305d8e535669e43f6be9680543f19e7a.ppt
- Количество слайдов: 119
 Introduction to the Mole and Molar Mass Revised 8/13/14
Introduction to the Mole and Molar Mass Revised 8/13/14
 Purpose At the completion of this unit students will • Have a conceptual understanding of the mole as the method of “counting” items and finding the mass of items that can’t be seen. • Be able to calculate the number of items (molecules, atoms, ions, and formula units) if given the number of moles. • Be able to calculate the number of moles if given the number of items. • Be able to calculate the mass of a sample if given the number of moles in a sample. • Be able to calculate the number of moles in a sample if given the mass of a sample. • Be able to prepare a sample containing a given number of moles. • Be able to determine the molar mass of a compound.
Purpose At the completion of this unit students will • Have a conceptual understanding of the mole as the method of “counting” items and finding the mass of items that can’t be seen. • Be able to calculate the number of items (molecules, atoms, ions, and formula units) if given the number of moles. • Be able to calculate the number of moles if given the number of items. • Be able to calculate the mass of a sample if given the number of moles in a sample. • Be able to calculate the number of moles in a sample if given the mass of a sample. • Be able to prepare a sample containing a given number of moles. • Be able to determine the molar mass of a compound.
 Background • When you buy eggs you usually ask for a _______ eggs. • You know that one dozen of any item is ______.
Background • When you buy eggs you usually ask for a _______ eggs. • You know that one dozen of any item is ______.
 Paper • Paper is packaged by a ream. • A ream of paper has 500 sheets. • Why is it useful to use units like a dozen or a ream?
Paper • Paper is packaged by a ream. • A ream of paper has 500 sheets. • Why is it useful to use units like a dozen or a ream?
 • What determines how many items should make up a particular unit?
• What determines how many items should make up a particular unit?
 • If you were asked to design a new unit to count something, what would you consider when choosing how many items should be included in your new counting unit?
• If you were asked to design a new unit to count something, what would you consider when choosing how many items should be included in your new counting unit?
 Materials • 3 packages of different types of candy, balance, worksheet, calculator, pencil.
Materials • 3 packages of different types of candy, balance, worksheet, calculator, pencil.
 Part 1 1. 2. 3. 4. Record the number of items in each package. Measure the mass of each package. Record the mass of each package in the data table. Answer questions in Analysis and Interpretations. Record masses your group measured on the board summarizing class data.
Part 1 1. 2. 3. 4. Record the number of items in each package. Measure the mass of each package. Record the mass of each package in the data table. Answer questions in Analysis and Interpretations. Record masses your group measured on the board summarizing class data.
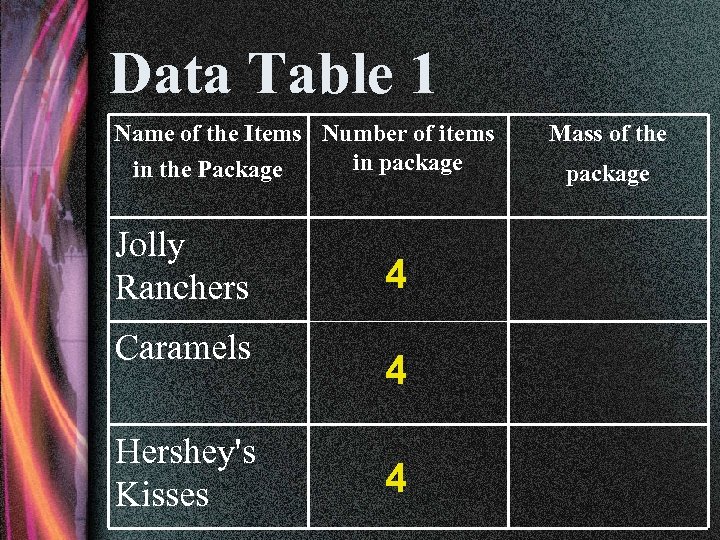 Data Table 1 Name of the Items Number of items in package in the Package Jolly Ranchers Caramels Hershey's Kisses 4 4 4 Mass of the package
Data Table 1 Name of the Items Number of items in package in the Package Jolly Ranchers Caramels Hershey's Kisses 4 4 4 Mass of the package
 ANALYSIS AND INTERPRETATION • As you know, a dozen represents 12 items. • Since I did not have enough items to make a dozen, I decided to make a new counting unit. I called this unit an DART. Each of your packages contains 4 _____ items.
ANALYSIS AND INTERPRETATION • As you know, a dozen represents 12 items. • Since I did not have enough items to make a dozen, I decided to make a new counting unit. I called this unit an DART. Each of your packages contains 4 _____ items.
 1. A KNIGHT of oranges will have _____ 4 oranges.
1. A KNIGHT of oranges will have _____ 4 oranges.
 2. A KNIGHT of pretzels has ____ pretzels.
2. A KNIGHT of pretzels has ____ pretzels.
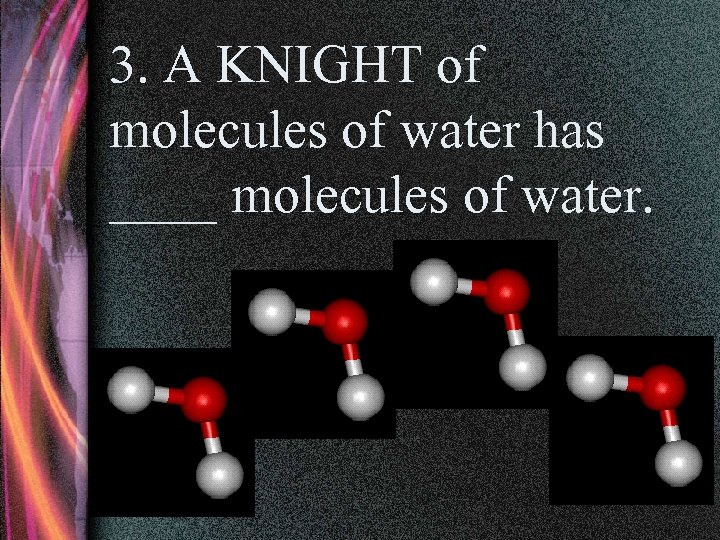 3. A KNIGHT of molecules of water has ____ molecules of water.
3. A KNIGHT of molecules of water has ____ molecules of water.
 4. A KNIGHT of particles has ___ particles.
4. A KNIGHT of particles has ___ particles.
 5. A KNIGHT of atoms of iron has _____ atoms of iron. 26 Fe 55. 85
5. A KNIGHT of atoms of iron has _____ atoms of iron. 26 Fe 55. 85
 6. A KNIGHT of formula units of salt has _______ formula units of salt.
6. A KNIGHT of formula units of salt has _______ formula units of salt.
 7. How many Hershey’s Kisses are in 2 KNIGHTS? _______
7. How many Hershey’s Kisses are in 2 KNIGHTS? _______
 8. How many caramels are in 10 KNIGHTS? _______
8. How many caramels are in 10 KNIGHTS? _______
 9. How many Dum Pops are in 400 KNIGHTS? _______
9. How many Dum Pops are in 400 KNIGHTS? _______
 10. How many Starbursts are in 1/2 KNIGHT? _____
10. How many Starbursts are in 1/2 KNIGHT? _____
 11. Write the directions for finding the number of items if given the number of KNIGHTS
11. Write the directions for finding the number of items if given the number of KNIGHTS
 12. How many oranges are in 5 KNIGHTS? _______
12. How many oranges are in 5 KNIGHTS? _______
 13. How many apples are in 0. 5 KNIGHTS? _______
13. How many apples are in 0. 5 KNIGHTS? _______
 14. How many pencils are in 0. 25 (1/4) KNIGHTS? ___
14. How many pencils are in 0. 25 (1/4) KNIGHTS? ___
 15. How many atoms of silver are in 20 KNIGHTS? _______ 47 Ag 107. 9
15. How many atoms of silver are in 20 KNIGHTS? _______ 47 Ag 107. 9
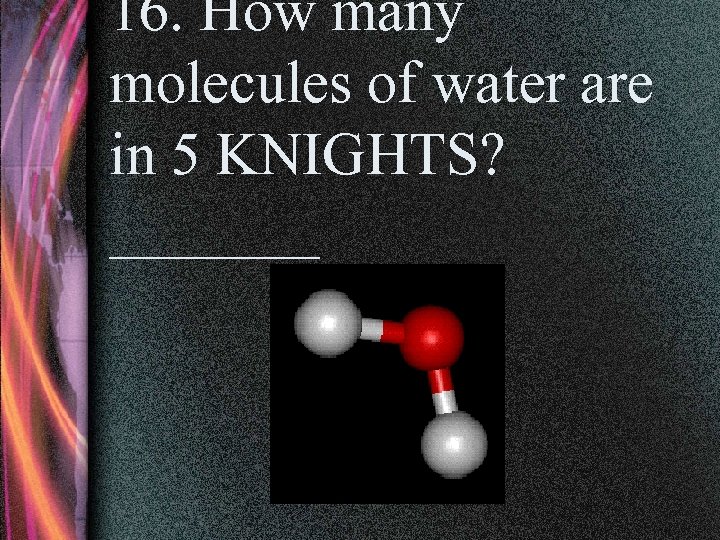 16. How many molecules of water are in 5 KNIGHTS? _______
16. How many molecules of water are in 5 KNIGHTS? _______
 17. How many KNIGHTS are 16 Hershey’s Kisses? ______
17. How many KNIGHTS are 16 Hershey’s Kisses? ______
 18. How many KNIGHTS are 100 pretzels? ____
18. How many KNIGHTS are 100 pretzels? ____
 19. How many KNIGHTS are 400 Starbursts? ____
19. How many KNIGHTS are 400 Starbursts? ____
 20. How many KNIGHTS is 1 orange? ____ (Write a fraction or a decimal. )
20. How many KNIGHTS is 1 orange? ____ (Write a fraction or a decimal. )
 21. How many KNIGHTS are 2 caramels? _____
21. How many KNIGHTS are 2 caramels? _____
 22. Write your own directions for finding the number of KNIGHTS given the number of pieces
22. Write your own directions for finding the number of KNIGHTS given the number of pieces
 23. How many KNIGHTS are 48 Hershey’s Kisses? ____
23. How many KNIGHTS are 48 Hershey’s Kisses? ____
 24. How many KNIGHTS are 32 Hershey’s Kisses? ______
24. How many KNIGHTS are 32 Hershey’s Kisses? ______
 25. How many KNIGHTS are 24 Jolly Ranchers? ______
25. How many KNIGHTS are 24 Jolly Ranchers? ______
 26. How many KNIGHTS are 2 Gobstoppers? ______
26. How many KNIGHTS are 2 Gobstoppers? ______
 27. How many KNIGHTS are 20 caramels? ______
27. How many KNIGHTS are 20 caramels? ______
 KNIGHTS • KNIGHT of molecules of water would be too small to see. • Scientists had to select a bigger unit for counting molecules of substances. • The unit scientists use is called a MOLE.
KNIGHTS • KNIGHT of molecules of water would be too small to see. • Scientists had to select a bigger unit for counting molecules of substances. • The unit scientists use is called a MOLE.
 One MOLE of anything has 602, 200, 000, 000 items.
One MOLE of anything has 602, 200, 000, 000 items.
 AVOGADRO’S NUMBER • 6. 022 x 23 10 • One MOLE of anything 23 items. has 6. 022 x 10
AVOGADRO’S NUMBER • 6. 022 x 23 10 • One MOLE of anything 23 items. has 6. 022 x 10
 28. How many Hershey’s Kisses make up 1 MOLE?
28. How many Hershey’s Kisses make up 1 MOLE?
 29. How many caramels make up 10 MOLES?
29. How many caramels make up 10 MOLES?
 30. Find the number of Jolly Ranchers in 4 MOLES.
30. Find the number of Jolly Ranchers in 4 MOLES.
 31. Find the number of atoms of sodium in 2 MOLES. 11 Na 22. 99
31. Find the number of atoms of sodium in 2 MOLES. 11 Na 22. 99
 32. Find the number of molecules of water in 6 MOLES.
32. Find the number of molecules of water in 6 MOLES.
 33. Find the number of caramels in 0. 5 MOLES.
33. Find the number of caramels in 0. 5 MOLES.
 34. How many moles of 23 caramels is 6. 022 x 10 of caramels? ___
34. How many moles of 23 caramels is 6. 022 x 10 of caramels? ___
 35. How many moles of 23 Starbursts is 6. 022 x 10 of Starbursts? ___
35. How many moles of 23 Starbursts is 6. 022 x 10 of Starbursts? ___
 36. How many moles of 23 Gobstoppers is 12. 04 x 10 of Gobstoppers? ___
36. How many moles of 23 Gobstoppers is 12. 04 x 10 of Gobstoppers? ___
 37. How many atoms of potassium make up one MOLE? 19 K 39. 10
37. How many atoms of potassium make up one MOLE? 19 K 39. 10
 38. How many atoms of potassium make up 2 MOLES? 19 K 39. 10
38. How many atoms of potassium make up 2 MOLES? 19 K 39. 10
 39. How many molecules of water make up 1 MOLE?
39. How many molecules of water make up 1 MOLE?
 40. How many molecules of water make up 5 MOLES?
40. How many molecules of water make up 5 MOLES?
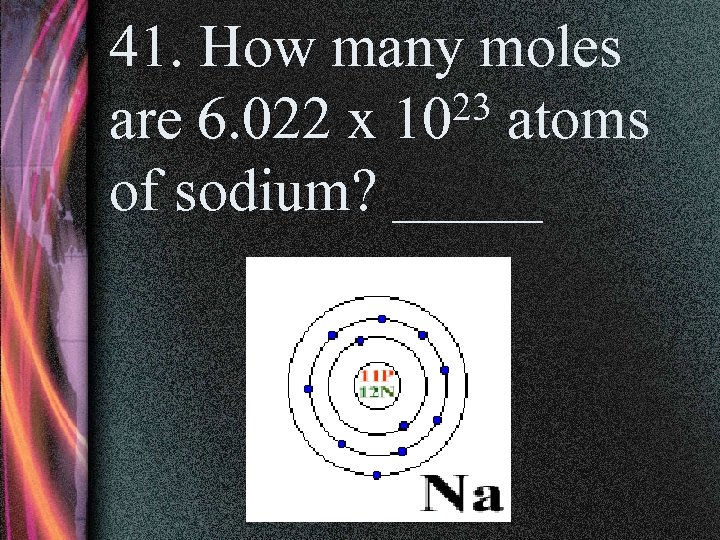 41. How many moles 23 atoms are 6. 022 x 10 of sodium? _____
41. How many moles 23 atoms are 6. 022 x 10 of sodium? _____
 42. How many moles 23 atoms are 12. 04 x 10 of carbon? _
42. How many moles 23 atoms are 12. 04 x 10 of carbon? _
 43. How many moles 23 atoms are 18. 06 x 10 of sodium? _____
43. How many moles 23 atoms are 18. 06 x 10 of sodium? _____
 44. How many moles 23 atoms are 60. 22 x 10 of sodium? _____
44. How many moles 23 atoms are 60. 22 x 10 of sodium? _____
 45. How many moles are 23 molecules 6. 022 x 10 of water? _____
45. How many moles are 23 molecules 6. 022 x 10 of water? _____
 46. How many moles are 23 molecules 12. 04 x 10 of water? _____
46. How many moles are 23 molecules 12. 04 x 10 of water? _____
 47. How many moles are 23 molecules 30. 10 x 10 of water? ____
47. How many moles are 23 molecules 30. 10 x 10 of water? ____
 Part 2
Part 2
 Mass • In addition to being able to tell the number of items in an KNIGHT, you can now tell the mass of an KNIGHT of Hershey’s Kisses, Jolly Ranchers, and caramels.
Mass • In addition to being able to tell the number of items in an KNIGHT, you can now tell the mass of an KNIGHT of Hershey’s Kisses, Jolly Ranchers, and caramels.
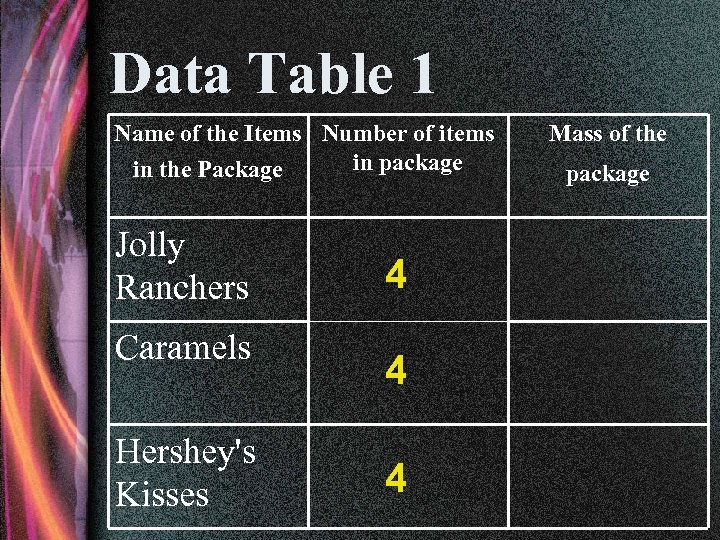 Data Table 1 Name of the Items Number of items in package in the Package Jolly Ranchers Caramels Hershey's Kisses 4 4 4 Mass of the package
Data Table 1 Name of the Items Number of items in package in the Package Jolly Ranchers Caramels Hershey's Kisses 4 4 4 Mass of the package
 48. Based on your measurements, the mass of 1 KNIGHT of Hershey’s Kisses is ______ g.
48. Based on your measurements, the mass of 1 KNIGHT of Hershey’s Kisses is ______ g.
 49. The mass of 2 KNIGHTS of Hershey’s Kisses is ______ g.
49. The mass of 2 KNIGHTS of Hershey’s Kisses is ______ g.
 50. The mass of 1 KNIGHT of Jolly Ranchers is ___ g.
50. The mass of 1 KNIGHT of Jolly Ranchers is ___ g.
 51. The mass of 1 KNIGHT of caramels is ____g
51. The mass of 1 KNIGHT of caramels is ____g
 52. The mass of 100 KNIGHTS of Hershey’s Kisses is ____ g.
52. The mass of 100 KNIGHTS of Hershey’s Kisses is ____ g.
 53. The mass of 1/2 KNIGHTS of Hershey’s Kisses is ____ g.
53. The mass of 1/2 KNIGHTS of Hershey’s Kisses is ____ g.
 54. Complete the directions for finding mass of a given number of KNIGHTS
54. Complete the directions for finding mass of a given number of KNIGHTS
 55. The mass of 4 KNIGHTS of Jolly Ranchers is ____ g.
55. The mass of 4 KNIGHTS of Jolly Ranchers is ____ g.
 56. The mass of 0. 5 KNIGHTS of Jolly Ranchers is ____ g.
56. The mass of 0. 5 KNIGHTS of Jolly Ranchers is ____ g.
 57. The mass of 100 KNIGHTS of caramels is _____ g.
57. The mass of 100 KNIGHTS of caramels is _____ g.
 58. The mass of 1/5 KNIGHTS of caramels is _____ g.
58. The mass of 1/5 KNIGHTS of caramels is _____ g.
 59. The mass of 60 KNIGHTS of Hershey’s Kisses is _____ g.
59. The mass of 60 KNIGHTS of Hershey’s Kisses is _____ g.
 60. The mass of 0. 1 KNIGHTS of Hershey’s Kisses is _____ g.
60. The mass of 0. 1 KNIGHTS of Hershey’s Kisses is _____ g.
 61. How would you calculate the number of KNIGHTS if you know the mass of a package of Hershey’s Kisses?
61. How would you calculate the number of KNIGHTS if you know the mass of a package of Hershey’s Kisses?
 62. How would you calculate the number of KNIGHTS if you know the mass of a package of caramels?
62. How would you calculate the number of KNIGHTS if you know the mass of a package of caramels?
 63. How would you calculate the number of KNIGHTS if you know the mass of a package of Jolly Ranchers?
63. How would you calculate the number of KNIGHTS if you know the mass of a package of Jolly Ranchers?
 Molar Mass • Scientists use the Periodic Table to determine the mass of a mole of atom of an element. • For example, a mole of Iron (Fe) atoms would have a mass of 55. 85 g.
Molar Mass • Scientists use the Periodic Table to determine the mass of a mole of atom of an element. • For example, a mole of Iron (Fe) atoms would have a mass of 55. 85 g.
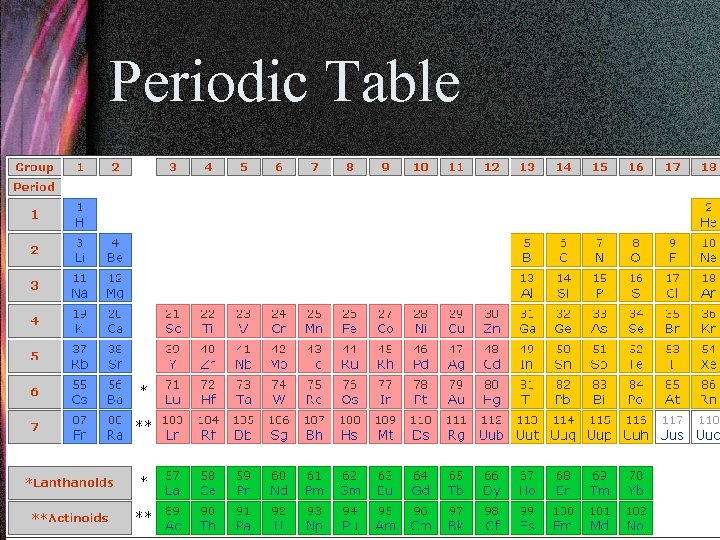 Periodic Table
Periodic Table
 64. The mass of 1 mole of Strontium (Sr) is ___ g. 38 Sr 87. 62
64. The mass of 1 mole of Strontium (Sr) is ___ g. 38 Sr 87. 62
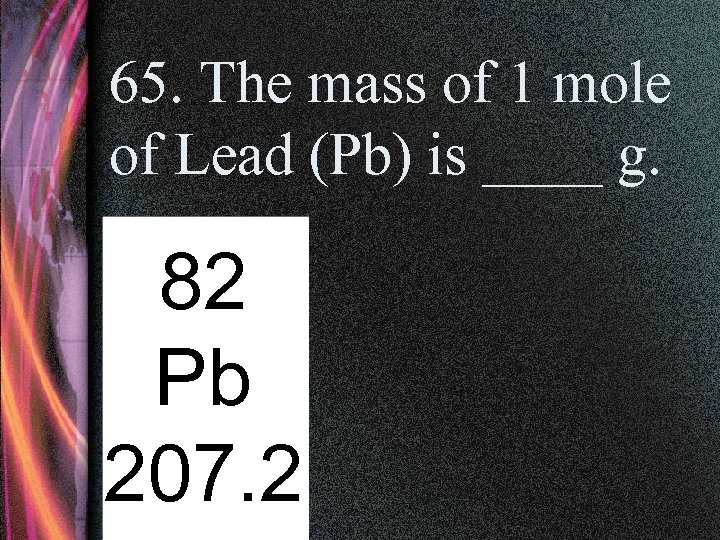 65. The mass of 1 mole of Lead (Pb) is ____ g. 82 Pb 207. 2
65. The mass of 1 mole of Lead (Pb) is ____ g. 82 Pb 207. 2
 66. The mass of 1 mole of Nickel (Ni) is ____ g. 28 Ni 58. 69
66. The mass of 1 mole of Nickel (Ni) is ____ g. 28 Ni 58. 69
 67. The mass of 4 moles of Strontium (Sr) is _____g. 38 Sr 87. 62
67. The mass of 4 moles of Strontium (Sr) is _____g. 38 Sr 87. 62
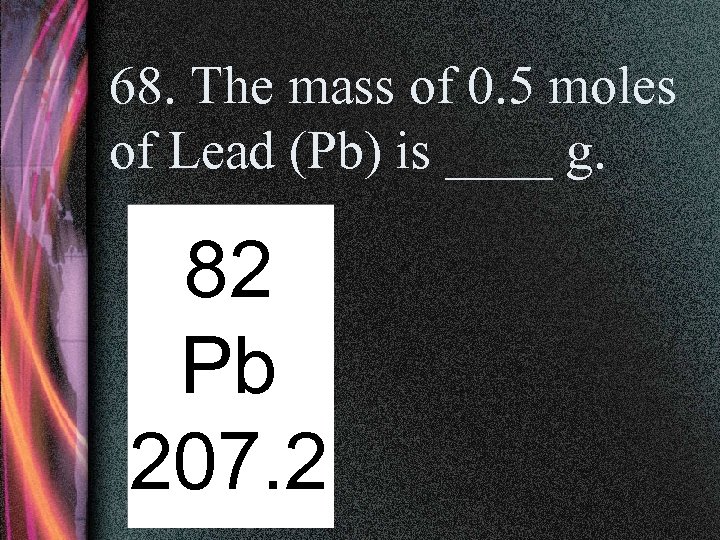 68. The mass of 0. 5 moles of Lead (Pb) is ____ g. 82 Pb 207. 2
68. The mass of 0. 5 moles of Lead (Pb) is ____ g. 82 Pb 207. 2
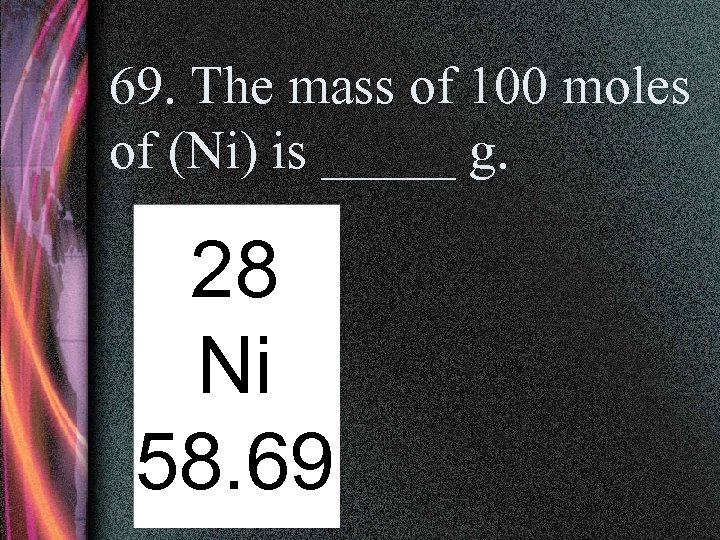 69. The mass of 100 moles of (Ni) is _____ g. 28 Ni 58. 69
69. The mass of 100 moles of (Ni) is _____ g. 28 Ni 58. 69
 Molar Mass • Scientists also use the Periodic Table to determine the molar mass (formula weight) of compounds. • The molar mass of the compound is the sum of the molar masses of the elements that make up the compound.
Molar Mass • Scientists also use the Periodic Table to determine the molar mass (formula weight) of compounds. • The molar mass of the compound is the sum of the molar masses of the elements that make up the compound.
 Potassium Chloride • The molar mass of Potassium Chloride is: Potassium (K) 39. 10 g/mole Chloride (Cl) +34. 45 g/mole 73. 55 g/mole
Potassium Chloride • The molar mass of Potassium Chloride is: Potassium (K) 39. 10 g/mole Chloride (Cl) +34. 45 g/mole 73. 55 g/mole
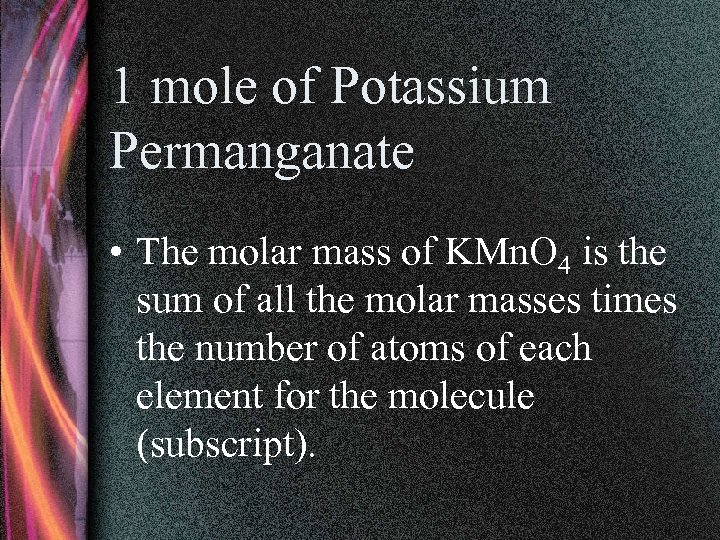 1 mole of Potassium Permanganate • The molar mass of KMn. O 4 is the sum of all the molar masses times the number of atoms of each element for the molecule (subscript).
1 mole of Potassium Permanganate • The molar mass of KMn. O 4 is the sum of all the molar masses times the number of atoms of each element for the molecule (subscript).
 Potassium Permanganate KMn. O 4 • Potassium (K) 39. 10 g/mole • Manganese (Mn) 54. 94 g/mole • Oxygen (O) 4[16. 00 g/mole] 158. 04 g/mole.
Potassium Permanganate KMn. O 4 • Potassium (K) 39. 10 g/mole • Manganese (Mn) 54. 94 g/mole • Oxygen (O) 4[16. 00 g/mole] 158. 04 g/mole.
 70. The molar mass of 1 mole of Sodium Chloride (Na. Cl) is____ g.
70. The molar mass of 1 mole of Sodium Chloride (Na. Cl) is____ g.
 71. The molar mass of 1 mole of Lead Iodide (Pb. I 2) is _____ g.
71. The molar mass of 1 mole of Lead Iodide (Pb. I 2) is _____ g.
 72. The mass of 100 moles of Nickel Sulfide (Ni. S) is ____ g.
72. The mass of 100 moles of Nickel Sulfide (Ni. S) is ____ g.
 73. The mass of 1 mole of is Glucose (C 6 H 12 O 6) is ______ g.
73. The mass of 1 mole of is Glucose (C 6 H 12 O 6) is ______ g.
 74. The mass of 1 mole of is Sucrose (C 12 H 24 O 12 ) _______ g.
74. The mass of 1 mole of is Sucrose (C 12 H 24 O 12 ) _______ g.
 75. The mass of 4 moles of Glucose (C 6 H 12 O 6) is _____ g.
75. The mass of 4 moles of Glucose (C 6 H 12 O 6) is _____ g.
 Mole Conversions • In chemistry, the mole is the standard measurement of amount. • However, balances DO NOT give readings in moles. Balances give readings in grams. • So the problem is that, while we compare amounts of one substance to another using moles, we must also use grams, since this is the information we get from balances.
Mole Conversions • In chemistry, the mole is the standard measurement of amount. • However, balances DO NOT give readings in moles. Balances give readings in grams. • So the problem is that, while we compare amounts of one substance to another using moles, we must also use grams, since this is the information we get from balances.
 There are three steps to converting grams of a substance to moles. 1. Determine how many grams are given in the problem. 2. Calculate the molar mass of the substance. 3. Divide step one by step two.
There are three steps to converting grams of a substance to moles. 1. Determine how many grams are given in the problem. 2. Calculate the molar mass of the substance. 3. Divide step one by step two.
 The three steps above can be expressed in the following proportion: Grams -------Molar Mass = Moles -------1 Mole
The three steps above can be expressed in the following proportion: Grams -------Molar Mass = Moles -------1 Mole
 Example #1 - Convert 25. 0 grams of KMn. O 4 to moles. Step One: The problem will tell you how many grams are present. Look for the unit of grams. The problem gives us 25. 0 grams. Step Two: You need to know the molar mass of the substance. The molar mass of KMn. O 4 is 158. 034 grams/mole. Potassium (K) = 39. 10 x 1 = 39. 10 g Manganese (Mn) = 54. 94 x 1 = 54. 94 g Oxygen (O) = 16. 00 x 4 = 64. 00 g -------158. 04 g Step Three: You divide the grams given by the substance's molar mass (25. 0/158. 04) The answer of 0. 16 mole has been rounded off.
Example #1 - Convert 25. 0 grams of KMn. O 4 to moles. Step One: The problem will tell you how many grams are present. Look for the unit of grams. The problem gives us 25. 0 grams. Step Two: You need to know the molar mass of the substance. The molar mass of KMn. O 4 is 158. 034 grams/mole. Potassium (K) = 39. 10 x 1 = 39. 10 g Manganese (Mn) = 54. 94 x 1 = 54. 94 g Oxygen (O) = 16. 00 x 4 = 64. 00 g -------158. 04 g Step Three: You divide the grams given by the substance's molar mass (25. 0/158. 04) The answer of 0. 16 mole has been rounded off.
 Example #2 - Calculate how many moles are in 57. 0 grams of Mg(NO 3)2 Step One: 57. 0 grams are given in the text of the problem. Step Two: The molar mass is 148 grams/mole. Mg (Magnesium) = 24 x 1= 24 g N (Nitrogen) = 14 x 2= 28 g O (Oxygen) = 16 x 6= 96 g Step Three: Again you divide the grams by the substances molar mass (57. 0 g/148 g). This answer has been rounded to 0. 39 moles.
Example #2 - Calculate how many moles are in 57. 0 grams of Mg(NO 3)2 Step One: 57. 0 grams are given in the text of the problem. Step Two: The molar mass is 148 grams/mole. Mg (Magnesium) = 24 x 1= 24 g N (Nitrogen) = 14 x 2= 28 g O (Oxygen) = 16 x 6= 96 g Step Three: Again you divide the grams by the substances molar mass (57. 0 g/148 g). This answer has been rounded to 0. 39 moles.
 Practice Problems
Practice Problems
 1. Calculate the moles present in: 2. 00 grams of H 2 O
1. Calculate the moles present in: 2. 00 grams of H 2 O
 2. Calculate the moles present in: 75. 57 grams of KBr
2. Calculate the moles present in: 75. 57 grams of KBr
 3. Calculate the moles present in: 100. 0 grams of KCl. O 4
3. Calculate the moles present in: 100. 0 grams of KCl. O 4
 4. Calculate the moles present in: 225. 5 grams of Sucrose (C 12 H 24 O 12)
4. Calculate the moles present in: 225. 5 grams of Sucrose (C 12 H 24 O 12)
 5. Calculate the moles present in: 350. 0 grams of Glucose (C 6 H 12 O 6)
5. Calculate the moles present in: 350. 0 grams of Glucose (C 6 H 12 O 6)
 Homework
Homework
 1. Calculate the moles present in: 3. 00 grams of Na. Cl
1. Calculate the moles present in: 3. 00 grams of Na. Cl
 2. Calculate the moles present in: 25. 0 grams of NH 3
2. Calculate the moles present in: 25. 0 grams of NH 3
 3. Calculate the moles present in: 16. 0 grams of KCl
3. Calculate the moles present in: 16. 0 grams of KCl
 4. Calculate the moles present in: 30. 25 grams of Be. Cl 2
4. Calculate the moles present in: 30. 25 grams of Be. Cl 2
 5. Calculate the moles present in: 175. 25 grams of Li 2 S
5. Calculate the moles present in: 175. 25 grams of Li 2 S
 6. Calculate the moles present in: 75. 62 grams of CO 2
6. Calculate the moles present in: 75. 62 grams of CO 2
 7. Calculate the moles present in: 56. 5 grams of H 2 O
7. Calculate the moles present in: 56. 5 grams of H 2 O
 8. Calculate the moles present in: 22. 6 grams of CH 3 COOH
8. Calculate the moles present in: 22. 6 grams of CH 3 COOH
 9. Calculate the moles present in: 18. 4 grams of Ag. Cl
9. Calculate the moles present in: 18. 4 grams of Ag. Cl
 10. Calculate the moles present in: 68. 3 grams of HCN
10. Calculate the moles present in: 68. 3 grams of HCN


