38f30eca9c0b7489927de9208a32513b.ppt
- Количество слайдов: 44
 Introduction to Quality Assurance and Quality Control Module 10 Center for Global Health
Introduction to Quality Assurance and Quality Control Module 10 Center for Global Health
 Learning Objectives Upon completion of this lesson you will be able to: • Define: Quality Assurance, Quality Control, Quality Indicators, analytical errors, Laboratory Best. Practices, Calibrator, Controls, mean, median, mode, standard deviation, coefficient of variation, Levey. Jennings chart, Gaussian curve, Control Range, Normal or Reference Range • Describe the purpose and key components of a quality assurance program • Differentiate quality assurance from quality control
Learning Objectives Upon completion of this lesson you will be able to: • Define: Quality Assurance, Quality Control, Quality Indicators, analytical errors, Laboratory Best. Practices, Calibrator, Controls, mean, median, mode, standard deviation, coefficient of variation, Levey. Jennings chart, Gaussian curve, Control Range, Normal or Reference Range • Describe the purpose and key components of a quality assurance program • Differentiate quality assurance from quality control
 Learning Objectives Upon completion of this lesson you will be able to: • Explain strategies for minimizing errors in laboratory results • Calculate the mean, standard deviation, coefficient of variation, and +(-) 3 Std Dev Range using a given set of data points • Construct a Levey-Jennings chart and plot a given set of data points
Learning Objectives Upon completion of this lesson you will be able to: • Explain strategies for minimizing errors in laboratory results • Calculate the mean, standard deviation, coefficient of variation, and +(-) 3 Std Dev Range using a given set of data points • Construct a Levey-Jennings chart and plot a given set of data points
 What is Quality Assurance? Quality Assurance (QA) is…. A set of proactive policies and procedures ascribed to, in order to create and maintain good quality practices and therefore accurate and precise outputs (laboratory results).
What is Quality Assurance? Quality Assurance (QA) is…. A set of proactive policies and procedures ascribed to, in order to create and maintain good quality practices and therefore accurate and precise outputs (laboratory results).
 Definitions http: //www. westgard. com/glossary. htm#r-s • Quality Control: Procedures regularly practiced throughout the laboratory to verify that procedures, practices and laboratory results are accurate and precise • Pre-analytical: processes that occur to obtain and prepare specimens before testing • Analytical: processes within the testing phase • Post-analytical: processes that take place after testing and until results are released
Definitions http: //www. westgard. com/glossary. htm#r-s • Quality Control: Procedures regularly practiced throughout the laboratory to verify that procedures, practices and laboratory results are accurate and precise • Pre-analytical: processes that occur to obtain and prepare specimens before testing • Analytical: processes within the testing phase • Post-analytical: processes that take place after testing and until results are released
 Definitions • Certified reference material, CRM: “A reference material that has one or more values certified by a technically valid procedure and is accompanied by, or is traceable to, a certificate or other document that is issued by a certifying body. ” [CLSI] • Control Material: Aqueous material with a protein base similar in composition to human plasma/serum. Can be ordered assayed (with values) or un-assayed (with no values)
Definitions • Certified reference material, CRM: “A reference material that has one or more values certified by a technically valid procedure and is accompanied by, or is traceable to, a certificate or other document that is issued by a certifying body. ” [CLSI] • Control Material: Aqueous material with a protein base similar in composition to human plasma/serum. Can be ordered assayed (with values) or un-assayed (with no values)
 Definitions • Calibration. “The process of testing and adjustment of an instrument, kit, or test system, to provide a known relationship between the measurement response and the value of the substance being measured by the test procedure. ” [CLSI]
Definitions • Calibration. “The process of testing and adjustment of an instrument, kit, or test system, to provide a known relationship between the measurement response and the value of the substance being measured by the test procedure. ” [CLSI]
 Definitions • Calibration verification. “The assaying of calibration materials in the same manner as patient samples to confirm that the calibration of the instrument, kit, or test system has remained stable throughout the laboratory’s reportable range for patient test results. ” [CLSI] • Quality Indicators: Measures (qualitative or quantitative) that indicate the test system and lab operation are functioning as expected
Definitions • Calibration verification. “The assaying of calibration materials in the same manner as patient samples to confirm that the calibration of the instrument, kit, or test system has remained stable throughout the laboratory’s reportable range for patient test results. ” [CLSI] • Quality Indicators: Measures (qualitative or quantitative) that indicate the test system and lab operation are functioning as expected
 Definitions • Laboratory Best-Practices: Utilization of quality policies and procedures to produce the best laboratory outcomes • Process Mapping: Breaking down a process step by step to define what is done, who is responsible, and what might fail or need improvement at each step
Definitions • Laboratory Best-Practices: Utilization of quality policies and procedures to produce the best laboratory outcomes • Process Mapping: Breaking down a process step by step to define what is done, who is responsible, and what might fail or need improvement at each step
 Definitions • Mean : Common expression for the mean of a statistical distribution with a discrete random variable is the mathematical average of all the terms. To calculate it, add up the values of all the terms and then divide by the number of terms. • Std Deviation: For a measurement procedure, it is generally expected that the distribution of control results will be normal or Gaussian. • Coefficient of Variation (%CV): Describes the standard deviation as a percentage of the mean, as shown in the following equation: CV = (s / m )100
Definitions • Mean : Common expression for the mean of a statistical distribution with a discrete random variable is the mathematical average of all the terms. To calculate it, add up the values of all the terms and then divide by the number of terms. • Std Deviation: For a measurement procedure, it is generally expected that the distribution of control results will be normal or Gaussian. • Coefficient of Variation (%CV): Describes the standard deviation as a percentage of the mean, as shown in the following equation: CV = (s / m )100
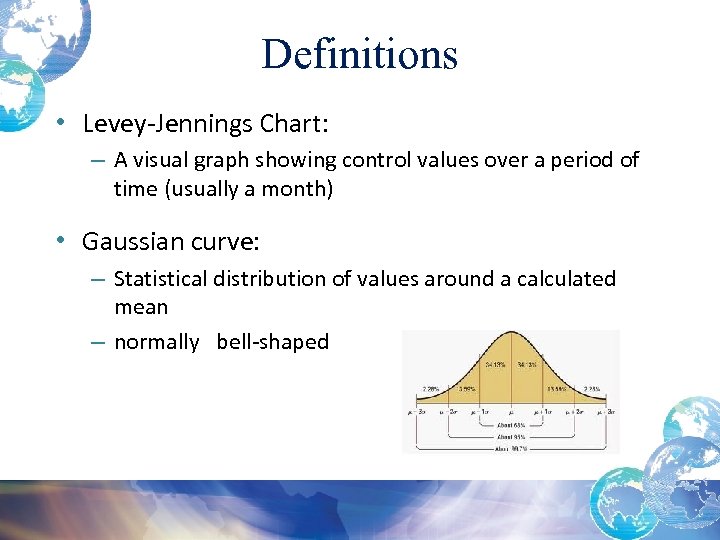 Definitions • Levey-Jennings Chart: – A visual graph showing control values over a period of time (usually a month) • Gaussian curve: – Statistical distribution of values around a calculated mean – normally bell-shaped
Definitions • Levey-Jennings Chart: – A visual graph showing control values over a period of time (usually a month) • Gaussian curve: – Statistical distribution of values around a calculated mean – normally bell-shaped
 Definitions • Control Range: – The accepted values of a control material – Includes a mean and +(-) 3 std dev • Normal or Reference Range: – The values of a specific analyte obtained from individuals considered to be healthy (usually 95% range)
Definitions • Control Range: – The accepted values of a control material – Includes a mean and +(-) 3 std dev • Normal or Reference Range: – The values of a specific analyte obtained from individuals considered to be healthy (usually 95% range)
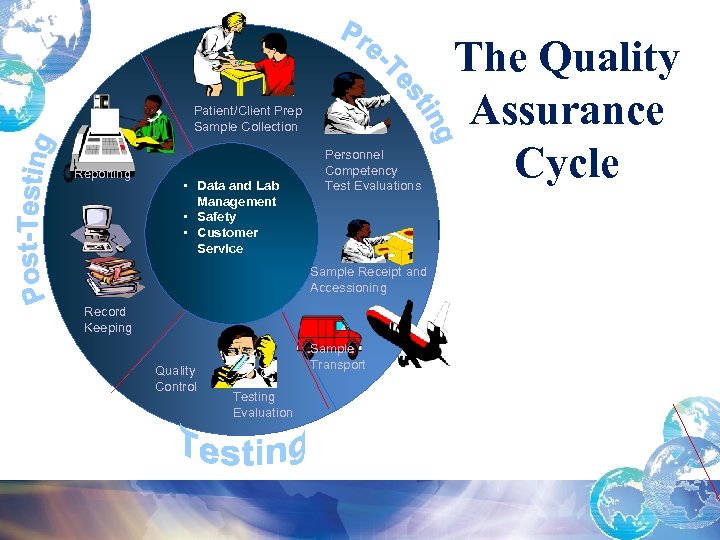 Patient/Client Prep Sample Collection Reporting • Data and Lab Management • Safety • Customer Service Personnel Competency Test Evaluations Sample Receipt and Accessioning Record Keeping Quality Control Sample Transport Testing Evaluation The Quality Assurance Cycle
Patient/Client Prep Sample Collection Reporting • Data and Lab Management • Safety • Customer Service Personnel Competency Test Evaluations Sample Receipt and Accessioning Record Keeping Quality Control Sample Transport Testing Evaluation The Quality Assurance Cycle
 Purpose of Quality Assurance Benefits in the Laboratory: • Provides the highest quality results for the patient • Quality Assurance: – Assists physicians in patient diagnosis, treatment and the monitoring of treatment – Builds physician trust for the laboratory and prevents complaints - which also motivates staff – Is the most cost-effective in the long run
Purpose of Quality Assurance Benefits in the Laboratory: • Provides the highest quality results for the patient • Quality Assurance: – Assists physicians in patient diagnosis, treatment and the monitoring of treatment – Builds physician trust for the laboratory and prevents complaints - which also motivates staff – Is the most cost-effective in the long run
 Four Key Components of Quality Assurance 1. External quality assessment (EQA) 2. Internal quality control (IQC) 3. Standardization of processes and procedures (preanalytic, analytic and post-analytic phases) 4. Management and 0 rganization (documentation and retention of all records including monitoring Quality Indicators and corrective action).
Four Key Components of Quality Assurance 1. External quality assessment (EQA) 2. Internal quality control (IQC) 3. Standardization of processes and procedures (preanalytic, analytic and post-analytic phases) 4. Management and 0 rganization (documentation and retention of all records including monitoring Quality Indicators and corrective action).
 External Quality Assessment Unknown specimens provided by an outside agency and sent to laboratories for analysis to check the lab’s accuracy. Proficiency Testing • For all tests done on site • Handling rules apply
External Quality Assessment Unknown specimens provided by an outside agency and sent to laboratories for analysis to check the lab’s accuracy. Proficiency Testing • For all tests done on site • Handling rules apply
 Internal Quality Control • All checks and procedures a laboratory regularly carries out to ensure test results are accurate • A well functioning internal assurance scheme results in good performance in External Quality Assessment
Internal Quality Control • All checks and procedures a laboratory regularly carries out to ensure test results are accurate • A well functioning internal assurance scheme results in good performance in External Quality Assessment
 Internal Quality Control: Pre-Analytical Activities Examples: • Specimen Collection and Management
Internal Quality Control: Pre-Analytical Activities Examples: • Specimen Collection and Management
 Internal Quality Control: Analytical Activities Examples: • Instrument calibration • Equipment maintenance • Analysis of known test samples (QC)
Internal Quality Control: Analytical Activities Examples: • Instrument calibration • Equipment maintenance • Analysis of known test samples (QC)
 Internal Quality Control: Post-Analytical Activities Examples: • i. e. crosschecking and validation of results
Internal Quality Control: Post-Analytical Activities Examples: • i. e. crosschecking and validation of results
 Quality Assurance vs. Quality Control • Quality Assurance: Proactive policies and procedures ascribed to in order to create and maintain good quality practices • Quality Control: Procedures regularly practiced throughout the laboratory to verify that procedures, practices and therefore laboratory results are accurate and precise
Quality Assurance vs. Quality Control • Quality Assurance: Proactive policies and procedures ascribed to in order to create and maintain good quality practices • Quality Control: Procedures regularly practiced throughout the laboratory to verify that procedures, practices and therefore laboratory results are accurate and precise
 Differences in QA and QC Quality Assurance Quality Control • Process–oriented • Designed to make sure processes are sufficient to meet objectives • Ensures a product or service is manufactured, implemented, created, or produced in the right way • Concerned with the product • Involves evaluating a product, activity, process, or service • Evaluates whether or not the end result is satisfactory
Differences in QA and QC Quality Assurance Quality Control • Process–oriented • Designed to make sure processes are sufficient to meet objectives • Ensures a product or service is manufactured, implemented, created, or produced in the right way • Concerned with the product • Involves evaluating a product, activity, process, or service • Evaluates whether or not the end result is satisfactory
 Standardization of Processes and Procedures When test processes are performed the same each time, procedure errors are eliminated. This is accomplished by: SOP for all phases of testing: Pre-analytic Analytic Post-analytic
Standardization of Processes and Procedures When test processes are performed the same each time, procedure errors are eliminated. This is accomplished by: SOP for all phases of testing: Pre-analytic Analytic Post-analytic
 Management & Organization • All QC and QA activities should be documented when done. • Corrective action, if needed, should be carried out timely and legibly documented. • Records should be reviewed regularly by the laboratory supervisor and retained within the laboratory’s organized archives for the required retention period.
Management & Organization • All QC and QA activities should be documented when done. • Corrective action, if needed, should be carried out timely and legibly documented. • Records should be reviewed regularly by the laboratory supervisor and retained within the laboratory’s organized archives for the required retention period.
 Analytic Errors During the testing phase errors that may occur can be detected by: • Equipment monitoring • Error codes from analyzers • Quality control sample result rejection signals using selected criteria (Westgard Rules)
Analytic Errors During the testing phase errors that may occur can be detected by: • Equipment monitoring • Error codes from analyzers • Quality control sample result rejection signals using selected criteria (Westgard Rules)
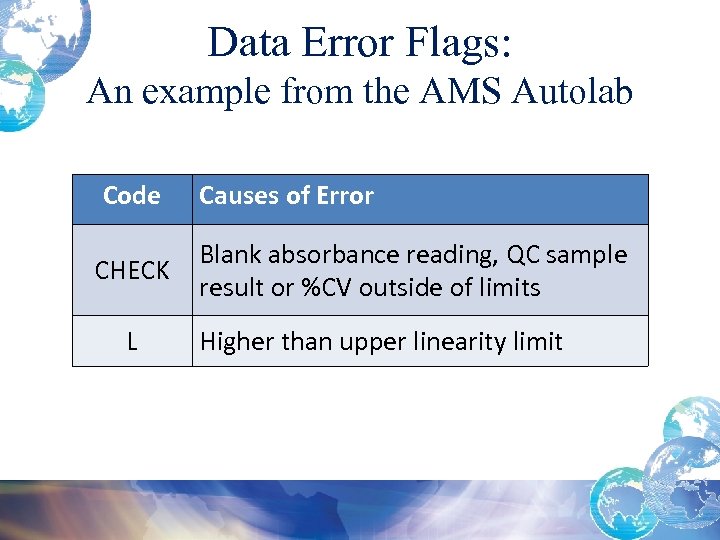 Data Error Flags: An example from the AMS Autolab Code Causes of Error CHECK Blank absorbance reading, QC sample result or %CV outside of limits L Higher than upper linearity limit
Data Error Flags: An example from the AMS Autolab Code Causes of Error CHECK Blank absorbance reading, QC sample result or %CV outside of limits L Higher than upper linearity limit
 Examples of Quality Control & Quality Assurance Documentation of temperature control Documentation of pipette calibration Quality Control samples analysis Use of Standardized procedures (SOPs) Documentation of competency checks for staff Documentation of preventive maintenance of equipment • Cross check and verification of lab reports • • •
Examples of Quality Control & Quality Assurance Documentation of temperature control Documentation of pipette calibration Quality Control samples analysis Use of Standardized procedures (SOPs) Documentation of competency checks for staff Documentation of preventive maintenance of equipment • Cross check and verification of lab reports • • •
 IS QUALITY CONTROL and QUALITY ASSURANCE IMPORTANT?
IS QUALITY CONTROL and QUALITY ASSURANCE IMPORTANT?
 Use of QC Materials • To monitor the analytical test system by running DAILY QC • Acceptance versus rejection of patient results • 2 or 3 control materials – Normal, high and/or low
Use of QC Materials • To monitor the analytical test system by running DAILY QC • Acceptance versus rejection of patient results • 2 or 3 control materials – Normal, high and/or low
 Post-Analytic Errors • Wrong reference ranges • Not reporting in a timely manner • Not using correct method of reporting • Not retaining record of patient results and report • Not crosschecking for transposition or clerical errors • No validation of results for illogical or incompatible results
Post-Analytic Errors • Wrong reference ranges • Not reporting in a timely manner • Not using correct method of reporting • Not retaining record of patient results and report • Not crosschecking for transposition or clerical errors • No validation of results for illogical or incompatible results
 Reporting Patient Results • Use Correct Format – Proper alignment, – Placement of decimal points and zeros • Legible if handwritten • Maintain Confidentiality • Retain Records
Reporting Patient Results • Use Correct Format – Proper alignment, – Placement of decimal points and zeros • Legible if handwritten • Maintain Confidentiality • Retain Records
 Interactive Opportunity • Who is responsible for QA and QC? • What is External Quality Assessment? • Why is this important? • How should EQA specimens be handled? • Name the aspects of the analytical testing phase that can cause inaccurate results but would be revealed with unacceptable QC sample results?
Interactive Opportunity • Who is responsible for QA and QC? • What is External Quality Assessment? • Why is this important? • How should EQA specimens be handled? • Name the aspects of the analytical testing phase that can cause inaccurate results but would be revealed with unacceptable QC sample results?
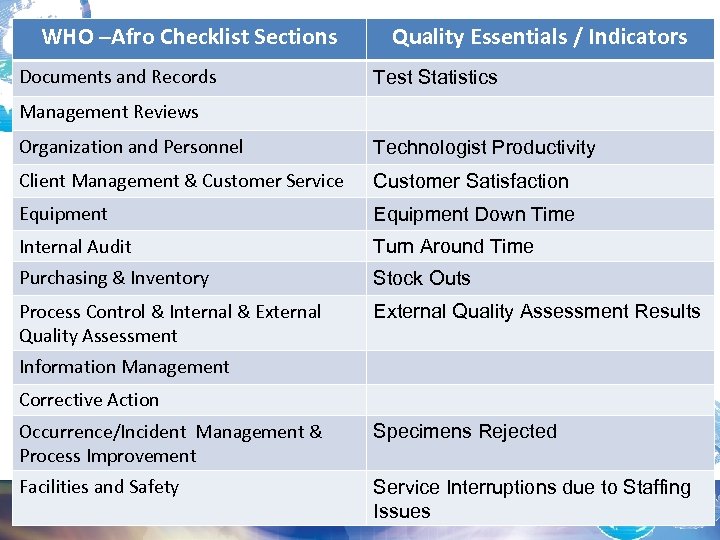 WHO –Afro Checklist Sections Documents and Records Quality Essentials / Indicators Test Statistics Management Reviews Organization and Personnel Technologist Productivity Client Management & Customer Service Customer Satisfaction Equipment Down Time Internal Audit Turn Around Time Purchasing & Inventory Stock Outs Process Control & Internal & External Quality Assessment Results Information Management Corrective Action Occurrence/Incident Management & Process Improvement Specimens Rejected Facilities and Safety Service Interruptions due to Staffing Issues
WHO –Afro Checklist Sections Documents and Records Quality Essentials / Indicators Test Statistics Management Reviews Organization and Personnel Technologist Productivity Client Management & Customer Service Customer Satisfaction Equipment Down Time Internal Audit Turn Around Time Purchasing & Inventory Stock Outs Process Control & Internal & External Quality Assessment Results Information Management Corrective Action Occurrence/Incident Management & Process Improvement Specimens Rejected Facilities and Safety Service Interruptions due to Staffing Issues
 Lab Exercise: Quality Control and Construction Levey-Jennings Charts of
Lab Exercise: Quality Control and Construction Levey-Jennings Charts of
 Levy-Jennings Activity Objectives At the completion of this activity, you will be able to: 1. 2. 3. 4. Perform statistical analysis on analyte values from the previous month’s control values to determine Mean, Std Dev, %CV. Draw a Levy-Jennings chart, label the x and y axis correctly, and properly label the LJ chart for documentation and filing. Plot current month’s values daily and evaluate LJ chart for Westgard violations, trends, shifts. Discuss the necessity of Quality Control and how they assess precision and accuracy
Levy-Jennings Activity Objectives At the completion of this activity, you will be able to: 1. 2. 3. 4. Perform statistical analysis on analyte values from the previous month’s control values to determine Mean, Std Dev, %CV. Draw a Levy-Jennings chart, label the x and y axis correctly, and properly label the LJ chart for documentation and filing. Plot current month’s values daily and evaluate LJ chart for Westgard violations, trends, shifts. Discuss the necessity of Quality Control and how they assess precision and accuracy
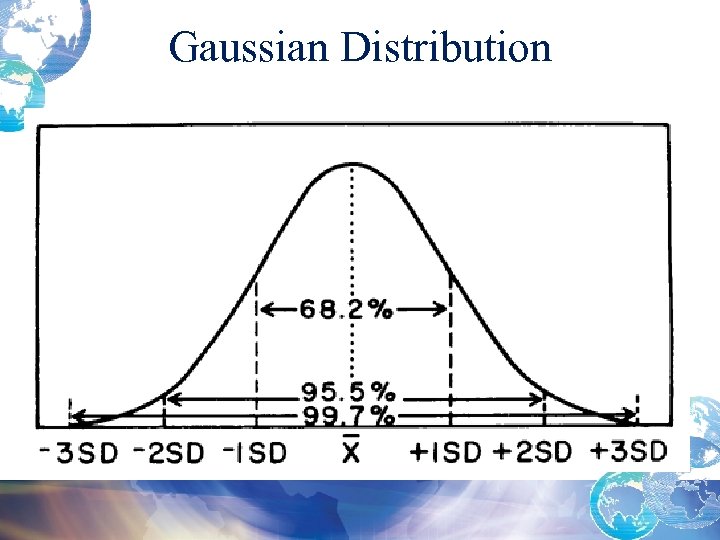 Gaussian Distribution
Gaussian Distribution
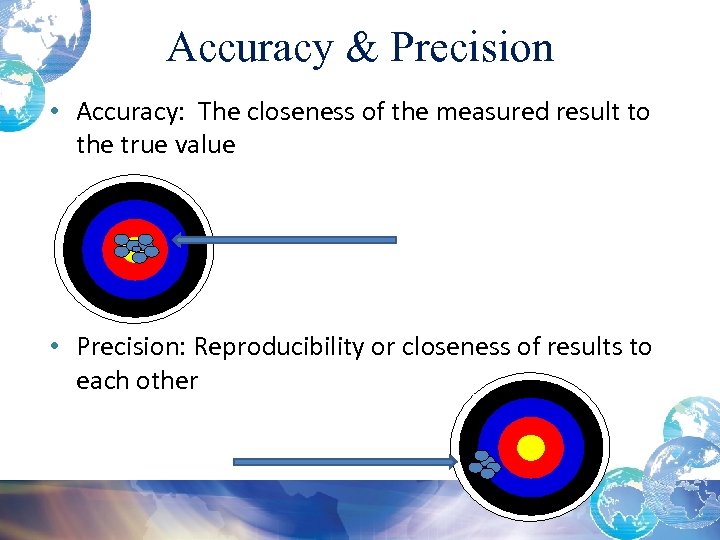 Accuracy & Precision • Accuracy: The closeness of the measured result to the true value • Precision: Reproducibility or closeness of results to each other
Accuracy & Precision • Accuracy: The closeness of the measured result to the true value • Precision: Reproducibility or closeness of results to each other
 Inaccurate and Imprecise
Inaccurate and Imprecise
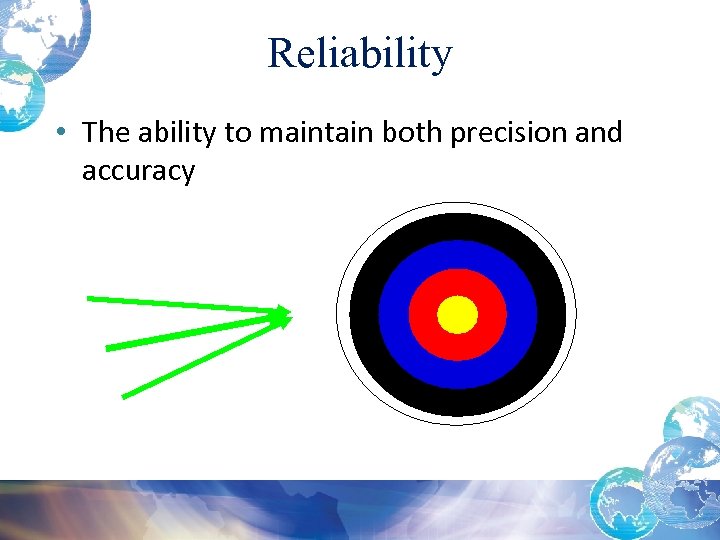 Reliability • The ability to maintain both precision and accuracy
Reliability • The ability to maintain both precision and accuracy
 Quality Control • Quality control is used to test the analytical phase of patient testing • It is a process or system for monitoring the quality of laboratory testing, and the accuracy and precision of results
Quality Control • Quality control is used to test the analytical phase of patient testing • It is a process or system for monitoring the quality of laboratory testing, and the accuracy and precision of results
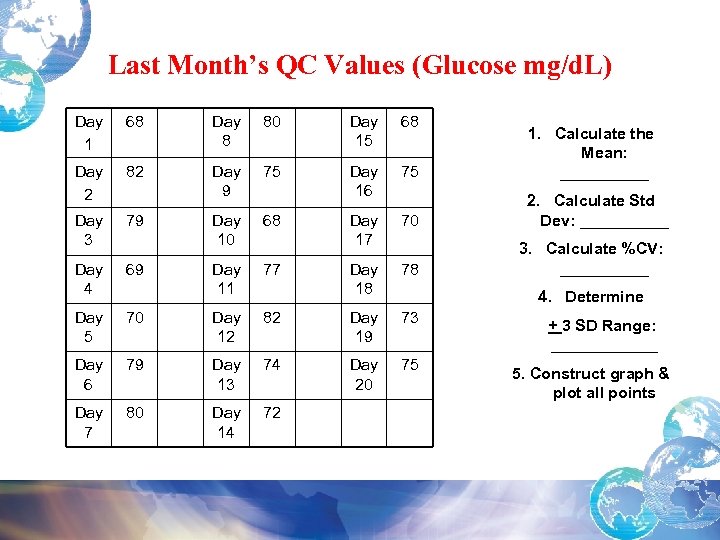 Last Month’s QC Values (Glucose mg/d. L) Day 1 68 Day 8 80 Day 15 68 Day 2 82 Day 9 75 Day 16 75 1. Calculate the Mean: _____ Day 3 79 Day 10 68 Day 17 70 2. Calculate Std Dev: _____ Day 4 69 Day 11 77 Day 18 78 Day 5 70 Day 12 82 Day 19 73 Day 6 79 Day 13 74 Day 20 75 Day 7 80 Day 14 72 3. Calculate %CV: _____ 4. Determine + 3 SD Range: ______ 5. Construct graph & plot all points
Last Month’s QC Values (Glucose mg/d. L) Day 1 68 Day 8 80 Day 15 68 Day 2 82 Day 9 75 Day 16 75 1. Calculate the Mean: _____ Day 3 79 Day 10 68 Day 17 70 2. Calculate Std Dev: _____ Day 4 69 Day 11 77 Day 18 78 Day 5 70 Day 12 82 Day 19 73 Day 6 79 Day 13 74 Day 20 75 Day 7 80 Day 14 72 3. Calculate %CV: _____ 4. Determine + 3 SD Range: ______ 5. Construct graph & plot all points
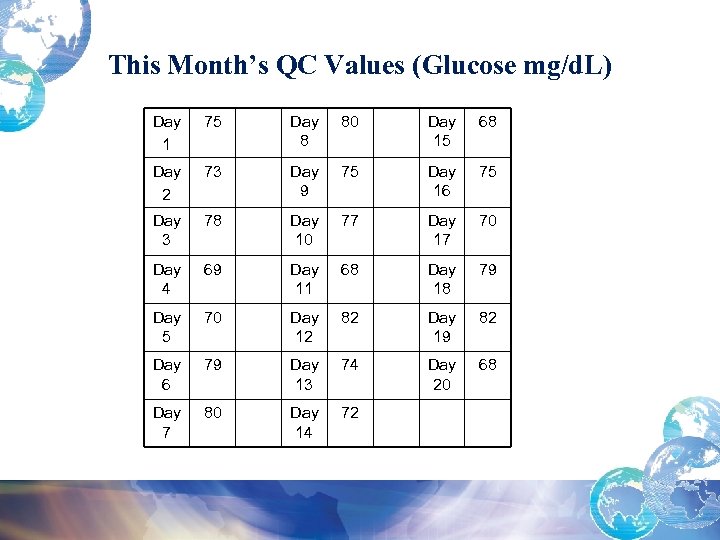 This Month’s QC Values (Glucose mg/d. L) Day 1 75 Day 8 80 Day 15 68 Day 2 73 Day 9 75 Day 16 75 Day 3 78 Day 10 77 Day 17 70 Day 4 69 Day 11 68 Day 18 79 Day 5 70 Day 12 82 Day 19 82 Day 6 79 Day 13 74 Day 20 68 Day 7 80 Day 14 72
This Month’s QC Values (Glucose mg/d. L) Day 1 75 Day 8 80 Day 15 68 Day 2 73 Day 9 75 Day 16 75 Day 3 78 Day 10 77 Day 17 70 Day 4 69 Day 11 68 Day 18 79 Day 5 70 Day 12 82 Day 19 82 Day 6 79 Day 13 74 Day 20 68 Day 7 80 Day 14 72
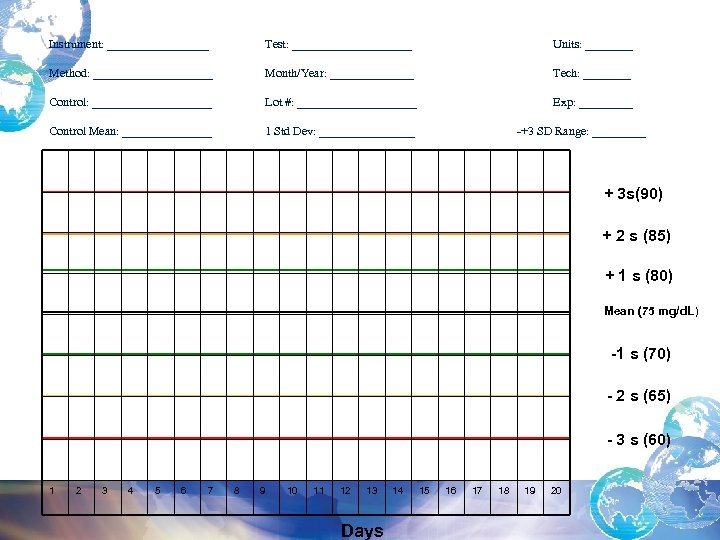 Instrument: _________ Test: __________ Units: ____ Method: __________ Month/Year: _______ Tech: ____ Control: __________ Lot #: __________ Exp: _____ Control Mean: ________ 1 Std Dev: ________ -+3 SD Range: _____ + 3 s(90) + 2 s (85) + 1 s (80) Mean (75 mg/d. L) -1 s (70) - 2 s (65) - 3 s (60) 1 2 3 4 5 6 7 8 9 10 11 12 13 Days 14 15 16 17 18 19 20
Instrument: _________ Test: __________ Units: ____ Method: __________ Month/Year: _______ Tech: ____ Control: __________ Lot #: __________ Exp: _____ Control Mean: ________ 1 Std Dev: ________ -+3 SD Range: _____ + 3 s(90) + 2 s (85) + 1 s (80) Mean (75 mg/d. L) -1 s (70) - 2 s (65) - 3 s (60) 1 2 3 4 5 6 7 8 9 10 11 12 13 Days 14 15 16 17 18 19 20
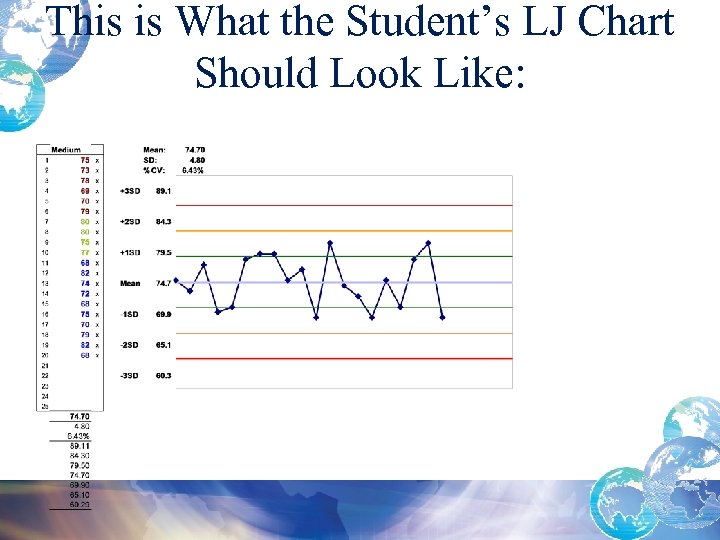 This is What the Student’s LJ Chart Should Look Like:
This is What the Student’s LJ Chart Should Look Like:


