81b1f85e5737337b1f5231a92b8717fa.ppt
- Количество слайдов: 65
 Introduction to Using CRS for GPRA & PART Reporting Stephanie Klepacki, CRS Federal Lead, IHS Stephanie. Klepacki@ihs. gov
Introduction to Using CRS for GPRA & PART Reporting Stephanie Klepacki, CRS Federal Lead, IHS Stephanie. Klepacki@ihs. gov
 Agenda • • Introduction to CRS Demonstrations Hands-on Session Question and Answer Session
Agenda • • Introduction to CRS Demonstrations Hands-on Session Question and Answer Session
 User Manual Help • The CRS 2009 User Manual contains information on GPRA and complete instructions for using CRS • In today’s presentation, this symbol on the lower right side of a slide indicates information for this subject is available in the CRS 2009 User Manual – The numbers indicate the sections of the User Manual 4. 3. 2. 1 4. 3. 2. 2
User Manual Help • The CRS 2009 User Manual contains information on GPRA and complete instructions for using CRS • In today’s presentation, this symbol on the lower right side of a slide indicates information for this subject is available in the CRS 2009 User Manual – The numbers indicate the sections of the User Manual 4. 3. 2. 1 4. 3. 2. 2
 INTRODUCTION TO THE CLINICAL REPORTING SYSTEM (CRS)
INTRODUCTION TO THE CLINICAL REPORTING SYSTEM (CRS)
 Relationship Between GPRA & CRS • The IHS Director has designated the Clinical Reporting System (CRS) as the national tool for reporting of all GPRA clinical measures – Federal (IHS) facilities are required to use CRS for GPRA reporting – Urban facilities are required to use CRS for GPRA reporting – Tribal facilities are not required to use CRS but are encouraged to use it • 4 th quarter report is used to compile IHS’ national performance measure rates for all clinical GPRA measures in the Annual Performance Report
Relationship Between GPRA & CRS • The IHS Director has designated the Clinical Reporting System (CRS) as the national tool for reporting of all GPRA clinical measures – Federal (IHS) facilities are required to use CRS for GPRA reporting – Urban facilities are required to use CRS for GPRA reporting – Tribal facilities are not required to use CRS but are encouraged to use it • 4 th quarter report is used to compile IHS’ national performance measure rates for all clinical GPRA measures in the Annual Performance Report
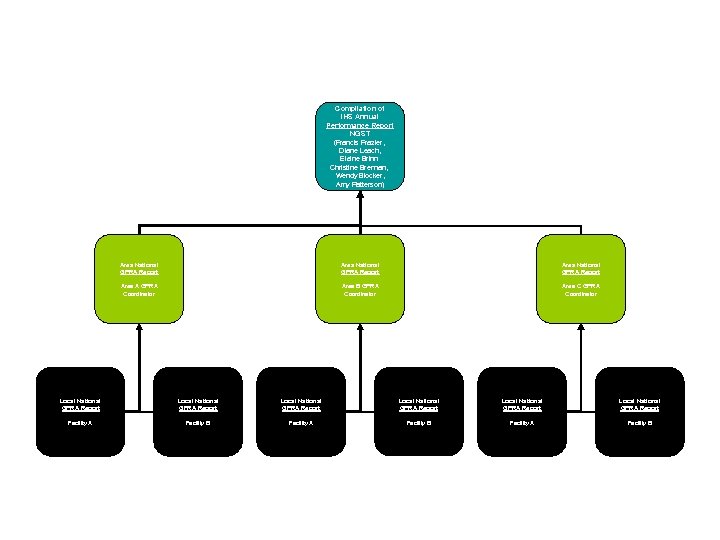 CRS GPRA Reporting Process Compilation of IHS Annual Performance Report NGST (Francis Frazier, Diane Leach, Elaine Brinn Christine Brennan, Wendy Blocker, Amy Patterson) Area National GPRA Report Area A GPRA Coordinator Area B GPRA Coordinator Area C GPRA Coordinator Local National GPRA Report Local National GPRA Report Facility A Facility B
CRS GPRA Reporting Process Compilation of IHS Annual Performance Report NGST (Francis Frazier, Diane Leach, Elaine Brinn Christine Brennan, Wendy Blocker, Amy Patterson) Area National GPRA Report Area A GPRA Coordinator Area B GPRA Coordinator Area C GPRA Coordinator Local National GPRA Report Local National GPRA Report Facility A Facility B
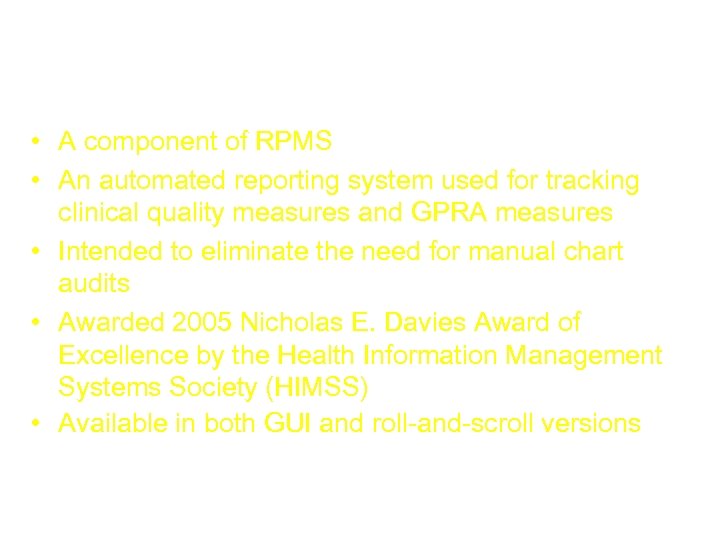 CRS (Clinical Reporting System) • A component of RPMS • An automated reporting system used for tracking clinical quality measures and GPRA measures • Intended to eliminate the need for manual chart audits • Awarded 2005 Nicholas E. Davies Award of Excellence by the Health Information Management Systems Society (HIMSS) • Available in both GUI and roll-and-scroll versions
CRS (Clinical Reporting System) • A component of RPMS • An automated reporting system used for tracking clinical quality measures and GPRA measures • Intended to eliminate the need for manual chart audits • Awarded 2005 Nicholas E. Davies Award of Excellence by the Health Information Management Systems Society (HIMSS) • Available in both GUI and roll-and-scroll versions
 CRS (Clinical Reporting System)
CRS (Clinical Reporting System)
 From Where Does CRS Gets its Data?
From Where Does CRS Gets its Data?
 Clinical Reporting System (CRS) • Based on software developed by Aberdeen Area in 2000 • Provides automated local, regional (Area) and national tracking of clinical performance on demand • Uses identical logic, thus ensuring comparable performance data is reported across all facilities • Updated annually to reflect changes in the logic descriptions and to add new topics 3. 2
Clinical Reporting System (CRS) • Based on software developed by Aberdeen Area in 2000 • Provides automated local, regional (Area) and national tracking of clinical performance on demand • Uses identical logic, thus ensuring comparable performance data is reported across all facilities • Updated annually to reflect changes in the logic descriptions and to add new topics 3. 2
 CRS Mines its Data from RPMS • Resource and Patient Management System (RPMS) – IHS’ Health Information Solution since 1984 – Comprised of over 50 component applications
CRS Mines its Data from RPMS • Resource and Patient Management System (RPMS) – IHS’ Health Information Solution since 1984 – Comprised of over 50 component applications
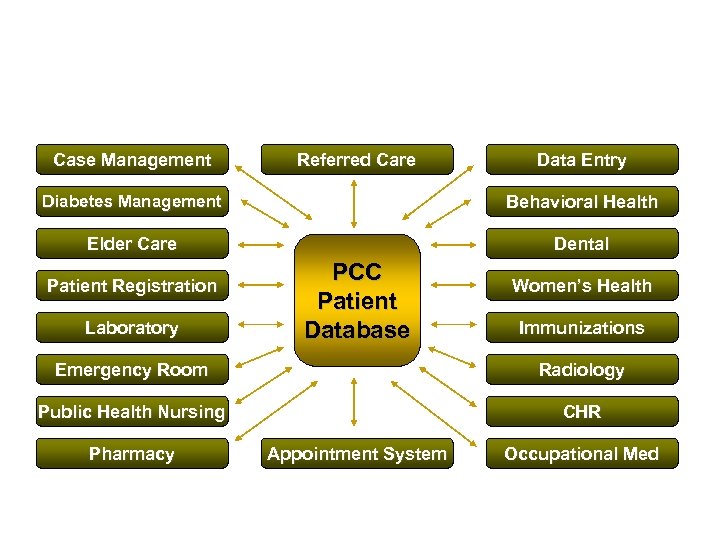 RPMS Integrates Multiple Clinical Systems into One Database Case Management Referred Care Data Entry Diabetes Management Behavioral Health Elder Care Dental Patient Registration Laboratory PCC Patient Database Women’s Health Immunizations Emergency Room Radiology Public Health Nursing CHR Pharmacy Appointment System Occupational Med
RPMS Integrates Multiple Clinical Systems into One Database Case Management Referred Care Data Entry Diabetes Management Behavioral Health Elder Care Dental Patient Registration Laboratory PCC Patient Database Women’s Health Immunizations Emergency Room Radiology Public Health Nursing CHR Pharmacy Appointment System Occupational Med
 RPMS Applications that CRS Mines • CRS mines data from these RPMS applications: – Majority of the Data • PCC (Patient Care Component) – Other Data • Behavioral Health (looks for BHS problem codes) • Women’s Health (looks for Pap Smears & Mammograms) • Immunization (gets children 19 -35 months who are active in the Immunization Package) • All RPMS applications have a link from the application to PCC – If that link is turned on, the data is passed from the application to PCC, where CRS will find it. (Default setting for these links is “on. ”)
RPMS Applications that CRS Mines • CRS mines data from these RPMS applications: – Majority of the Data • PCC (Patient Care Component) – Other Data • Behavioral Health (looks for BHS problem codes) • Women’s Health (looks for Pap Smears & Mammograms) • Immunization (gets children 19 -35 months who are active in the Immunization Package) • All RPMS applications have a link from the application to PCC – If that link is turned on, the data is passed from the application to PCC, where CRS will find it. (Default setting for these links is “on. ”)
 What About the RPMS EHR? • Since the Electronic Health Record (EHR) updates the PCC database and other applications that pass data to PCC (e. g. Immunizations, Lab, Pharmacy), CRS will find that data in PCC
What About the RPMS EHR? • Since the Electronic Health Record (EHR) updates the PCC database and other applications that pass data to PCC (e. g. Immunizations, Lab, Pharmacy), CRS will find that data in PCC
 CRS Data CRS does not update the PCC database; it reports on data it mines from PCC and the Behavioral Health, Women’s Health, and Immunization packages.
CRS Data CRS does not update the PCC database; it reports on data it mines from PCC and the Behavioral Health, Women’s Health, and Immunization packages.
 Types of Data CRS Mines • Patient Demographic Data – Name – Age – Sex – Community of Residence – Chart Number • Patient Health Data – Standard Codes – Site-Populated Codes
Types of Data CRS Mines • Patient Demographic Data – Name – Age – Sex – Community of Residence – Chart Number • Patient Health Data – Standard Codes – Site-Populated Codes
 Types of Data CRS Mines (cont’d) • Standard codes, which are written into the CRS programs and may not be edited – Industry-standard Codes • • ICD-9 codes (diagnosis and procedure) CPT codes (billing) CVX codes (immunizations) LOINC codes (standard coding for lab tests) – IHS-exclusive Codes • Exam codes (e. g. 03 Diabetic Retinal Exam) • Patient Education codes (e. g. DM-M: Diabetes Mellitus – Medications education) • Health Factors (e. g. Alcohol or Tobacco User)
Types of Data CRS Mines (cont’d) • Standard codes, which are written into the CRS programs and may not be edited – Industry-standard Codes • • ICD-9 codes (diagnosis and procedure) CPT codes (billing) CVX codes (immunizations) LOINC codes (standard coding for lab tests) – IHS-exclusive Codes • Exam codes (e. g. 03 Diabetic Retinal Exam) • Patient Education codes (e. g. DM-M: Diabetes Mellitus – Medications education) • Health Factors (e. g. Alcohol or Tobacco User)
 Types of Data CRS Mines (cont’d) • Site-populated codes, which are stored in taxonomies that are maintained by each site • Lab Tests – Examples: Hemoglobin A 1 c, LDL Cholesterol, Pap Smear, FOBT • Medications – Examples: Beta-blockers, ACEIs/ARBs, Aspirin, Statins – Most medication taxonomies are pre- populated either by NDC or VA Drug Class codes – Sites need to update their taxonomies in CRS periodically to add new lab tests and medications
Types of Data CRS Mines (cont’d) • Site-populated codes, which are stored in taxonomies that are maintained by each site • Lab Tests – Examples: Hemoglobin A 1 c, LDL Cholesterol, Pap Smear, FOBT • Medications – Examples: Beta-blockers, ACEIs/ARBs, Aspirin, Statins – Most medication taxonomies are pre- populated either by NDC or VA Drug Class codes – Sites need to update their taxonomies in CRS periodically to add new lab tests and medications
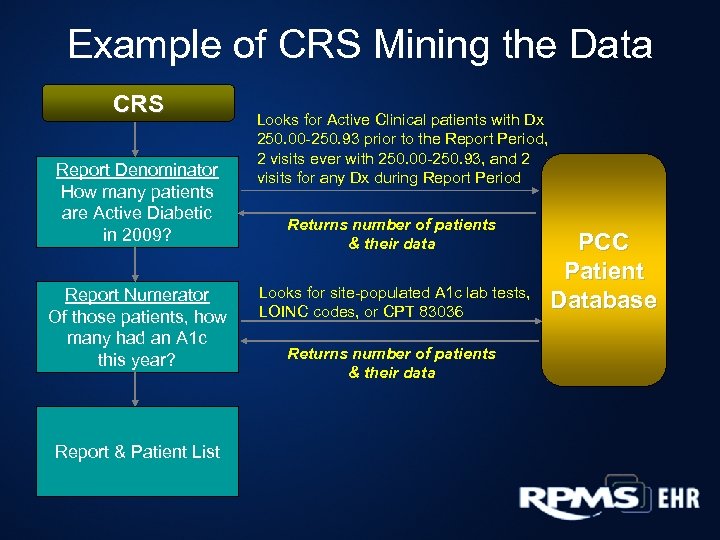 Example of CRS Mining the Data CRS Report Denominator How many patients are Active Diabetic in 2009? Report Numerator Of those patients, how many had an A 1 c this year? Report & Patient List Looks for Active Clinical patients with Dx 250. 00 -250. 93 prior to the Report Period, 2 visits ever with 250. 00 -250. 93, and 2 visits for any Dx during Report Period Returns number of patients & their data Looks for site-populated A 1 c lab tests, LOINC codes, or CPT 83036 Returns number of patients & their data PCC Patient Database
Example of CRS Mining the Data CRS Report Denominator How many patients are Active Diabetic in 2009? Report Numerator Of those patients, how many had an A 1 c this year? Report & Patient List Looks for Active Clinical patients with Dx 250. 00 -250. 93 prior to the Report Period, 2 visits ever with 250. 00 -250. 93, and 2 visits for any Dx during Report Period Returns number of patients & their data Looks for site-populated A 1 c lab tests, LOINC codes, or CPT 83036 Returns number of patients & their data PCC Patient Database
 CRS Lingo
CRS Lingo
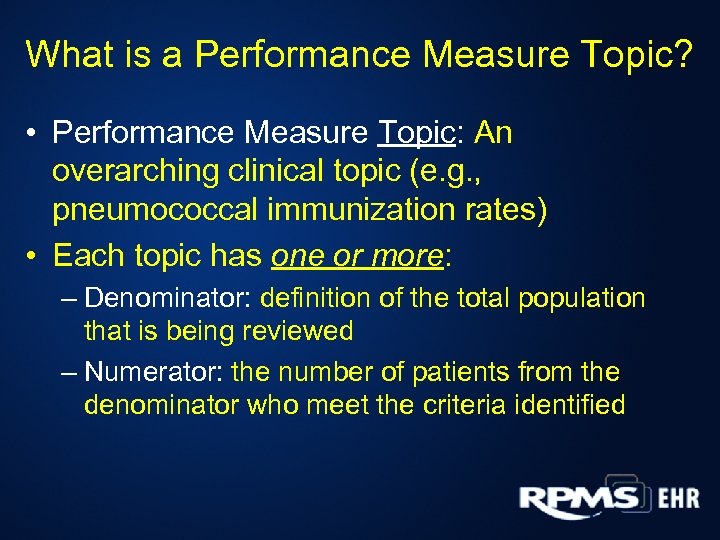 What is a Performance Measure Topic? • Performance Measure Topic: An overarching clinical topic (e. g. , pneumococcal immunization rates) • Each topic has one or more: – Denominator: definition of the total population that is being reviewed – Numerator: the number of patients from the denominator who meet the criteria identified
What is a Performance Measure Topic? • Performance Measure Topic: An overarching clinical topic (e. g. , pneumococcal immunization rates) • Each topic has one or more: – Denominator: definition of the total population that is being reviewed – Numerator: the number of patients from the denominator who meet the criteria identified
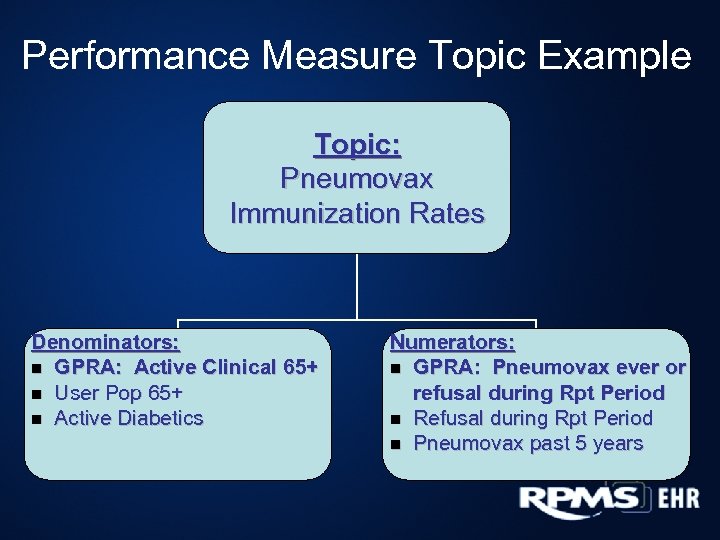 Performance Measure Topic Example Topic: Pneumovax Immunization Rates Denominators: n GPRA: Active Clinical 65+ n User Pop 65+ n Active Diabetics Numerators: n GPRA: Pneumovax ever or refusal during Rpt Period n Refusal during Rpt Period n Pneumovax past 5 years
Performance Measure Topic Example Topic: Pneumovax Immunization Rates Denominators: n GPRA: Active Clinical 65+ n User Pop 65+ n Active Diabetics Numerators: n GPRA: Pneumovax ever or refusal during Rpt Period n Refusal during Rpt Period n Pneumovax past 5 years
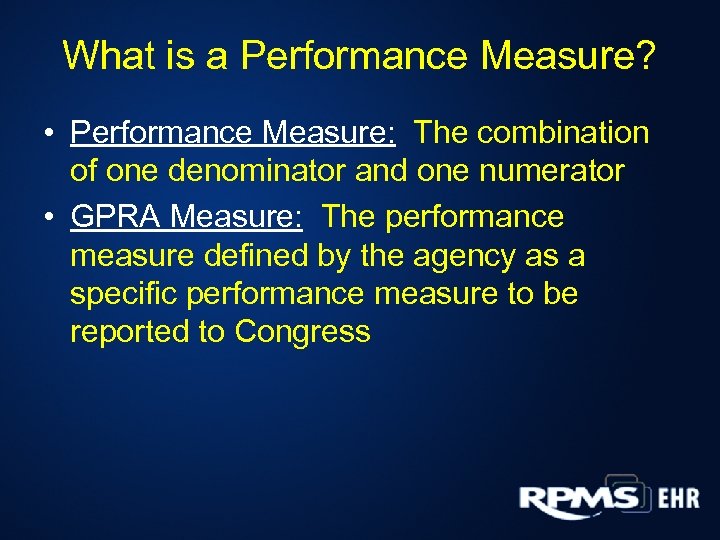 What is a Performance Measure? • Performance Measure: The combination of one denominator and one numerator • GPRA Measure: The performance measure defined by the agency as a specific performance measure to be reported to Congress
What is a Performance Measure? • Performance Measure: The combination of one denominator and one numerator • GPRA Measure: The performance measure defined by the agency as a specific performance measure to be reported to Congress
 Example: CRS GPRA Measure Active Clinical patients 65 or older (denominator) with Pneumococcal vaccine documented at any time before the end of the Report Period, including refusals in past year (numerator).
Example: CRS GPRA Measure Active Clinical patients 65 or older (denominator) with Pneumococcal vaccine documented at any time before the end of the Report Period, including refusals in past year (numerator).
 User Population Denominator • For GPRA, defined as: – Must be Indian/Alaska Native, based on Beneficiary classification 01, and – Must reside in a community specified in the site’s GPRA community taxonomy, and – Must be alive on last day of Report Period, and – Must have 1 visit to any clinic in the past 3 years 3. 2. 3. 3
User Population Denominator • For GPRA, defined as: – Must be Indian/Alaska Native, based on Beneficiary classification 01, and – Must reside in a community specified in the site’s GPRA community taxonomy, and – Must be alive on last day of Report Period, and – Must have 1 visit to any clinic in the past 3 years 3. 2. 3. 3
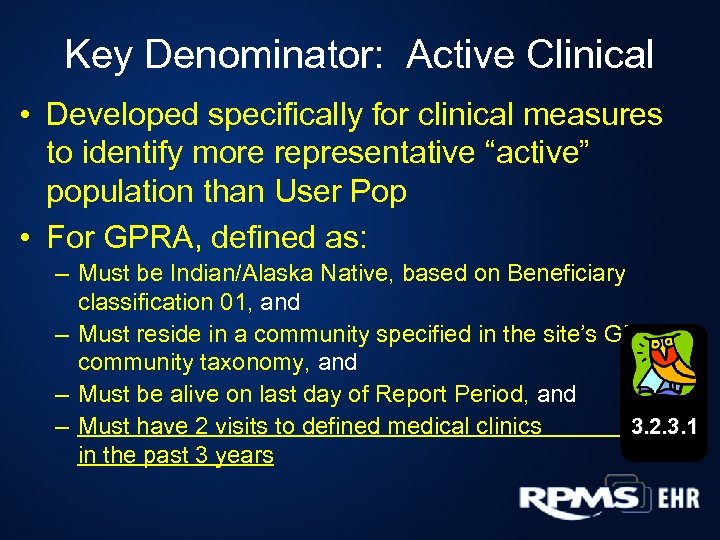 Key Denominator: Active Clinical • Developed specifically for clinical measures to identify more representative “active” population than User Pop • For GPRA, defined as: – Must be Indian/Alaska Native, based on Beneficiary classification 01, and – Must reside in a community specified in the site’s GPRA community taxonomy, and – Must be alive on last day of Report Period, and 3. 2. 3. 1 – Must have 2 visits to defined medical clinics in the past 3 years
Key Denominator: Active Clinical • Developed specifically for clinical measures to identify more representative “active” population than User Pop • For GPRA, defined as: – Must be Indian/Alaska Native, based on Beneficiary classification 01, and – Must reside in a community specified in the site’s GPRA community taxonomy, and – Must be alive on last day of Report Period, and 3. 2. 3. 1 – Must have 2 visits to defined medical clinics in the past 3 years
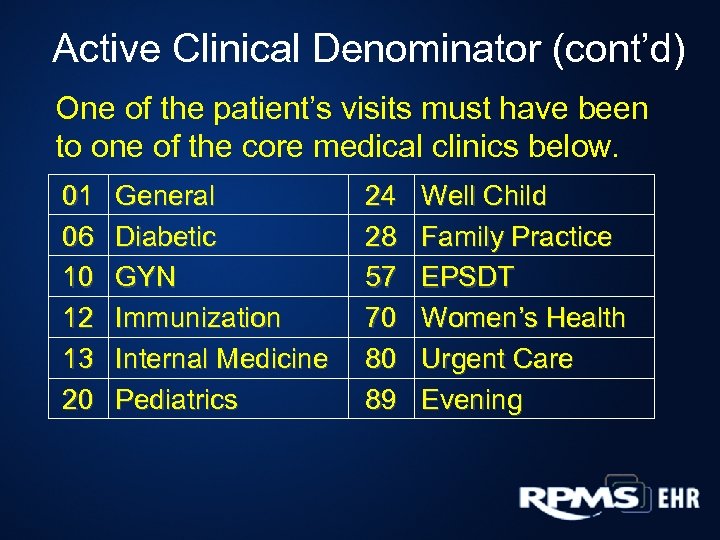 Active Clinical Denominator (cont’d) One of the patient’s visits must have been to one of the core medical clinics below. 01 06 10 12 13 20 General Diabetic GYN Immunization Internal Medicine Pediatrics 24 28 57 70 80 89 Well Child Family Practice EPSDT Women’s Health Urgent Care Evening
Active Clinical Denominator (cont’d) One of the patient’s visits must have been to one of the core medical clinics below. 01 06 10 12 13 20 General Diabetic GYN Immunization Internal Medicine Pediatrics 24 28 57 70 80 89 Well Child Family Practice EPSDT Women’s Health Urgent Care Evening
 Active Clinical Denominator (cont’d) The second visit must be to one of the core clinics (previous slide) or to one of the clinics listed below. 02 03 Cardiac Chest and TB 37 38 Neurology Rheumatology 05 Dermatology 49 Nephrology 07 08 ENT Family Planning 50 69 Chronic Disease Endocrinology 16 19 23 25 26 27 31 32 Obstetrics Orthopedic Surgical Other High Risk General Preventive Hypertension Postpartum 75 81 85 88 B 9 C 3 Urology Men’s Health Screening Teen Clinic Sports Medicine Gastroenterology – Hepatology Oncology – Hematology Colposcopy
Active Clinical Denominator (cont’d) The second visit must be to one of the core clinics (previous slide) or to one of the clinics listed below. 02 03 Cardiac Chest and TB 37 38 Neurology Rheumatology 05 Dermatology 49 Nephrology 07 08 ENT Family Planning 50 69 Chronic Disease Endocrinology 16 19 23 25 26 27 31 32 Obstetrics Orthopedic Surgical Other High Risk General Preventive Hypertension Postpartum 75 81 85 88 B 9 C 3 Urology Men’s Health Screening Teen Clinic Sports Medicine Gastroenterology – Hepatology Oncology – Hematology Colposcopy
 CRS Access & Security Keys
CRS Access & Security Keys
 Who Should Have Access to CRS? • Anyone who will perform any of the following functions: – Set up the Site Parameters – Edit the site-populated lab or drug taxonomies – Run the National GPRA & PART Report and generate export files – Run other CRS reports – Run patient lists • Access to the above functions should be limited to the needs of the user
Who Should Have Access to CRS? • Anyone who will perform any of the following functions: – Set up the Site Parameters – Edit the site-populated lab or drug taxonomies – Run the National GPRA & PART Report and generate export files – Run other CRS reports – Run patient lists • Access to the above functions should be limited to the needs of the user
 CRS Security Keys – BGPZ MENU: Enables user to run all reports except the CMS Report. Does not give user any of the functionality listed below. – BGPZ PATIENT LISTS: Enables a user to run lists of patients that contain patient identifiers and medical information. – BGPZ SITE PARAMETERS: Enables a user to edit the site parameters. – BGPZ TAXONOMY EDIT: Enables a user to edit the site-populated lab and medication taxonomies. – BGPZAREA: Provides a user with access to the Area Office menu, where Area Aggregate reports may be run. 3. 2. 2
CRS Security Keys – BGPZ MENU: Enables user to run all reports except the CMS Report. Does not give user any of the functionality listed below. – BGPZ PATIENT LISTS: Enables a user to run lists of patients that contain patient identifiers and medical information. – BGPZ SITE PARAMETERS: Enables a user to edit the site parameters. – BGPZ TAXONOMY EDIT: Enables a user to edit the site-populated lab and medication taxonomies. – BGPZAREA: Provides a user with access to the Area Office menu, where Area Aggregate reports may be run. 3. 2. 2
 Standard Codes Used in CRS Logic
Standard Codes Used in CRS Logic
 Standard Codes • Hard-coded in CRS program logic; users cannot change the codes • Types of Standard Codes – CPT: to report diagnostic and therapeutic procedures for billing – ICD: • Diagnoses (POV, Problem List) • Procedure codes – LOINC: for laboratory tests, etc. – IHS National Patient Education Codes – IHS Health Factors (e. g. tobacco or alcohol user) – IHS Exam Codes (e. g. dental exam, diabetic foot exam)
Standard Codes • Hard-coded in CRS program logic; users cannot change the codes • Types of Standard Codes – CPT: to report diagnostic and therapeutic procedures for billing – ICD: • Diagnoses (POV, Problem List) • Procedure codes – LOINC: for laboratory tests, etc. – IHS National Patient Education Codes – IHS Health Factors (e. g. tobacco or alcohol user) – IHS Exam Codes (e. g. dental exam, diabetic foot exam)
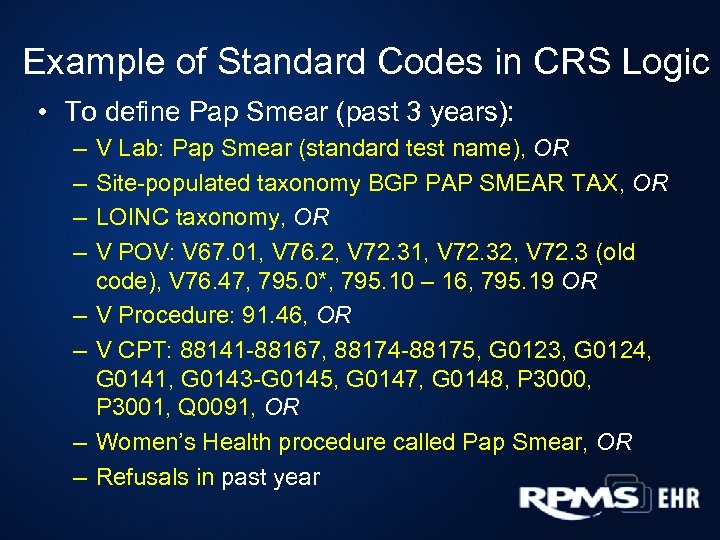 Example of Standard Codes in CRS Logic • To define Pap Smear (past 3 years): – – – – V Lab: Pap Smear (standard test name), OR Site-populated taxonomy BGP PAP SMEAR TAX, OR LOINC taxonomy, OR V POV: V 67. 01, V 76. 2, V 72. 31, V 72. 32, V 72. 3 (old code), V 76. 47, 795. 0*, 795. 10 – 16, 795. 19 OR V Procedure: 91. 46, OR V CPT: 88141 -88167, 88174 -88175, G 0123, G 0124, G 0141, G 0143 -G 0145, G 0147, G 0148, P 3000, P 3001, Q 0091, OR Women’s Health procedure called Pap Smear, OR Refusals in past year
Example of Standard Codes in CRS Logic • To define Pap Smear (past 3 years): – – – – V Lab: Pap Smear (standard test name), OR Site-populated taxonomy BGP PAP SMEAR TAX, OR LOINC taxonomy, OR V POV: V 67. 01, V 76. 2, V 72. 31, V 72. 32, V 72. 3 (old code), V 76. 47, 795. 0*, 795. 10 – 16, 795. 19 OR V Procedure: 91. 46, OR V CPT: 88141 -88167, 88174 -88175, G 0123, G 0124, G 0141, G 0143 -G 0145, G 0147, G 0148, P 3000, P 3001, Q 0091, OR Women’s Health procedure called Pap Smear, OR Refusals in past year
 10 Minute Break
10 Minute Break
 Taxonomies
Taxonomies
 Taxonomies • Groupings of similar things – Lab Tests – Drugs – CPT codes – ICD-9 codes – Others 4. 3 • Used by RPMS applications, including CRS, to find data items in PCC
Taxonomies • Groupings of similar things – Lab Tests – Drugs – CPT codes – ICD-9 codes – Others 4. 3 • Used by RPMS applications, including CRS, to find data items in PCC
 Taxonomies • 2 Types of Taxonomies in CRS – Hard-coded • Users cannot update 4. 3. 1 • LOINCs are included in these 4. 3. 2 – Site-populated • Users update with System Setup menu option • All non-LOINC lab tests are included in these
Taxonomies • 2 Types of Taxonomies in CRS – Hard-coded • Users cannot update 4. 3. 1 • LOINCs are included in these 4. 3. 2 – Site-populated • Users update with System Setup menu option • All non-LOINC lab tests are included in these
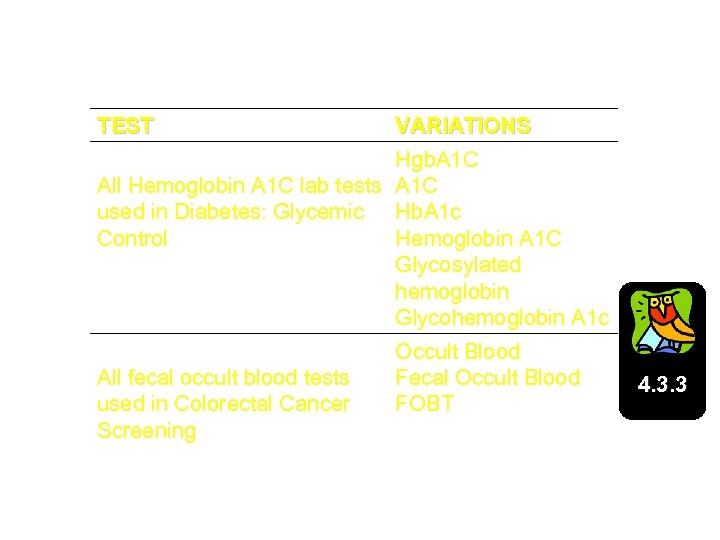 Site-Populated Taxonomy Examples TEST DM AUDIT HGB A 1 C TAX All Hemoglobin A 1 C lab tests used in Diabetes: Glycemic Control VARIATIONS Hgb. A 1 C Hb. A 1 c Hemoglobin A 1 C Glycosylated hemoglobin Glycohemoglobin A 1 c BGP GPRA FOB TESTS All fecal occult blood tests used in Colorectal Cancer Screening Occult Blood Fecal Occult Blood FOBT 4. 3. 3
Site-Populated Taxonomy Examples TEST DM AUDIT HGB A 1 C TAX All Hemoglobin A 1 C lab tests used in Diabetes: Glycemic Control VARIATIONS Hgb. A 1 C Hb. A 1 c Hemoglobin A 1 C Glycosylated hemoglobin Glycohemoglobin A 1 c BGP GPRA FOB TESTS All fecal occult blood tests used in Colorectal Cancer Screening Occult Blood Fecal Occult Blood FOBT 4. 3. 3
 Taxonomy Tips • You must work with your Lab & Pharmacy staff to identify all test and drug names – Run the Lab & Medication Taxonomy Reports and give to your Lab & Pharmacy Supervisors • Include ALL test names used by your facility since 1995, even if codes are currently inactive 4. 3. 2 4. 3. 3 4. 3. 4 – GPRA reports use a baseline year of 2000 and some measures look back 5 years – Must include tests that were active at that time if you want good baseline data
Taxonomy Tips • You must work with your Lab & Pharmacy staff to identify all test and drug names – Run the Lab & Medication Taxonomy Reports and give to your Lab & Pharmacy Supervisors • Include ALL test names used by your facility since 1995, even if codes are currently inactive 4. 3. 2 4. 3. 3 4. 3. 4 – GPRA reports use a baseline year of 2000 and some measures look back 5 years – Must include tests that were active at that time if you want good baseline data
 Taxonomy Tips (cont’d) • Do not include names of lab panels in taxonomies for specific tests that look at results (e. g. , “Lipid Panel” should not be included in LDL taxonomy) – For LDL cholesterol, include a lipid panel if it is the test that is normally performed for diabetes patients instead of an LDL cholesterol test – Panels do not report the test result, only that the test was done
Taxonomy Tips (cont’d) • Do not include names of lab panels in taxonomies for specific tests that look at results (e. g. , “Lipid Panel” should not be included in LDL taxonomy) – For LDL cholesterol, include a lipid panel if it is the test that is normally performed for diabetes patients instead of an LDL cholesterol test – Panels do not report the test result, only that the test was done
 Reports and Patient Lists
Reports and Patient Lists
 Report Parameters • Report Period – 1 -year time period (e. g. July 1, 2009 – June 30, 2010, Jan 1, 2009 – Dec 31, 2009) • Baseline Year – 1 -year time period (e. g. July 1, 1999 – June 30, 2000) • Patient Population – AI/AN patients only – Non-AI/AN patients only – Both AI/AN and non-AI/AN • Community Taxonomy – All of the communities included in the report • Patients must reside in one of these communities; otherwise, they are not reported
Report Parameters • Report Period – 1 -year time period (e. g. July 1, 2009 – June 30, 2010, Jan 1, 2009 – Dec 31, 2009) • Baseline Year – 1 -year time period (e. g. July 1, 1999 – June 30, 2000) • Patient Population – AI/AN patients only – Non-AI/AN patients only – Both AI/AN and non-AI/AN • Community Taxonomy – All of the communities included in the report • Patients must reside in one of these communities; otherwise, they are not reported
 Types of CRS Reports • National GPRA & PART Report – GPRA measures, GPRA developmental measures (e. g. measure counts excluding refusals) and several non-GPRA measures included for context, preset to current GPRA year. Breastfeeding Rates are included as a PART measure in this national report. • GPRA & PART Performance Report – Same as National GPRA & PART except users can choose the report parameters • Other National Measures (ONM) Report – 20 non-GPRA topics reported nationally. • Executive Order Quality Transparency Reports – 11 non-GPRA topics reported nationally. 5. 0
Types of CRS Reports • National GPRA & PART Report – GPRA measures, GPRA developmental measures (e. g. measure counts excluding refusals) and several non-GPRA measures included for context, preset to current GPRA year. Breastfeeding Rates are included as a PART measure in this national report. • GPRA & PART Performance Report – Same as National GPRA & PART except users can choose the report parameters • Other National Measures (ONM) Report – 20 non-GPRA topics reported nationally. • Executive Order Quality Transparency Reports – 11 non-GPRA topics reported nationally. 5. 0
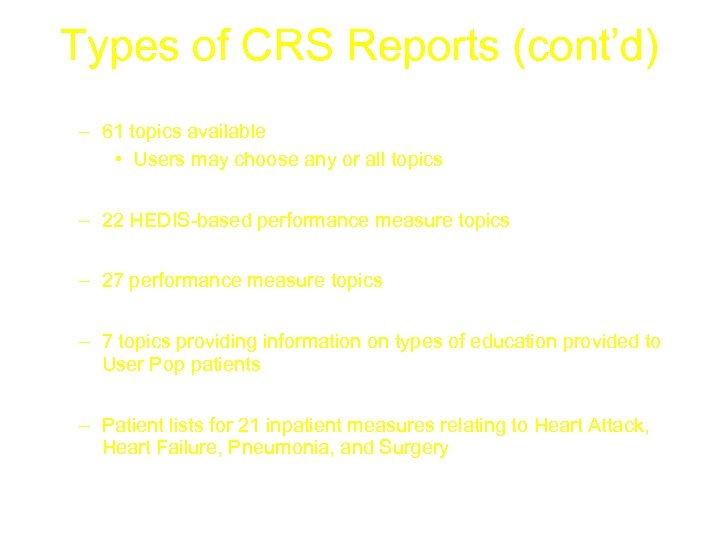 Types of CRS Reports (cont’d) • Selected Measures Report – 61 topics available • Users may choose any or all topics • HEDIS Report – 22 HEDIS-based performance measure topics • Elder Care Report – 27 performance measure topics • Patient Education Report – 7 topics providing information on types of education provided to User Pop patients • CMS Report – Patient lists for 21 inpatient measures relating to Heart Attack, Heart Failure, Pneumonia, and Surgery
Types of CRS Reports (cont’d) • Selected Measures Report – 61 topics available • Users may choose any or all topics • HEDIS Report – 22 HEDIS-based performance measure topics • Elder Care Report – 27 performance measure topics • Patient Education Report – 7 topics providing information on types of education provided to User Pop patients • CMS Report – Patient lists for 21 inpatient measures relating to Heart Attack, Heart Failure, Pneumonia, and Surgery
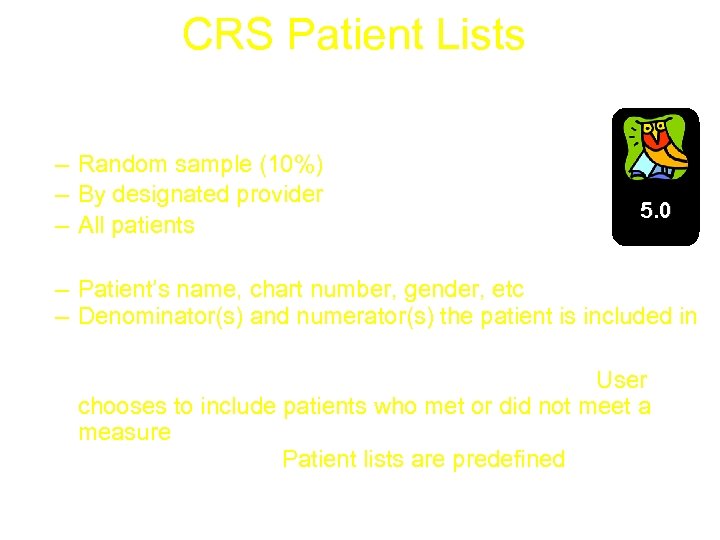 CRS Patient Lists • Show the detail behind the report • List options – Random sample (10%) – By designated provider – All patients 5. 0 • Display information about the patient – Patient’s name, chart number, gender, etc – Denominator(s) and numerator(s) the patient is included in • Available for all reports – National GPRA & PART Report & ONM Report: User chooses to include patients who met or did not meet a measure – All Other Reports: Patient lists are predefined
CRS Patient Lists • Show the detail behind the report • List options – Random sample (10%) – By designated provider – All patients 5. 0 • Display information about the patient – Patient’s name, chart number, gender, etc – Denominator(s) and numerator(s) the patient is included in • Available for all reports – National GPRA & PART Report & ONM Report: User chooses to include patients who met or did not meet a measure – All Other Reports: Patient lists are predefined
 Patient Lists Can Be Used For. . . • Verifying RPMS data against patient’s chart info • Identifying patients who need certain screenings/procedures – e. g. , tobacco screening, flu shot • Identifying “at risk” patients – e. g. , high LDL, high BP, obese • Delimited files are most useful output for patient lists!
Patient Lists Can Be Used For. . . • Verifying RPMS data against patient’s chart info • Identifying patients who need certain screenings/procedures – e. g. , tobacco screening, flu shot • Identifying “at risk” patients – e. g. , high LDL, high BP, obese • Delimited files are most useful output for patient lists!
 Review of Sample National GPRA & PART Report
Review of Sample National GPRA & PART Report
 Review of Sample Patient Lists
Review of Sample Patient Lists
 10 Minute Break
10 Minute Break
 Demo of Site Parameters Setup CRS Hands-On Scripts
Demo of Site Parameters Setup CRS Hands-On Scripts
 CRS Disclaimer • CRS is not a workload application, nor is it intended for managing patient care • Its purpose is to report on the quality of care IHS is providing to its patient population as defined by specific performance measures • Unless specifically stated in the logic, the test found (i. e. numerator value) does not indicate the most recent test – If the date of the most recent test is needed, check PCC • Cannot compare CRS results with results of a QMan search unless the EXACT SAME LOGIC is used!
CRS Disclaimer • CRS is not a workload application, nor is it intended for managing patient care • Its purpose is to report on the quality of care IHS is providing to its patient population as defined by specific performance measures • Unless specifically stated in the logic, the test found (i. e. numerator value) does not indicate the most recent test – If the date of the most recent test is needed, check PCC • Cannot compare CRS results with results of a QMan search unless the EXACT SAME LOGIC is used!
 CRS Disclaimer (cont’d) • Software is not a solution not • Software is a tool to assist users in identifying and aggregating comparable clinical information comparabl • Software can help identify problems help – with data – with clinical documentation process – with clinical care Bottom Line: CRS cannot fix a facility’s problems; an active QI program is needed. Users must run and review the CRS reports to see if the rates are reasonable. If they are not, need to research the patient’s data and get the patient the needed test/screenings/care.
CRS Disclaimer (cont’d) • Software is not a solution not • Software is a tool to assist users in identifying and aggregating comparable clinical information comparabl • Software can help identify problems help – with data – with clinical documentation process – with clinical care Bottom Line: CRS cannot fix a facility’s problems; an active QI program is needed. Users must run and review the CRS reports to see if the rates are reasonable. If they are not, need to research the patient’s data and get the patient the needed test/screenings/care.
 QUESTION & ANSWER SESSION
QUESTION & ANSWER SESSION
 Optional Discussion: Tips for Improvement
Optional Discussion: Tips for Improvement
 Report Results • Low or “incorrect” results on your CRS reports does not necessarily mean that you are not performing the appropriate procedures, screenings, etc • It does mean that the data cannot be located in RPMS • First, check what’s in the chart against what’s in RPMS – Use Patient Lists
Report Results • Low or “incorrect” results on your CRS reports does not necessarily mean that you are not performing the appropriate procedures, screenings, etc • It does mean that the data cannot be located in RPMS • First, check what’s in the chart against what’s in RPMS – Use Patient Lists
 Tips for Improvement • Ensure Data Entry is up-to-date – Final GPRA reports normally are due to CAO the first week of August. The end of the GPRA report period is June 30. – Reports at local facilities will be run in July. – If data entry is >4 weeks behind, none of the data that is entered after July will be counted in your GPRA report!!!
Tips for Improvement • Ensure Data Entry is up-to-date – Final GPRA reports normally are due to CAO the first week of August. The end of the GPRA report period is June 30. – Reports at local facilities will be run in July. – If data entry is >4 weeks behind, none of the data that is entered after July will be counted in your GPRA report!!!
 Tips for Improvement (cont’d) • Review your GPRA community taxonomy – Ensure all communities assigned within your service area are included in the GPRA taxonomy • Your site or Area Planning Officer or Statistician should be able to assist in defining appropriate communities • Only Area Planning Officers should edit the GPRA community taxonomy – Removing or adding taxonomies could have a negative impact on the GPRA & PART measures – If you want to add or delete communities for testing purposes, then create a new taxonomy but leave the GPRA taxonomy as is – Find out if any name changes have been made to communities in your taxonomy • If yes, need to change taxonomy to delete old community and add new community
Tips for Improvement (cont’d) • Review your GPRA community taxonomy – Ensure all communities assigned within your service area are included in the GPRA taxonomy • Your site or Area Planning Officer or Statistician should be able to assist in defining appropriate communities • Only Area Planning Officers should edit the GPRA community taxonomy – Removing or adding taxonomies could have a negative impact on the GPRA & PART measures – If you want to add or delete communities for testing purposes, then create a new taxonomy but leave the GPRA taxonomy as is – Find out if any name changes have been made to communities in your taxonomy • If yes, need to change taxonomy to delete old community and add new community
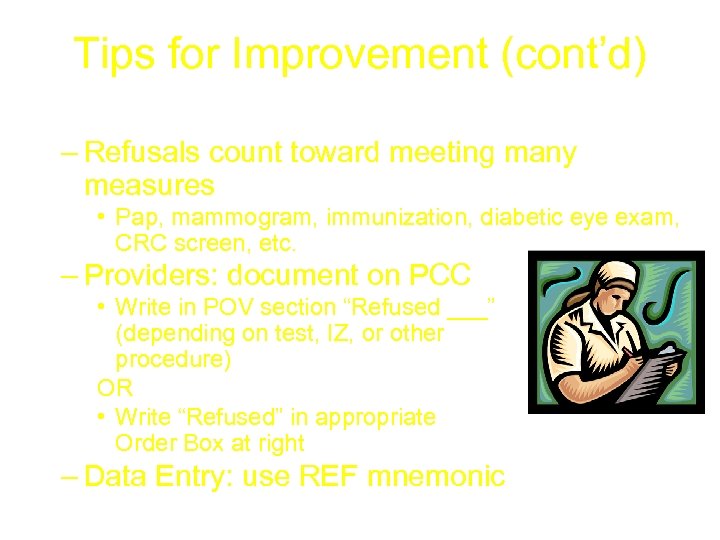 Tips for Improvement (cont’d) • Document and enter refusals – Refusals count toward meeting many measures • Pap, mammogram, immunization, diabetic eye exam, CRC screen, etc. – Providers: document on PCC • Write in POV section “Refused ___” (depending on test, IZ, or other procedure) OR • Write “Refused” in appropriate Order Box at right – Data Entry: use REF mnemonic
Tips for Improvement (cont’d) • Document and enter refusals – Refusals count toward meeting many measures • Pap, mammogram, immunization, diabetic eye exam, CRC screen, etc. – Providers: document on PCC • Write in POV section “Refused ___” (depending on test, IZ, or other procedure) OR • Write “Refused” in appropriate Order Box at right – Data Entry: use REF mnemonic
 Tips for Improvement (cont’d) • Document historical lab tests and procedures – Providers: Ask about and record historical information on PCC • Ask patients about common off-site procedures (e. g. , IZ type, date received, location) • Document telephone visits • Verbal or written lab or other referral reports – Data Entry: Use Historical Mnemonics HIM (Immunization) HPAP (Pap Smear) HRAD (Radiology) 76090 -76092 for mammogram HBE (Barium Enema) HCOL (Colonoscopy) HFOB (FOBT, guaiac) HSIG (Sigmoidoscopy)
Tips for Improvement (cont’d) • Document historical lab tests and procedures – Providers: Ask about and record historical information on PCC • Ask patients about common off-site procedures (e. g. , IZ type, date received, location) • Document telephone visits • Verbal or written lab or other referral reports – Data Entry: Use Historical Mnemonics HIM (Immunization) HPAP (Pap Smear) HRAD (Radiology) 76090 -76092 for mammogram HBE (Barium Enema) HCOL (Colonoscopy) HFOB (FOBT, guaiac) HSIG (Sigmoidoscopy)
 Tips for Improvement (cont’d) • Download the Clinical Cheat Sheet from the CRS web site (Performance Improvement Toolbox page) – Contains detailed instructions for providers and data entry on documenting and entering information for: • Historical Data • Refusals • Exams
Tips for Improvement (cont’d) • Download the Clinical Cheat Sheet from the CRS web site (Performance Improvement Toolbox page) – Contains detailed instructions for providers and data entry on documenting and entering information for: • Historical Data • Refusals • Exams
 Tips for Improvement (cont’d) • Include all relevant lab tests for taxonomies – Update taxonomies at least annually because the Lab updates lab profile and codes periodically throughout the year – Include changed, inactive, deleted and current tests in your taxonomy because CRS looks at tests as far back as 1995 – Coordinate with lab tech to assure ALL codes identified. They may know names of tests you wouldn’t know. • Document reference lab results – If labs are sent out, ensure that test completion and result are entered in PCC when returned
Tips for Improvement (cont’d) • Include all relevant lab tests for taxonomies – Update taxonomies at least annually because the Lab updates lab profile and codes periodically throughout the year – Include changed, inactive, deleted and current tests in your taxonomy because CRS looks at tests as far back as 1995 – Coordinate with lab tech to assure ALL codes identified. They may know names of tests you wouldn’t know. • Document reference lab results – If labs are sent out, ensure that test completion and result are entered in PCC when returned
 For more info, visit: www. ihs. gov/cio/crs
For more info, visit: www. ihs. gov/cio/crs
 Join the CRS Listserv 1. Go the CRS Listserv page (URL shown below). http: //www. ihs. gov/cio/crs/index. cfm? module=crs_listserv 2. Click “Subscribe” link. 3. It brings up an e-mail window. Click the “Send” button. 4. You will receive an email response with the following message in the text. Your command: SUBSCRIBE CRS [Your Last Name, Your First Name] (IHS/NPA) has been received. You must now reply to this message (as explained below) to complete your subscription. The purpose of this confirmation procedure is to make sure that you have indeed requested to be added to the list. To confirm the execution of your command, simply click on the following URL http: //listserv. ihs. gov/scripts/wa. exe
Join the CRS Listserv 1. Go the CRS Listserv page (URL shown below). http: //www. ihs. gov/cio/crs/index. cfm? module=crs_listserv 2. Click “Subscribe” link. 3. It brings up an e-mail window. Click the “Send” button. 4. You will receive an email response with the following message in the text. Your command: SUBSCRIBE CRS [Your Last Name, Your First Name] (IHS/NPA) has been received. You must now reply to this message (as explained below) to complete your subscription. The purpose of this confirmation procedure is to make sure that you have indeed requested to be added to the list. To confirm the execution of your command, simply click on the following URL http: //listserv. ihs. gov/scripts/wa. exe
 CRS Contacts GPRA Lead Francis Frazier FNP, MPH (301) 443 -4700 Francis. Frazier@ihs. gov OIT Clinical Lead Chris Lamer (828) 497 -9163 Chris. Lamer@ihs. gov OIT Federal Lead Stephanie Klepacki (505) 821 -4480 Stephanie. Klepacki@ihs. gov Lead Developer Lori Butcher (520) 577 -2146 butcherla@aol. com GUI Developer Mark Williams (928) 774 -6200 cim@att. net
CRS Contacts GPRA Lead Francis Frazier FNP, MPH (301) 443 -4700 Francis. Frazier@ihs. gov OIT Clinical Lead Chris Lamer (828) 497 -9163 Chris. Lamer@ihs. gov OIT Federal Lead Stephanie Klepacki (505) 821 -4480 Stephanie. Klepacki@ihs. gov Lead Developer Lori Butcher (520) 577 -2146 butcherla@aol. com GUI Developer Mark Williams (928) 774 -6200 cim@att. net


