c40d0d4b86fbaa6127ca4020b40d9112.ppt
- Количество слайдов: 31
 Introduction to Environmental Engineering Dr. Kagan ERYURUK
Introduction to Environmental Engineering Dr. Kagan ERYURUK
 Water Supply and Treatment Why Treat Water? • Human health and the welfare of the general population are improved if public water supplies are treated prior to use. • Nearly all structures require a water supply. • Appropriate flow rate, pressure, and water quality are necessary for effective use.
Water Supply and Treatment Why Treat Water? • Human health and the welfare of the general population are improved if public water supplies are treated prior to use. • Nearly all structures require a water supply. • Appropriate flow rate, pressure, and water quality are necessary for effective use.
 Uses of Water • • Bathing Toilets Cleaning Food preparation Cooling Fire protection Industrial purposes Drinking water = Potable water ©i. Stockphoto. com
Uses of Water • • Bathing Toilets Cleaning Food preparation Cooling Fire protection Industrial purposes Drinking water = Potable water ©i. Stockphoto. com
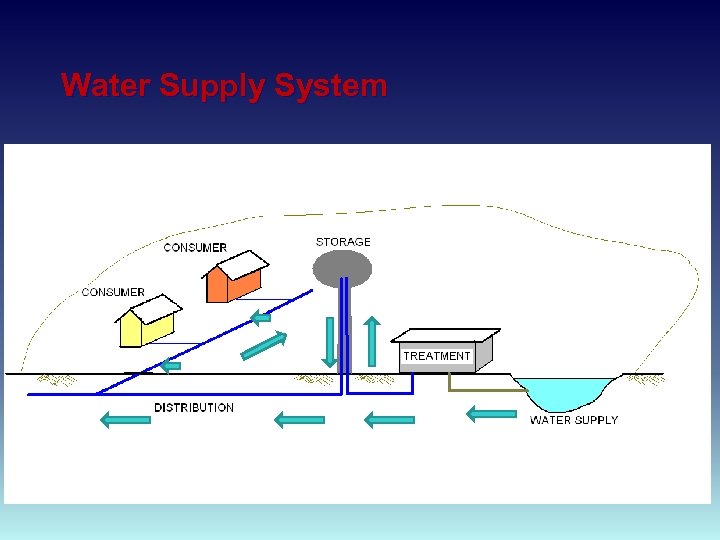 Water Supply System
Water Supply System
 THE HYDROLOGIC CYCLE AND WATER AVAILABILITY Precipitation; applied to all forms of moisture originating in the atmosphere and falling to the ground (e. g. , rain, sleet, and snow). Evaporation and transpiration are the two ways water reenters the atmosphere. Evaporation is loss from free water surfaces while transpiration is loss by plants. Water on the surface of earth that is exposed to the atmosphere is called surface water. Surface waters include rivers, lakes, oceans, etc. Through the process of percolation, some surface water (especially during a precipitation event) seeps into the ground and becomes groundwater. 5
THE HYDROLOGIC CYCLE AND WATER AVAILABILITY Precipitation; applied to all forms of moisture originating in the atmosphere and falling to the ground (e. g. , rain, sleet, and snow). Evaporation and transpiration are the two ways water reenters the atmosphere. Evaporation is loss from free water surfaces while transpiration is loss by plants. Water on the surface of earth that is exposed to the atmosphere is called surface water. Surface waters include rivers, lakes, oceans, etc. Through the process of percolation, some surface water (especially during a precipitation event) seeps into the ground and becomes groundwater. 5
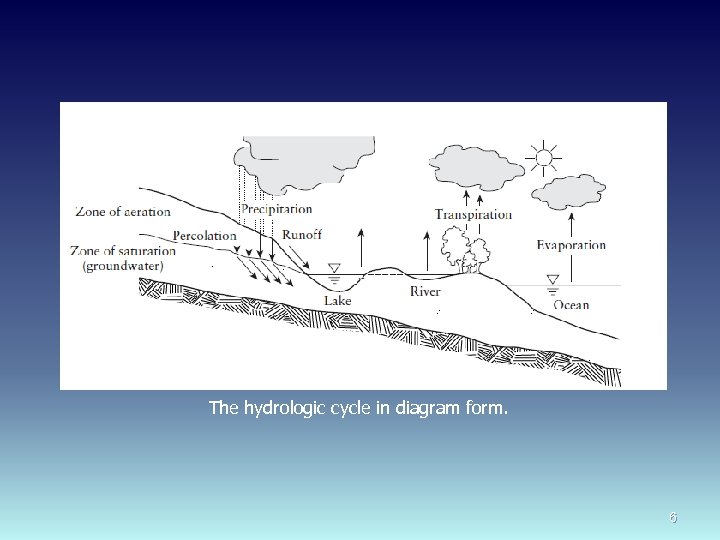 The hydrologic cycle in diagram form. 6
The hydrologic cycle in diagram form. 6
 Sources of Water Groundwater Supplies (Aquifers) • • • Primary source of drinking water Porous consolidated rock or unconsolidated soil Groundwater fills spaces Wells and pumps used to remove water Porosity; The fraction of voids volume to total volume of the soil is termed porosity. This image was reproduced from groundwater. org with the permission of The Groundwater Foundation. © 2010 The Groundwater Foundation. All Rights Reserved 7
Sources of Water Groundwater Supplies (Aquifers) • • • Primary source of drinking water Porous consolidated rock or unconsolidated soil Groundwater fills spaces Wells and pumps used to remove water Porosity; The fraction of voids volume to total volume of the soil is termed porosity. This image was reproduced from groundwater. org with the permission of The Groundwater Foundation. © 2010 The Groundwater Foundation. All Rights Reserved 7
 Sources of Water Surface Water • Lakes, reservoirs, rivers • Rivers dammed to create reservoirs • Reservoirs store water during heavy rain/snow Courtesy USDA http: //www. ks. nrcs. usda. gov/news/highlights/2006_april. html ©i. Stockphoto. com Courtesy NASA http: //www. ghcc. msfc. nasa. gov/surface_hydrology/water_ma nagement. html Lake Tuscaloosa Dam
Sources of Water Surface Water • Lakes, reservoirs, rivers • Rivers dammed to create reservoirs • Reservoirs store water during heavy rain/snow Courtesy USDA http: //www. ks. nrcs. usda. gov/news/highlights/2006_april. html ©i. Stockphoto. com Courtesy NASA http: //www. ghcc. msfc. nasa. gov/surface_hydrology/water_ma nagement. html Lake Tuscaloosa Dam
 Water Treatment • Amount of treatment • depends on quality of the source Ground water requires less treatment than surface water Courtesty USGS http: //pubs. usgs. gov/fs/2004/3069/ The city of Salem water treatment facility withdraws water from the North Santiam River.
Water Treatment • Amount of treatment • depends on quality of the source Ground water requires less treatment than surface water Courtesty USGS http: //pubs. usgs. gov/fs/2004/3069/ The city of Salem water treatment facility withdraws water from the North Santiam River.
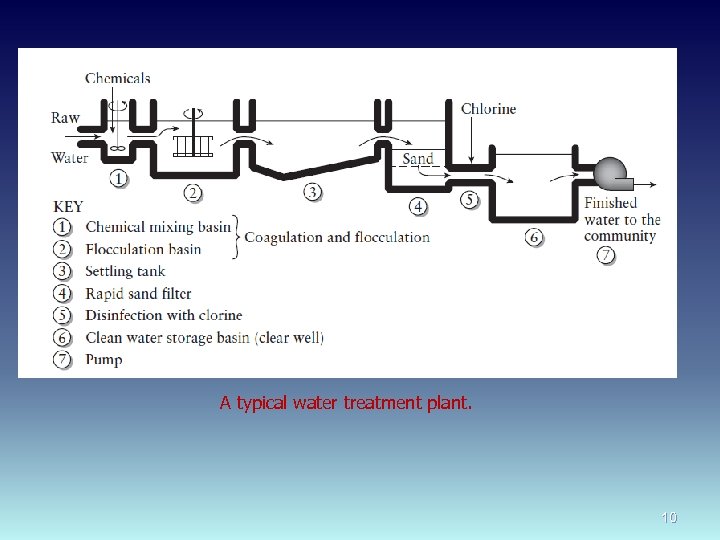 A typical water treatment plant. 10
A typical water treatment plant. 10
 Water Treatment Processes 1. Softening Some waters need hardness removed to use them as a potable water source. Hardness is caused by multivalent cations (or minerals)—such as calcium, magnesium, and iron—that dissolve from soil and rocks (particularly limestone). Not cause health problems but reduces the effectiveness of soaps and cause scale formation. Total Hardness (TH) can be calculated as below. 11
Water Treatment Processes 1. Softening Some waters need hardness removed to use them as a potable water source. Hardness is caused by multivalent cations (or minerals)—such as calcium, magnesium, and iron—that dissolve from soil and rocks (particularly limestone). Not cause health problems but reduces the effectiveness of soaps and cause scale formation. Total Hardness (TH) can be calculated as below. 11
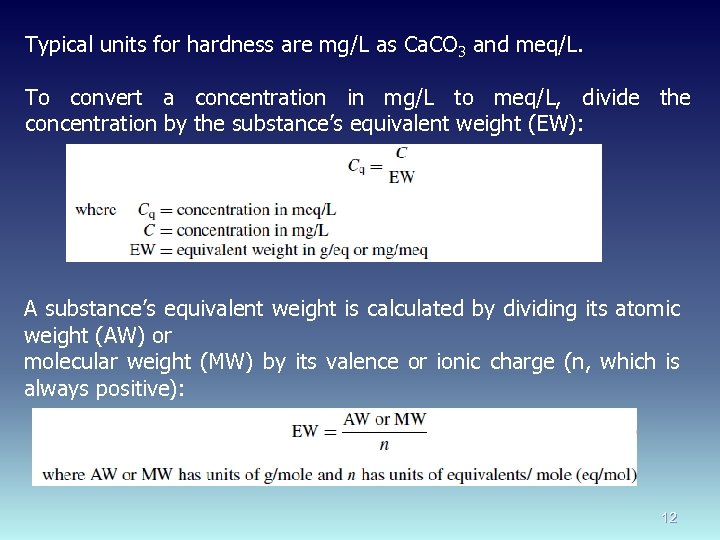 Typical units for hardness are mg/L as Ca. CO 3 and meq/L. To convert a concentration in mg/L to meq/L, divide the concentration by the substance’s equivalent weight (EW): A substance’s equivalent weight is calculated by dividing its atomic weight (AW) or molecular weight (MW) by its valence or ionic charge (n, which is always positive): 12
Typical units for hardness are mg/L as Ca. CO 3 and meq/L. To convert a concentration in mg/L to meq/L, divide the concentration by the substance’s equivalent weight (EW): A substance’s equivalent weight is calculated by dividing its atomic weight (AW) or molecular weight (MW) by its valence or ionic charge (n, which is always positive): 12
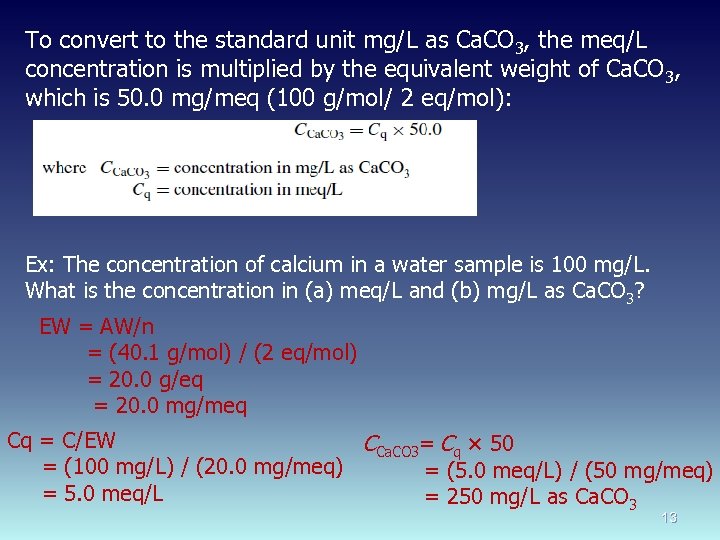 To convert to the standard unit mg/L as Ca. CO 3, the meq/L concentration is multiplied by the equivalent weight of Ca. CO 3, which is 50. 0 mg/meq (100 g/mol/ 2 eq/mol): Ex: The concentration of calcium in a water sample is 100 mg/L. What is the concentration in (a) meq/L and (b) mg/L as Ca. CO 3? EW = AW/n = (40. 1 g/mol) / (2 eq/mol) = 20. 0 g/eq = 20. 0 mg/meq Cq = C/EW CCa. CO 3= Cq × 50 = (100 mg/L) / (20. 0 mg/meq) = (5. 0 meq/L) / (50 mg/meq) = 5. 0 meq/L = 250 mg/L as Ca. CO 3 13
To convert to the standard unit mg/L as Ca. CO 3, the meq/L concentration is multiplied by the equivalent weight of Ca. CO 3, which is 50. 0 mg/meq (100 g/mol/ 2 eq/mol): Ex: The concentration of calcium in a water sample is 100 mg/L. What is the concentration in (a) meq/L and (b) mg/L as Ca. CO 3? EW = AW/n = (40. 1 g/mol) / (2 eq/mol) = 20. 0 g/eq = 20. 0 mg/meq Cq = C/EW CCa. CO 3= Cq × 50 = (100 mg/L) / (20. 0 mg/meq) = (5. 0 meq/L) / (50 mg/meq) = 5. 0 meq/L = 250 mg/L as Ca. CO 3 13
 2. Coagulation and Flocculation Tiny (colloidal) clay and silt particles, which have a natural electrostatic charge that keeps them continually in motion and prevents them from colliding and sticking together, cause turbidity. Coagulants, such as alum (aluminum sulfate), and coagulant aids, such as lime and polymers, are added to the water, first to neutralize the charge on the particles and then to aid in making the tiny particles “sticky” so they can coalesce and form large, quick-settling particles. 14
2. Coagulation and Flocculation Tiny (colloidal) clay and silt particles, which have a natural electrostatic charge that keeps them continually in motion and prevents them from colliding and sticking together, cause turbidity. Coagulants, such as alum (aluminum sulfate), and coagulant aids, such as lime and polymers, are added to the water, first to neutralize the charge on the particles and then to aid in making the tiny particles “sticky” so they can coalesce and form large, quick-settling particles. 14
 3. Settling After flocs have been formed, flocs must be separated from the water. This is invariably done in gravity settling tanks that simply allow the heavier-than-water particles to settle to the bottom. Settling tanks work because the density of the solids exceeds that of the liquid. The movement of a solid particle through a fluid under the pull of gravity is governed by a number of variables, including; • • • particle size (volume) particle shape particle density fluid viscosity. 15
3. Settling After flocs have been formed, flocs must be separated from the water. This is invariably done in gravity settling tanks that simply allow the heavier-than-water particles to settle to the bottom. Settling tanks work because the density of the solids exceeds that of the liquid. The movement of a solid particle through a fluid under the pull of gravity is governed by a number of variables, including; • • • particle size (volume) particle shape particle density fluid viscosity. 15
 4. Filtration Rapid sand filters; The operation of a rapid sand filter involves two phases: filtration and washing. Water from the settling basins enters the filter and seeps through the sand gravel bed, through a false floor, and out into a clear well that stores the finished water. The suspended solids that escape the flocculation and settling steps are caught on the filter. The rapid sand filter becomes clogged in time ad backwashing is performed to clean the filter. 16
4. Filtration Rapid sand filters; The operation of a rapid sand filter involves two phases: filtration and washing. Water from the settling basins enters the filter and seeps through the sand gravel bed, through a false floor, and out into a clear well that stores the finished water. The suspended solids that escape the flocculation and settling steps are caught on the filter. The rapid sand filter becomes clogged in time ad backwashing is performed to clean the filter. 16
 5. Disinfection Disinfected to destroy whatever pathogenic organisms might remain. Commonly, disinfection is accomplished by using chlorine. The presence of a residual of active chlorine in the water is an indication that no further organics remain to be oxidized and that the water can be assumed to be free of disease causing organisms. 17
5. Disinfection Disinfected to destroy whatever pathogenic organisms might remain. Commonly, disinfection is accomplished by using chlorine. The presence of a residual of active chlorine in the water is an indication that no further organics remain to be oxidized and that the water can be assumed to be free of disease causing organisms. 17
 Distribution of Water Storage Pumped to Storage Tank • Storage • Water pressure opsi o 1 psi = 0. 70 m of water NOAA http: //www. csc. noaa. gov/alternatives/infrastructure. html
Distribution of Water Storage Pumped to Storage Tank • Storage • Water pressure opsi o 1 psi = 0. 70 m of water NOAA http: //www. csc. noaa. gov/alternatives/infrastructure. html
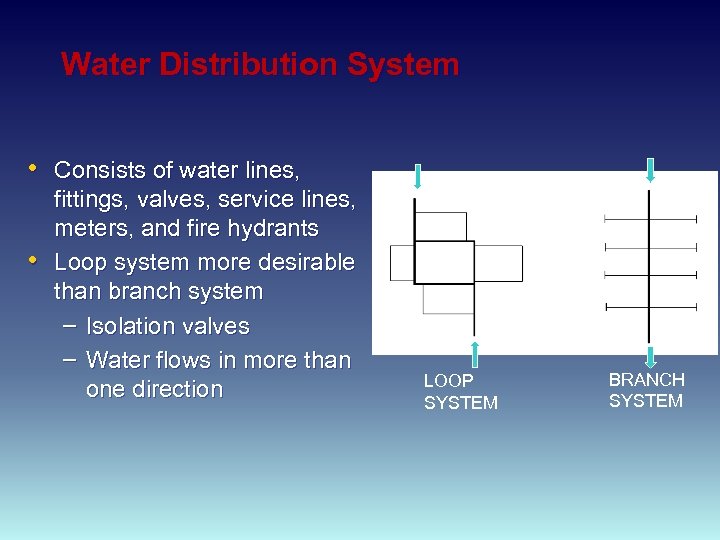 Water Distribution System • Consists of water lines, • fittings, valves, service lines, meters, and fire hydrants Loop system more desirable than branch system – Isolation valves – Water flows in more than one direction LOOP SYSTEM BRANCH SYSTEM
Water Distribution System • Consists of water lines, • fittings, valves, service lines, meters, and fire hydrants Loop system more desirable than branch system – Isolation valves – Water flows in more than one direction LOOP SYSTEM BRANCH SYSTEM
 Water Distribution System • Typical new system pipe • • • – Thermoplastic or ductile iron – Reinforced concrete in larger mains Older system pipe – Cast-iron or asbestos cement Typical distribution pressure of 65 – 75 psi Designed for less than 150 psi wikimedia
Water Distribution System • Typical new system pipe • • • – Thermoplastic or ductile iron – Reinforced concrete in larger mains Older system pipe – Cast-iron or asbestos cement Typical distribution pressure of 65 – 75 psi Designed for less than 150 psi wikimedia
 Consumer • Residential, commercial, and • • industrial facilities Residential – Min. distribution pressure = 40 psi – Max. distribution pressure = 80 psi • Pressure-reducing valve Commercial or industrial facilities – May require higher pressure – Pumps can increase pressure ©i. Stockphoto. com
Consumer • Residential, commercial, and • • industrial facilities Residential – Min. distribution pressure = 40 psi – Max. distribution pressure = 80 psi • Pressure-reducing valve Commercial or industrial facilities – May require higher pressure – Pumps can increase pressure ©i. Stockphoto. com
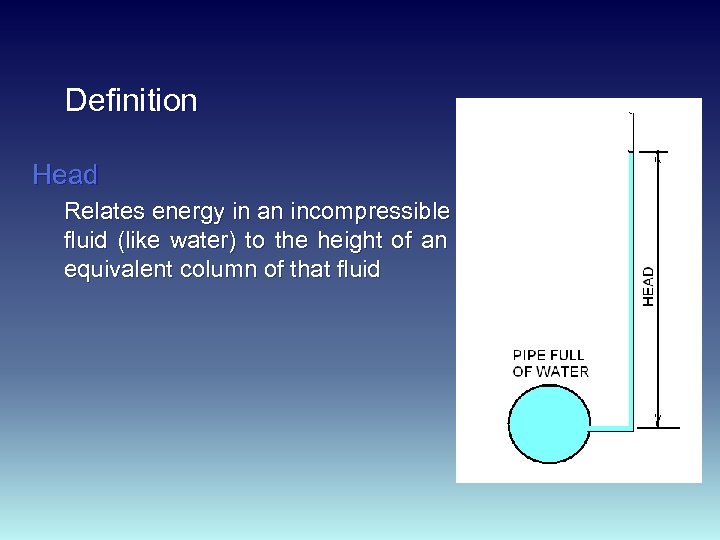 Definition Head Relates energy in an incompressible fluid (like water) to the height of an equivalent column of that fluid
Definition Head Relates energy in an incompressible fluid (like water) to the height of an equivalent column of that fluid
 Definition Static Head • • • Potential energy of the water at rest Measured in feet of water Change in elevation between source and discharge • Ex: What is the static head at a residential supply line if the water level in the elevated tank is 287 m and the elevation at the supply line is 271 m? 287 m – 271 m = 16 m of water EPA at http: //www. epa. gov/region 02/superfund/npl/mohonkr oad/images. html
Definition Static Head • • • Potential energy of the water at rest Measured in feet of water Change in elevation between source and discharge • Ex: What is the static head at a residential supply line if the water level in the elevated tank is 287 m and the elevation at the supply line is 271 m? 287 m – 271 m = 16 m of water EPA at http: //www. epa. gov/region 02/superfund/npl/mohonkr oad/images. html
 Definition Static Pressure • • Pressure of water at rest Measured in pounds per square inch (psi) 0. 70 m of water = 1 psi Ex: What is the static pressure at distribution if the static head is 16 m of water? • Is this the pressure at which water would exit a faucet in the house?
Definition Static Pressure • • Pressure of water at rest Measured in pounds per square inch (psi) 0. 70 m of water = 1 psi Ex: What is the static pressure at distribution if the static head is 16 m of water? • Is this the pressure at which water would exit a faucet in the house?
 Water Pressure Calculations • How far above the supply line must the water level in a water tower be in order to provide a minimum 40 psi? • Except water loses pressure as it travels through pipe. NOAA http: //www. csc. noaa. gov/alternatives/in frastructure. html
Water Pressure Calculations • How far above the supply line must the water level in a water tower be in order to provide a minimum 40 psi? • Except water loses pressure as it travels through pipe. NOAA http: //www. csc. noaa. gov/alternatives/in frastructure. html
 Definitions Head Loss • Energy loss due to friction as water moves through the distribution system − Pipes − Fittings • Elbows, tees, reducers, etc. − Equipment (pumps, etc. ) • Major losses = head loss associated with friction per length of pipe • Minor losses = head loss associated with bends, fittings, valves, etc.
Definitions Head Loss • Energy loss due to friction as water moves through the distribution system − Pipes − Fittings • Elbows, tees, reducers, etc. − Equipment (pumps, etc. ) • Major losses = head loss associated with friction per length of pipe • Minor losses = head loss associated with bends, fittings, valves, etc.
 Calculating Head Loss Darcy Equation The head loss in a pipeline with Newtonian fluids can be determined using the Darcy equation; Where: hf = head loss due to friction (m); L = the length of the pipe (m); D = the hydraulic diameter of the pipe (m); V = the average flow velocity (m/s); g = the local acceleration due to gravity (m/s 2); fd= a dimensionless parameter called the Darcy friction factor
Calculating Head Loss Darcy Equation The head loss in a pipeline with Newtonian fluids can be determined using the Darcy equation; Where: hf = head loss due to friction (m); L = the length of the pipe (m); D = the hydraulic diameter of the pipe (m); V = the average flow velocity (m/s); g = the local acceleration due to gravity (m/s 2); fd= a dimensionless parameter called the Darcy friction factor
 Definition Dynamic Head • Head of a moving fluid • Measured in feet of water Dynamic Head = Static Head – Head Loss Courtesy Constructionphotographs. com
Definition Dynamic Head • Head of a moving fluid • Measured in feet of water Dynamic Head = Static Head – Head Loss Courtesy Constructionphotographs. com
 Definition Dynamic / Actual Pressure • Measured in psi Dynamic Pressure = Actual Pressure = Dynamic Head x (1 psi/0. 70 m)
Definition Dynamic / Actual Pressure • Measured in psi Dynamic Pressure = Actual Pressure = Dynamic Head x (1 psi/0. 70 m)
 Water Pressure Calculations Example The water level in the water tower supplying the home is 450 m. The elevation of the supply line at the residence is 380 m. Find the static head, the static pressure, the dynamic head, and the actual pressure of the water as it enters the residence. Take the head loss as 0. 90 m.
Water Pressure Calculations Example The water level in the water tower supplying the home is 450 m. The elevation of the supply line at the residence is 380 m. Find the static head, the static pressure, the dynamic head, and the actual pressure of the water as it enters the residence. Take the head loss as 0. 90 m.
 Example Static Head= 450 m – 380 m = 70 m Static Pressure = 70 m x (1 psi/0. 70 m) = 100 psi Head Loss (major and minor) = 0. 90 m Dynamic Head = Static Head – Head Loss = 70 m – 0. 90 m= 69. 1 m Dynamic Pressure = 69. 1 m x (1 psi/0. 70 m) = 98. 7 psi
Example Static Head= 450 m – 380 m = 70 m Static Pressure = 70 m x (1 psi/0. 70 m) = 100 psi Head Loss (major and minor) = 0. 90 m Dynamic Head = Static Head – Head Loss = 70 m – 0. 90 m= 69. 1 m Dynamic Pressure = 69. 1 m x (1 psi/0. 70 m) = 98. 7 psi


