лекция_Миняев.ppt
- Количество слайдов: 28
 Introduction to Lanthanide Chemistry Dr. Mikhail Minyaev
Introduction to Lanthanide Chemistry Dr. Mikhail Minyaev
 Rare-earth metals Sc Y La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Abundance: widespread, 0. 015% in earth crust (except Pm) Pm is an artificial element, radioactive. Sc, Lu, Tm are more or less “rare” elements, but almost all “rare-earth metals” are more abundant than Pt, Hg, Ag ! Why all of them are called rare-earth metals? Sc, Y and lanthanides are very dispersed over earth crust and are not easy to separate. 2
Rare-earth metals Sc Y La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Abundance: widespread, 0. 015% in earth crust (except Pm) Pm is an artificial element, radioactive. Sc, Lu, Tm are more or less “rare” elements, but almost all “rare-earth metals” are more abundant than Pt, Hg, Ag ! Why all of them are called rare-earth metals? Sc, Y and lanthanides are very dispersed over earth crust and are not easy to separate. 2
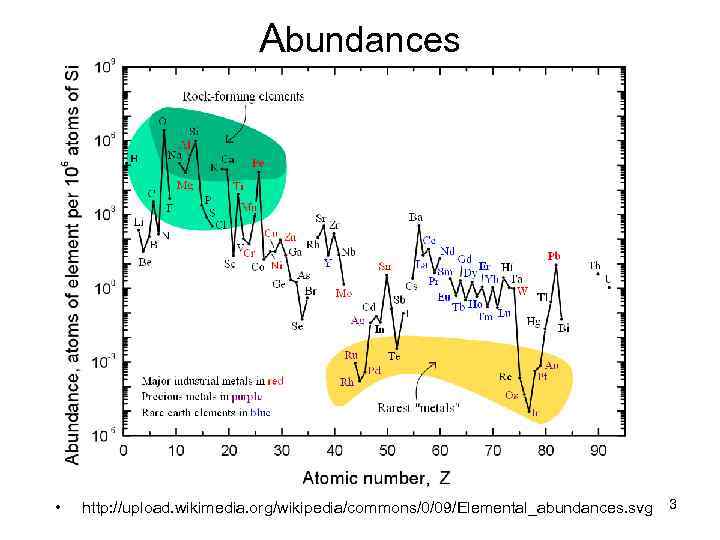 Abundances • http: //upload. wikimedia. org/wikipedia/commons/0/09/Elemental_abundances. svg 3
Abundances • http: //upload. wikimedia. org/wikipedia/commons/0/09/Elemental_abundances. svg 3
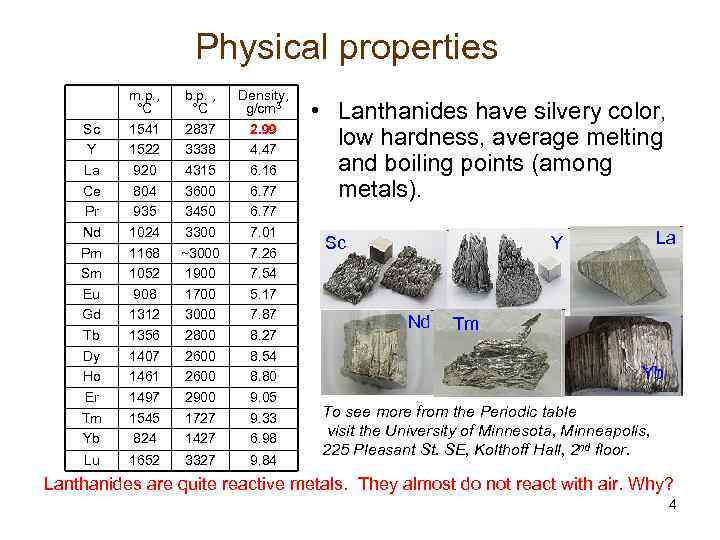 Physical properties Sc Y La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb m. p. , °C 1541 1522 920 804 935 1024 1168 1052 908 1312 1356 1407 1461 1497 1545 824 b. p. , °C 2837 3338 4315 3600 3450 3300 ~3000 1900 1700 3000 2800 2600 2900 1727 1427 Density, g/cm 3 2. 99 4. 47 6. 16 6. 77 7. 01 7. 26 7. 54 5. 17 7. 87 8. 27 8. 54 8. 80 9. 05 9. 33 6. 98 Lu 1652 3327 9. 84 • Lanthanides have silvery color, low hardness, average melting and boiling points (among metals). La Y Sc Nd Tm Yb To see more from the Periodic table visit the University of Minnesota, Minneapolis, 225 Pleasant St. SE, Kolthoff Hall, 2 nd floor. Lanthanides are quite reactive metals. They almost do not react with air. Why? 4
Physical properties Sc Y La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb m. p. , °C 1541 1522 920 804 935 1024 1168 1052 908 1312 1356 1407 1461 1497 1545 824 b. p. , °C 2837 3338 4315 3600 3450 3300 ~3000 1900 1700 3000 2800 2600 2900 1727 1427 Density, g/cm 3 2. 99 4. 47 6. 16 6. 77 7. 01 7. 26 7. 54 5. 17 7. 87 8. 27 8. 54 8. 80 9. 05 9. 33 6. 98 Lu 1652 3327 9. 84 • Lanthanides have silvery color, low hardness, average melting and boiling points (among metals). La Y Sc Nd Tm Yb To see more from the Periodic table visit the University of Minnesota, Minneapolis, 225 Pleasant St. SE, Kolthoff Hall, 2 nd floor. Lanthanides are quite reactive metals. They almost do not react with air. Why? 4
 Some applications rare-earth metals • • Electronics Optics Organic synthesis (as reagents and catalysts) Probes in biochemistry (Ln 3+ is harmless to human body) • Nanoporous material for various applications • New generation catalysts for polymerization so on… • Sc – building light bicycles 5
Some applications rare-earth metals • • Electronics Optics Organic synthesis (as reagents and catalysts) Probes in biochemistry (Ln 3+ is harmless to human body) • Nanoporous material for various applications • New generation catalysts for polymerization so on… • Sc – building light bicycles 5
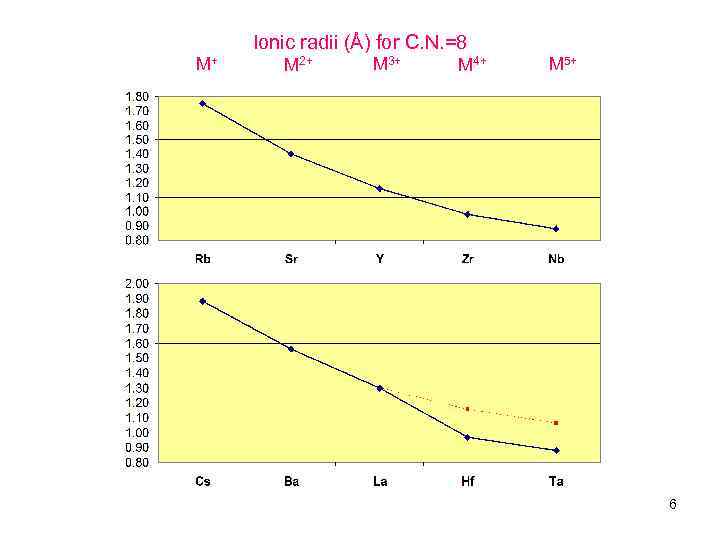 Ionic radii (Å) for C. N. =8 M+ M 2+ M 3+ M 4+ M 5+ 6
Ionic radii (Å) for C. N. =8 M+ M 2+ M 3+ M 4+ M 5+ 6
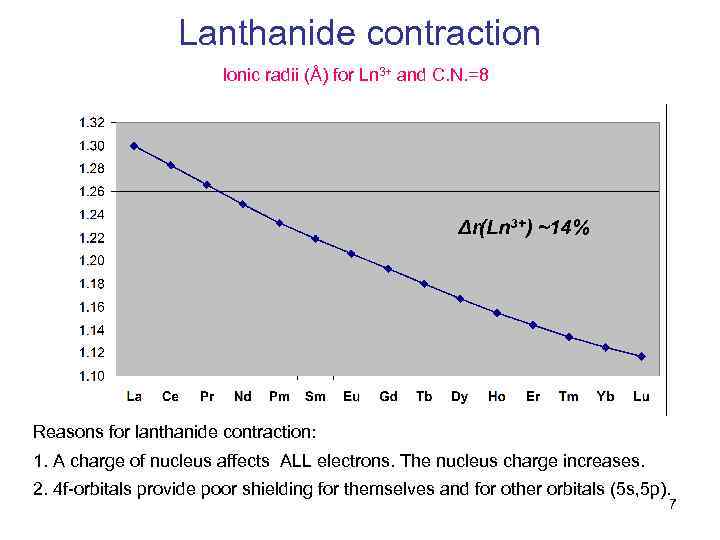 Lanthanide contraction Ionic radii (Å) for Ln 3+ and C. N. =8 Δr(Ln 3+) ~14% Reasons for lanthanide contraction: 1. A charge of nucleus affects ALL electrons. The nucleus charge increases. 2. 4 f-orbitals provide poor shielding for themselves and for other orbitals (5 s, 5 p). 7
Lanthanide contraction Ionic radii (Å) for Ln 3+ and C. N. =8 Δr(Ln 3+) ~14% Reasons for lanthanide contraction: 1. A charge of nucleus affects ALL electrons. The nucleus charge increases. 2. 4 f-orbitals provide poor shielding for themselves and for other orbitals (5 s, 5 p). 7
 The difference in size of Ln 3+ is c. a. 14% (small), including Y 3+ has an ionic radius of 1. 16Å (C. N. =8) (like the size of Ho 3+). Just compare: r(Li+) – 1. 06 Å, r(Na+) – 1. 32Å, r(К+) – 1. 65 Å (C. N. =8) The differences in ionic radii between Li+ and Na+ or between Na+ and K+ are 20% (!) – more than for lanthanides. r(Mg 2+) – 1. 03 Å, r(Ca 2+) – 1. 26 Å, r(Sr 2+) – 1. 40 Å (C. N. =8) All ionic radius values were taken from Acta crystallographyca, 1976, A 32, pp. 751 -767, Supplementary data. Up to middle 1950 -s, lanthanides are consider to be (1) very similar to each other (14% differences in size) (2) and analogous to alkaline-earth metals (Ca-Ba), only bearing charge of 3+ and also forming ionic compounds. 8
The difference in size of Ln 3+ is c. a. 14% (small), including Y 3+ has an ionic radius of 1. 16Å (C. N. =8) (like the size of Ho 3+). Just compare: r(Li+) – 1. 06 Å, r(Na+) – 1. 32Å, r(К+) – 1. 65 Å (C. N. =8) The differences in ionic radii between Li+ and Na+ or between Na+ and K+ are 20% (!) – more than for lanthanides. r(Mg 2+) – 1. 03 Å, r(Ca 2+) – 1. 26 Å, r(Sr 2+) – 1. 40 Å (C. N. =8) All ionic radius values were taken from Acta crystallographyca, 1976, A 32, pp. 751 -767, Supplementary data. Up to middle 1950 -s, lanthanides are consider to be (1) very similar to each other (14% differences in size) (2) and analogous to alkaline-earth metals (Ca-Ba), only bearing charge of 3+ and also forming ionic compounds. 8
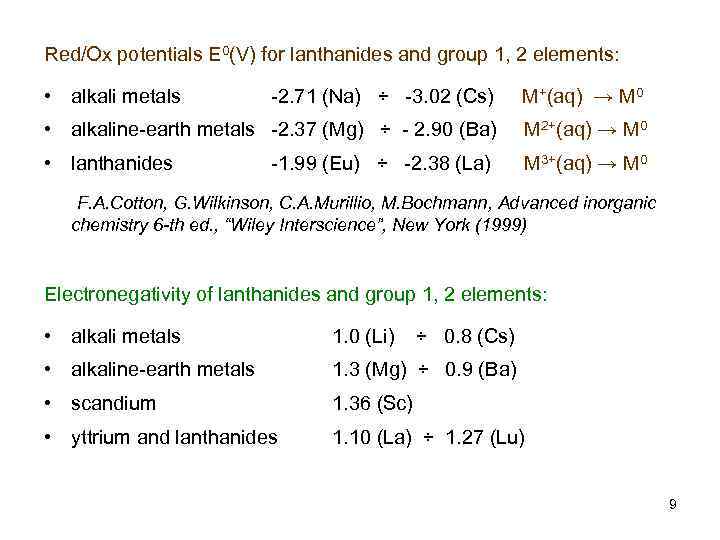 Red/Ox potentials E 0(V) for lanthanides and group 1, 2 elements: • alkali metals -2. 71 (Na) ÷ -3. 02 (Cs) M+(aq) → M 0 • alkaline-earth metals -2. 37 (Mg) ÷ - 2. 90 (Ba) M 2+(aq) → M 0 • lanthanides M 3+(aq) → M 0 -1. 99 (Eu) ÷ -2. 38 (La) F. A. Cotton, G. Wilkinson, C. A. Murillio, M. Bochmann, Advanced inorganic chemistry 6 -th ed. , “Wiley Interscience”, New York (1999) Electronegativity of lanthanides and group 1, 2 elements: • alkali metals 1. 0 (Li) • alkaline-earth metals 1. 3 (Mg) ÷ 0. 9 (Ba) • scandium 1. 36 (Sc) • yttrium and lanthanides 1. 10 (La) ÷ 1. 27 (Lu) ÷ 0. 8 (Cs) 9
Red/Ox potentials E 0(V) for lanthanides and group 1, 2 elements: • alkali metals -2. 71 (Na) ÷ -3. 02 (Cs) M+(aq) → M 0 • alkaline-earth metals -2. 37 (Mg) ÷ - 2. 90 (Ba) M 2+(aq) → M 0 • lanthanides M 3+(aq) → M 0 -1. 99 (Eu) ÷ -2. 38 (La) F. A. Cotton, G. Wilkinson, C. A. Murillio, M. Bochmann, Advanced inorganic chemistry 6 -th ed. , “Wiley Interscience”, New York (1999) Electronegativity of lanthanides and group 1, 2 elements: • alkali metals 1. 0 (Li) • alkaline-earth metals 1. 3 (Mg) ÷ 0. 9 (Ba) • scandium 1. 36 (Sc) • yttrium and lanthanides 1. 10 (La) ÷ 1. 27 (Lu) ÷ 0. 8 (Cs) 9
 • Lanthanides have common oxidation state +3 • • • Consider Ce. I 2 and La. I 2 salts. They conduct electricity. Formal oxidation state +2 ? Ce 3+(I-)2·ē and La 3+(I-)2·ē Metallic phases. Oxidation state +3 • However, Eu and Yb metals dissolve in liquid ammonia like aklali and alkaline-earth metals: M 0 M 2+ + 2ē Stable configurations: Eu 2+ - 5 d 04 f 7, Yb 2+ - 5 d 04 f 14 10
• Lanthanides have common oxidation state +3 • • • Consider Ce. I 2 and La. I 2 salts. They conduct electricity. Formal oxidation state +2 ? Ce 3+(I-)2·ē and La 3+(I-)2·ē Metallic phases. Oxidation state +3 • However, Eu and Yb metals dissolve in liquid ammonia like aklali and alkaline-earth metals: M 0 M 2+ + 2ē Stable configurations: Eu 2+ - 5 d 04 f 7, Yb 2+ - 5 d 04 f 14 10
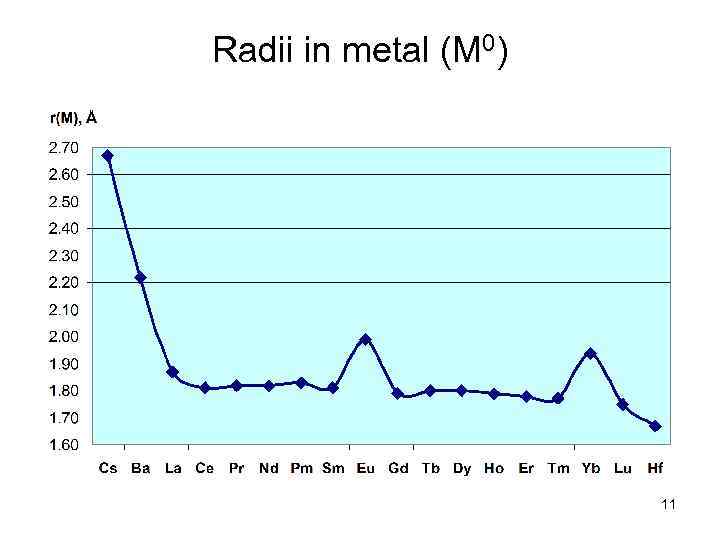 Radii in metal (M 0) 11
Radii in metal (M 0) 11
 Common oxidation states • Sc, Y, La-Lu -- Ln 3+ (in water and other solvents) • Eu 2+ , Yb 2+, Sm 2+ (non-aqueous media) Reducing agents • Ce 4+ (only with inorganic ligands such as NO 3 -, SO 42 -, F-, and with few organic ligands acac- (CH 3 COCH 3 -), but not RCOO-) Very strong oxidizing agent Other oxidation states are very uncommon. 12
Common oxidation states • Sc, Y, La-Lu -- Ln 3+ (in water and other solvents) • Eu 2+ , Yb 2+, Sm 2+ (non-aqueous media) Reducing agents • Ce 4+ (only with inorganic ligands such as NO 3 -, SO 42 -, F-, and with few organic ligands acac- (CH 3 COCH 3 -), but not RCOO-) Very strong oxidizing agent Other oxidation states are very uncommon. 12
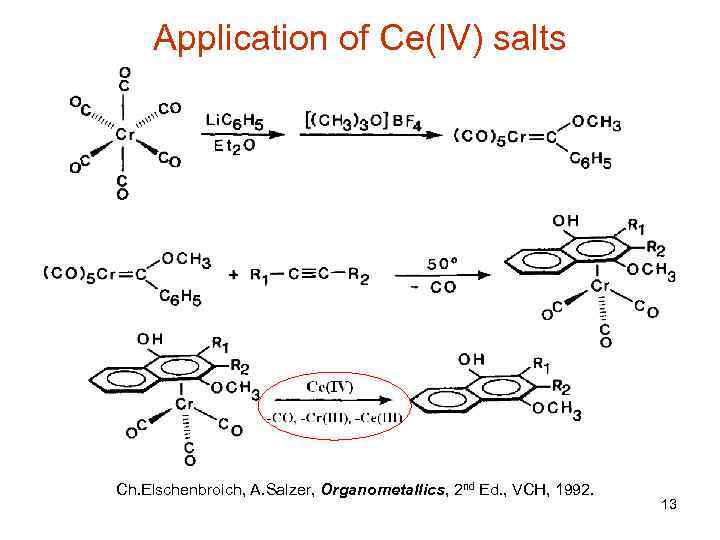 Application of Ce(IV) salts Ch. Elschenbroich, A. Salzer, Organometallics, 2 nd Ed. , VCH, 1992. 13
Application of Ce(IV) salts Ch. Elschenbroich, A. Salzer, Organometallics, 2 nd Ed. , VCH, 1992. 13
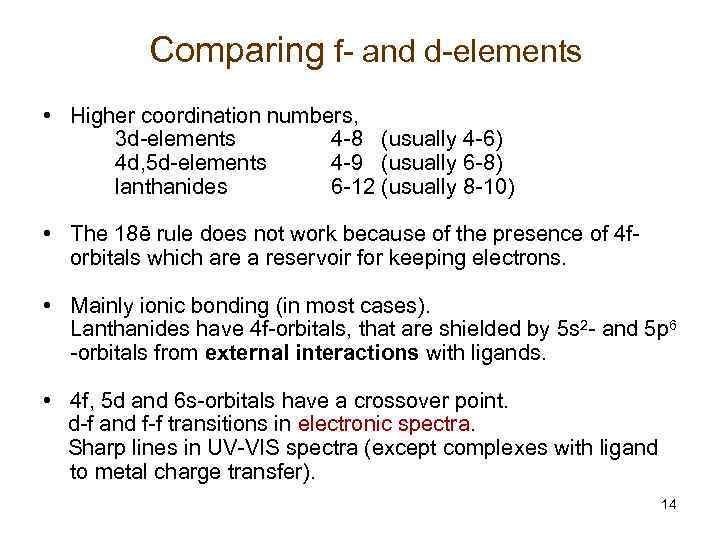 Comparing f- and d-elements • Higher coordination numbers, 3 d-elements 4 -8 (usually 4 -6) 4 d, 5 d-elements 4 -9 (usually 6 -8) lanthanides 6 -12 (usually 8 -10) • The 18ē rule does not work because of the presence of 4 forbitals which are a reservoir for keeping electrons. • Mainly ionic bonding (in most cases). Lanthanides have 4 f-orbitals, that are shielded by 5 s 2 - and 5 p 6 -orbitals from external interactions with ligands. • 4 f, 5 d and 6 s-orbitals have a crossover point. d-f and f-f transitions in electronic spectra. Sharp lines in UV-VIS spectra (except complexes with ligand to metal charge transfer). 14
Comparing f- and d-elements • Higher coordination numbers, 3 d-elements 4 -8 (usually 4 -6) 4 d, 5 d-elements 4 -9 (usually 6 -8) lanthanides 6 -12 (usually 8 -10) • The 18ē rule does not work because of the presence of 4 forbitals which are a reservoir for keeping electrons. • Mainly ionic bonding (in most cases). Lanthanides have 4 f-orbitals, that are shielded by 5 s 2 - and 5 p 6 -orbitals from external interactions with ligands. • 4 f, 5 d and 6 s-orbitals have a crossover point. d-f and f-f transitions in electronic spectra. Sharp lines in UV-VIS spectra (except complexes with ligand to metal charge transfer). 14
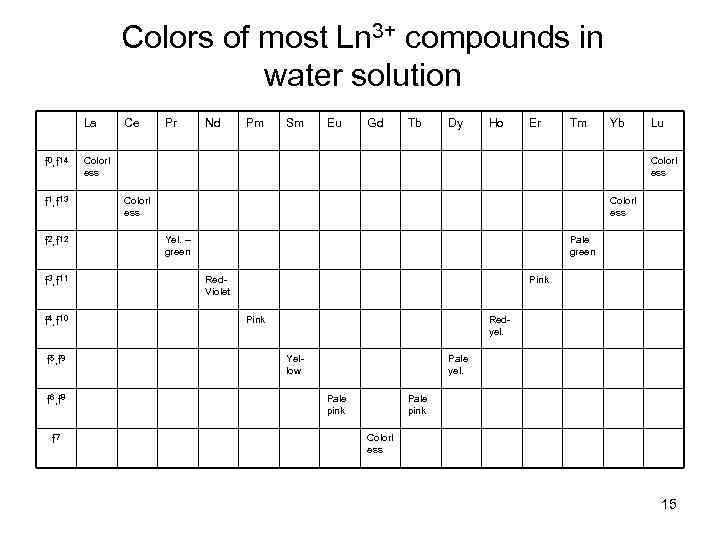 Colors of most Ln 3+ compounds in water solution La f 0, f 14 f 1, f 13 f 2, f 12 f 3, f 11 f 4, f 10 f 5, f 9 f 6, f 8 f 7 Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Colorl ess Lu Colorl ess Yel. – green Pale green Red. Violet Pink Redyel. Yellow Pale yel. Pale pink Colorl ess 15
Colors of most Ln 3+ compounds in water solution La f 0, f 14 f 1, f 13 f 2, f 12 f 3, f 11 f 4, f 10 f 5, f 9 f 6, f 8 f 7 Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Colorl ess Lu Colorl ess Yel. – green Pale green Red. Violet Pink Redyel. Yellow Pale yel. Pale pink Colorl ess 15
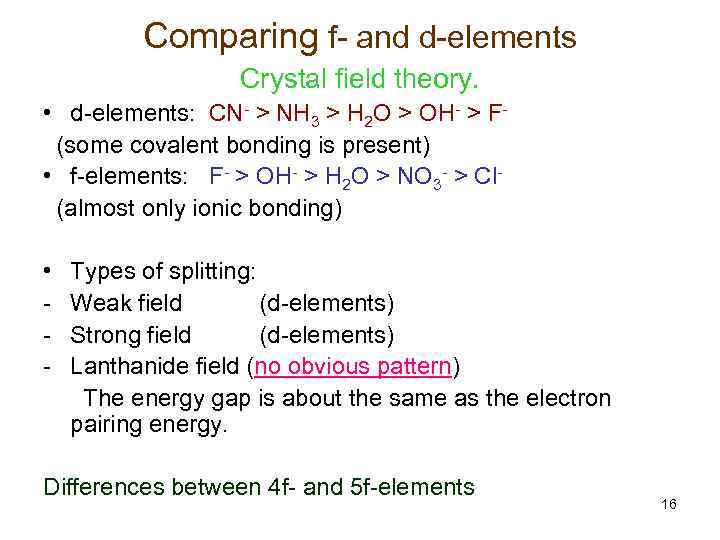 Comparing f- and d-elements Crystal field theory. • d-elements: CN- > NH 3 > H 2 O > OH- > F(some covalent bonding is present) • f-elements: F- > OH- > H 2 O > NO 3 - > Cl(almost only ionic bonding) • - Types of splitting: Weak field (d-elements) Strong field (d-elements) Lanthanide field (no obvious pattern) The energy gap is about the same as the electron pairing energy. Differences between 4 f- and 5 f-elements 16
Comparing f- and d-elements Crystal field theory. • d-elements: CN- > NH 3 > H 2 O > OH- > F(some covalent bonding is present) • f-elements: F- > OH- > H 2 O > NO 3 - > Cl(almost only ionic bonding) • - Types of splitting: Weak field (d-elements) Strong field (d-elements) Lanthanide field (no obvious pattern) The energy gap is about the same as the electron pairing energy. Differences between 4 f- and 5 f-elements 16
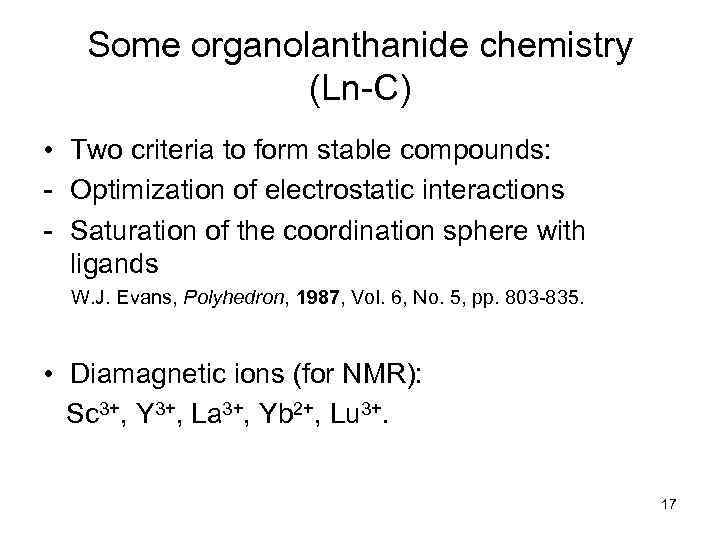 Some organolanthanide chemistry (Ln-C) • Two criteria to form stable compounds: - Optimization of electrostatic interactions - Saturation of the coordination sphere with ligands W. J. Evans, Polyhedron, 1987, Vol. 6, No. 5, pp. 803 -835. • Diamagnetic ions (for NMR): Sc 3+, Y 3+, La 3+, Yb 2+, Lu 3+. 17
Some organolanthanide chemistry (Ln-C) • Two criteria to form stable compounds: - Optimization of electrostatic interactions - Saturation of the coordination sphere with ligands W. J. Evans, Polyhedron, 1987, Vol. 6, No. 5, pp. 803 -835. • Diamagnetic ions (for NMR): Sc 3+, Y 3+, La 3+, Yb 2+, Lu 3+. 17
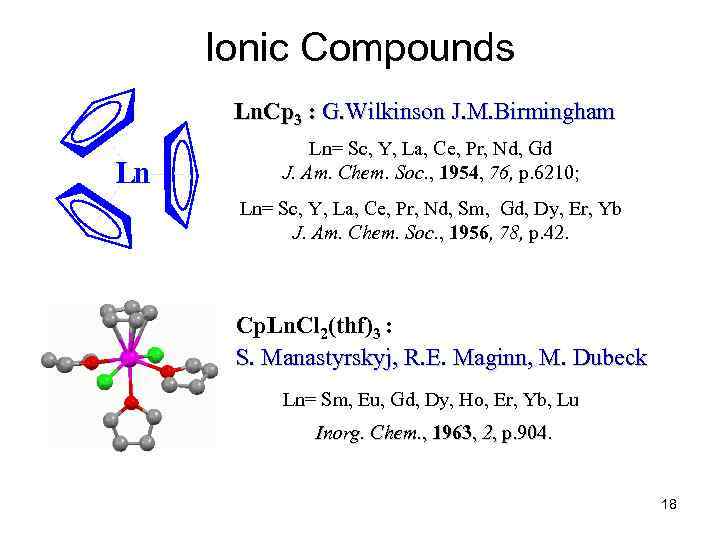 Ionic Compounds Ln. Cp 3 : G. Wilkinson J. M. Birmingham Ln Ln= Sc, Y, La, Ce, Pr, Nd, Gd J. Am. Chem. Soc. , 1954, 76, p. 6210; Ln= Sc, Y, La, Ce, Pr, Nd, Sm, Gd, Dy, Er, Yb J. Am. Chem. Soc. , 1956, 78, p. 42. Cp. Ln. Cl 2(thf)3 : S. Manastyrskyj, R. E. Maginn, M. Dubeck Ln= Sm, Eu, Gd, Dy, Ho, Er, Yb, Lu Inorg. Chem. , 1963, 2, p. 904. 18
Ionic Compounds Ln. Cp 3 : G. Wilkinson J. M. Birmingham Ln Ln= Sc, Y, La, Ce, Pr, Nd, Gd J. Am. Chem. Soc. , 1954, 76, p. 6210; Ln= Sc, Y, La, Ce, Pr, Nd, Sm, Gd, Dy, Er, Yb J. Am. Chem. Soc. , 1956, 78, p. 42. Cp. Ln. Cl 2(thf)3 : S. Manastyrskyj, R. E. Maginn, M. Dubeck Ln= Sm, Eu, Gd, Dy, Ho, Er, Yb, Lu Inorg. Chem. , 1963, 2, p. 904. 18
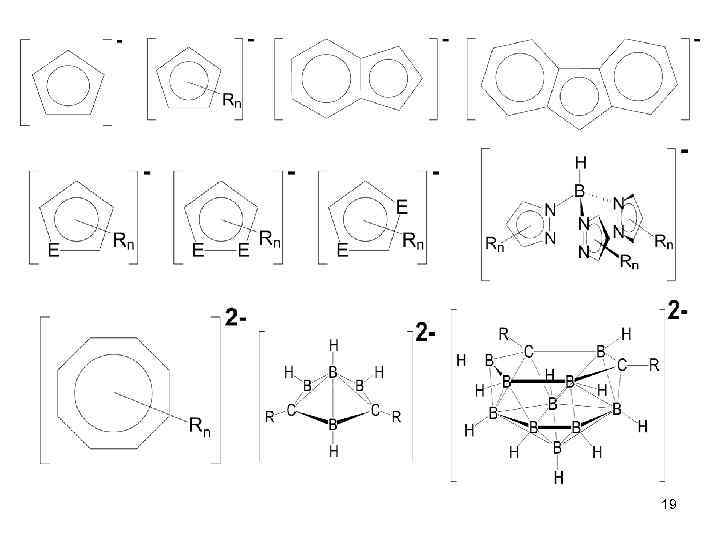 19
19
![[( 5 -C 2 B 9 H 11)2 La(THF)2]- [K(THF)3]2[Eu( 8 -C 8 H [( 5 -C 2 B 9 H 11)2 La(THF)2]- [K(THF)3]2[Eu( 8 -C 8 H](https://present5.com/presentation/7211841_92301838/image-20.jpg) [( 5 -C 2 B 9 H 11)2 La(THF)2]- [K(THF)3]2[Eu( 8 -C 8 H 8)2] ( 8 -C 8 H 8)Tm. I(THF)2 ( 8 -C 8 H 8)Lu( 5 -C 5 Me 5) 20
[( 5 -C 2 B 9 H 11)2 La(THF)2]- [K(THF)3]2[Eu( 8 -C 8 H 8)2] ( 8 -C 8 H 8)Tm. I(THF)2 ( 8 -C 8 H 8)Lu( 5 -C 5 Me 5) 20
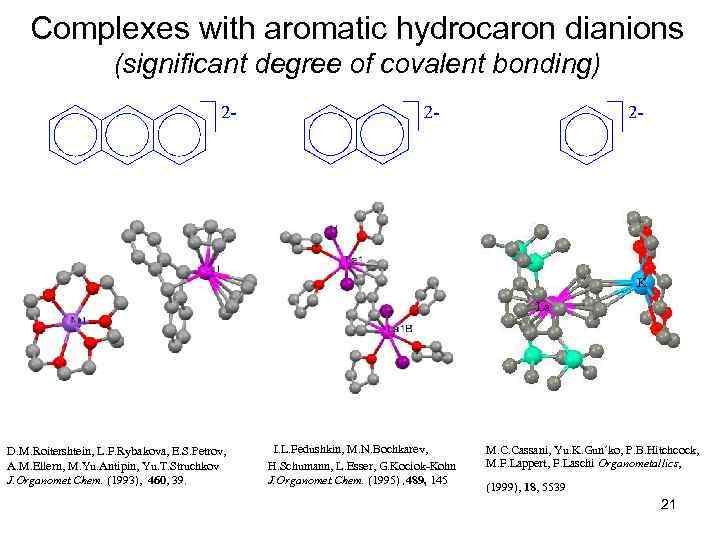 Complexes with aromatic hydrocaron dianions (significant degree of covalent bonding) K La D. M. Roitershtein, L. F. Rybakova, E. S. Petrov, A. M. Ellern, M. Yu. Antipin, Yu. T. Struchkov J. Organomet. Chem. (1993), 460, 39. I. L. Fedushkin, M. N. Bochkarev, H. Schumann, L. Esser, G. Kociok-Kohn J. Organomet. Chem. (1995) , 489, 145 M. C. Cassani, Yu. K. Gun´ko, P. B. Hitchcock, M. F. Lappert, F. Laschi Organometallics, (1999), 18, 5539 21
Complexes with aromatic hydrocaron dianions (significant degree of covalent bonding) K La D. M. Roitershtein, L. F. Rybakova, E. S. Petrov, A. M. Ellern, M. Yu. Antipin, Yu. T. Struchkov J. Organomet. Chem. (1993), 460, 39. I. L. Fedushkin, M. N. Bochkarev, H. Schumann, L. Esser, G. Kociok-Kohn J. Organomet. Chem. (1995) , 489, 145 M. C. Cassani, Yu. K. Gun´ko, P. B. Hitchcock, M. F. Lappert, F. Laschi Organometallics, (1999), 18, 5539 21
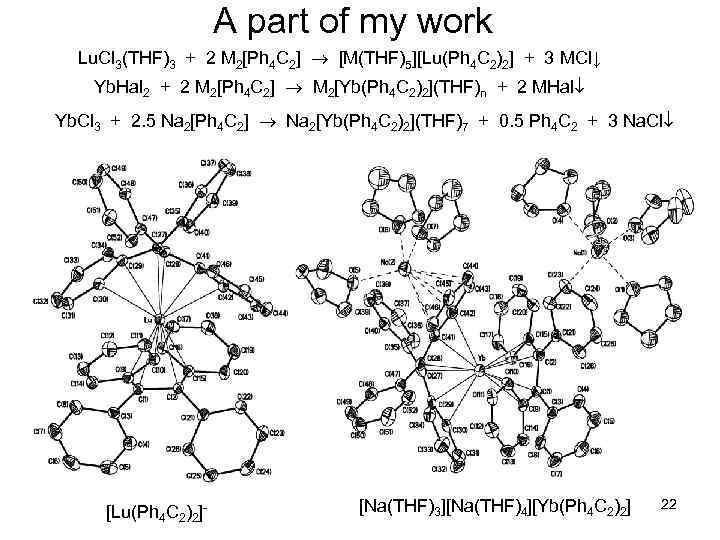 A part of my work Lu. Cl 3(THF)3 + 2 M 2[Ph 4 C 2] [M(THF)5][Lu(Ph 4 C 2)2] + 3 MCl↓ Yb. Hal 2 + 2 M 2[Ph 4 C 2] M 2[Yb(Ph 4 C 2)2](THF)n + 2 MHal Yb. Cl 3 + 2. 5 Na 2[Ph 4 C 2] Na 2[Yb(Ph 4 C 2)2](THF)7 + 0. 5 Ph 4 C 2 + 3 Na. Cl [Lu(Ph 4 C 2)2]- [Na(THF)3][Na(THF)4][Yb(Ph 4 C 2)2] 22
A part of my work Lu. Cl 3(THF)3 + 2 M 2[Ph 4 C 2] [M(THF)5][Lu(Ph 4 C 2)2] + 3 MCl↓ Yb. Hal 2 + 2 M 2[Ph 4 C 2] M 2[Yb(Ph 4 C 2)2](THF)n + 2 MHal Yb. Cl 3 + 2. 5 Na 2[Ph 4 C 2] Na 2[Yb(Ph 4 C 2)2](THF)7 + 0. 5 Ph 4 C 2 + 3 Na. Cl [Lu(Ph 4 C 2)2]- [Na(THF)3][Na(THF)4][Yb(Ph 4 C 2)2] 22
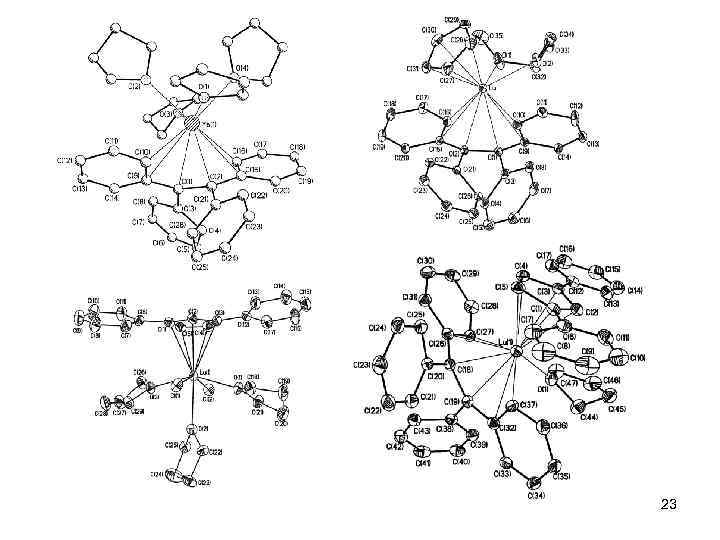 23
23
 24
24
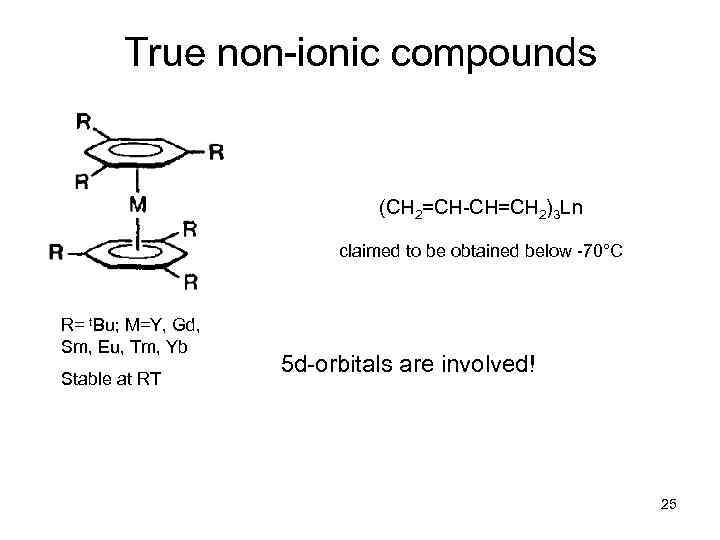 True non-ionic compounds (CH 2=CH-CH=CH 2)3 Ln claimed to be obtained below -70°C R= t. Bu; M=Y, Gd, Sm, Eu, Tm, Yb Stable at RT 5 d-orbitals are involved! 25
True non-ionic compounds (CH 2=CH-CH=CH 2)3 Ln claimed to be obtained below -70°C R= t. Bu; M=Y, Gd, Sm, Eu, Tm, Yb Stable at RT 5 d-orbitals are involved! 25
 Thank you for your attention • The organolanthanide chemistry have had an exploding growth during last 25 -30 years. • There are much to explore! • There is no clear conception of Ln-C bonding yet! 26
Thank you for your attention • The organolanthanide chemistry have had an exploding growth during last 25 -30 years. • There are much to explore! • There is no clear conception of Ln-C bonding yet! 26
 Designing of a new organolanthanide compound 27
Designing of a new organolanthanide compound 27
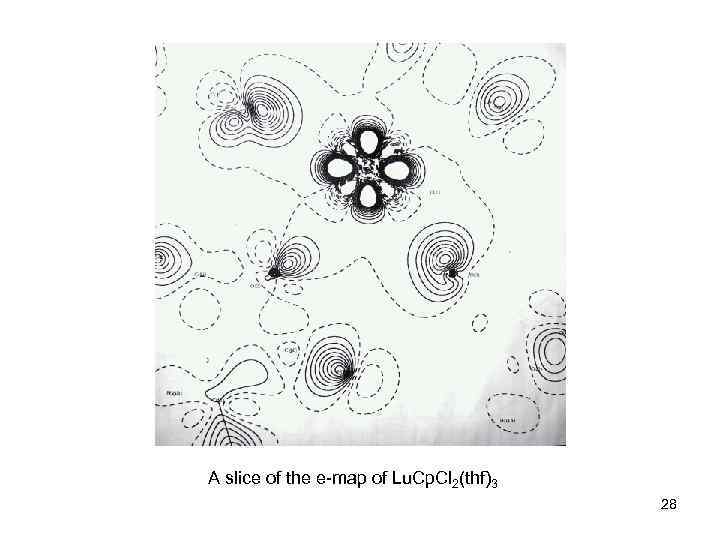 A slice of the e-map of Lu. Cp. Cl 2(thf)3 28
A slice of the e-map of Lu. Cp. Cl 2(thf)3 28


