 01 g, 1 mol = 6. 022 x 1023 Solution: Check: Since the given amount is more than Avogadro’s number, the mass > 46 g makes sense. Tro's "Introductory Chemistry", Chapter 6 30
Example 6. 5—What Is the Mass of 4. 78 x 1024 NO 2 Molecules? Given: 4. 78 x 1024 NO 2 molecules Find: g NO 2 Solution Map: molecules Relationships: mol NO 2 g NO 2 1 mol NO 2 = 46. 01 g, 1 mol = 6. 022 x 1023 Solution: Check: Since the given amount is more than Avogadro’s number, the mass > 46 g makes sense. Tro's "Introductory Chemistry", Chapter 6 30
 Counting and ratio’s It takes me. 2 gal of gas to get to IVC. It is a very simple ratio: = What if I only had. 1 gal 200 2 X 4’s, 3 sinks, 2 showers, you can make a house with 3 bathrooms and 3 bedrooms. What if you had 12 sinks…how many houses could you make. 200 3 2 = 1 3 3
Counting and ratio’s It takes me. 2 gal of gas to get to IVC. It is a very simple ratio: = What if I only had. 1 gal 200 2 X 4’s, 3 sinks, 2 showers, you can make a house with 3 bathrooms and 3 bedrooms. What if you had 12 sinks…how many houses could you make. 200 3 2 = 1 3 3
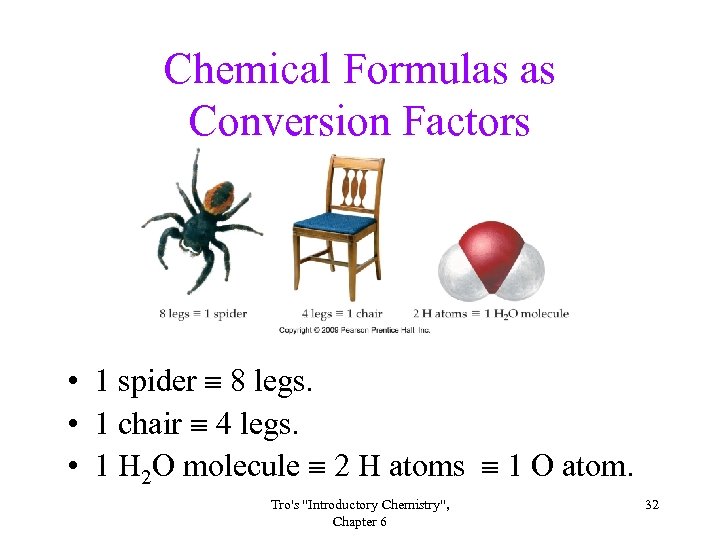 Chemical Formulas as Conversion Factors • 1 spider 8 legs. • 1 chair 4 legs. • 1 H 2 O molecule 2 H atoms 1 O atom. Tro's "Introductory Chemistry", Chapter 6 32
Chemical Formulas as Conversion Factors • 1 spider 8 legs. • 1 chair 4 legs. • 1 H 2 O molecule 2 H atoms 1 O atom. Tro's "Introductory Chemistry", Chapter 6 32
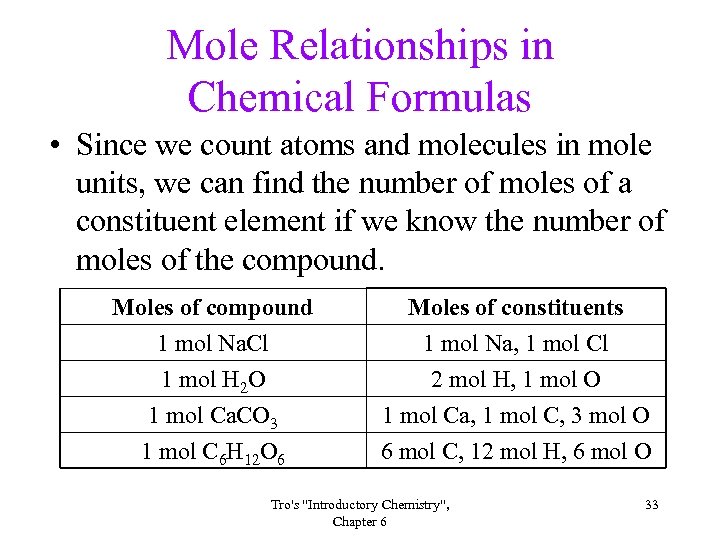 Mole Relationships in Chemical Formulas • Since we count atoms and molecules in mole units, we can find the number of moles of a constituent element if we know the number of moles of the compound. Moles of compound 1 mol Na. Cl 1 mol H 2 O 1 mol Ca. CO 3 Moles of constituents 1 mol Na, 1 mol Cl 2 mol H, 1 mol O 1 mol Ca, 1 mol C, 3 mol O 1 mol C 6 H 12 O 6 6 mol C, 12 mol H, 6 mol O Tro's "Introductory Chemistry", Chapter 6 33
Mole Relationships in Chemical Formulas • Since we count atoms and molecules in mole units, we can find the number of moles of a constituent element if we know the number of moles of the compound. Moles of compound 1 mol Na. Cl 1 mol H 2 O 1 mol Ca. CO 3 Moles of constituents 1 mol Na, 1 mol Cl 2 mol H, 1 mol O 1 mol Ca, 1 mol C, 3 mol O 1 mol C 6 H 12 O 6 6 mol C, 12 mol H, 6 mol O Tro's "Introductory Chemistry", Chapter 6 33
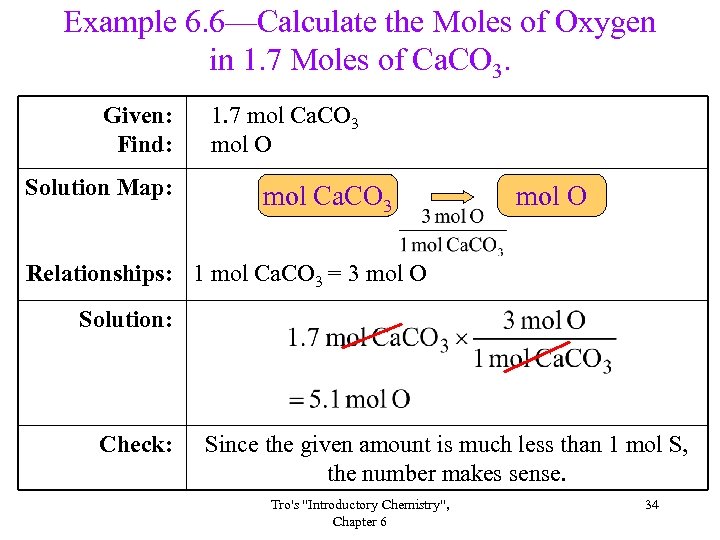 Example 6. 6—Calculate the Moles of Oxygen in 1. 7 Moles of Ca. CO 3. Given: Find: Solution Map: 1. 7 mol Ca. CO 3 mol O Relationships: 1 mol Ca. CO 3 = 3 mol O Solution: Check: Since the given amount is much less than 1 mol S, the number makes sense. Tro's "Introductory Chemistry", Chapter 6 34
Example 6. 6—Calculate the Moles of Oxygen in 1. 7 Moles of Ca. CO 3. Given: Find: Solution Map: 1. 7 mol Ca. CO 3 mol O Relationships: 1 mol Ca. CO 3 = 3 mol O Solution: Check: Since the given amount is much less than 1 mol S, the number makes sense. Tro's "Introductory Chemistry", Chapter 6 34
 Example 6. 7: • Carvone (C 10 H 14 O) is the main component in spearmint oil. It has a pleasant odor and mint flavor. It is often added to chewing gum, liqueurs, soaps, and perfumes. Find the mass of carbon in 55. 4 g of carvone. Tro's "Introductory Chemistry", Chapter 6 35
Example 6. 7: • Carvone (C 10 H 14 O) is the main component in spearmint oil. It has a pleasant odor and mint flavor. It is often added to chewing gum, liqueurs, soaps, and perfumes. Find the mass of carbon in 55. 4 g of carvone. Tro's "Introductory Chemistry", Chapter 6 35
 Example: Find the mass of carbon in 55. 4 g of carvone, (C 10 H 14 O). • Write down the given quantity and its units. Given: 55. 4 g C 10 H 14 O Tro's "Introductory Chemistry", Chapter 6 36
Example: Find the mass of carbon in 55. 4 g of carvone, (C 10 H 14 O). • Write down the given quantity and its units. Given: 55. 4 g C 10 H 14 O Tro's "Introductory Chemistry", Chapter 6 36
 Example: Find the mass of carbon in 55. 4 g of carvone, (C 10 H 14 O). Information: Given: 55. 4 g C 10 H 14 O • Write down the quantity to find and/or its units. Find: ? g C Tro's "Introductory Chemistry", Chapter 6 37
Example: Find the mass of carbon in 55. 4 g of carvone, (C 10 H 14 O). Information: Given: 55. 4 g C 10 H 14 O • Write down the quantity to find and/or its units. Find: ? g C Tro's "Introductory Chemistry", Chapter 6 37
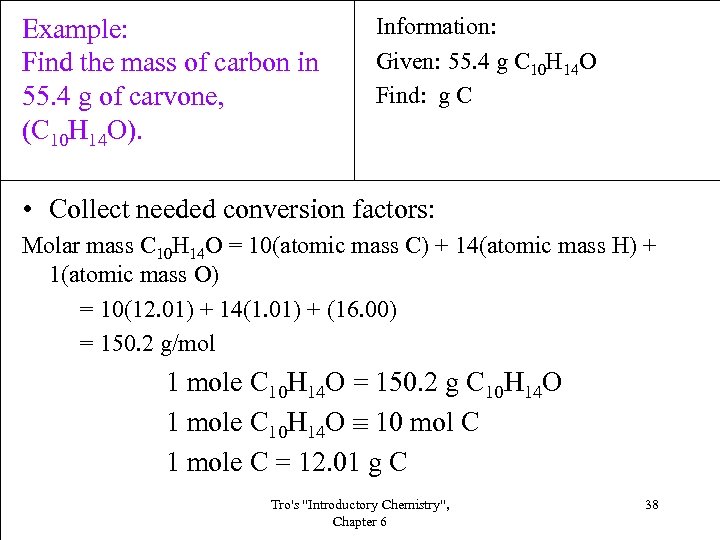 Example: Find the mass of carbon in 55. 4 g of carvone, (C 10 H 14 O). Information: Given: 55. 4 g C 10 H 14 O Find: g C • Collect needed conversion factors: Molar mass C 10 H 14 O = 10(atomic mass C) + 14(atomic mass H) + 1(atomic mass O) = 10(12. 01) + 14(1. 01) + (16. 00) = 150. 2 g/mol 1 mole C 10 H 14 O = 150. 2 g C 10 H 14 O 1 mole C 10 H 14 O 10 mol C 1 mole C = 12. 01 g C Tro's "Introductory Chemistry", Chapter 6 38
Example: Find the mass of carbon in 55. 4 g of carvone, (C 10 H 14 O). Information: Given: 55. 4 g C 10 H 14 O Find: g C • Collect needed conversion factors: Molar mass C 10 H 14 O = 10(atomic mass C) + 14(atomic mass H) + 1(atomic mass O) = 10(12. 01) + 14(1. 01) + (16. 00) = 150. 2 g/mol 1 mole C 10 H 14 O = 150. 2 g C 10 H 14 O 1 mole C 10 H 14 O 10 mol C 1 mole C = 12. 01 g C Tro's "Introductory Chemistry", Chapter 6 38
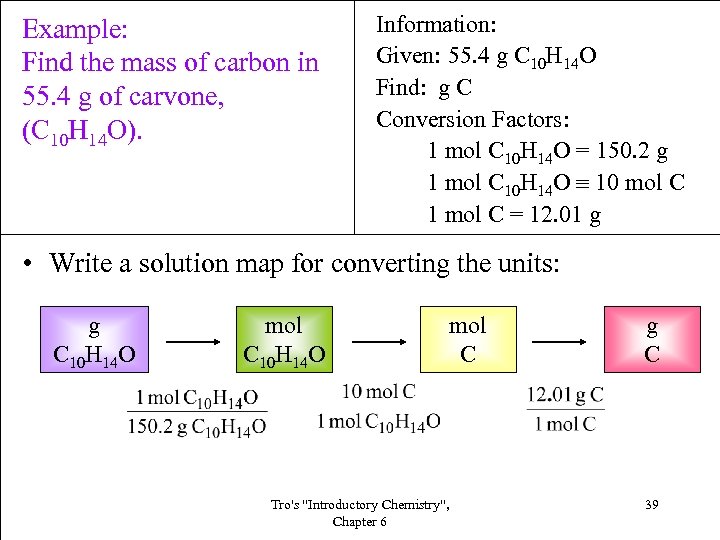 Example: Find the mass of carbon in 55. 4 g of carvone, (C 10 H 14 O). Information: Given: 55. 4 g C 10 H 14 O Find: g C Conversion Factors: 1 mol C 10 H 14 O = 150. 2 g 1 mol C 10 H 14 O 10 mol C 1 mol C = 12. 01 g • Write a solution map for converting the units: g C 10 H 14 O mol C 10 H 14 O Tro's "Introductory Chemistry", Chapter 6 mol C g C 39
Example: Find the mass of carbon in 55. 4 g of carvone, (C 10 H 14 O). Information: Given: 55. 4 g C 10 H 14 O Find: g C Conversion Factors: 1 mol C 10 H 14 O = 150. 2 g 1 mol C 10 H 14 O 10 mol C 1 mol C = 12. 01 g • Write a solution map for converting the units: g C 10 H 14 O mol C 10 H 14 O Tro's "Introductory Chemistry", Chapter 6 mol C g C 39
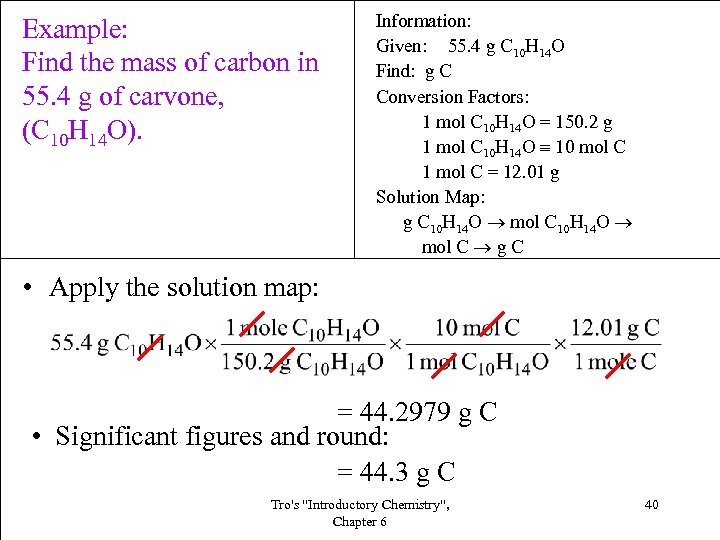 Example: Find the mass of carbon in 55. 4 g of carvone, (C 10 H 14 O). Information: Given: 55. 4 g C 10 H 14 O Find: g C Conversion Factors: 1 mol C 10 H 14 O = 150. 2 g 1 mol C 10 H 14 O 10 mol C 1 mol C = 12. 01 g Solution Map: g C 10 H 14 O mol C g C • Apply the solution map: = 44. 2979 g C • Significant figures and round: = 44. 3 g C Tro's "Introductory Chemistry", Chapter 6 40
Example: Find the mass of carbon in 55. 4 g of carvone, (C 10 H 14 O). Information: Given: 55. 4 g C 10 H 14 O Find: g C Conversion Factors: 1 mol C 10 H 14 O = 150. 2 g 1 mol C 10 H 14 O 10 mol C 1 mol C = 12. 01 g Solution Map: g C 10 H 14 O mol C g C • Apply the solution map: = 44. 2979 g C • Significant figures and round: = 44. 3 g C Tro's "Introductory Chemistry", Chapter 6 40
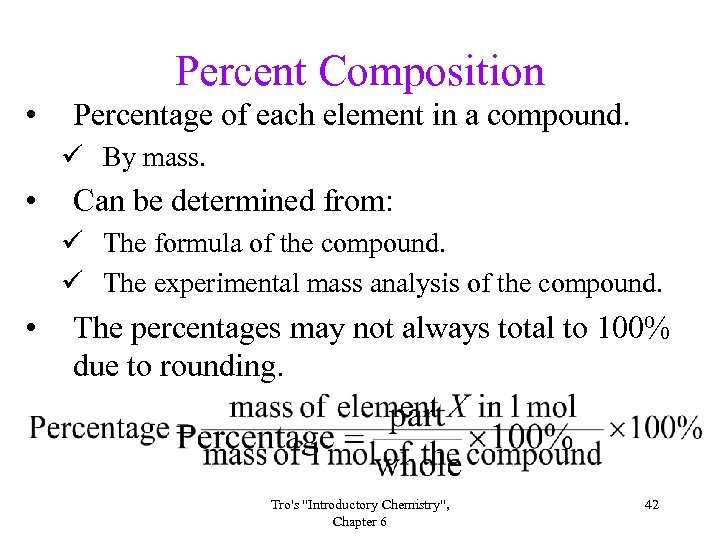 Percent Composition • Percentage of each element in a compound. ü By mass. • Can be determined from: ü The formula of the compound. ü The experimental mass analysis of the compound. • The percentages may not always total to 100% due to rounding. Tro's "Introductory Chemistry", Chapter 6 42
Percent Composition • Percentage of each element in a compound. ü By mass. • Can be determined from: ü The formula of the compound. ü The experimental mass analysis of the compound. • The percentages may not always total to 100% due to rounding. Tro's "Introductory Chemistry", Chapter 6 42
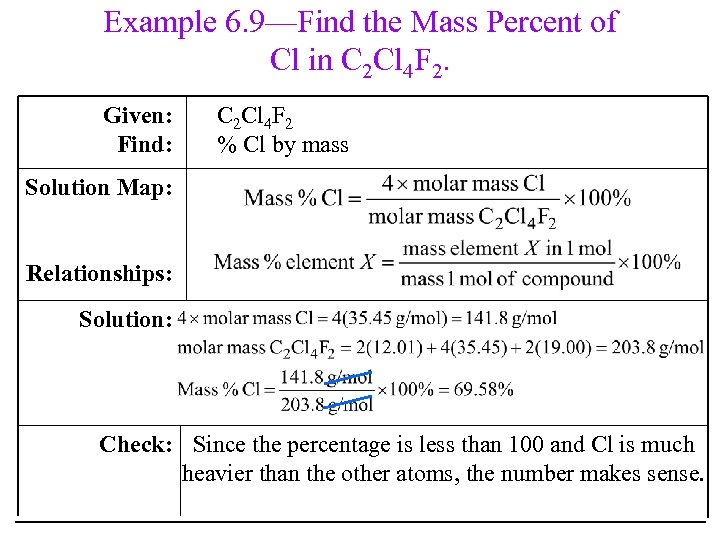 Example 6. 9—Find the Mass Percent of Cl in C 2 Cl 4 F 2. Given: Find: C 2 Cl 4 F 2 % Cl by mass Solution Map: Relationships: Solution: Check: Since the percentage is less than 100 and Cl is much heavier than the other atoms, the number makes sense.
Example 6. 9—Find the Mass Percent of Cl in C 2 Cl 4 F 2. Given: Find: C 2 Cl 4 F 2 % Cl by mass Solution Map: Relationships: Solution: Check: Since the percentage is less than 100 and Cl is much heavier than the other atoms, the number makes sense.
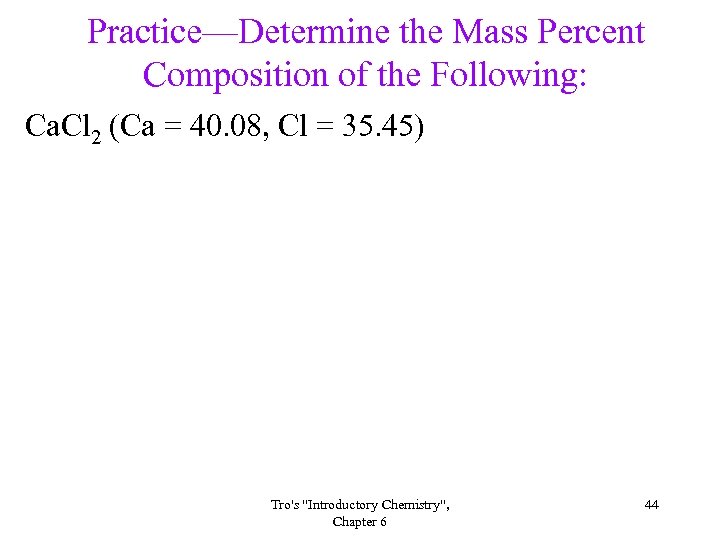 Practice—Determine the Mass Percent Composition of the Following: Ca. Cl 2 (Ca = 40. 08, Cl = 35. 45) Tro's "Introductory Chemistry", Chapter 6 44
Practice—Determine the Mass Percent Composition of the Following: Ca. Cl 2 (Ca = 40. 08, Cl = 35. 45) Tro's "Introductory Chemistry", Chapter 6 44
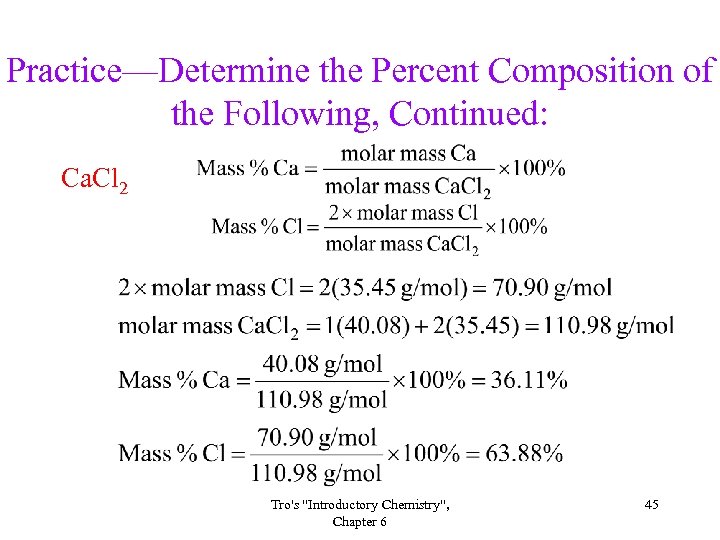 Practice—Determine the Percent Composition of the Following, Continued: Ca. Cl 2 Tro's "Introductory Chemistry", Chapter 6 45
Practice—Determine the Percent Composition of the Following, Continued: Ca. Cl 2 Tro's "Introductory Chemistry", Chapter 6 45
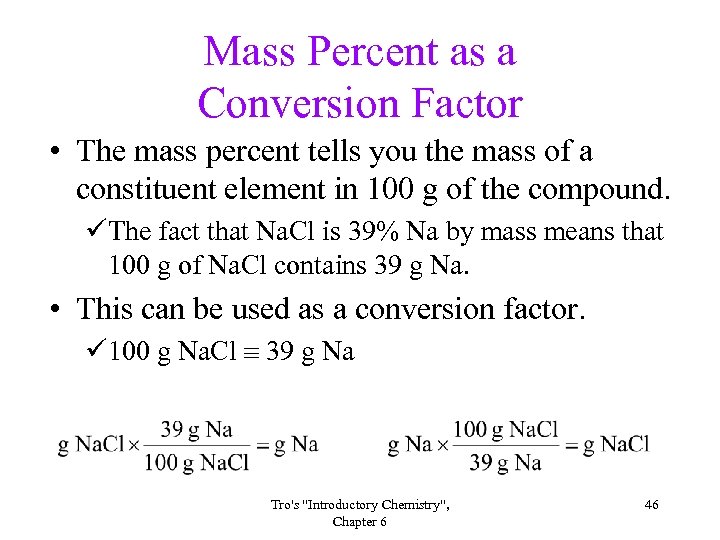 Mass Percent as a Conversion Factor • The mass percent tells you the mass of a constituent element in 100 g of the compound. üThe fact that Na. Cl is 39% Na by mass means that 100 g of Na. Cl contains 39 g Na. • This can be used as a conversion factor. ü 100 g Na. Cl 39 g Na Tro's "Introductory Chemistry", Chapter 6 46
Mass Percent as a Conversion Factor • The mass percent tells you the mass of a constituent element in 100 g of the compound. üThe fact that Na. Cl is 39% Na by mass means that 100 g of Na. Cl contains 39 g Na. • This can be used as a conversion factor. ü 100 g Na. Cl 39 g Na Tro's "Introductory Chemistry", Chapter 6 46
 Empirical Formulas • The simplest, whole-number ratio of atoms in a molecule is called the empirical formula. üCan be determined from percent composition or combining masses. • The molecular formula is a multiple of the empirical formula. Tro's "Introductory Chemistry", Chapter 6 47
Empirical Formulas • The simplest, whole-number ratio of atoms in a molecule is called the empirical formula. üCan be determined from percent composition or combining masses. • The molecular formula is a multiple of the empirical formula. Tro's "Introductory Chemistry", Chapter 6 47
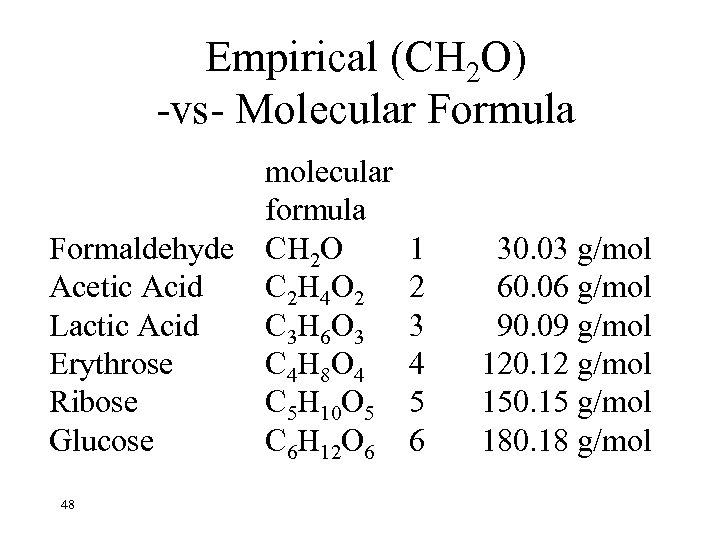 Empirical (CH 2 O) -vs- Molecular Formula molecular formula Formaldehyde CH 2 O Acetic Acid C 2 H 4 O 2 Lactic Acid C 3 H 6 O 3 Erythrose C 4 H 8 O 4 Ribose C 5 H 10 O 5 Glucose C 6 H 12 O 6 48 1 2 3 4 5 6 30. 03 g/mol 60. 06 g/mol 90. 09 g/mol 120. 12 g/mol 150. 15 g/mol 180. 18 g/mol
Empirical (CH 2 O) -vs- Molecular Formula molecular formula Formaldehyde CH 2 O Acetic Acid C 2 H 4 O 2 Lactic Acid C 3 H 6 O 3 Erythrose C 4 H 8 O 4 Ribose C 5 H 10 O 5 Glucose C 6 H 12 O 6 48 1 2 3 4 5 6 30. 03 g/mol 60. 06 g/mol 90. 09 g/mol 120. 12 g/mol 150. 15 g/mol 180. 18 g/mol
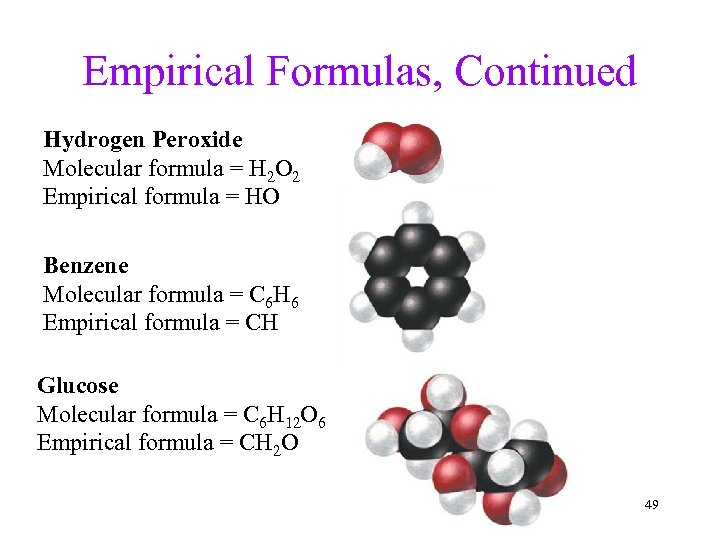 Empirical Formulas, Continued Hydrogen Peroxide Molecular formula = H 2 O 2 Empirical formula = HO Benzene Molecular formula = C 6 H 6 Empirical formula = CH Glucose Molecular formula = C 6 H 12 O 6 Empirical formula = CH 2 O 49
Empirical Formulas, Continued Hydrogen Peroxide Molecular formula = H 2 O 2 Empirical formula = HO Benzene Molecular formula = C 6 H 6 Empirical formula = CH Glucose Molecular formula = C 6 H 12 O 6 Empirical formula = CH 2 O 49
 Example 6. 11—Finding an Empirical Formula from Experimental Data Tro's "Introductory Chemistry", Chapter 6 50
Example 6. 11—Finding an Empirical Formula from Experimental Data Tro's "Introductory Chemistry", Chapter 6 50
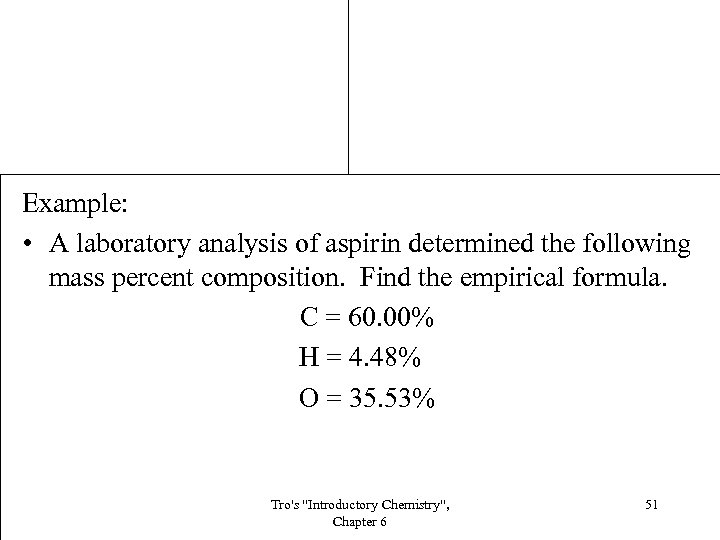 Example: • A laboratory analysis of aspirin determined the following mass percent composition. Find the empirical formula. C = 60. 00% H = 4. 48% O = 35. 53% Tro's "Introductory Chemistry", Chapter 6 51
Example: • A laboratory analysis of aspirin determined the following mass percent composition. Find the empirical formula. C = 60. 00% H = 4. 48% O = 35. 53% Tro's "Introductory Chemistry", Chapter 6 51
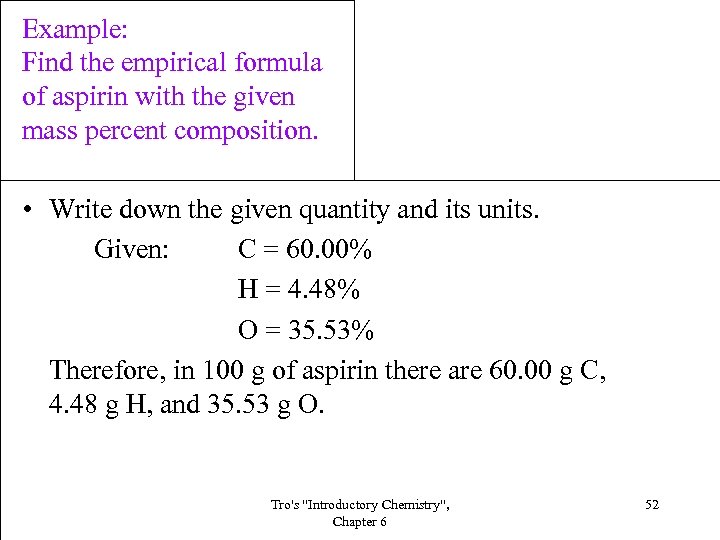 Example: Find the empirical formula of aspirin with the given mass percent composition. • Write down the given quantity and its units. Given: C = 60. 00% H = 4. 48% O = 35. 53% Therefore, in 100 g of aspirin there are 60. 00 g C, 4. 48 g H, and 35. 53 g O. Tro's "Introductory Chemistry", Chapter 6 52
Example: Find the empirical formula of aspirin with the given mass percent composition. • Write down the given quantity and its units. Given: C = 60. 00% H = 4. 48% O = 35. 53% Therefore, in 100 g of aspirin there are 60. 00 g C, 4. 48 g H, and 35. 53 g O. Tro's "Introductory Chemistry", Chapter 6 52
 Example: Find the empirical formula of aspirin with the given mass percent composition. Information: Given: 60. 00 g C, 4. 48 g H, 35. 53 g O • Write down the quantity to find and/or its units. Find: empirical formula, Cx. Hy. Oz Tro's "Introductory Chemistry", Chapter 6 53
Example: Find the empirical formula of aspirin with the given mass percent composition. Information: Given: 60. 00 g C, 4. 48 g H, 35. 53 g O • Write down the quantity to find and/or its units. Find: empirical formula, Cx. Hy. Oz Tro's "Introductory Chemistry", Chapter 6 53
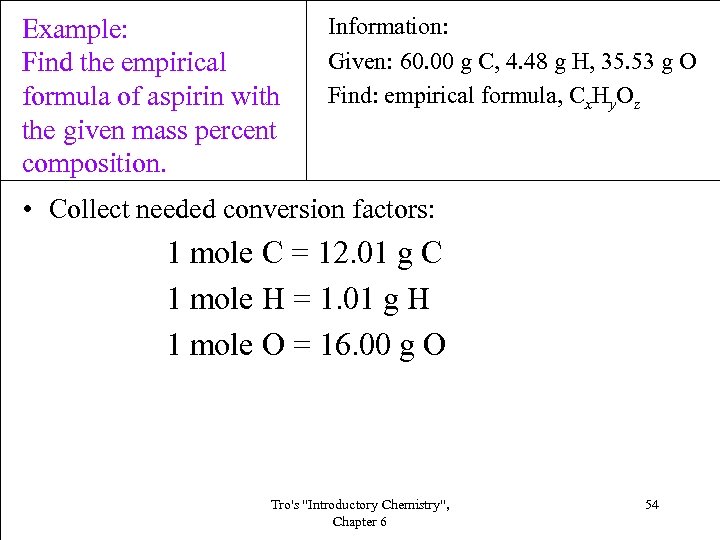 Example: Find the empirical formula of aspirin with the given mass percent composition. Information: Given: 60. 00 g C, 4. 48 g H, 35. 53 g O Find: empirical formula, Cx. Hy. Oz • Collect needed conversion factors: 1 mole C = 12. 01 g C 1 mole H = 1. 01 g H 1 mole O = 16. 00 g O Tro's "Introductory Chemistry", Chapter 6 54
Example: Find the empirical formula of aspirin with the given mass percent composition. Information: Given: 60. 00 g C, 4. 48 g H, 35. 53 g O Find: empirical formula, Cx. Hy. Oz • Collect needed conversion factors: 1 mole C = 12. 01 g C 1 mole H = 1. 01 g H 1 mole O = 16. 00 g O Tro's "Introductory Chemistry", Chapter 6 54
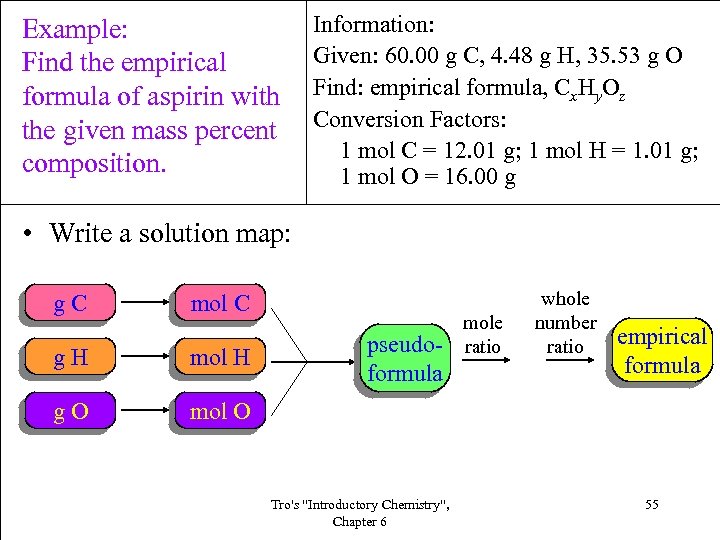 Example: Find the empirical formula of aspirin with the given mass percent composition. Information: Given: 60. 00 g C, 4. 48 g H, 35. 53 g O Find: empirical formula, Cx. Hy. Oz Conversion Factors: 1 mol C = 12. 01 g; 1 mol H = 1. 01 g; 1 mol O = 16. 00 g • Write a solution map: g. C mol C g. H mol H g. O pseudoformula mole ratio whole number ratio empirical formula mol O Tro's "Introductory Chemistry", Chapter 6 55
Example: Find the empirical formula of aspirin with the given mass percent composition. Information: Given: 60. 00 g C, 4. 48 g H, 35. 53 g O Find: empirical formula, Cx. Hy. Oz Conversion Factors: 1 mol C = 12. 01 g; 1 mol H = 1. 01 g; 1 mol O = 16. 00 g • Write a solution map: g. C mol C g. H mol H g. O pseudoformula mole ratio whole number ratio empirical formula mol O Tro's "Introductory Chemistry", Chapter 6 55
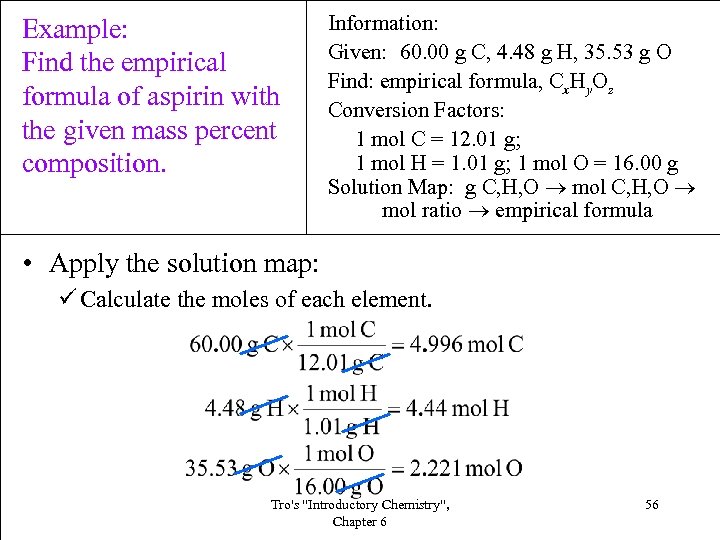 Example: Find the empirical formula of aspirin with the given mass percent composition. Information: Given: 60. 00 g C, 4. 48 g H, 35. 53 g O Find: empirical formula, Cx. Hy. Oz Conversion Factors: 1 mol C = 12. 01 g; 1 mol H = 1. 01 g; 1 mol O = 16. 00 g Solution Map: g C, H, O mol ratio empirical formula • Apply the solution map: ü Calculate the moles of each element. Tro's "Introductory Chemistry", Chapter 6 56
Example: Find the empirical formula of aspirin with the given mass percent composition. Information: Given: 60. 00 g C, 4. 48 g H, 35. 53 g O Find: empirical formula, Cx. Hy. Oz Conversion Factors: 1 mol C = 12. 01 g; 1 mol H = 1. 01 g; 1 mol O = 16. 00 g Solution Map: g C, H, O mol ratio empirical formula • Apply the solution map: ü Calculate the moles of each element. Tro's "Introductory Chemistry", Chapter 6 56
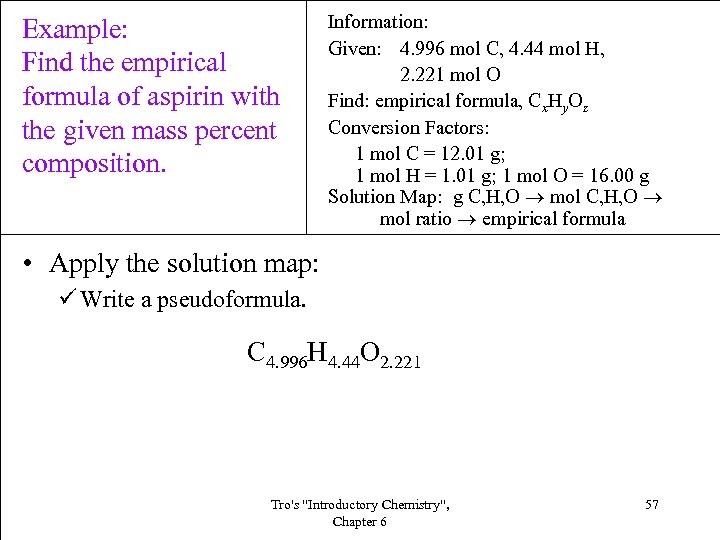 Example: Find the empirical formula of aspirin with the given mass percent composition. Information: Given: 4. 996 mol C, 4. 44 mol H, 2. 221 mol O Find: empirical formula, Cx. Hy. Oz Conversion Factors: 1 mol C = 12. 01 g; 1 mol H = 1. 01 g; 1 mol O = 16. 00 g Solution Map: g C, H, O mol ratio empirical formula • Apply the solution map: ü Write a pseudoformula. C 4. 996 H 4. 44 O 2. 221 Tro's "Introductory Chemistry", Chapter 6 57
Example: Find the empirical formula of aspirin with the given mass percent composition. Information: Given: 4. 996 mol C, 4. 44 mol H, 2. 221 mol O Find: empirical formula, Cx. Hy. Oz Conversion Factors: 1 mol C = 12. 01 g; 1 mol H = 1. 01 g; 1 mol O = 16. 00 g Solution Map: g C, H, O mol ratio empirical formula • Apply the solution map: ü Write a pseudoformula. C 4. 996 H 4. 44 O 2. 221 Tro's "Introductory Chemistry", Chapter 6 57
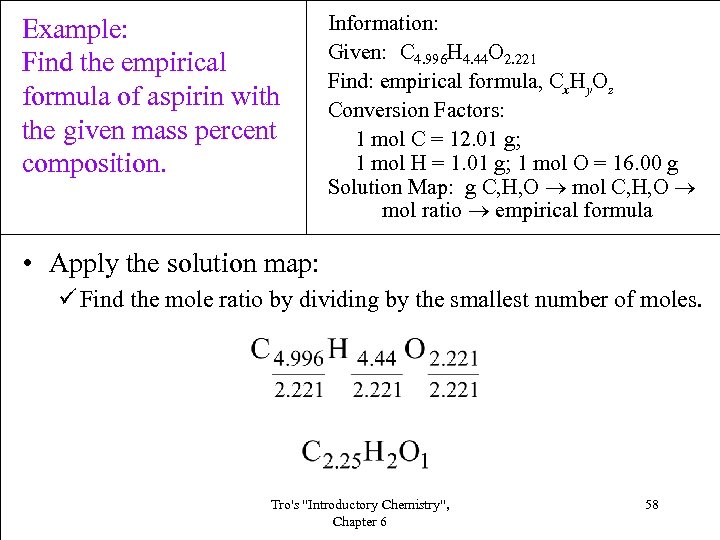 Example: Find the empirical formula of aspirin with the given mass percent composition. Information: Given: C 4. 996 H 4. 44 O 2. 221 Find: empirical formula, Cx. Hy. Oz Conversion Factors: 1 mol C = 12. 01 g; 1 mol H = 1. 01 g; 1 mol O = 16. 00 g Solution Map: g C, H, O mol ratio empirical formula • Apply the solution map: ü Find the mole ratio by dividing by the smallest number of moles. Tro's "Introductory Chemistry", Chapter 6 58
Example: Find the empirical formula of aspirin with the given mass percent composition. Information: Given: C 4. 996 H 4. 44 O 2. 221 Find: empirical formula, Cx. Hy. Oz Conversion Factors: 1 mol C = 12. 01 g; 1 mol H = 1. 01 g; 1 mol O = 16. 00 g Solution Map: g C, H, O mol ratio empirical formula • Apply the solution map: ü Find the mole ratio by dividing by the smallest number of moles. Tro's "Introductory Chemistry", Chapter 6 58
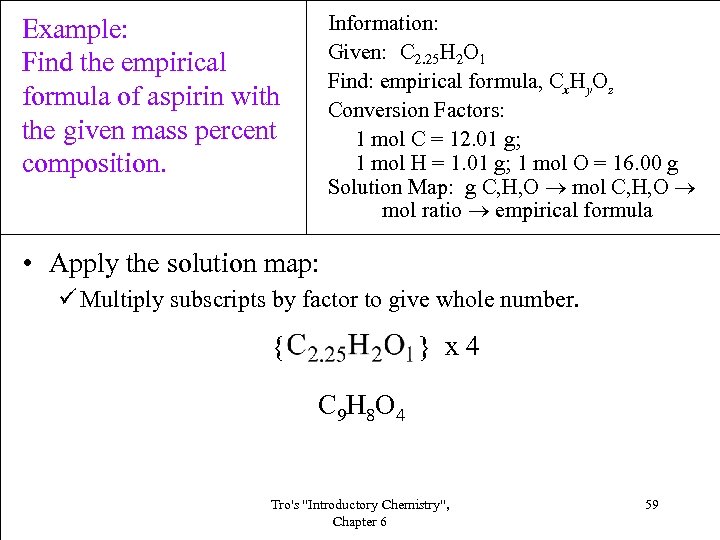 Information: Given: C 2. 25 H 2 O 1 Find: empirical formula, Cx. Hy. Oz Conversion Factors: 1 mol C = 12. 01 g; 1 mol H = 1. 01 g; 1 mol O = 16. 00 g Solution Map: g C, H, O mol ratio empirical formula Example: Find the empirical formula of aspirin with the given mass percent composition. • Apply the solution map: ü Multiply subscripts by factor to give whole number. { } x 4 C 9 H 8 O 4 Tro's "Introductory Chemistry", Chapter 6 59
Information: Given: C 2. 25 H 2 O 1 Find: empirical formula, Cx. Hy. Oz Conversion Factors: 1 mol C = 12. 01 g; 1 mol H = 1. 01 g; 1 mol O = 16. 00 g Solution Map: g C, H, O mol ratio empirical formula Example: Find the empirical formula of aspirin with the given mass percent composition. • Apply the solution map: ü Multiply subscripts by factor to give whole number. { } x 4 C 9 H 8 O 4 Tro's "Introductory Chemistry", Chapter 6 59
 Example 6. 12—Finding an Empirical Formula from Experimental Data Tro's "Introductory Chemistry", Chapter 6 60
Example 6. 12—Finding an Empirical Formula from Experimental Data Tro's "Introductory Chemistry", Chapter 6 60
 Example: • A 3. 24 -g sample of titanium reacts with oxygen to form 5. 40 g of the metal oxide. What is the formula of the oxide? Tro's "Introductory Chemistry", Chapter 6 61
Example: • A 3. 24 -g sample of titanium reacts with oxygen to form 5. 40 g of the metal oxide. What is the formula of the oxide? Tro's "Introductory Chemistry", Chapter 6 61
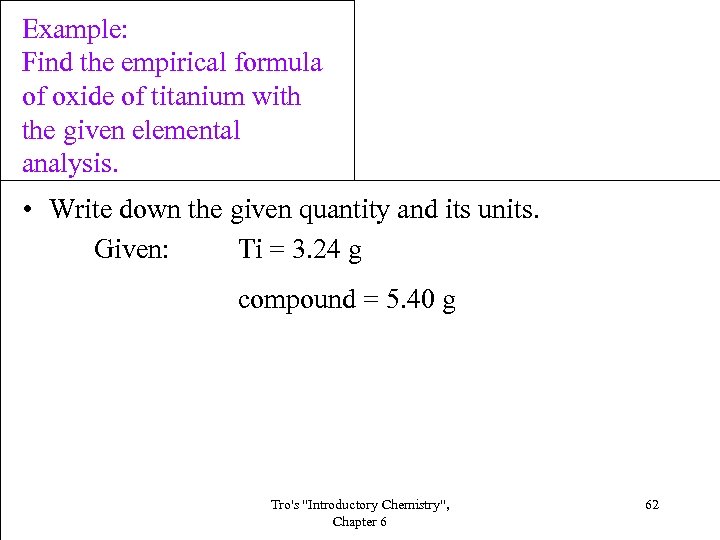 Example: Find the empirical formula of oxide of titanium with the given elemental analysis. • Write down the given quantity and its units. Given: Ti = 3. 24 g compound = 5. 40 g Tro's "Introductory Chemistry", Chapter 6 62
Example: Find the empirical formula of oxide of titanium with the given elemental analysis. • Write down the given quantity and its units. Given: Ti = 3. 24 g compound = 5. 40 g Tro's "Introductory Chemistry", Chapter 6 62
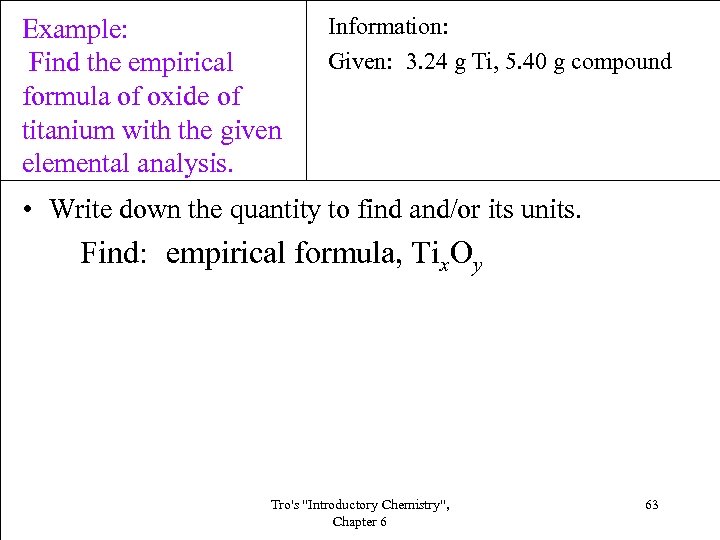 Example: Find the empirical formula of oxide of titanium with the given elemental analysis. Information: Given: 3. 24 g Ti, 5. 40 g compound • Write down the quantity to find and/or its units. Find: empirical formula, Tix. Oy Tro's "Introductory Chemistry", Chapter 6 63
Example: Find the empirical formula of oxide of titanium with the given elemental analysis. Information: Given: 3. 24 g Ti, 5. 40 g compound • Write down the quantity to find and/or its units. Find: empirical formula, Tix. Oy Tro's "Introductory Chemistry", Chapter 6 63
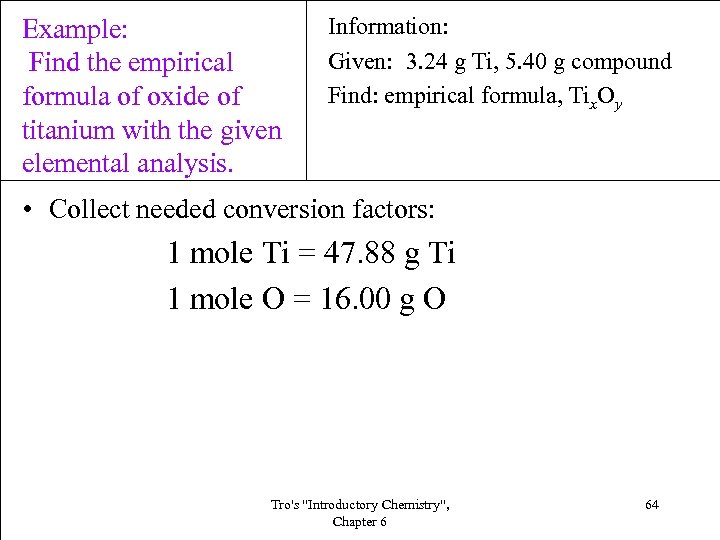 Example: Find the empirical formula of oxide of titanium with the given elemental analysis. Information: Given: 3. 24 g Ti, 5. 40 g compound Find: empirical formula, Tix. Oy • Collect needed conversion factors: 1 mole Ti = 47. 88 g Ti 1 mole O = 16. 00 g O Tro's "Introductory Chemistry", Chapter 6 64
Example: Find the empirical formula of oxide of titanium with the given elemental analysis. Information: Given: 3. 24 g Ti, 5. 40 g compound Find: empirical formula, Tix. Oy • Collect needed conversion factors: 1 mole Ti = 47. 88 g Ti 1 mole O = 16. 00 g O Tro's "Introductory Chemistry", Chapter 6 64
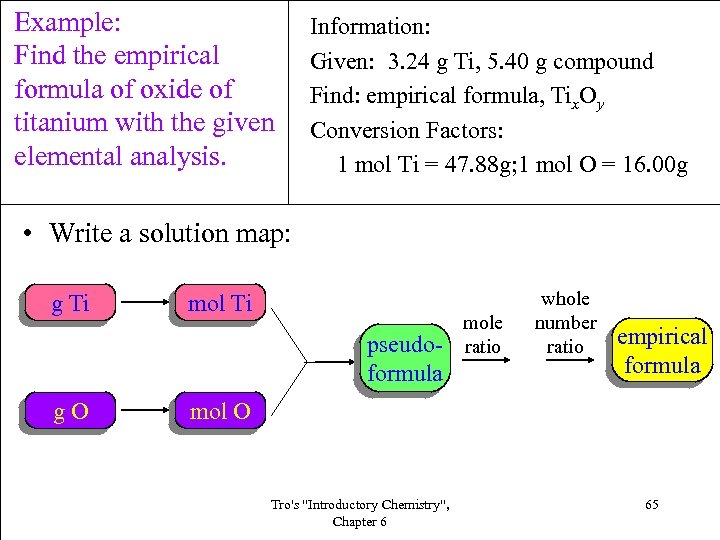 Example: Find the empirical formula of oxide of titanium with the given elemental analysis. Information: Given: 3. 24 g Ti, 5. 40 g compound Find: empirical formula, Tix. Oy Conversion Factors: 1 mol Ti = 47. 88 g; 1 mol O = 16. 00 g • Write a solution map: g Ti mol Ti pseudoformula g. O mole ratio whole number ratio empirical formula mol O Tro's "Introductory Chemistry", Chapter 6 65
Example: Find the empirical formula of oxide of titanium with the given elemental analysis. Information: Given: 3. 24 g Ti, 5. 40 g compound Find: empirical formula, Tix. Oy Conversion Factors: 1 mol Ti = 47. 88 g; 1 mol O = 16. 00 g • Write a solution map: g Ti mol Ti pseudoformula g. O mole ratio whole number ratio empirical formula mol O Tro's "Introductory Chemistry", Chapter 6 65
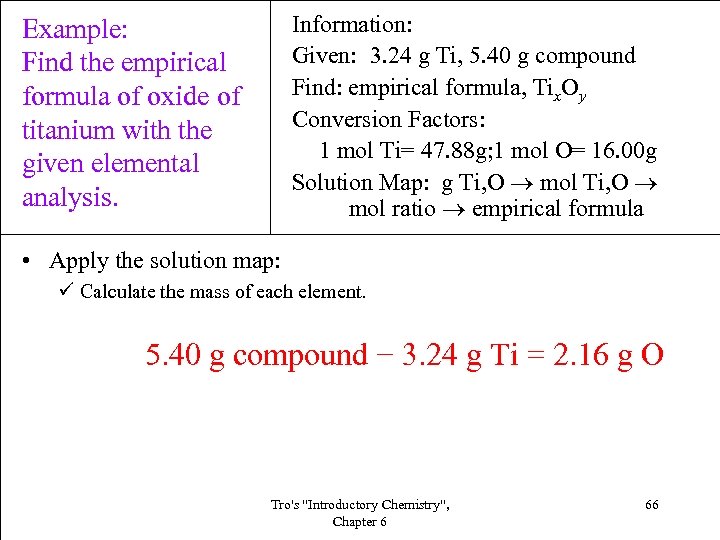 Information: Given: 3. 24 g Ti, 5. 40 g compound Find: empirical formula, Tix. Oy Conversion Factors: 1 mol Ti= 47. 88 g; 1 mol O= 16. 00 g Solution Map: g Ti, O mol ratio empirical formula Example: Find the empirical formula of oxide of titanium with the given elemental analysis. • Apply the solution map: ü Calculate the mass of each element. 5. 40 g compound − 3. 24 g Ti = 2. 16 g O Tro's "Introductory Chemistry", Chapter 6 66
Information: Given: 3. 24 g Ti, 5. 40 g compound Find: empirical formula, Tix. Oy Conversion Factors: 1 mol Ti= 47. 88 g; 1 mol O= 16. 00 g Solution Map: g Ti, O mol ratio empirical formula Example: Find the empirical formula of oxide of titanium with the given elemental analysis. • Apply the solution map: ü Calculate the mass of each element. 5. 40 g compound − 3. 24 g Ti = 2. 16 g O Tro's "Introductory Chemistry", Chapter 6 66
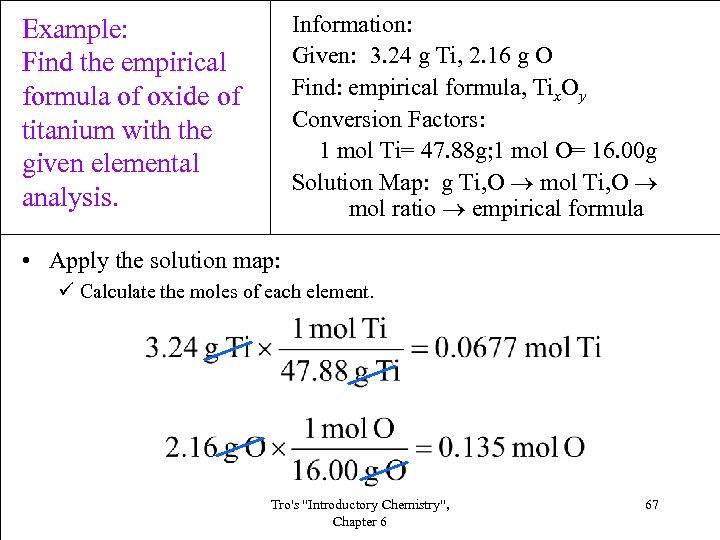 Information: Given: 3. 24 g Ti, 2. 16 g O Find: empirical formula, Tix. Oy Conversion Factors: 1 mol Ti= 47. 88 g; 1 mol O= 16. 00 g Solution Map: g Ti, O mol ratio empirical formula Example: Find the empirical formula of oxide of titanium with the given elemental analysis. • Apply the solution map: ü Calculate the moles of each element. Tro's "Introductory Chemistry", Chapter 6 67
Information: Given: 3. 24 g Ti, 2. 16 g O Find: empirical formula, Tix. Oy Conversion Factors: 1 mol Ti= 47. 88 g; 1 mol O= 16. 00 g Solution Map: g Ti, O mol ratio empirical formula Example: Find the empirical formula of oxide of titanium with the given elemental analysis. • Apply the solution map: ü Calculate the moles of each element. Tro's "Introductory Chemistry", Chapter 6 67
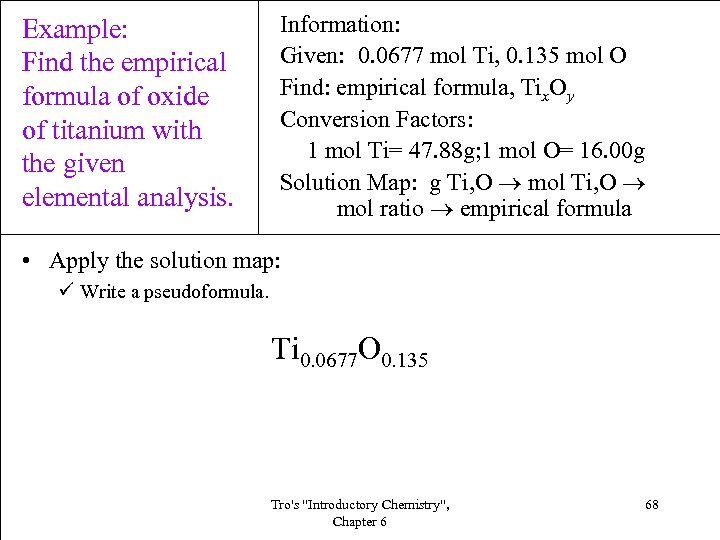 Example: Find the empirical formula of oxide of titanium with the given elemental analysis. Information: Given: 0. 0677 mol Ti, 0. 135 mol O Find: empirical formula, Tix. Oy Conversion Factors: 1 mol Ti= 47. 88 g; 1 mol O= 16. 00 g Solution Map: g Ti, O mol ratio empirical formula • Apply the solution map: ü Write a pseudoformula. Ti 0. 0677 O 0. 135 Tro's "Introductory Chemistry", Chapter 6 68
Example: Find the empirical formula of oxide of titanium with the given elemental analysis. Information: Given: 0. 0677 mol Ti, 0. 135 mol O Find: empirical formula, Tix. Oy Conversion Factors: 1 mol Ti= 47. 88 g; 1 mol O= 16. 00 g Solution Map: g Ti, O mol ratio empirical formula • Apply the solution map: ü Write a pseudoformula. Ti 0. 0677 O 0. 135 Tro's "Introductory Chemistry", Chapter 6 68
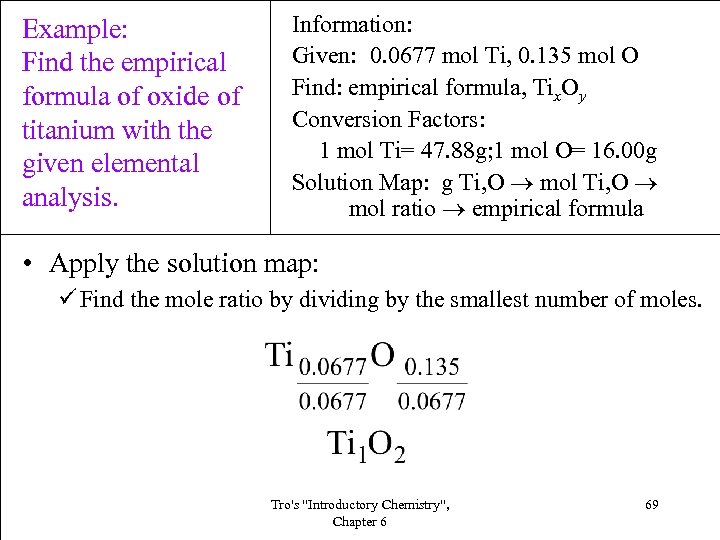 Example: Find the empirical formula of oxide of titanium with the given elemental analysis. Information: Given: 0. 0677 mol Ti, 0. 135 mol O Find: empirical formula, Tix. Oy Conversion Factors: 1 mol Ti= 47. 88 g; 1 mol O= 16. 00 g Solution Map: g Ti, O mol ratio empirical formula • Apply the solution map: ü Find the mole ratio by dividing by the smallest number of moles. Tro's "Introductory Chemistry", Chapter 6 69
Example: Find the empirical formula of oxide of titanium with the given elemental analysis. Information: Given: 0. 0677 mol Ti, 0. 135 mol O Find: empirical formula, Tix. Oy Conversion Factors: 1 mol Ti= 47. 88 g; 1 mol O= 16. 00 g Solution Map: g Ti, O mol ratio empirical formula • Apply the solution map: ü Find the mole ratio by dividing by the smallest number of moles. Tro's "Introductory Chemistry", Chapter 6 69
 Practice—Determine the Empirical Formula of Tin (II) Fluoride, which Contains 75. 7% Sn (118. 70) and the Rest Fluorine (19. 00). Tro's "Introductory Chemistry", Chapter 6 70
Practice—Determine the Empirical Formula of Tin (II) Fluoride, which Contains 75. 7% Sn (118. 70) and the Rest Fluorine (19. 00). Tro's "Introductory Chemistry", Chapter 6 70
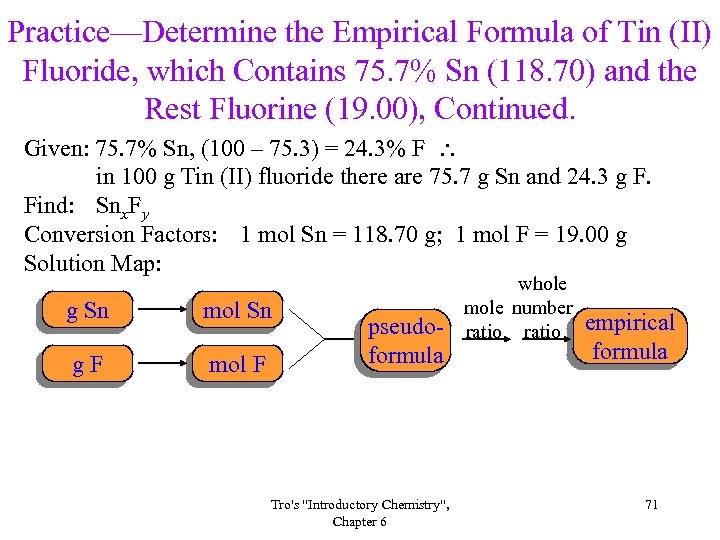 Practice—Determine the Empirical Formula of Tin (II) Fluoride, which Contains 75. 7% Sn (118. 70) and the Rest Fluorine (19. 00), Continued. Given: 75. 7% Sn, (100 – 75. 3) = 24. 3% F in 100 g Tin (II) fluoride there are 75. 7 g Sn and 24. 3 g F. Find: Snx. Fy Conversion Factors: 1 mol Sn = 118. 70 g; 1 mol F = 19. 00 g Solution Map: g Sn mol Sn g. F mol F pseudoformula Tro's "Introductory Chemistry", Chapter 6 whole mole number ratio empirical formula 71
Practice—Determine the Empirical Formula of Tin (II) Fluoride, which Contains 75. 7% Sn (118. 70) and the Rest Fluorine (19. 00), Continued. Given: 75. 7% Sn, (100 – 75. 3) = 24. 3% F in 100 g Tin (II) fluoride there are 75. 7 g Sn and 24. 3 g F. Find: Snx. Fy Conversion Factors: 1 mol Sn = 118. 70 g; 1 mol F = 19. 00 g Solution Map: g Sn mol Sn g. F mol F pseudoformula Tro's "Introductory Chemistry", Chapter 6 whole mole number ratio empirical formula 71
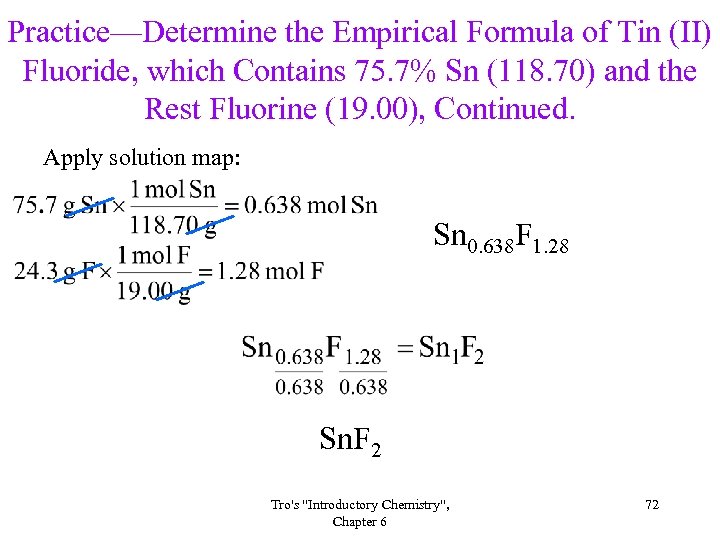 Practice—Determine the Empirical Formula of Tin (II) Fluoride, which Contains 75. 7% Sn (118. 70) and the Rest Fluorine (19. 00), Continued. Apply solution map: Sn 0. 638 F 1. 28 Sn. F 2 Tro's "Introductory Chemistry", Chapter 6 72
Practice—Determine the Empirical Formula of Tin (II) Fluoride, which Contains 75. 7% Sn (118. 70) and the Rest Fluorine (19. 00), Continued. Apply solution map: Sn 0. 638 F 1. 28 Sn. F 2 Tro's "Introductory Chemistry", Chapter 6 72
 Practice—Determine the Empirical Formula of Hematite, which Contains 72. 4% Fe (55. 85) and the Rest Oxygen (16. 00). Tro's "Introductory Chemistry", Chapter 6 73
Practice—Determine the Empirical Formula of Hematite, which Contains 72. 4% Fe (55. 85) and the Rest Oxygen (16. 00). Tro's "Introductory Chemistry", Chapter 6 73
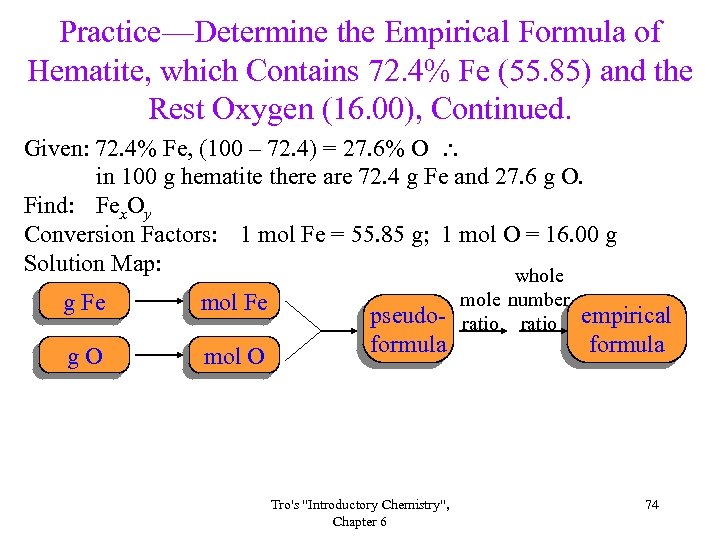 Practice—Determine the Empirical Formula of Hematite, which Contains 72. 4% Fe (55. 85) and the Rest Oxygen (16. 00), Continued. Given: 72. 4% Fe, (100 – 72. 4) = 27. 6% O in 100 g hematite there are 72. 4 g Fe and 27. 6 g O. Find: Fex. Oy Conversion Factors: 1 mol Fe = 55. 85 g; 1 mol O = 16. 00 g Solution Map: whole g Fe mol Fe g. O mol O pseudoformula Tro's "Introductory Chemistry", Chapter 6 mole number ratio empirical formula 74
Practice—Determine the Empirical Formula of Hematite, which Contains 72. 4% Fe (55. 85) and the Rest Oxygen (16. 00), Continued. Given: 72. 4% Fe, (100 – 72. 4) = 27. 6% O in 100 g hematite there are 72. 4 g Fe and 27. 6 g O. Find: Fex. Oy Conversion Factors: 1 mol Fe = 55. 85 g; 1 mol O = 16. 00 g Solution Map: whole g Fe mol Fe g. O mol O pseudoformula Tro's "Introductory Chemistry", Chapter 6 mole number ratio empirical formula 74
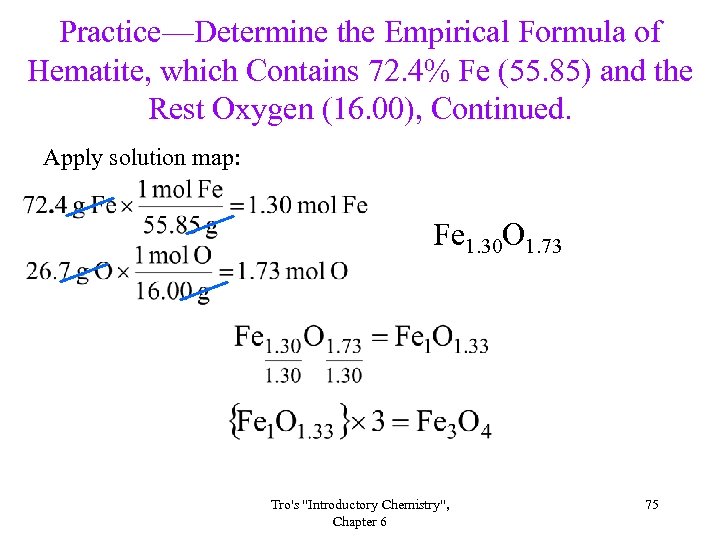 Practice—Determine the Empirical Formula of Hematite, which Contains 72. 4% Fe (55. 85) and the Rest Oxygen (16. 00), Continued. Apply solution map: Fe 1. 30 O 1. 73 Tro's "Introductory Chemistry", Chapter 6 75
Practice—Determine the Empirical Formula of Hematite, which Contains 72. 4% Fe (55. 85) and the Rest Oxygen (16. 00), Continued. Apply solution map: Fe 1. 30 O 1. 73 Tro's "Introductory Chemistry", Chapter 6 75
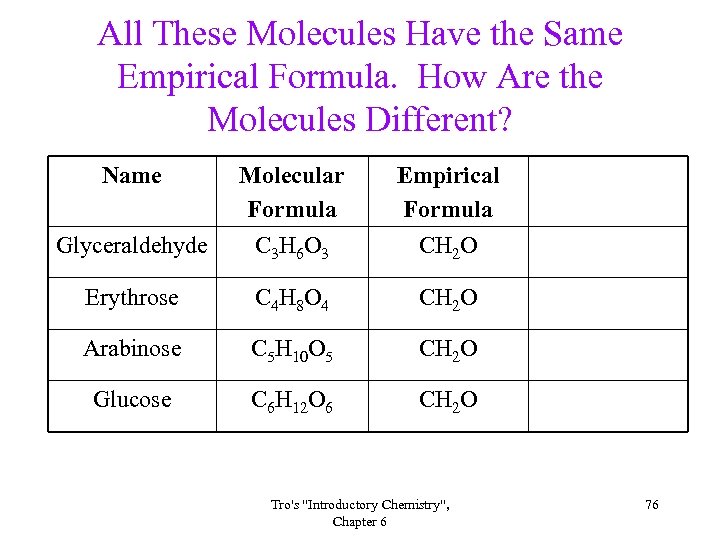 All These Molecules Have the Same Empirical Formula. How Are the Molecules Different? Name Glyceraldehyde Molecular Formula C 3 H 6 O 3 Empirical Formula CH 2 O Erythrose C 4 H 8 O 4 CH 2 O Arabinose C 5 H 10 O 5 CH 2 O Glucose C 6 H 12 O 6 CH 2 O Tro's "Introductory Chemistry", Chapter 6 76
All These Molecules Have the Same Empirical Formula. How Are the Molecules Different? Name Glyceraldehyde Molecular Formula C 3 H 6 O 3 Empirical Formula CH 2 O Erythrose C 4 H 8 O 4 CH 2 O Arabinose C 5 H 10 O 5 CH 2 O Glucose C 6 H 12 O 6 CH 2 O Tro's "Introductory Chemistry", Chapter 6 76
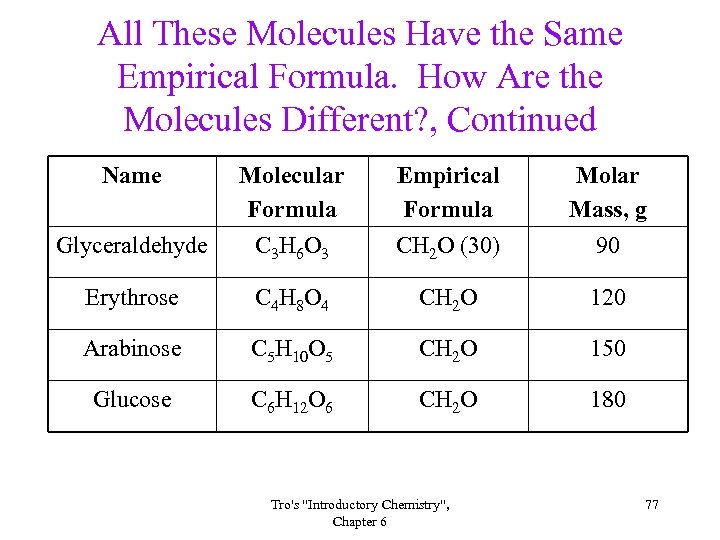 All These Molecules Have the Same Empirical Formula. How Are the Molecules Different? , Continued Name Glyceraldehyde Molecular Formula C 3 H 6 O 3 Empirical Formula CH 2 O (30) Molar Mass, g 90 Erythrose C 4 H 8 O 4 CH 2 O 120 Arabinose C 5 H 10 O 5 CH 2 O 150 Glucose C 6 H 12 O 6 CH 2 O 180 Tro's "Introductory Chemistry", Chapter 6 77
All These Molecules Have the Same Empirical Formula. How Are the Molecules Different? , Continued Name Glyceraldehyde Molecular Formula C 3 H 6 O 3 Empirical Formula CH 2 O (30) Molar Mass, g 90 Erythrose C 4 H 8 O 4 CH 2 O 120 Arabinose C 5 H 10 O 5 CH 2 O 150 Glucose C 6 H 12 O 6 CH 2 O 180 Tro's "Introductory Chemistry", Chapter 6 77
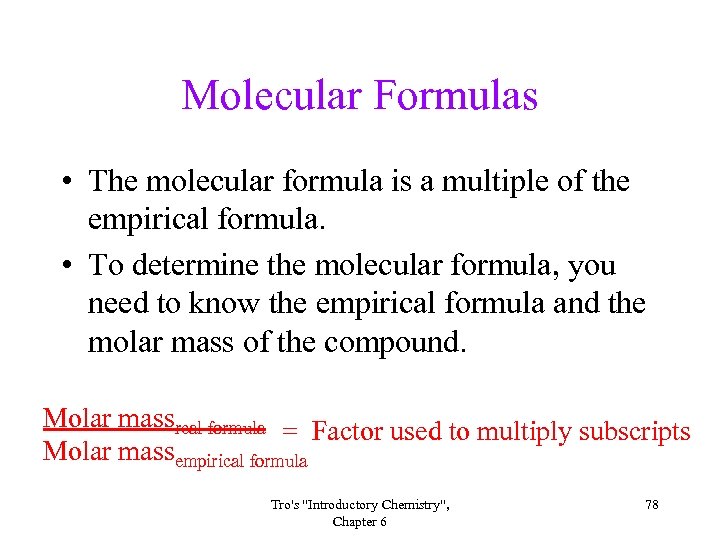 Molecular Formulas • The molecular formula is a multiple of the empirical formula. • To determine the molecular formula, you need to know the empirical formula and the molar mass of the compound. Molar massreal formula = Factor used to multiply subscripts Molar massempirical formula Tro's "Introductory Chemistry", Chapter 6 78
Molecular Formulas • The molecular formula is a multiple of the empirical formula. • To determine the molecular formula, you need to know the empirical formula and the molar mass of the compound. Molar massreal formula = Factor used to multiply subscripts Molar massempirical formula Tro's "Introductory Chemistry", Chapter 6 78
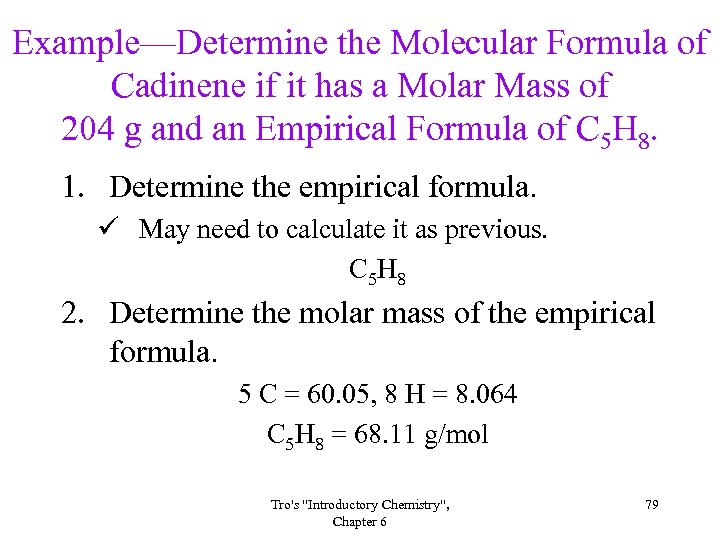 Example—Determine the Molecular Formula of Cadinene if it has a Molar Mass of 204 g and an Empirical Formula of C 5 H 8. 1. Determine the empirical formula. ü May need to calculate it as previous. C 5 H 8 2. Determine the molar mass of the empirical formula. 5 C = 60. 05, 8 H = 8. 064 C 5 H 8 = 68. 11 g/mol Tro's "Introductory Chemistry", Chapter 6 79
Example—Determine the Molecular Formula of Cadinene if it has a Molar Mass of 204 g and an Empirical Formula of C 5 H 8. 1. Determine the empirical formula. ü May need to calculate it as previous. C 5 H 8 2. Determine the molar mass of the empirical formula. 5 C = 60. 05, 8 H = 8. 064 C 5 H 8 = 68. 11 g/mol Tro's "Introductory Chemistry", Chapter 6 79
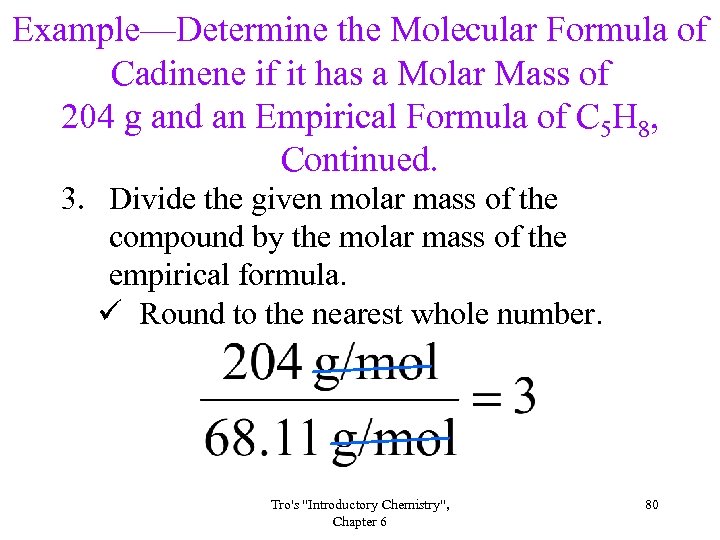 Example—Determine the Molecular Formula of Cadinene if it has a Molar Mass of 204 g and an Empirical Formula of C 5 H 8, Continued. 3. Divide the given molar mass of the compound by the molar mass of the empirical formula. ü Round to the nearest whole number. Tro's "Introductory Chemistry", Chapter 6 80
Example—Determine the Molecular Formula of Cadinene if it has a Molar Mass of 204 g and an Empirical Formula of C 5 H 8, Continued. 3. Divide the given molar mass of the compound by the molar mass of the empirical formula. ü Round to the nearest whole number. Tro's "Introductory Chemistry", Chapter 6 80
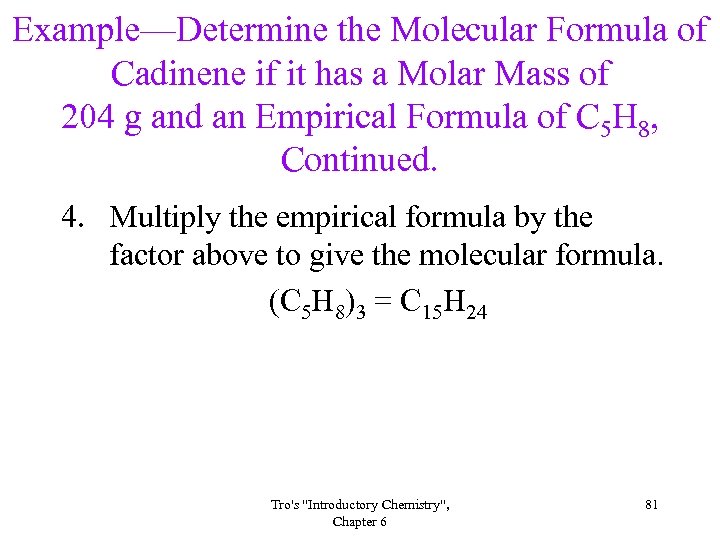 Example—Determine the Molecular Formula of Cadinene if it has a Molar Mass of 204 g and an Empirical Formula of C 5 H 8, Continued. 4. Multiply the empirical formula by the factor above to give the molecular formula. (C 5 H 8)3 = C 15 H 24 Tro's "Introductory Chemistry", Chapter 6 81
Example—Determine the Molecular Formula of Cadinene if it has a Molar Mass of 204 g and an Empirical Formula of C 5 H 8, Continued. 4. Multiply the empirical formula by the factor above to give the molecular formula. (C 5 H 8)3 = C 15 H 24 Tro's "Introductory Chemistry", Chapter 6 81
 Practice—Benzopyrene has a Molar Mass of 252 g and an Empirical Formula of C 5 H 3. What is its Molecular Formula? (C = 12. 01, H=1. 01) Tro's "Introductory Chemistry", Chapter 6 82
Practice—Benzopyrene has a Molar Mass of 252 g and an Empirical Formula of C 5 H 3. What is its Molecular Formula? (C = 12. 01, H=1. 01) Tro's "Introductory Chemistry", Chapter 6 82
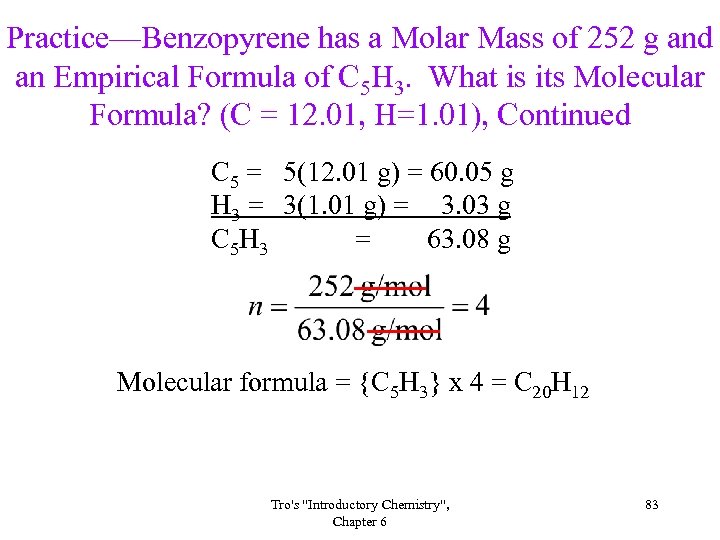 Practice—Benzopyrene has a Molar Mass of 252 g and an Empirical Formula of C 5 H 3. What is its Molecular Formula? (C = 12. 01, H=1. 01), Continued C 5 = 5(12. 01 g) = 60. 05 g H 3 = 3(1. 01 g) = 3. 03 g C 5 H 3 = 63. 08 g Molecular formula = {C 5 H 3} x 4 = C 20 H 12 Tro's "Introductory Chemistry", Chapter 6 83
Practice—Benzopyrene has a Molar Mass of 252 g and an Empirical Formula of C 5 H 3. What is its Molecular Formula? (C = 12. 01, H=1. 01), Continued C 5 = 5(12. 01 g) = 60. 05 g H 3 = 3(1. 01 g) = 3. 03 g C 5 H 3 = 63. 08 g Molecular formula = {C 5 H 3} x 4 = C 20 H 12 Tro's "Introductory Chemistry", Chapter 6 83
 Practice—Determine the Molecular Formula of Nicotine, which has a Molar Mass of 162 g and is 74. 0% C, 8. 7% H, and the Rest N. (C=12. 01, H=1. 01, N=14. 01) Tro's "Introductory Chemistry", Chapter 6 84
Practice—Determine the Molecular Formula of Nicotine, which has a Molar Mass of 162 g and is 74. 0% C, 8. 7% H, and the Rest N. (C=12. 01, H=1. 01, N=14. 01) Tro's "Introductory Chemistry", Chapter 6 84
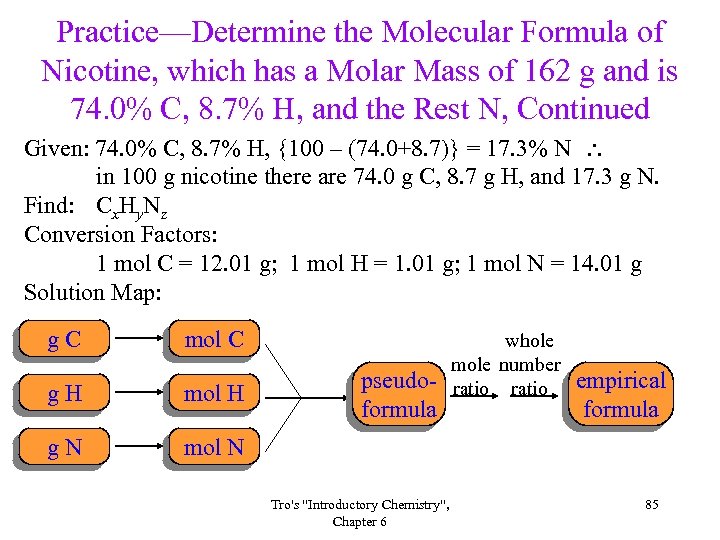 Practice—Determine the Molecular Formula of Nicotine, which has a Molar Mass of 162 g and is 74. 0% C, 8. 7% H, and the Rest N, Continued Given: 74. 0% C, 8. 7% H, {100 – (74. 0+8. 7)} = 17. 3% N in 100 g nicotine there are 74. 0 g C, 8. 7 g H, and 17. 3 g N. Find: Cx. Hy. Nz Conversion Factors: 1 mol C = 12. 01 g; 1 mol H = 1. 01 g; 1 mol N = 14. 01 g Solution Map: g. C mol C g. H mol H g. N mol N pseudoformula Tro's "Introductory Chemistry", Chapter 6 whole mole number ratio empirical formula 85
Practice—Determine the Molecular Formula of Nicotine, which has a Molar Mass of 162 g and is 74. 0% C, 8. 7% H, and the Rest N, Continued Given: 74. 0% C, 8. 7% H, {100 – (74. 0+8. 7)} = 17. 3% N in 100 g nicotine there are 74. 0 g C, 8. 7 g H, and 17. 3 g N. Find: Cx. Hy. Nz Conversion Factors: 1 mol C = 12. 01 g; 1 mol H = 1. 01 g; 1 mol N = 14. 01 g Solution Map: g. C mol C g. H mol H g. N mol N pseudoformula Tro's "Introductory Chemistry", Chapter 6 whole mole number ratio empirical formula 85
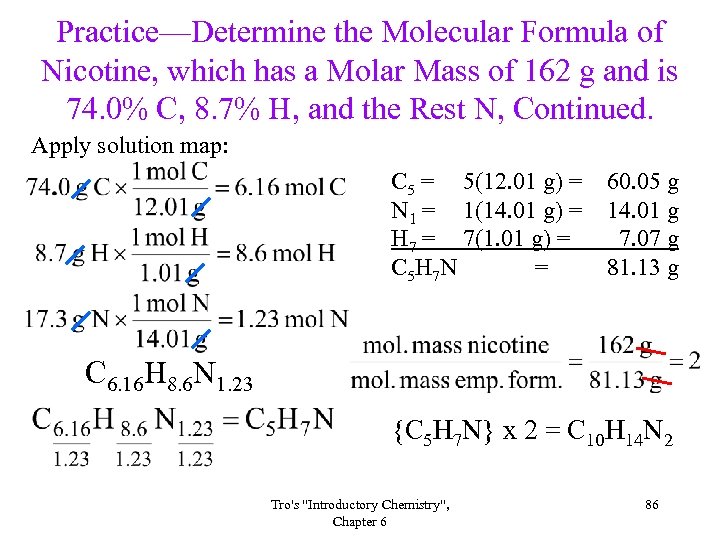 Practice—Determine the Molecular Formula of Nicotine, which has a Molar Mass of 162 g and is 74. 0% C, 8. 7% H, and the Rest N, Continued. Apply solution map: C 5 = 5(12. 01 g) = N 1 = 1(14. 01 g) = H 7 = 7(1. 01 g) = C 5 H 7 N = 60. 05 g 14. 01 g 7. 07 g 81. 13 g C 6. 16 H 8. 6 N 1. 23 {C 5 H 7 N} x 2 = C 10 H 14 N 2 Tro's "Introductory Chemistry", Chapter 6 86
Practice—Determine the Molecular Formula of Nicotine, which has a Molar Mass of 162 g and is 74. 0% C, 8. 7% H, and the Rest N, Continued. Apply solution map: C 5 = 5(12. 01 g) = N 1 = 1(14. 01 g) = H 7 = 7(1. 01 g) = C 5 H 7 N = 60. 05 g 14. 01 g 7. 07 g 81. 13 g C 6. 16 H 8. 6 N 1. 23 {C 5 H 7 N} x 2 = C 10 H 14 N 2 Tro's "Introductory Chemistry", Chapter 6 86


