2247074c2ba16246c4ace36b754913e7.ppt
- Количество слайдов: 13

Introduction to the Risk Assessment (Background, Scope, and Purpose) Sherri Dennis, Ph. D Food and Drug Administration Center for Food Safety and Applied Nutrition Interagency Risk Assessment - L. monocytogenes in Retail Delicatessens Public Meeting Washington, DC - May 22 nd, 2013

Interagency Retail Lm Risk Assessment • Objective: Ascertain the impact on public health of current practices and potential interventions that reduce or prevent Listeria monocytogenes contamination in ready-to-eat food sliced, prepared and/or packaged in retail facilities May 22 nd, 2013 Interagency Risk Assessment--Listeria monocytogenes in Retail Delicatessens Public Meeting 2

Risk Management Questions • “What is the exposure to Listeria monocytogenes from consuming ready-to-eat foods prepared in retail facilities? ” • “What are the key processes that increase ready-to-foods contamination at retails? ” • “How much is the relative risk per serving reduced according to specific risk management options? ” May 22 nd, 2013 Interagency Risk Assessment--Listeria monocytogenes in Retail Delicatessens Public Meeting 3

Risk Management Questions Further refined; a list of proposed ‘what if’ scenarios to evaluate: – Sanitation – Worker behavior – Growth inhibition – Cross contamination – Storage temperature & duration • Examples – What is the public health impact of temperature abuse in deli cases? – What would be the impact of separated slicers/counters for growth versus non-growth products? – What is the impact of the use of gloves in the retail environment? May 22 nd, 2013 Interagency Risk Assessment--Listeria monocytogenes in Retail Delicatessens Public Meeting 4

Steps to Conduct Risk Assessment • • Step 1: Commission (form teams, charge) Step 2: Collect & evaluate data (FRN) Step 3: Build & validate model; prepare report(s) Step 4: Review (includes external peer review; agency clearance) • Step 5: Issue (first as draft for public comment, then revised) May 22 nd, 2013 Interagency Risk Assessment--Listeria monocytogenes in Retail Delicatessens Public Meeting 5
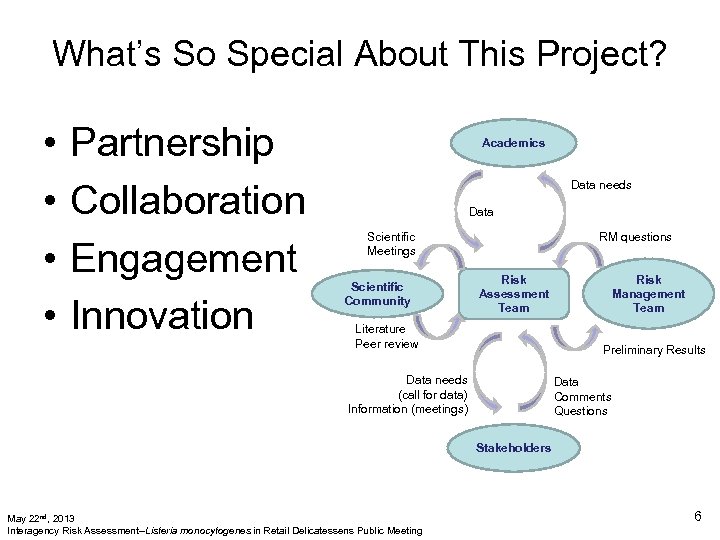
What’s So Special About This Project? • • Partnership Collaboration Engagement Innovation Academics Data needs Data Scientific Meetings Scientific Community RM questions Risk Assessment Team Literature Peer review Risk Management Team Preliminary Results Data needs (call for data) Information (meetings) Data Comments Questions Stakeholders May 22 nd, 2013 Interagency Risk Assessment--Listeria monocytogenes in Retail Delicatessens Public Meeting 6
Partnership • Interagency Risk Assessment Team – Members from both Agencies – Share & leverage resources • Value of team approach – Share resources • public meetings, data collection, peer review costs – Ensure that a variety of perspectives/ disciplines are taken into account – Identify and evaluate a wide range of risk management options May 22 nd, 2013 Interagency Risk Assessment--Listeria monocytogenes in Retail Delicatessens Public Meeting 7
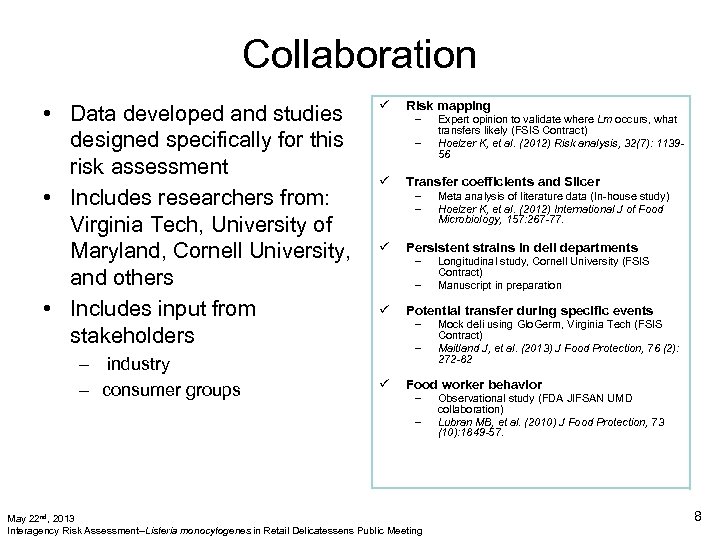
Collaboration • Data developed and studies designed specifically for this risk assessment • Includes researchers from: Virginia Tech, University of Maryland, Cornell University, and others • Includes input from stakeholders – industry – consumer groups ü Risk mapping – – ü Transfer coefficients and Slicer – – ü – Longitudinal study, Cornell University (FSIS Contract) Manuscript in preparation Potential transfer during specific events – – ü Meta analysis of literature data (In-house study) Hoelzer K, et al. (2012) International J of Food Microbiology, 157: 267 -77. Persistent strains in deli departments – ü Expert opinion to validate where Lm occurs, what transfers likely (FSIS Contract) Hoelzer K, et al. (2012) Risk analysis, 32(7): 113956 Mock deli using Glo. Germ, Virginia Tech (FSIS Contract) Maitland J, et al. (2013) J Food Protection, 76 (2): 272 -82 Food worker behavior – – May 22 nd, 2013 Interagency Risk Assessment--Listeria monocytogenes in Retail Delicatessens Public Meeting Observational study (FDA JIFSAN UMD collaboration) Lubran MB, et al. (2010) J Food Protection, 73 (10): 1849 -57. 8

Early Engagement of Stakeholders • Request for Scientific Data and Information – Federal Register Notices • Vol. 74, No. 12, Jan 21 st, 2009 • Reopened Aug 27 th, 2009 • Public meeting – Federal Register Notice • Vol. 74, No. 109, Jun 9 th, 2009 – Held June 23, 2009; Washington D. C. May 22 nd, 2013 Interagency Risk Assessment--Listeria monocytogenes in Retail Delicatessens Public Meeting 9

Continued Engagement of Stakeholders • Presentations at technical scientific meetings – JIFSAN workshops, SRA, IAFP, CDC/ATSDR symposium, ISOPOL, and others • Briefings and meetings (56+ ! From 2009 -2013) – Industry, trade associations, consumer advocacy groups – Other federal agencies – Universities • Peer review (January 2011) – Of the model framework/approach, data, assumptions, model components, scenarios, uncertainty/variability, – Responses to peer review comments available on FDA and FSIS website May 22 nd, 2013 Interagency Risk Assessment--Listeria monocytogenes in Retail Delicatessens Public Meeting 10
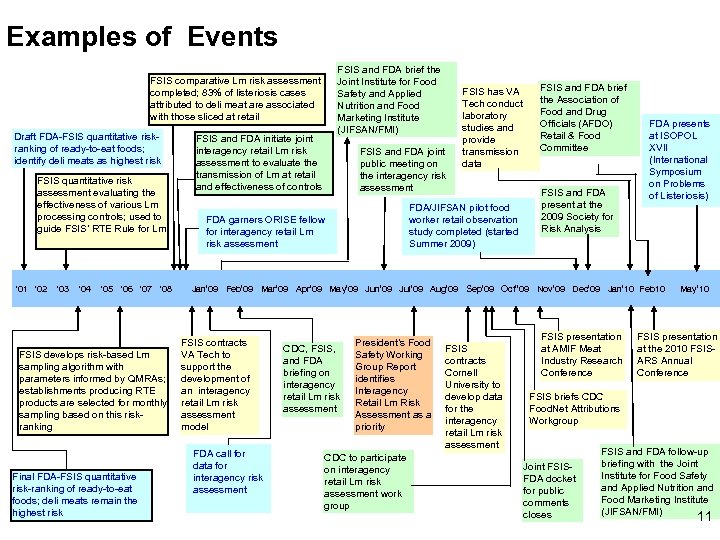
Examples of Events FSIS and FDA brief the Joint Institute for Food Safety and Applied Nutrition and Food Marketing Institute (JIFSAN/FMI) FSIS comparative Lm risk assessment completed; 83% of listeriosis cases attributed to deli meat are associated with those sliced at retail Draft FDA-FSIS quantitative riskranking of ready-to-eat foods; identify deli meats as highest risk FSIS quantitative risk assessment evaluating the effectiveness of various Lm processing controls; used to guide FSIS’ RTE Rule for Lm ‘ 01 ‘ 02 ‘ 03 ‘ 04 ‘ 05 ‘ 06 ‘ 07 ‘ 08 FSIS develops risk-based Lm sampling algorithm with parameters informed by QMRAs; establishments producing RTE products are selected for monthly sampling based on this riskranking Final FDA-FSIS quantitative risk-ranking of ready-to-eat foods; deli meats remain the highest risk FSIS and FDA initiate joint interagency retail Lm risk assessment to evaluate the transmission of Lm at retail and effectiveness of controls FSIS and FDA joint public meeting on the interagency risk assessment FSIS has VA Tech conduct laboratory studies and provide transmission data FDA/JIFSAN pilot food worker retail observation study completed (started Summer 2009) FDA garners ORISE fellow for interagency retail Lm risk assessment FSIS and FDA brief the Association of Food and Drug Officials (AFDO) Retail & Food Committee FSIS and FDA present at the 2009 Society for Risk Analysis FDA presents at ISOPOL XVII (International Symposium on Problems of Listeriosis) Jan’ 09 Feb’ 09 Mar’ 09 Apr’ 09 May’ 09 Jun’ 09 Jul’ 09 Aug’ 09 Sep’ 09 Oct’’ 09 Nov’ 09 Dec’ 09 Jan’ 10 Feb 10 FSIS contracts VA Tech to support the development of an interagency retail Lm risk assessment model FDA call for data for interagency risk assessment CDC, FSIS, and FDA briefing on interagency retail Lm risk assessment President’s Food Safety Working Group Report identifies Interagency Retail Lm Risk Assessment as a priority CDC to participate on interagency retail Lm risk assessment work group FSIS contracts Cornell University to develop data for the interagency retail Lm risk assessment FSIS presentation at AMIF Meat Industry Research Conference May’ 10 FSIS presentation at the 2010 FSISARS Annual Conference FSIS briefs CDC Food. Net Attributions Workgroup Joint FSISFDA docket for public comments closes FSIS and FDA follow-up briefing with the Joint Institute for Food Safety and Applied Nutrition and Food Marketing Institute (JIFSAN/FMI) 11
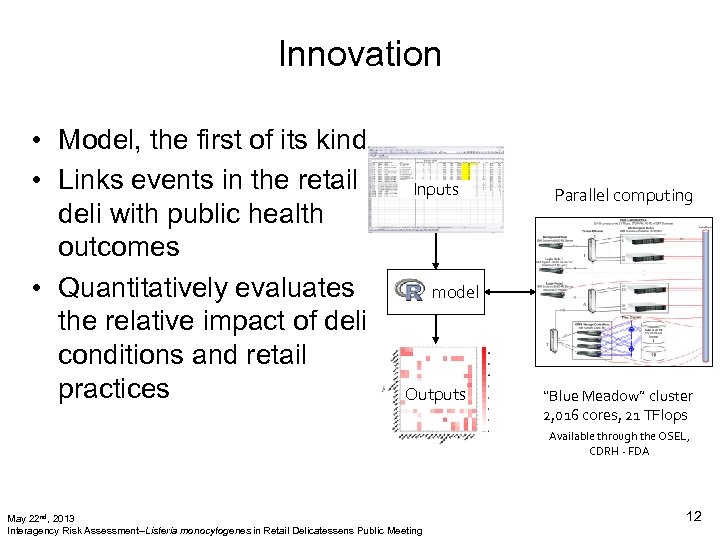
Innovation • Model, the first of its kind • Links events in the retail deli with public health outcomes • Quantitatively evaluates the relative impact of deli conditions and retail practices Inputs Parallel computing model Outputs “Blue Meadow” cluster 2, 016 cores, 21 TFlops Available through the OSEL, CDRH - FDA May 22 nd, 2013 Interagency Risk Assessment--Listeria monocytogenes in Retail Delicatessens Public Meeting 12

Thank you ! May 22 nd, 2013 Interagency Risk Assessment--Listeria monocytogenes in Retail Delicatessens Public Meeting 13
2247074c2ba16246c4ace36b754913e7.ppt