32b9cfe87dc0b2b1dd4fd9db34901d7d.ppt
- Количество слайдов: 130
 Introduction Clare Kahn, Ph. D. Vice President, US Regulatory Affairs Cardiovascular, Urogenital & Metabolic Products, Glaxo. Smith. Kline
Introduction Clare Kahn, Ph. D. Vice President, US Regulatory Affairs Cardiovascular, Urogenital & Metabolic Products, Glaxo. Smith. Kline
 Carvedilol Pharmacological Properties • • 2 Nonselective -adrenergic receptor antagonist 1 -adrenergic receptor antagonist
Carvedilol Pharmacological Properties • • 2 Nonselective -adrenergic receptor antagonist 1 -adrenergic receptor antagonist
 Myocardial Injury Chronic Heart Failure Hypertension LV dysfunction Approved 1995 3 Mild Moderate Severe
Myocardial Injury Chronic Heart Failure Hypertension LV dysfunction Approved 1995 3 Mild Moderate Severe
 Myocardial Injury Chronic Heart Failure Hypertension LV dysfunction Approved 1995 4 Mild Moderate Approved 1997 Severe
Myocardial Injury Chronic Heart Failure Hypertension LV dysfunction Approved 1995 4 Mild Moderate Approved 1997 Severe
 Myocardial Injury Chronic Heart Failure Hypertension LV dysfunction Approved 1995 Mild Moderate Severe Approved 1997 Approved 2001 5
Myocardial Injury Chronic Heart Failure Hypertension LV dysfunction Approved 1995 Mild Moderate Severe Approved 1997 Approved 2001 5
 Myocardial Injury Chronic Heart Failure Hypertension LV dysfunction Approved 1995 Moderate Severe Approved 1997 Proposed 2003 6 Mild Approved 2001
Myocardial Injury Chronic Heart Failure Hypertension LV dysfunction Approved 1995 Moderate Severe Approved 1997 Proposed 2003 6 Mild Approved 2001
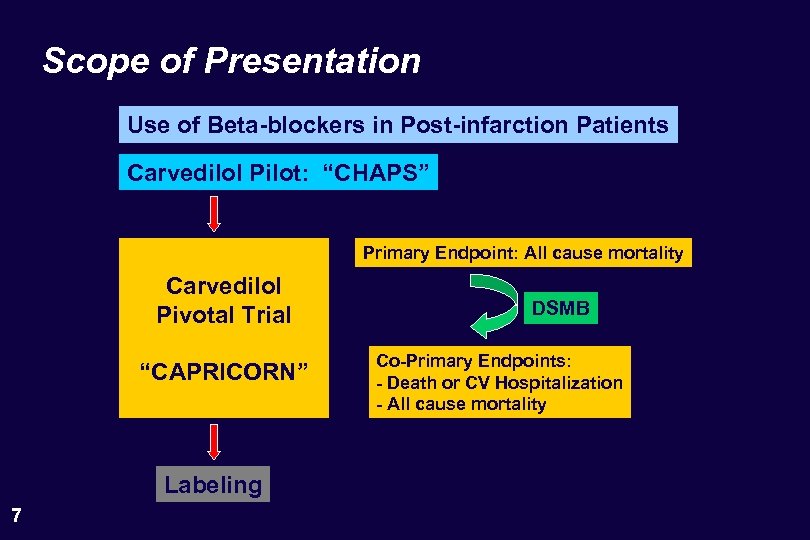 Scope of Presentation Use of Beta-blockers in Post-infarction Patients Carvedilol Pilot: “CHAPS” Primary Endpoint: All cause mortality Carvedilol Pivotal Trial “CAPRICORN” Labeling 7 DSMB Co-Primary Endpoints: - Death or CV Hospitalization - All cause mortality
Scope of Presentation Use of Beta-blockers in Post-infarction Patients Carvedilol Pilot: “CHAPS” Primary Endpoint: All cause mortality Carvedilol Pivotal Trial “CAPRICORN” Labeling 7 DSMB Co-Primary Endpoints: - Death or CV Hospitalization - All cause mortality
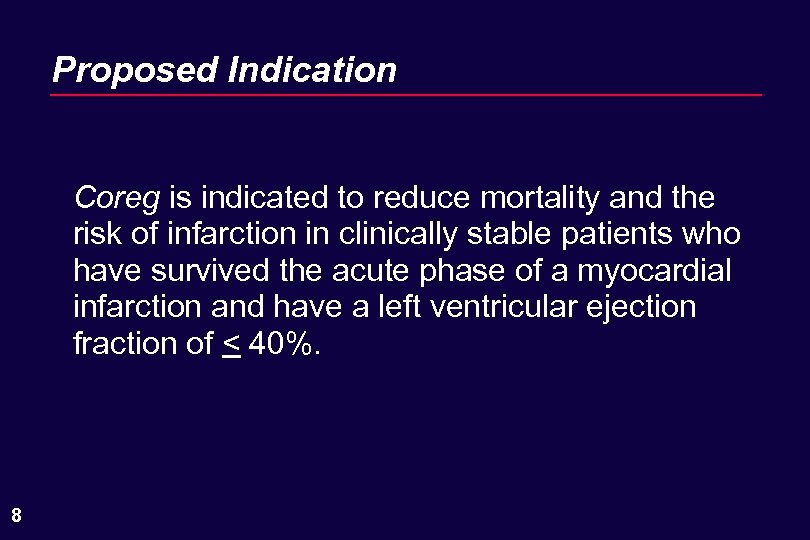 Proposed Indication Coreg is indicated to reduce mortality and the risk of infarction in clinically stable patients who have survived the acute phase of a myocardial infarction and have a left ventricular ejection fraction of < 40%. 8
Proposed Indication Coreg is indicated to reduce mortality and the risk of infarction in clinically stable patients who have survived the acute phase of a myocardial infarction and have a left ventricular ejection fraction of < 40%. 8
 Agenda Introduction Clare Kahn, Ph. D. Background to Mary Ann Lukas, M. D. the CAPRICORN Trial Primary Endpoints Why Are We Here? Henry Dargie, MB. , Ch. B. Milton Packer, M. D. CAPRICORN Trial Henry Dargie, MB. , Ch. B. Effect on Non-Fatal Events 9 Safety and Concluding Remarks Milton Packer, M. D.
Agenda Introduction Clare Kahn, Ph. D. Background to Mary Ann Lukas, M. D. the CAPRICORN Trial Primary Endpoints Why Are We Here? Henry Dargie, MB. , Ch. B. Milton Packer, M. D. CAPRICORN Trial Henry Dargie, MB. , Ch. B. Effect on Non-Fatal Events 9 Safety and Concluding Remarks Milton Packer, M. D.
 Consultants Henry Dargie, MB. , Ch. B. University of Glasgow Principal Investigator, CAPRICORN University of Glasgow Biostatistician, CAPRICORN Milton Packer, M. D. Jonathan Sackner-Bernstein, M. D. Columbia University Planning Committee, CAPRICORN Principal Investigator, COPERNICUS 10 Ian Ford, Ph. D. Columbia University Endpoint Committee, CAPRICORN
Consultants Henry Dargie, MB. , Ch. B. University of Glasgow Principal Investigator, CAPRICORN University of Glasgow Biostatistician, CAPRICORN Milton Packer, M. D. Jonathan Sackner-Bernstein, M. D. Columbia University Planning Committee, CAPRICORN Principal Investigator, COPERNICUS 10 Ian Ford, Ph. D. Columbia University Endpoint Committee, CAPRICORN
 Background to the CAPRICORN Trial Mary Ann Lukas, M. D.
Background to the CAPRICORN Trial Mary Ann Lukas, M. D.
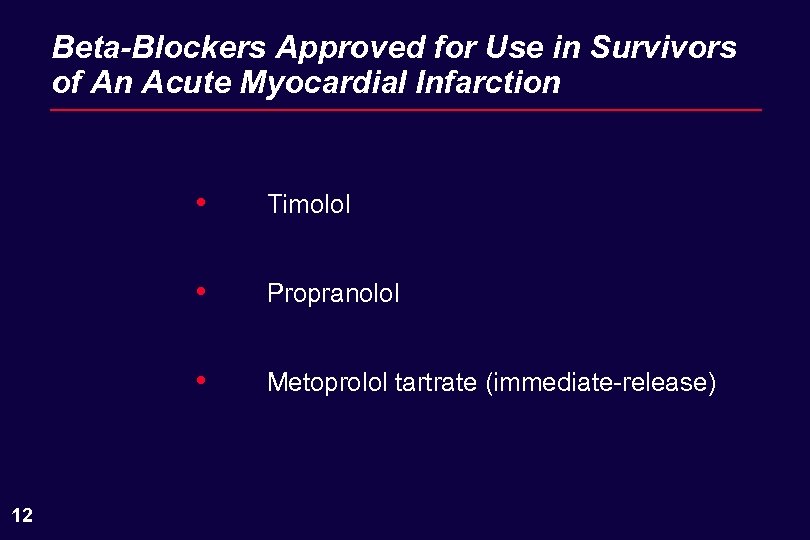 Beta-Blockers Approved for Use in Survivors of An Acute Myocardial Infarction • • Propranolol • 12 Timolol Metoprolol tartrate (immediate-release)
Beta-Blockers Approved for Use in Survivors of An Acute Myocardial Infarction • • Propranolol • 12 Timolol Metoprolol tartrate (immediate-release)
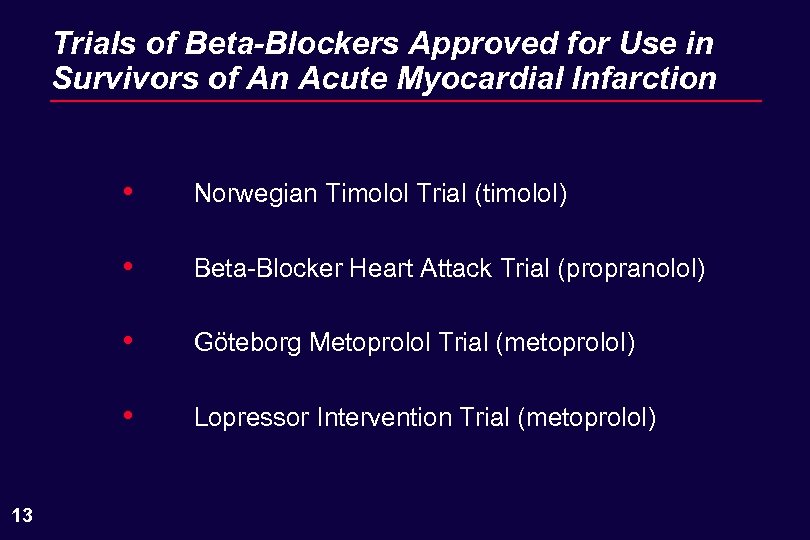 Trials of Beta-Blockers Approved for Use in Survivors of An Acute Myocardial Infarction • • Beta-Blocker Heart Attack Trial (propranolol) • Göteborg Metoprolol Trial (metoprolol) • 13 Norwegian Timolol Trial (timolol) Lopressor Intervention Trial (metoprolol)
Trials of Beta-Blockers Approved for Use in Survivors of An Acute Myocardial Infarction • • Beta-Blocker Heart Attack Trial (propranolol) • Göteborg Metoprolol Trial (metoprolol) • 13 Norwegian Timolol Trial (timolol) Lopressor Intervention Trial (metoprolol)
 Patient Populations Not Included in Earlier Post-Infarction Trials of b-Blockers • • Many currently available treatments for the immediate management of the post-infarction patient were not available or used (e. g. , ACE inhibitors, IV nitroglycerin, heparin, thrombolytics). • 14 High risk patients (e. g. , heart failure or systolic BP < 100 -110 mm Hg) were generally not enrolled. Many currently available treatments for the long-term management of the post-infarction patient were not allowed (e. g. , ACE inhibitors, aspirin, anticoagulants or lipid lowering drugs).
Patient Populations Not Included in Earlier Post-Infarction Trials of b-Blockers • • Many currently available treatments for the immediate management of the post-infarction patient were not available or used (e. g. , ACE inhibitors, IV nitroglycerin, heparin, thrombolytics). • 14 High risk patients (e. g. , heart failure or systolic BP < 100 -110 mm Hg) were generally not enrolled. Many currently available treatments for the long-term management of the post-infarction patient were not allowed (e. g. , ACE inhibitors, aspirin, anticoagulants or lipid lowering drugs).
 Should b-blockers Still Be Used in the Post. Infarction Patients in the Modern Era? Are -blockers still needed? • Trials carried out before advent of ACE inhibitors, thrombolytics, heparin, aspirin, anticoagulants or lipid lowering drugs. Are -blockers worth the risks? • 15 Concerns about risk of worsening heart failure (in patients with low EF) or hypotension (in patients with receiving ACE inhibitors or vasodilators).
Should b-blockers Still Be Used in the Post. Infarction Patients in the Modern Era? Are -blockers still needed? • Trials carried out before advent of ACE inhibitors, thrombolytics, heparin, aspirin, anticoagulants or lipid lowering drugs. Are -blockers worth the risks? • 15 Concerns about risk of worsening heart failure (in patients with low EF) or hypotension (in patients with receiving ACE inhibitors or vasodilators).
 To Complicate Matters Further. . . • The -blockers approved for use in post-infarction patients are not approved for heart failure and currently carry a contraindication to their use in heart failure. — Timolol, propranolol and immediate-release metoprolol • The -blockers approved for use in chronic heart failure are not approved for use following a recent myocardial infarction. — Carvedilol and sustained-release metoprolol 16
To Complicate Matters Further. . . • The -blockers approved for use in post-infarction patients are not approved for heart failure and currently carry a contraindication to their use in heart failure. — Timolol, propranolol and immediate-release metoprolol • The -blockers approved for use in chronic heart failure are not approved for use following a recent myocardial infarction. — Carvedilol and sustained-release metoprolol 16
 Timolol, propranolol, metoprolol IR Myocardial Injury Chronic Heart Failure LV dysfunction 17 Mild Moderate Severe
Timolol, propranolol, metoprolol IR Myocardial Injury Chronic Heart Failure LV dysfunction 17 Mild Moderate Severe
 Timolol, propranolol, metoprolol IR Myocardial Injury Chronic Heart Failure LV dysfunction 18 Mild Moderate Severe Metoprolol SR Carvedilol
Timolol, propranolol, metoprolol IR Myocardial Injury Chronic Heart Failure LV dysfunction 18 Mild Moderate Severe Metoprolol SR Carvedilol
 Timolol, propranolol, metoprolol IR Myocardial Injury Chronic Heart Failure No b-blocker LV dysfunction 19 Mild Moderate Severe Metoprolol SR Carvedilol
Timolol, propranolol, metoprolol IR Myocardial Injury Chronic Heart Failure No b-blocker LV dysfunction 19 Mild Moderate Severe Metoprolol SR Carvedilol
 Recent Myocardial Infarction Acute Post-MI MI LV dysfunction 20 Remote Myocardial Infarction Mild CHF Moderate CHF Severe CHF
Recent Myocardial Infarction Acute Post-MI MI LV dysfunction 20 Remote Myocardial Infarction Mild CHF Moderate CHF Severe CHF
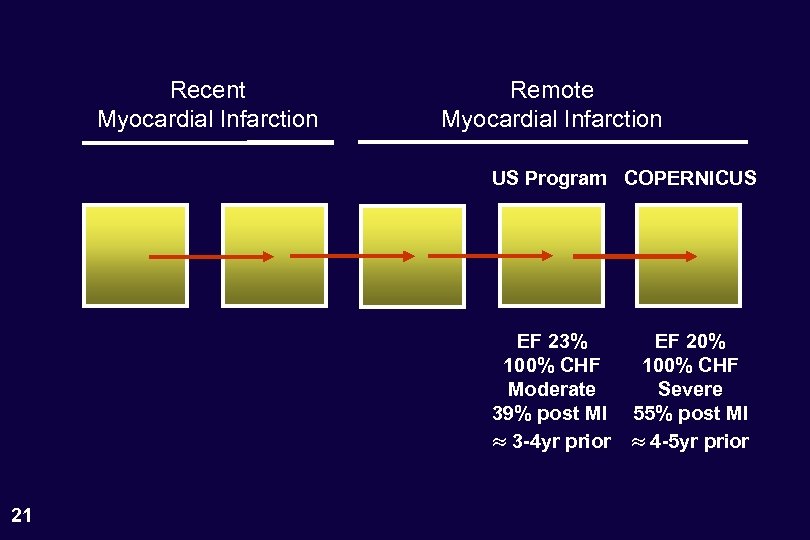 Recent Myocardial Infarction Remote Myocardial Infarction US Program COPERNICUS EF 23% 100% CHF Moderate 39% post MI 3 -4 yr prior 21 EF 20% 100% CHF Severe 55% post MI 4 -5 yr prior
Recent Myocardial Infarction Remote Myocardial Infarction US Program COPERNICUS EF 23% 100% CHF Moderate 39% post MI 3 -4 yr prior 21 EF 20% 100% CHF Severe 55% post MI 4 -5 yr prior
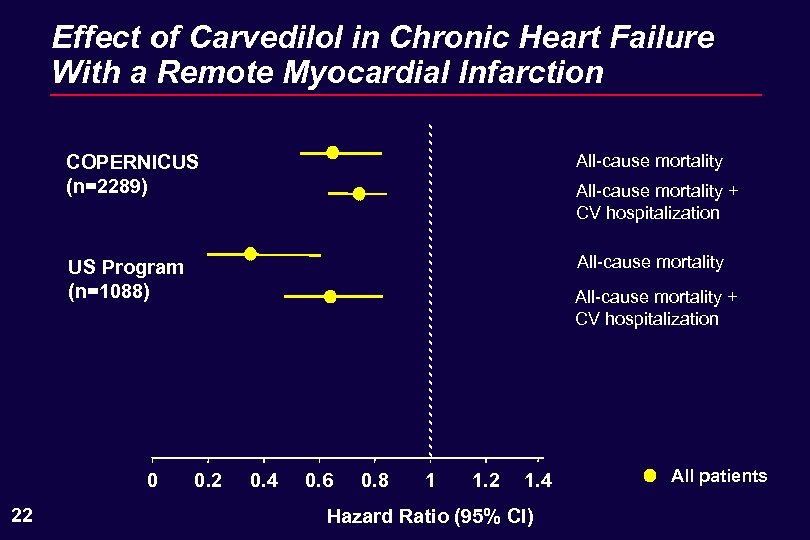 Effect of Carvedilol in Chronic Heart Failure With a Remote Myocardial Infarction COPERNICUS (n=2289) All-cause mortality US Program (n=1088) All-cause mortality 0 22 All-cause mortality + CV hospitalization 0. 2 0. 4 0. 6 0. 8 1 1. 2 1. 4 Hazard Ratio (95% CI) All patients
Effect of Carvedilol in Chronic Heart Failure With a Remote Myocardial Infarction COPERNICUS (n=2289) All-cause mortality US Program (n=1088) All-cause mortality 0 22 All-cause mortality + CV hospitalization 0. 2 0. 4 0. 6 0. 8 1 1. 2 1. 4 Hazard Ratio (95% CI) All patients
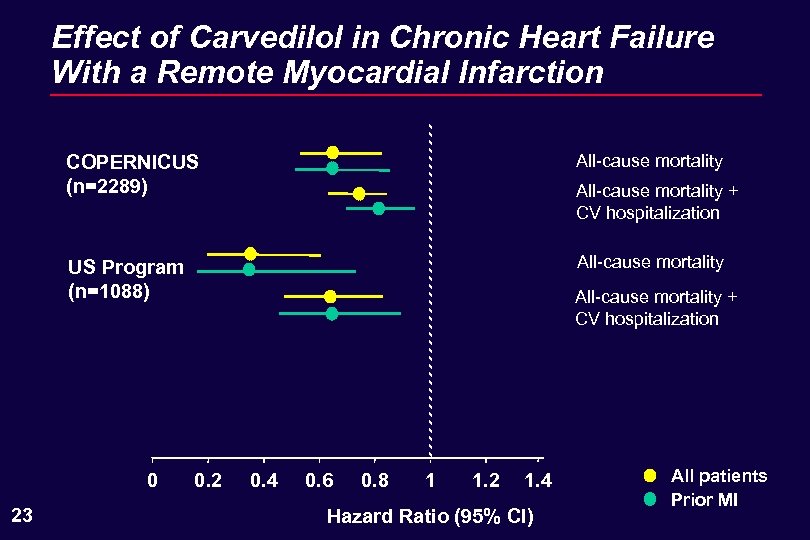 Effect of Carvedilol in Chronic Heart Failure With a Remote Myocardial Infarction COPERNICUS (n=2289) All-cause mortality US Program (n=1088) All-cause mortality 0 23 All-cause mortality + CV hospitalization 0. 2 0. 4 0. 6 0. 8 1 1. 2 1. 4 Hazard Ratio (95% CI) All patients Prior MI
Effect of Carvedilol in Chronic Heart Failure With a Remote Myocardial Infarction COPERNICUS (n=2289) All-cause mortality US Program (n=1088) All-cause mortality 0 23 All-cause mortality + CV hospitalization 0. 2 0. 4 0. 6 0. 8 1 1. 2 1. 4 Hazard Ratio (95% CI) All patients Prior MI
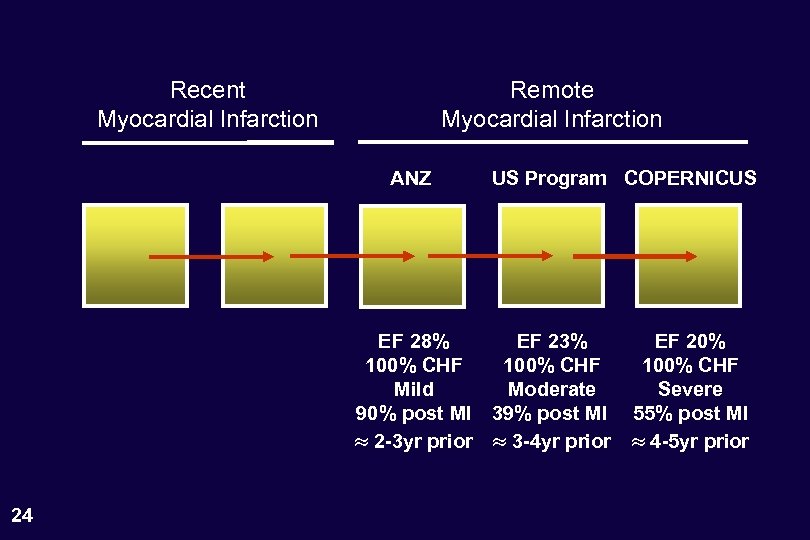 Recent Myocardial Infarction Remote Myocardial Infarction ANZ US Program COPERNICUS EF 28% EF 23% 100% CHF Mild Moderate 90% post MI 39% post MI 2 -3 yr prior 3 -4 yr prior 24 EF 20% 100% CHF Severe 55% post MI 4 -5 yr prior
Recent Myocardial Infarction Remote Myocardial Infarction ANZ US Program COPERNICUS EF 28% EF 23% 100% CHF Mild Moderate 90% post MI 39% post MI 2 -3 yr prior 3 -4 yr prior 24 EF 20% 100% CHF Severe 55% post MI 4 -5 yr prior
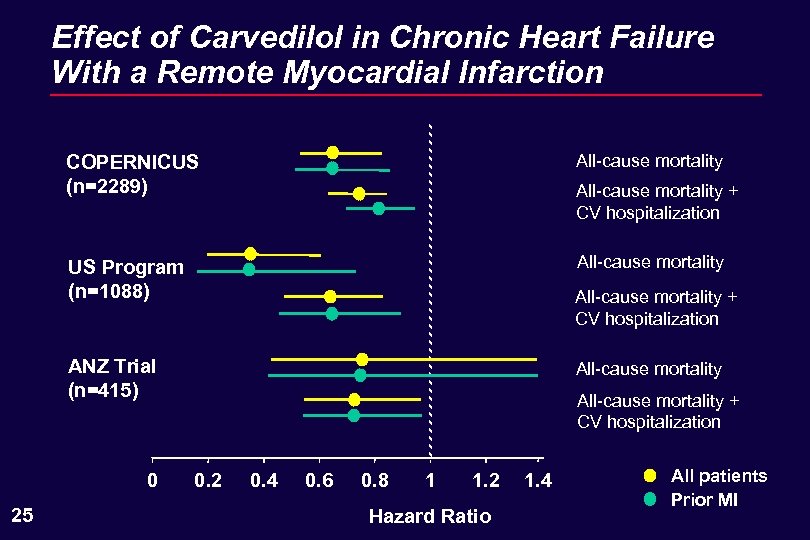 Effect of Carvedilol in Chronic Heart Failure With a Remote Myocardial Infarction COPERNICUS (n=2289) All-cause mortality US Program (n=1088) All-cause mortality + CV hospitalization ANZ Trial (n=415) 0 25 All-cause mortality + CV hospitalization 0. 2 0. 4 0. 6 0. 8 1 1. 2 Hazard Ratio 1. 4 All patients Prior MI
Effect of Carvedilol in Chronic Heart Failure With a Remote Myocardial Infarction COPERNICUS (n=2289) All-cause mortality US Program (n=1088) All-cause mortality + CV hospitalization ANZ Trial (n=415) 0 25 All-cause mortality + CV hospitalization 0. 2 0. 4 0. 6 0. 8 1 1. 2 Hazard Ratio 1. 4 All patients Prior MI
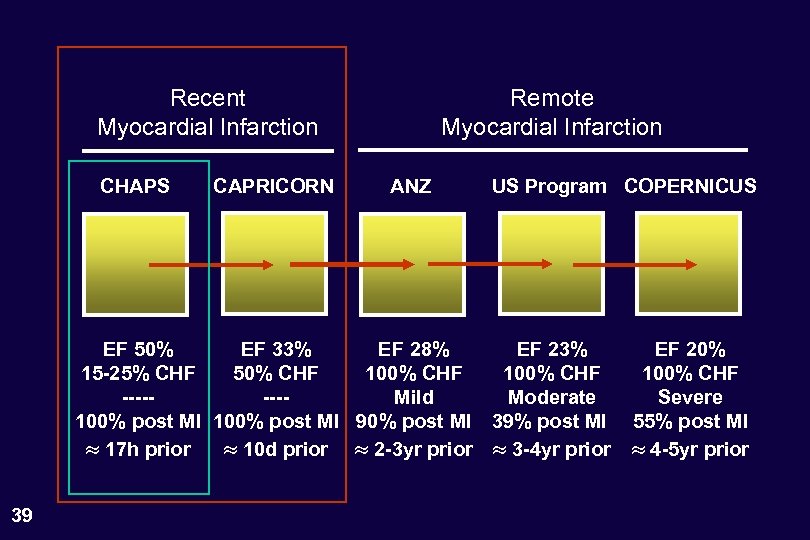
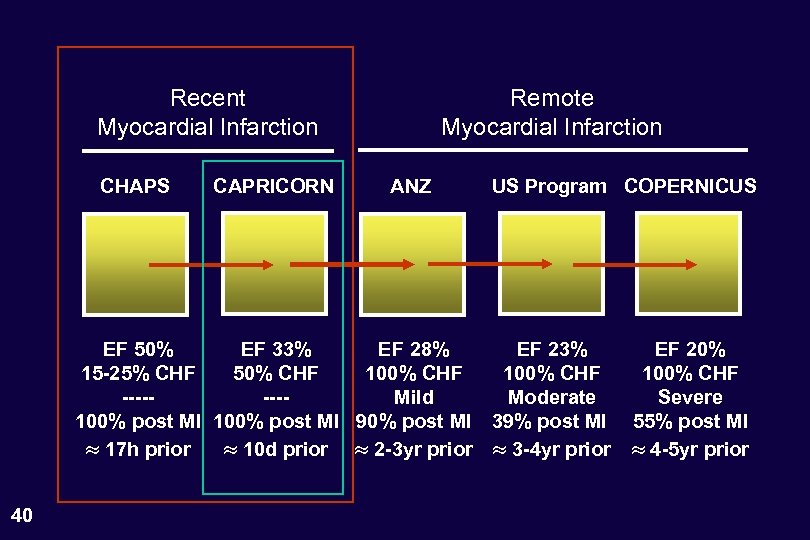
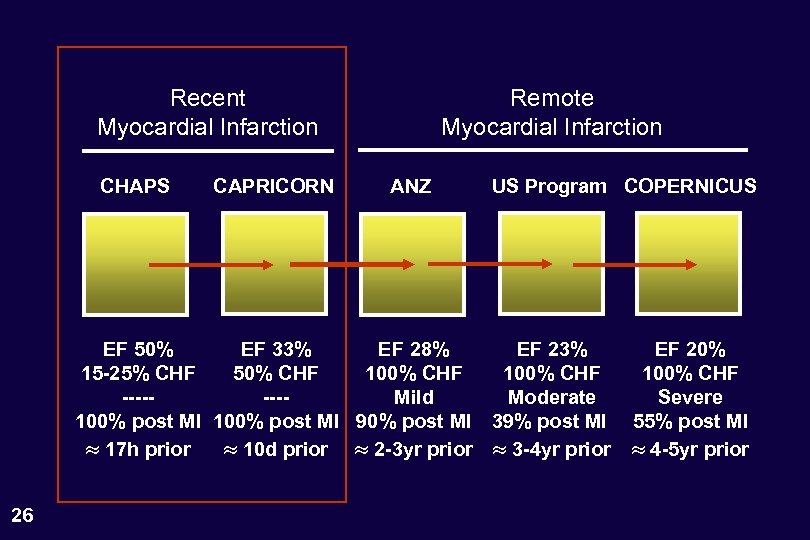 Recent Myocardial Infarction CHAPS CAPRICORN Remote Myocardial Infarction ANZ US Program COPERNICUS EF 50% EF 33% EF 28% EF 23% 15 -25% CHF 50% CHF 100% CHF -------Mild Moderate 100% post MI 90% post MI 39% post MI 17 h prior 10 d prior 2 -3 yr prior 3 -4 yr prior 26 EF 20% 100% CHF Severe 55% post MI 4 -5 yr prior
Recent Myocardial Infarction CHAPS CAPRICORN Remote Myocardial Infarction ANZ US Program COPERNICUS EF 50% EF 33% EF 28% EF 23% 15 -25% CHF 50% CHF 100% CHF -------Mild Moderate 100% post MI 90% post MI 39% post MI 17 h prior 10 d prior 2 -3 yr prior 3 -4 yr prior 26 EF 20% 100% CHF Severe 55% post MI 4 -5 yr prior
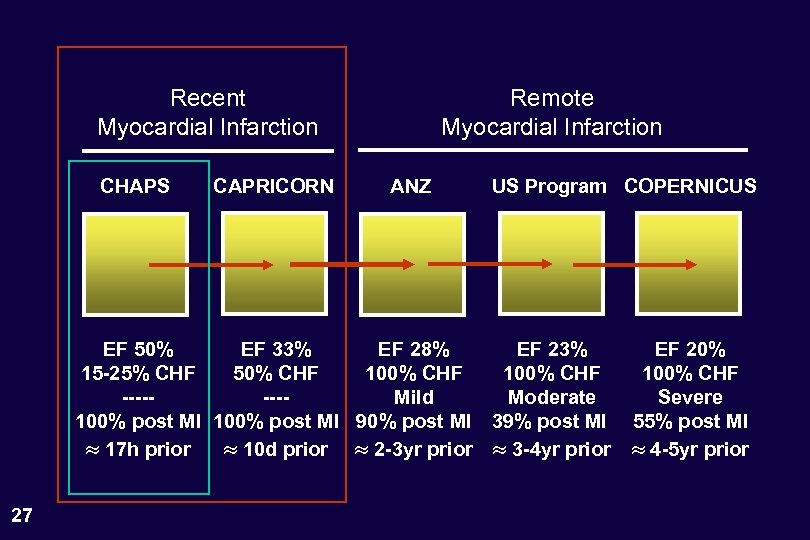 Recent Myocardial Infarction CHAPS CAPRICORN Remote Myocardial Infarction ANZ US Program COPERNICUS EF 50% EF 33% EF 28% EF 23% 15 -25% CHF 50% CHF 100% CHF -------Mild Moderate 100% post MI 90% post MI 39% post MI 17 h prior 10 d prior 2 -3 yr prior 3 -4 yr prior 27 EF 20% 100% CHF Severe 55% post MI 4 -5 yr prior
Recent Myocardial Infarction CHAPS CAPRICORN Remote Myocardial Infarction ANZ US Program COPERNICUS EF 50% EF 33% EF 28% EF 23% 15 -25% CHF 50% CHF 100% CHF -------Mild Moderate 100% post MI 90% post MI 39% post MI 17 h prior 10 d prior 2 -3 yr prior 3 -4 yr prior 27 EF 20% 100% CHF Severe 55% post MI 4 -5 yr prior
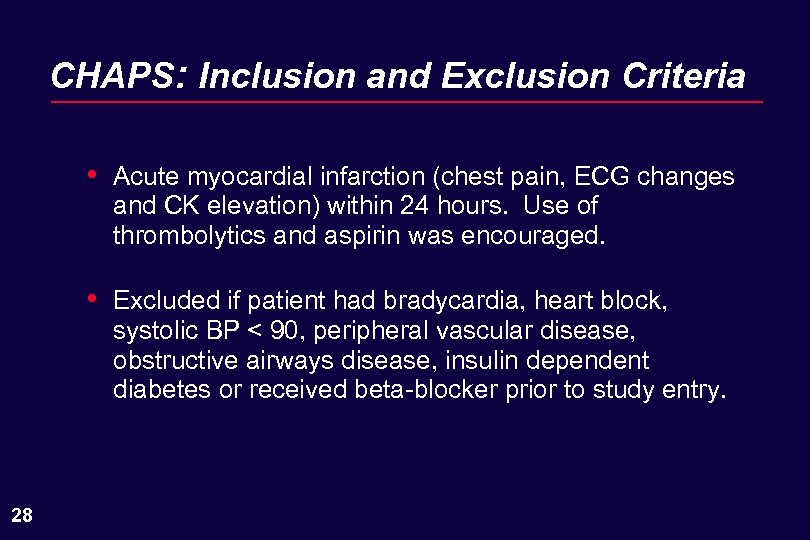 CHAPS: Inclusion and Exclusion Criteria • • 28 Acute myocardial infarction (chest pain, ECG changes and CK elevation) within 24 hours. Use of thrombolytics and aspirin was encouraged. Excluded if patient had bradycardia, heart block, systolic BP < 90, peripheral vascular disease, obstructive airways disease, insulin dependent diabetes or received beta-blocker prior to study entry.
CHAPS: Inclusion and Exclusion Criteria • • 28 Acute myocardial infarction (chest pain, ECG changes and CK elevation) within 24 hours. Use of thrombolytics and aspirin was encouraged. Excluded if patient had bradycardia, heart block, systolic BP < 90, peripheral vascular disease, obstructive airways disease, insulin dependent diabetes or received beta-blocker prior to study entry.
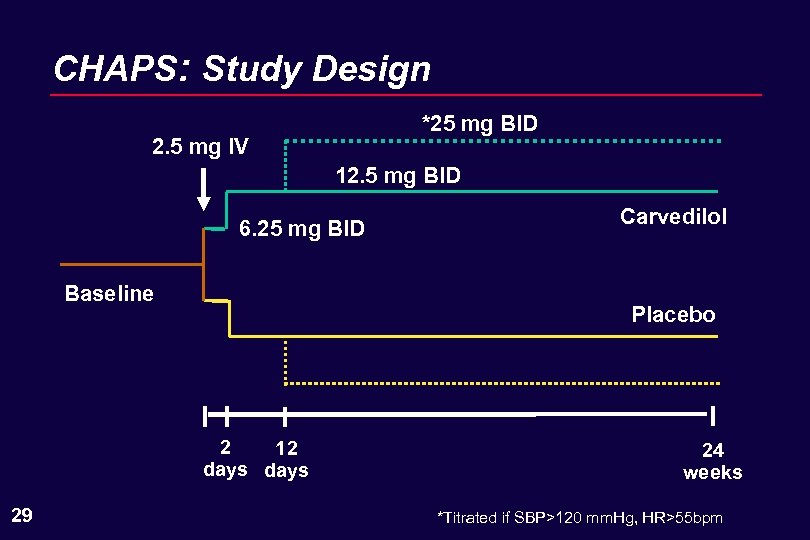 CHAPS: Study Design *25 mg BID 2. 5 mg IV 12. 5 mg BID 6. 25 mg BID Baseline Placebo 2 12 days 29 Carvedilol 24 weeks *Titrated if SBP>120 mm. Hg, HR>55 bpm
CHAPS: Study Design *25 mg BID 2. 5 mg IV 12. 5 mg BID 6. 25 mg BID Baseline Placebo 2 12 days 29 Carvedilol 24 weeks *Titrated if SBP>120 mm. Hg, HR>55 bpm
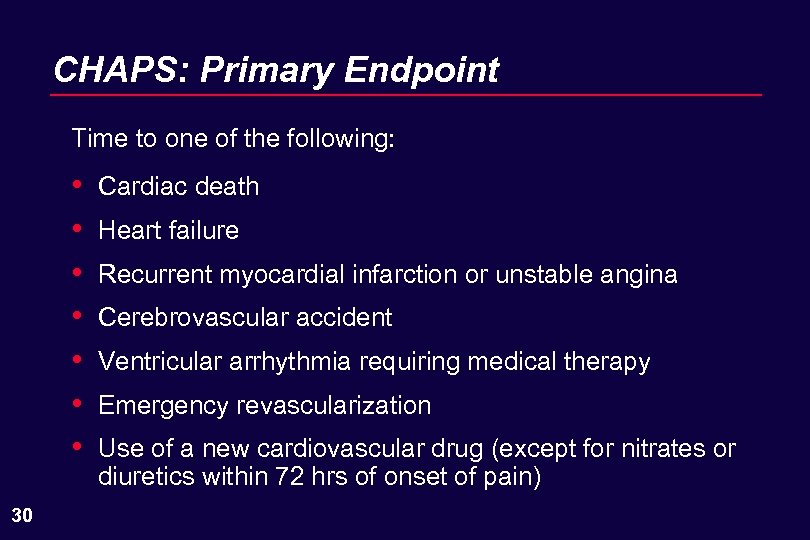 CHAPS: Primary Endpoint Time to one of the following: • • 30 Cardiac death Heart failure Recurrent myocardial infarction or unstable angina Cerebrovascular accident Ventricular arrhythmia requiring medical therapy Emergency revascularization Use of a new cardiovascular drug (except for nitrates or diuretics within 72 hrs of onset of pain)
CHAPS: Primary Endpoint Time to one of the following: • • 30 Cardiac death Heart failure Recurrent myocardial infarction or unstable angina Cerebrovascular accident Ventricular arrhythmia requiring medical therapy Emergency revascularization Use of a new cardiovascular drug (except for nitrates or diuretics within 72 hrs of onset of pain)
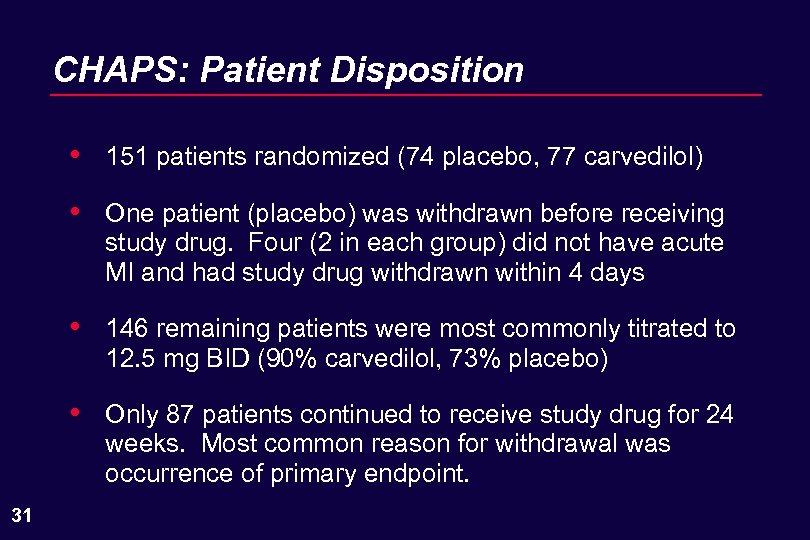 CHAPS: Patient Disposition • • One patient (placebo) was withdrawn before receiving study drug. Four (2 in each group) did not have acute MI and had study drug withdrawn within 4 days • 146 remaining patients were most commonly titrated to 12. 5 mg BID (90% carvedilol, 73% placebo) • 31 151 patients randomized (74 placebo, 77 carvedilol) Only 87 patients continued to receive study drug for 24 weeks. Most common reason for withdrawal was occurrence of primary endpoint.
CHAPS: Patient Disposition • • One patient (placebo) was withdrawn before receiving study drug. Four (2 in each group) did not have acute MI and had study drug withdrawn within 4 days • 146 remaining patients were most commonly titrated to 12. 5 mg BID (90% carvedilol, 73% placebo) • 31 151 patients randomized (74 placebo, 77 carvedilol) Only 87 patients continued to receive study drug for 24 weeks. Most common reason for withdrawal was occurrence of primary endpoint.
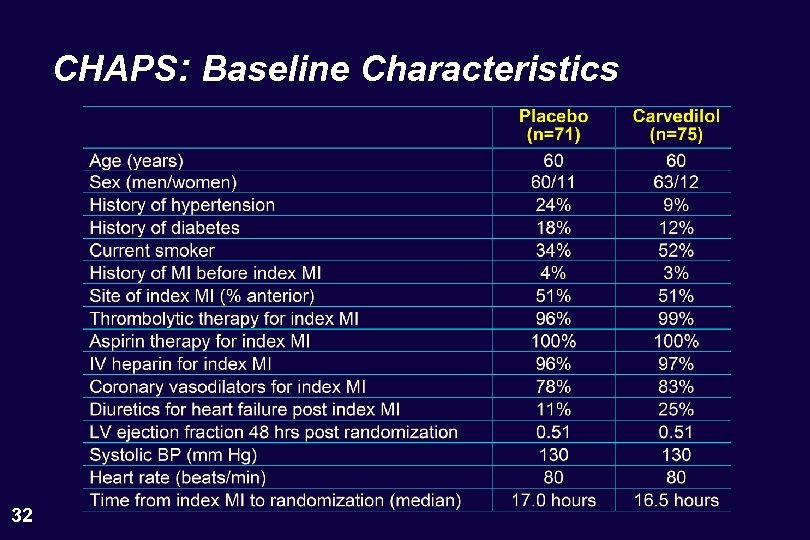 CHAPS: Baseline Characteristics 32
CHAPS: Baseline Characteristics 32
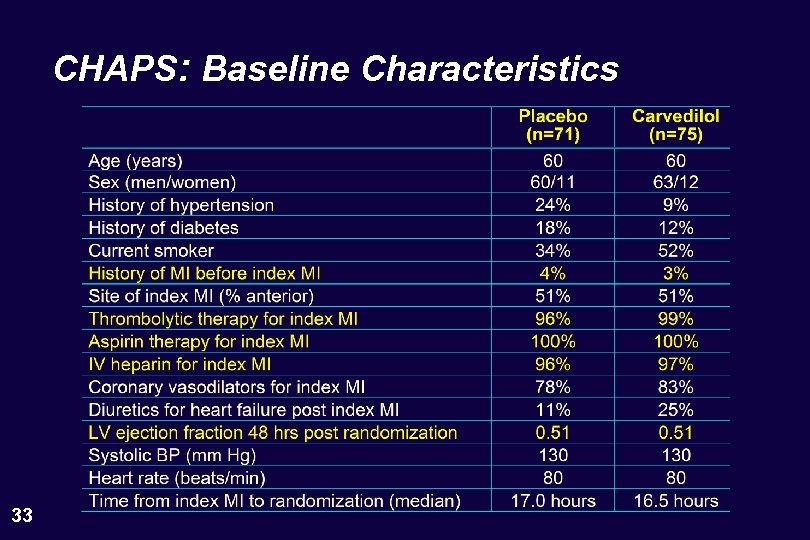 CHAPS: Baseline Characteristics 33
CHAPS: Baseline Characteristics 33
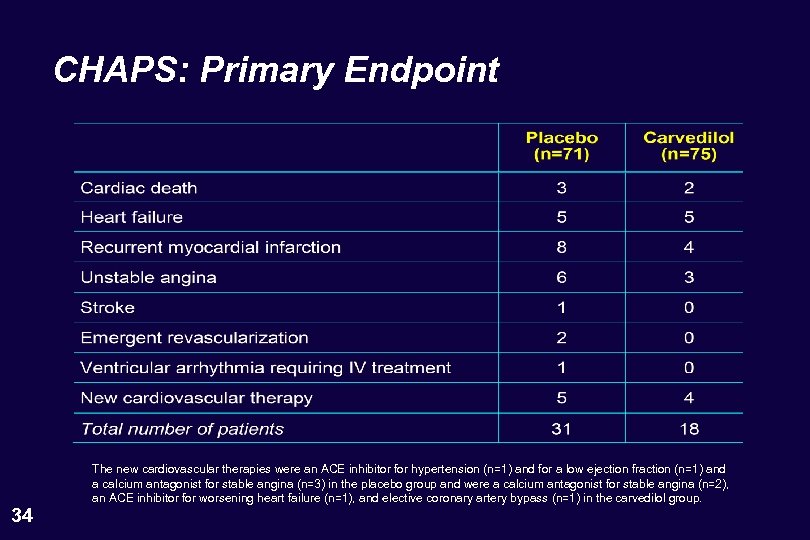 CHAPS: Primary Endpoint 34 The new cardiovascular therapies were an ACE inhibitor for hypertension (n=1) and for a low ejection fraction (n=1) and a calcium antagonist for stable angina (n=3) in the placebo group and were a calcium antagonist for stable angina (n=2), an ACE inhibitor for worsening heart failure (n=1), and elective coronary artery bypass (n=1) in the carvedilol group.
CHAPS: Primary Endpoint 34 The new cardiovascular therapies were an ACE inhibitor for hypertension (n=1) and for a low ejection fraction (n=1) and a calcium antagonist for stable angina (n=3) in the placebo group and were a calcium antagonist for stable angina (n=2), an ACE inhibitor for worsening heart failure (n=1), and elective coronary artery bypass (n=1) in the carvedilol group.
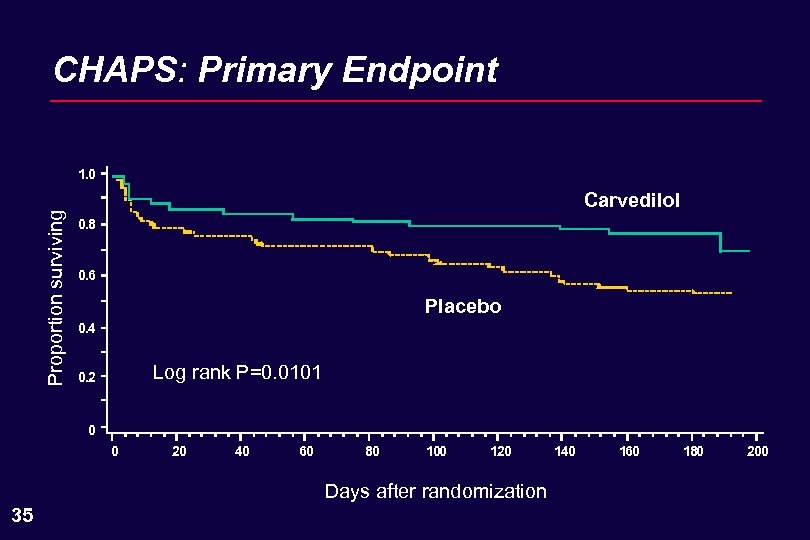 CHAPS: Primary Endpoint Proportion surviving 1. 0 Carvedilol 0. 8 0. 6 Placebo 0. 4 Log rank P=0. 0101 0. 2 0 0 20 40 60 80 100 120 Days after randomization 35 140 160 180 200
CHAPS: Primary Endpoint Proportion surviving 1. 0 Carvedilol 0. 8 0. 6 Placebo 0. 4 Log rank P=0. 0101 0. 2 0 0 20 40 60 80 100 120 Days after randomization 35 140 160 180 200
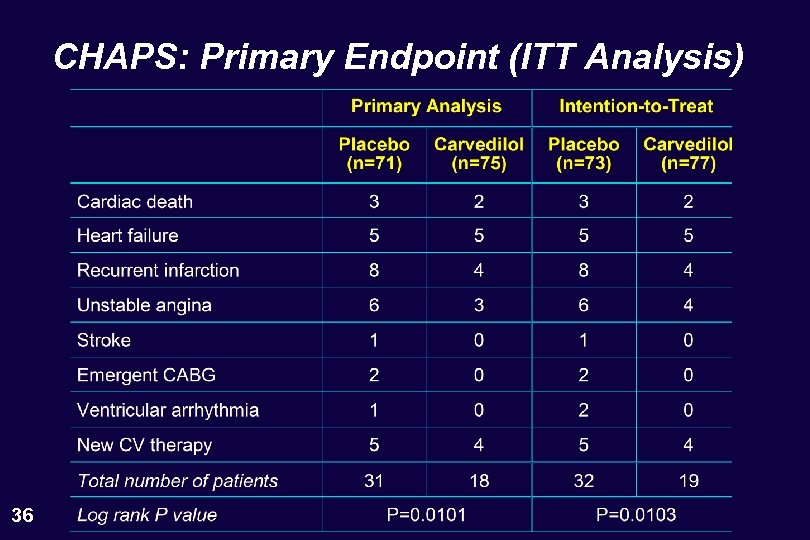 CHAPS: Primary Endpoint (ITT Analysis) 36
CHAPS: Primary Endpoint (ITT Analysis) 36
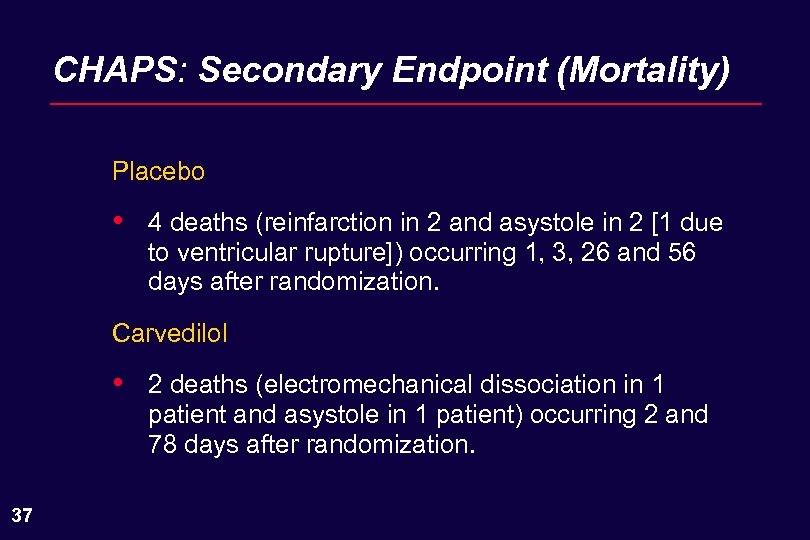 CHAPS: Secondary Endpoint (Mortality) Placebo • 4 deaths (reinfarction in 2 and asystole in 2 [1 due to ventricular rupture]) occurring 1, 3, 26 and 56 days after randomization. Carvedilol • 37 2 deaths (electromechanical dissociation in 1 patient and asystole in 1 patient) occurring 2 and 78 days after randomization.
CHAPS: Secondary Endpoint (Mortality) Placebo • 4 deaths (reinfarction in 2 and asystole in 2 [1 due to ventricular rupture]) occurring 1, 3, 26 and 56 days after randomization. Carvedilol • 37 2 deaths (electromechanical dissociation in 1 patient and asystole in 1 patient) occurring 2 and 78 days after randomization.
 CHAPS: Summary • • 38 The CHAPS study supports ability of carvedilol to reduce the risk of death and reinfarction in postinfarction patients. The CHAPS study demonstrates the tolerability of carvedilol in immediate post-infarction period.
CHAPS: Summary • • 38 The CHAPS study supports ability of carvedilol to reduce the risk of death and reinfarction in postinfarction patients. The CHAPS study demonstrates the tolerability of carvedilol in immediate post-infarction period.
 Recent Myocardial Infarction CHAPS CAPRICORN Remote Myocardial Infarction ANZ US Program COPERNICUS EF 50% EF 33% EF 28% EF 23% 15 -25% CHF 50% CHF 100% CHF -------Mild Moderate 100% post MI 90% post MI 39% post MI 17 h prior 10 d prior 2 -3 yr prior 3 -4 yr prior 39 EF 20% 100% CHF Severe 55% post MI 4 -5 yr prior
Recent Myocardial Infarction CHAPS CAPRICORN Remote Myocardial Infarction ANZ US Program COPERNICUS EF 50% EF 33% EF 28% EF 23% 15 -25% CHF 50% CHF 100% CHF -------Mild Moderate 100% post MI 90% post MI 39% post MI 17 h prior 10 d prior 2 -3 yr prior 3 -4 yr prior 39 EF 20% 100% CHF Severe 55% post MI 4 -5 yr prior
 Recent Myocardial Infarction CHAPS CAPRICORN Remote Myocardial Infarction ANZ US Program COPERNICUS EF 50% EF 33% EF 28% EF 23% 15 -25% CHF 50% CHF 100% CHF -------Mild Moderate 100% post MI 90% post MI 39% post MI 17 h prior 10 d prior 2 -3 yr prior 3 -4 yr prior 40 EF 20% 100% CHF Severe 55% post MI 4 -5 yr prior
Recent Myocardial Infarction CHAPS CAPRICORN Remote Myocardial Infarction ANZ US Program COPERNICUS EF 50% EF 33% EF 28% EF 23% 15 -25% CHF 50% CHF 100% CHF -------Mild Moderate 100% post MI 90% post MI 39% post MI 17 h prior 10 d prior 2 -3 yr prior 3 -4 yr prior 40 EF 20% 100% CHF Severe 55% post MI 4 -5 yr prior
 Primary Results of the CAPRICORN Trial Henry Dargie, MB. , Ch. B.
Primary Results of the CAPRICORN Trial Henry Dargie, MB. , Ch. B.
 CAPRICORN Objective/Study Design • • Multicenter, randomized, placebo-controlled, parallelgroup trial in patients with LV ejection fraction 40%, with or without heart failure • 42 To evaluate the effect of carvedilol on all-cause mortality in patients with LV dysfunction who have recently survived an acute myocardial infarction in the modern era Involved 163 centers in 17 countries (including those in Europe, Israel, North America, Australia, New Zealand)
CAPRICORN Objective/Study Design • • Multicenter, randomized, placebo-controlled, parallelgroup trial in patients with LV ejection fraction 40%, with or without heart failure • 42 To evaluate the effect of carvedilol on all-cause mortality in patients with LV dysfunction who have recently survived an acute myocardial infarction in the modern era Involved 163 centers in 17 countries (including those in Europe, Israel, North America, Australia, New Zealand)
 CAPRICORN Study Organization Steering Committee H Dargie (UK), W Colucci (US), JL Lopez-Sendon (Es), W Remme (NL), N Sharpe (NZ) Endpoint Committee J Mc. Murray (UK) , L Kober (DK), J Sackner-Bernstein (US), J Soler-Soler (Es), F Zannad(F) Data and Safety Monitoring Board D Julian (UK), B Massie (US), S Thompson (UK), L Wilhelmson (DK), I Ford (UK) 43
CAPRICORN Study Organization Steering Committee H Dargie (UK), W Colucci (US), JL Lopez-Sendon (Es), W Remme (NL), N Sharpe (NZ) Endpoint Committee J Mc. Murray (UK) , L Kober (DK), J Sackner-Bernstein (US), J Soler-Soler (Es), F Zannad(F) Data and Safety Monitoring Board D Julian (UK), B Massie (US), S Thompson (UK), L Wilhelmson (DK), I Ford (UK) 43
 CAPRICORN Inclusion and Exclusion Criteria • • LV ejection fraction 40% and receiving ACE inhibitor 48 hr; 80% hospitalized at time of study entry. • Excluded if unstable angina, uncontrolled ventricular arrhythmias or hypertension, bradycardia, heart block, systolic BP < 90 mm Hg, obstructive airways disease, unstable diabetes or requiring inotropic therapy. • 44 Acute myocardial infarction within 21 days. Use of all adjunctive therapy was encouraged. Clinically stable but may have had pulmonary edema or cardiogenic shock during index infarction.
CAPRICORN Inclusion and Exclusion Criteria • • LV ejection fraction 40% and receiving ACE inhibitor 48 hr; 80% hospitalized at time of study entry. • Excluded if unstable angina, uncontrolled ventricular arrhythmias or hypertension, bradycardia, heart block, systolic BP < 90 mm Hg, obstructive airways disease, unstable diabetes or requiring inotropic therapy. • 44 Acute myocardial infarction within 21 days. Use of all adjunctive therapy was encouraged. Clinically stable but may have had pulmonary edema or cardiogenic shock during index infarction.
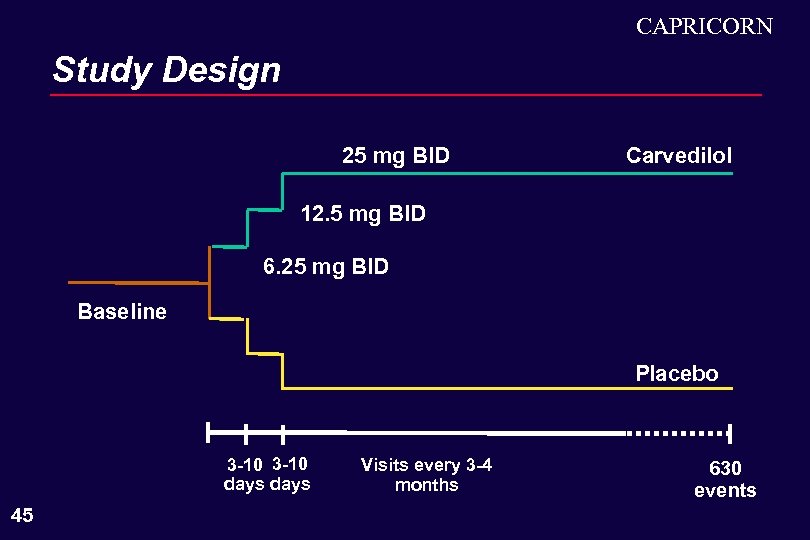 CAPRICORN Study Design 25 mg BID Carvedilol 12. 5 mg BID 6. 25 mg BID Baseline Placebo 3 -10 days 45 Visits every 3 -4 months 630 events
CAPRICORN Study Design 25 mg BID Carvedilol 12. 5 mg BID 6. 25 mg BID Baseline Placebo 3 -10 days 45 Visits every 3 -4 months 630 events
 CAPRICORN Protocol-Specified Endpoints • Primary Endpoint – All cause mortality • Secondary Endpoints – All-cause mortality or CV hospitalization – Sudden death – Progression of heart failure 46
CAPRICORN Protocol-Specified Endpoints • Primary Endpoint – All cause mortality • Secondary Endpoints – All-cause mortality or CV hospitalization – Sudden death – Progression of heart failure 46
 CAPRICORN Statistical Considerations • Sample size was 2600 based on assumption that 21 month mortality would be 29% in the placebo group and that risk of death would be altered by 20% as a result of treatment with carvedilol (90% power, =0. 05). – No allowance provided for open-label use of blockers. • • 47 Trial was to continue until 630 patients had died with minimum follow-up of 12 months. All patients were to be followed until end of study whether they continued taking the study medication
CAPRICORN Statistical Considerations • Sample size was 2600 based on assumption that 21 month mortality would be 29% in the placebo group and that risk of death would be altered by 20% as a result of treatment with carvedilol (90% power, =0. 05). – No allowance provided for open-label use of blockers. • • 47 Trial was to continue until 630 patients had died with minimum follow-up of 12 months. All patients were to be followed until end of study whether they continued taking the study medication
 CAPRICORN DSMB Recommendation (March 1999) • • Since high rate of open-label -blocker use might compromise study and in view of a lower than anticipated mortality rate DSMB recommended adoption of a new endpoint to allow accelerated completion of study. • 48 Enrollment began June 1997. Based on findings of CIBIS II and MERIT-HF (announced in late 1998 and early 1999), DSMB believed that patients developing heart failure should be considered for -blockade. DSMB recommendations made in March 1999 prior to any formal interim analysis of unblinded data.
CAPRICORN DSMB Recommendation (March 1999) • • Since high rate of open-label -blocker use might compromise study and in view of a lower than anticipated mortality rate DSMB recommended adoption of a new endpoint to allow accelerated completion of study. • 48 Enrollment began June 1997. Based on findings of CIBIS II and MERIT-HF (announced in late 1998 and early 1999), DSMB believed that patients developing heart failure should be considered for -blockade. DSMB recommendations made in March 1999 prior to any formal interim analysis of unblinded data.
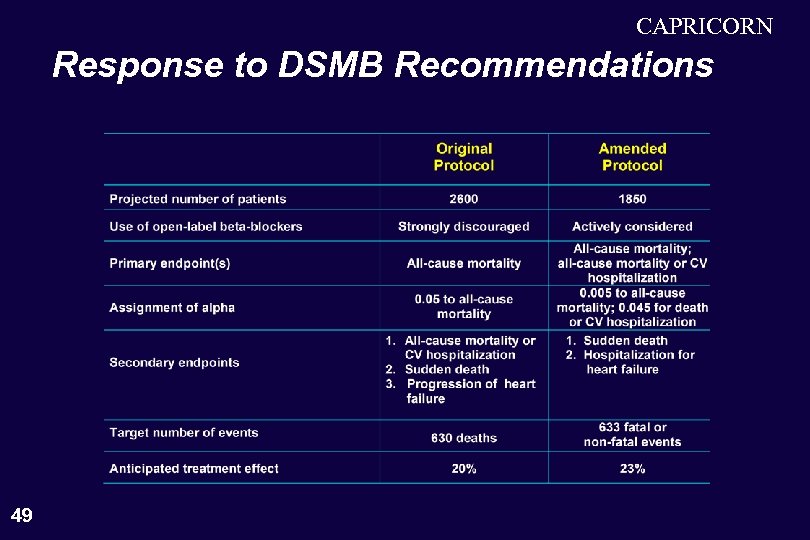 CAPRICORN Response to DSMB Recommendations 49
CAPRICORN Response to DSMB Recommendations 49
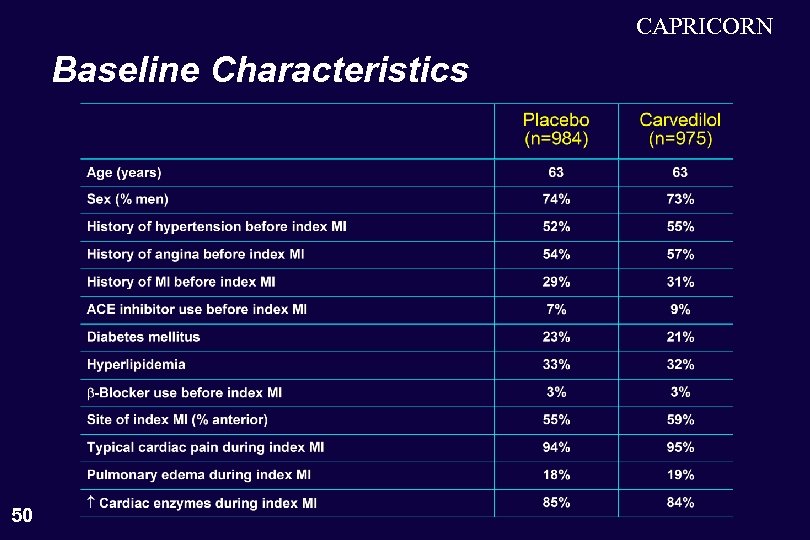 CAPRICORN Baseline Characteristics 50
CAPRICORN Baseline Characteristics 50
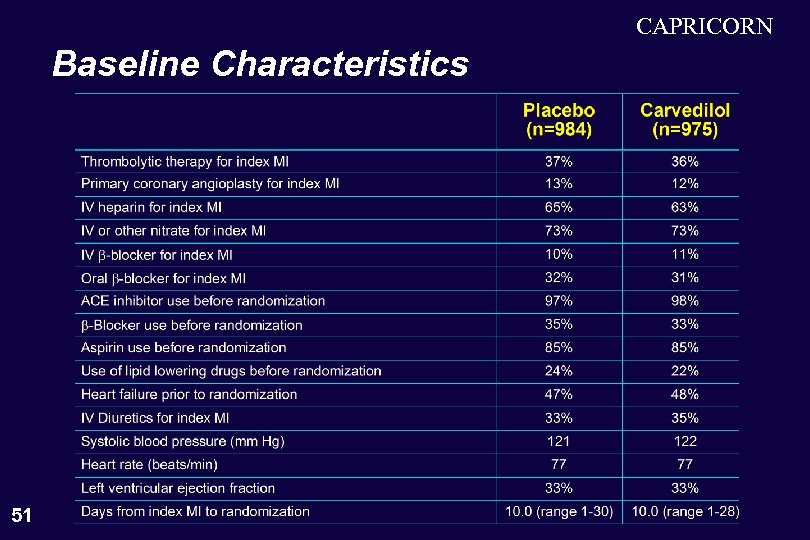 CAPRICORN Baseline Characteristics 51
CAPRICORN Baseline Characteristics 51
 CAPRICORN Patient Disposition • • Ten patients were randomized but did not receive the study drug (included in all analyses). • Target doses achieved in 84% of placebo and 78% of carvedilol patients within 12 weeks and generally maintained for duration of study. • 52 1959 patients randomized (984 placebo, 975 carvedilol) Duration of follow-up: 3 -33 months (mean 15 months)
CAPRICORN Patient Disposition • • Ten patients were randomized but did not receive the study drug (included in all analyses). • Target doses achieved in 84% of placebo and 78% of carvedilol patients within 12 weeks and generally maintained for duration of study. • 52 1959 patients randomized (984 placebo, 975 carvedilol) Duration of follow-up: 3 -33 months (mean 15 months)
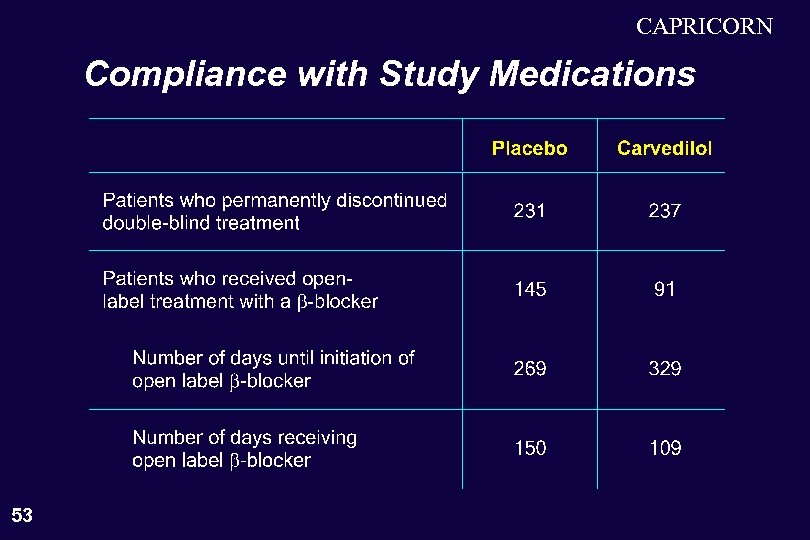 CAPRICORN Compliance with Study Medications 53
CAPRICORN Compliance with Study Medications 53
 CAPRICORN Results on Primary Endpoints • • 54 All-cause mortality or cardiovascular hospitalization All-cause mortality
CAPRICORN Results on Primary Endpoints • • 54 All-cause mortality or cardiovascular hospitalization All-cause mortality
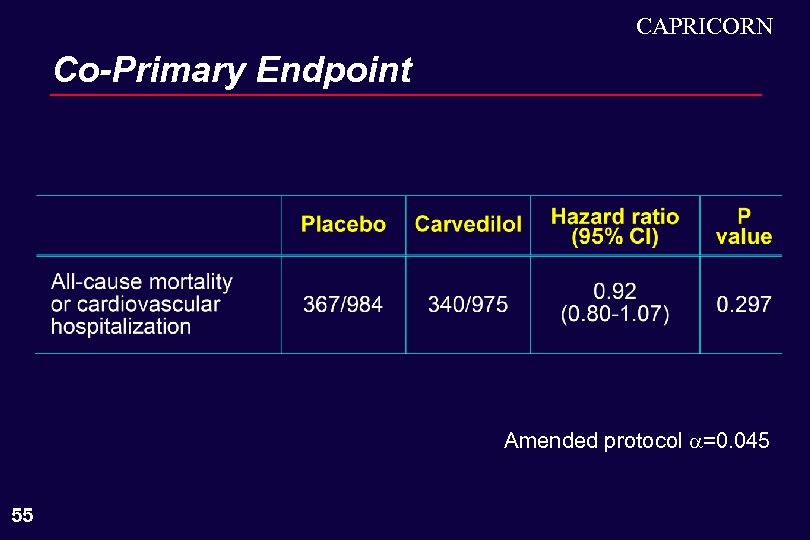 CAPRICORN Co-Primary Endpoint Amended protocol =0. 045 55
CAPRICORN Co-Primary Endpoint Amended protocol =0. 045 55
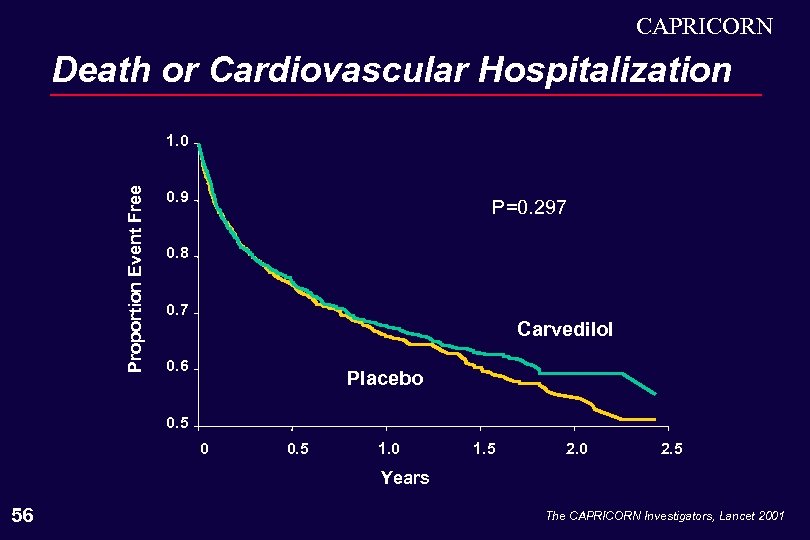 CAPRICORN Death or Cardiovascular Hospitalization Proportion Event Free 1. 0 0. 9 P=0. 297 0. 8 0. 7 Carvedilol 0. 6 Placebo 0. 5 0 0. 5 1. 0 1. 5 2. 0 2. 5 Years 56 The CAPRICORN Investigators, Lancet 2001
CAPRICORN Death or Cardiovascular Hospitalization Proportion Event Free 1. 0 0. 9 P=0. 297 0. 8 0. 7 Carvedilol 0. 6 Placebo 0. 5 0 0. 5 1. 0 1. 5 2. 0 2. 5 Years 56 The CAPRICORN Investigators, Lancet 2001
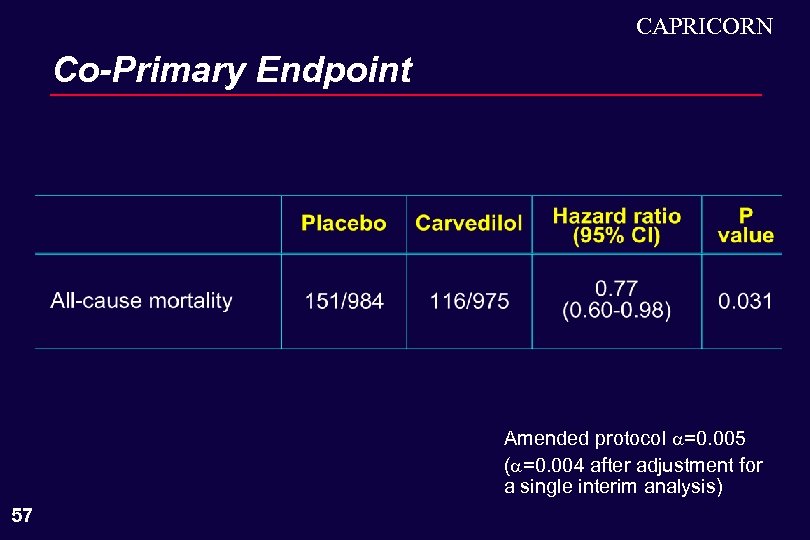 CAPRICORN Co-Primary Endpoint Amended protocol =0. 005 ( =0. 004 after adjustment for a single interim analysis) 57
CAPRICORN Co-Primary Endpoint Amended protocol =0. 005 ( =0. 004 after adjustment for a single interim analysis) 57
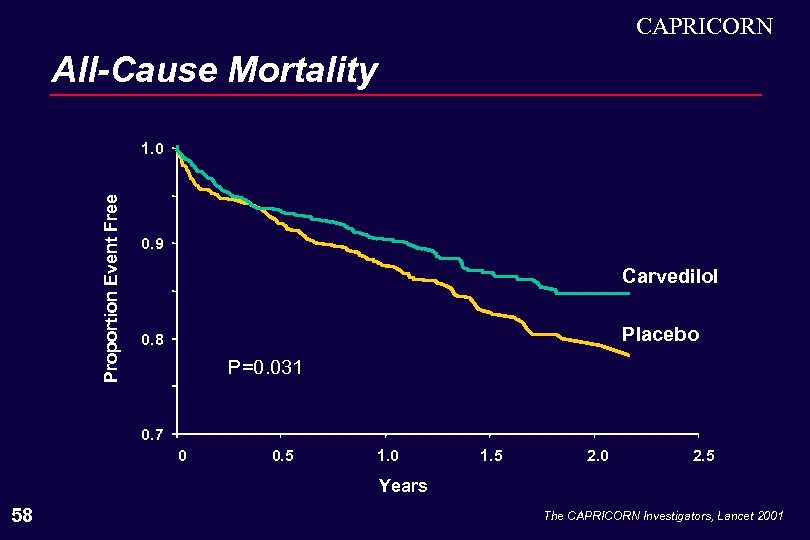 CAPRICORN All-Cause Mortality Proportion Event Free 1. 0 0. 9 Carvedilol Placebo 0. 8 P=0. 031 0. 7 0 0. 5 1. 0 1. 5 2. 0 2. 5 Years 58 The CAPRICORN Investigators, Lancet 2001
CAPRICORN All-Cause Mortality Proportion Event Free 1. 0 0. 9 Carvedilol Placebo 0. 8 P=0. 031 0. 7 0 0. 5 1. 0 1. 5 2. 0 2. 5 Years 58 The CAPRICORN Investigators, Lancet 2001
 Why Are We Here? Milton Packer, M. D.
Why Are We Here? Milton Packer, M. D.
 Critical Questions to the Committee • • 60 Can the findings from a trial that did NOT meet its primary endpoint be used as the primary basis for labeling? If so, what criteria should the data supporting such a finding fulfill to justify incorporation into labeling?
Critical Questions to the Committee • • 60 Can the findings from a trial that did NOT meet its primary endpoint be used as the primary basis for labeling? If so, what criteria should the data supporting such a finding fulfill to justify incorporation into labeling?
 Drugs Approved Based on the Results of Trials That Did Not Achieve Primary Endpoint Digoxin (Lanoxin®) • • 61 Indicated for treatment of mild-to-moderate heart failure to reduce heart failure-related hospitalizations. The trial that observed this benefit (DIG) did not achieve its primary endpoint (all-cause mortality), P=0. 80.
Drugs Approved Based on the Results of Trials That Did Not Achieve Primary Endpoint Digoxin (Lanoxin®) • • 61 Indicated for treatment of mild-to-moderate heart failure to reduce heart failure-related hospitalizations. The trial that observed this benefit (DIG) did not achieve its primary endpoint (all-cause mortality), P=0. 80.
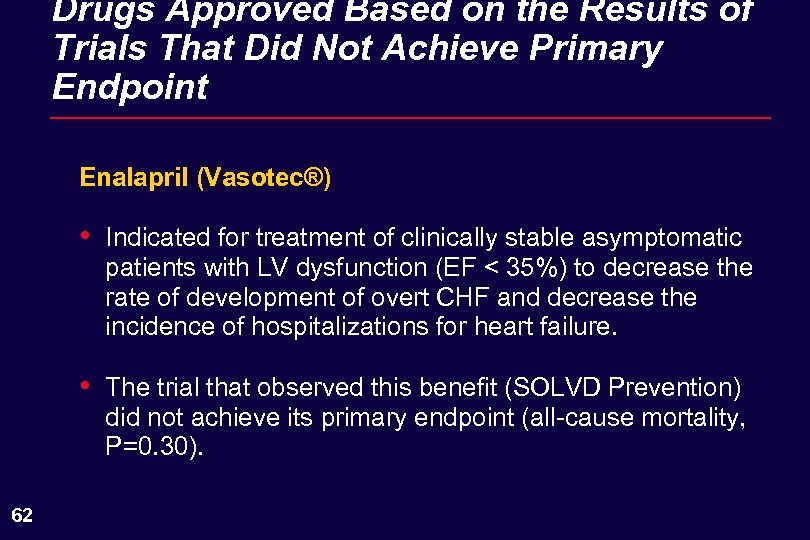 Drugs Approved Based on the Results of Trials That Did Not Achieve Primary Endpoint Enalapril (Vasotec®) • • 62 Indicated for treatment of clinically stable asymptomatic patients with LV dysfunction (EF < 35%) to decrease the rate of development of overt CHF and decrease the incidence of hospitalizations for heart failure. The trial that observed this benefit (SOLVD Prevention) did not achieve its primary endpoint (all-cause mortality, P=0. 30).
Drugs Approved Based on the Results of Trials That Did Not Achieve Primary Endpoint Enalapril (Vasotec®) • • 62 Indicated for treatment of clinically stable asymptomatic patients with LV dysfunction (EF < 35%) to decrease the rate of development of overt CHF and decrease the incidence of hospitalizations for heart failure. The trial that observed this benefit (SOLVD Prevention) did not achieve its primary endpoint (all-cause mortality, P=0. 30).
 Critical Questions to the Committee • 63 Can the findings from a trial that did NOT meet its primary endpoint be used as the primary basis for labeling?
Critical Questions to the Committee • 63 Can the findings from a trial that did NOT meet its primary endpoint be used as the primary basis for labeling?
 Critical Questions to the Committee • • 64 Can the findings from a trial that did NOT meet its primary endpoint be used as the primary basis for labeling? If so, what criteria should the data supporting such a finding fulfill to justify incorporation into labeling?
Critical Questions to the Committee • • 64 Can the findings from a trial that did NOT meet its primary endpoint be used as the primary basis for labeling? If so, what criteria should the data supporting such a finding fulfill to justify incorporation into labeling?
 What Rules Should Guide the Decision to Allow the Inclusion of a “Discovery” Into Labeling? 65
What Rules Should Guide the Decision to Allow the Inclusion of a “Discovery” Into Labeling? 65
 What Rules Should Guide the Decision to Allow the Inclusion of a “Discovery” Into Labeling? • 66 The criteria that would allow inclusion of a “discovery” into labeling should have strength of evidence comparable to that which would allow labeling based on a trial or trials that achieved their primary endpoints.
What Rules Should Guide the Decision to Allow the Inclusion of a “Discovery” Into Labeling? • 66 The criteria that would allow inclusion of a “discovery” into labeling should have strength of evidence comparable to that which would allow labeling based on a trial or trials that achieved their primary endpoints.
 What Rules Should Guide the Decision to Allow the Inclusion of a “Discovery” Into Labeling? • • 67 The criteria that would allow inclusion of a “discovery” into labeling should have strength of evidence comparable to that which would allow labeling based on a trial or trials that achieved their primary endpoints. Do such criteria allow one to conclude that carvedilol reduces mortality in post-infarction patients with LV dysfunction?
What Rules Should Guide the Decision to Allow the Inclusion of a “Discovery” Into Labeling? • • 67 The criteria that would allow inclusion of a “discovery” into labeling should have strength of evidence comparable to that which would allow labeling based on a trial or trials that achieved their primary endpoints. Do such criteria allow one to conclude that carvedilol reduces mortality in post-infarction patients with LV dysfunction?
 Death is a Unique Endpoint • • 68 The finding of a reduction in the risk of death associated with treatment is always compelling, since death is an unbiased endpoint of paramount clinical importance. FDA review: “FDA has acted as if all clinical trials implicitly have =0. 05 assigned to an analysis of mortality, independent of the primary end point. ”
Death is a Unique Endpoint • • 68 The finding of a reduction in the risk of death associated with treatment is always compelling, since death is an unbiased endpoint of paramount clinical importance. FDA review: “FDA has acted as if all clinical trials implicitly have =0. 05 assigned to an analysis of mortality, independent of the primary end point. ”
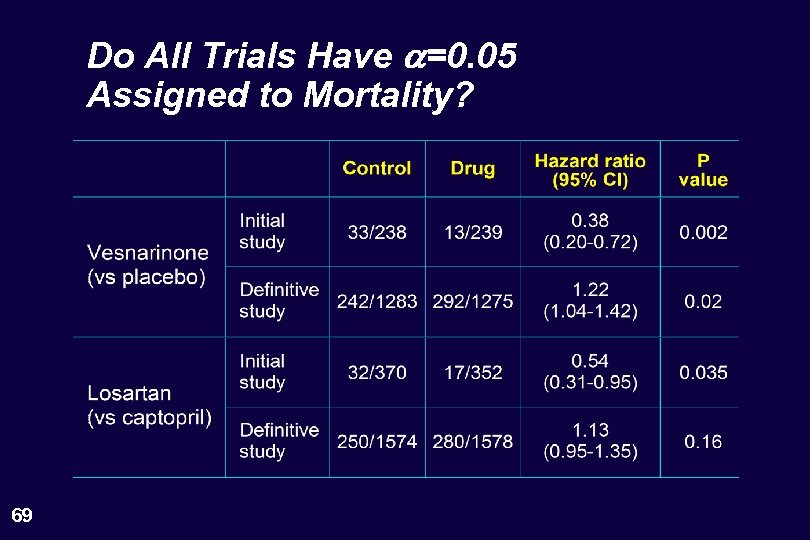 Do All Trials Have a=0. 05 Assigned to Mortality? 69
Do All Trials Have a=0. 05 Assigned to Mortality? 69
 CAPRICORN • • The CAPRICORN Trial was designed specifically to evaluate the effect of carvedilol on mortality. • 70 The mortality effect seen in CAPRICORN was not a accidental “discovery”. Large number of events (n=267) with high annual placebo mortality rate (12. 1%).
CAPRICORN • • The CAPRICORN Trial was designed specifically to evaluate the effect of carvedilol on mortality. • 70 The mortality effect seen in CAPRICORN was not a accidental “discovery”. Large number of events (n=267) with high annual placebo mortality rate (12. 1%).
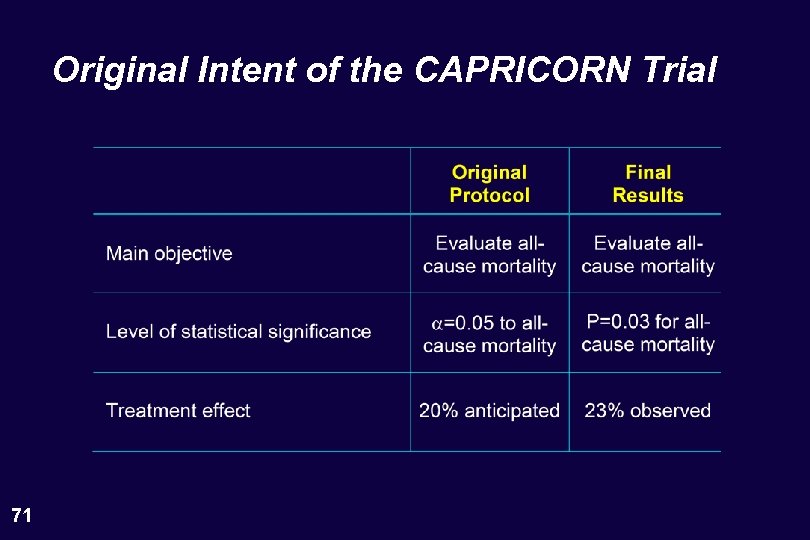 Original Intent of the CAPRICORN Trial 71
Original Intent of the CAPRICORN Trial 71
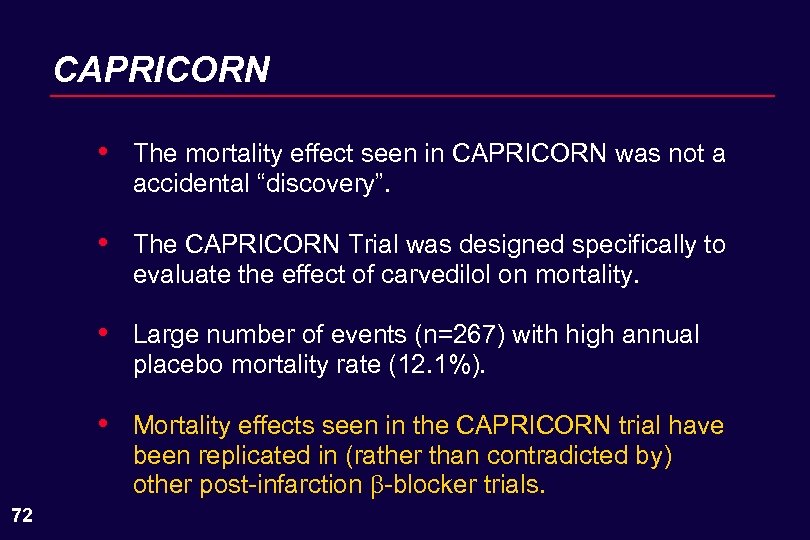 CAPRICORN • • The CAPRICORN Trial was designed specifically to evaluate the effect of carvedilol on mortality. • Large number of events (n=267) with high annual placebo mortality rate (12. 1%). • 72 The mortality effect seen in CAPRICORN was not a accidental “discovery”. Mortality effects seen in the CAPRICORN trial have been replicated in (rather than contradicted by) other post-infarction -blocker trials.
CAPRICORN • • The CAPRICORN Trial was designed specifically to evaluate the effect of carvedilol on mortality. • Large number of events (n=267) with high annual placebo mortality rate (12. 1%). • 72 The mortality effect seen in CAPRICORN was not a accidental “discovery”. Mortality effects seen in the CAPRICORN trial have been replicated in (rather than contradicted by) other post-infarction -blocker trials.
 Do All Trials Have an Implicit a Assigned to the Analysis of Mortality? • Even if one assumes that all trials implicitly have an =0. 05 assigned to mortality, how persuasive is the P=0. 031 observed for the mortality effect of carvedilol in the CAPRICORN trial? • The =0. 005 assigned to mortality in CAPRICORN set an extremely high standard of reproducibility — achieved by one trial with a very small P value or two or more trials with P < 0. 05. 73
Do All Trials Have an Implicit a Assigned to the Analysis of Mortality? • Even if one assumes that all trials implicitly have an =0. 05 assigned to mortality, how persuasive is the P=0. 031 observed for the mortality effect of carvedilol in the CAPRICORN trial? • The =0. 005 assigned to mortality in CAPRICORN set an extremely high standard of reproducibility — achieved by one trial with a very small P value or two or more trials with P < 0. 05. 73
 b-Blockers Shown to Reduce Mortality in a Large-Scale Controlled Clinical Trial • • Metoprolol • Propranolol • Acebutolol • 74 Timolol Practolol
b-Blockers Shown to Reduce Mortality in a Large-Scale Controlled Clinical Trial • • Metoprolol • Propranolol • Acebutolol • 74 Timolol Practolol
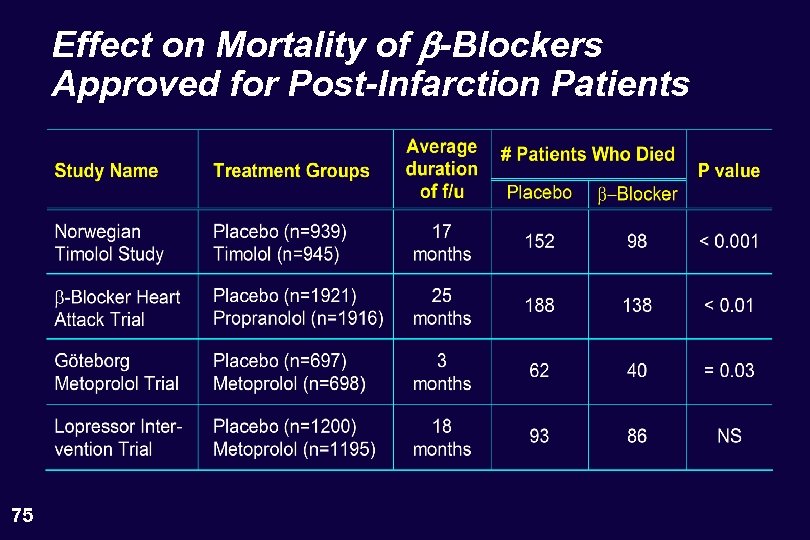 Effect on Mortality of b-Blockers Approved for Post-Infarction Patients 75
Effect on Mortality of b-Blockers Approved for Post-Infarction Patients 75
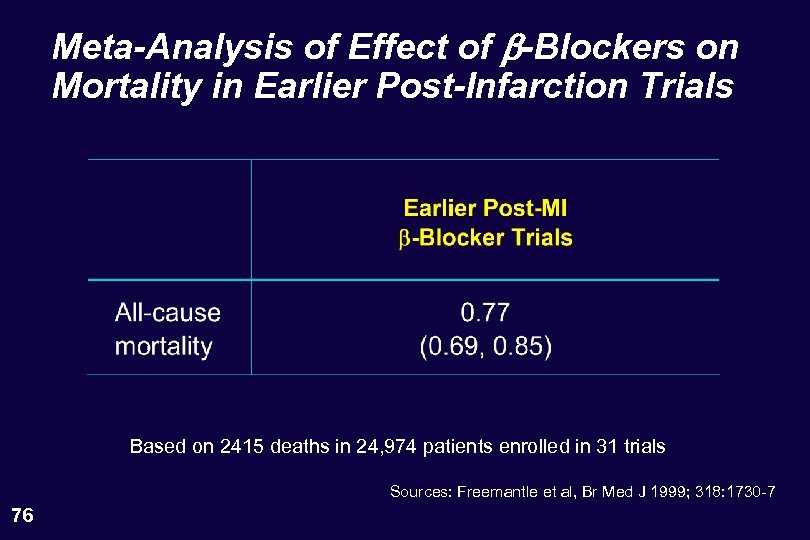 Meta-Analysis of Effect of b-Blockers on Mortality in Earlier Post-Infarction Trials Based on 2415 deaths in 24, 974 patients enrolled in 31 trials Sources: Freemantle et al, Br Med J 1999; 318: 1730 -7 76
Meta-Analysis of Effect of b-Blockers on Mortality in Earlier Post-Infarction Trials Based on 2415 deaths in 24, 974 patients enrolled in 31 trials Sources: Freemantle et al, Br Med J 1999; 318: 1730 -7 76
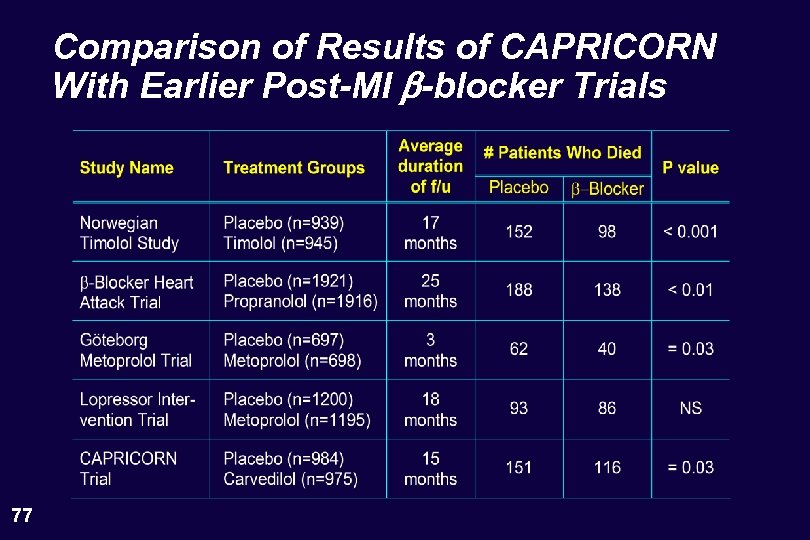 Comparison of Results of CAPRICORN With Earlier Post-MI b-blocker Trials 77
Comparison of Results of CAPRICORN With Earlier Post-MI b-blocker Trials 77
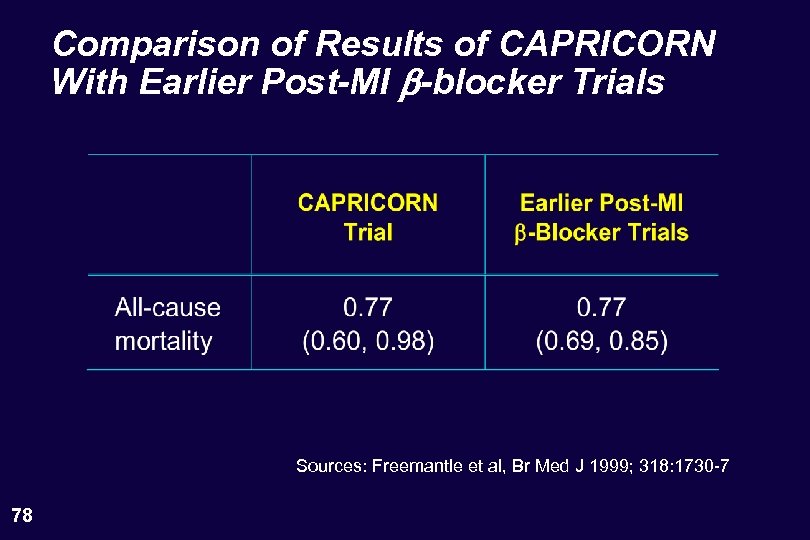 Comparison of Results of CAPRICORN With Earlier Post-MI b-blocker Trials Sources: Freemantle et al, Br Med J 1999; 318: 1730 -7 78
Comparison of Results of CAPRICORN With Earlier Post-MI b-blocker Trials Sources: Freemantle et al, Br Med J 1999; 318: 1730 -7 78
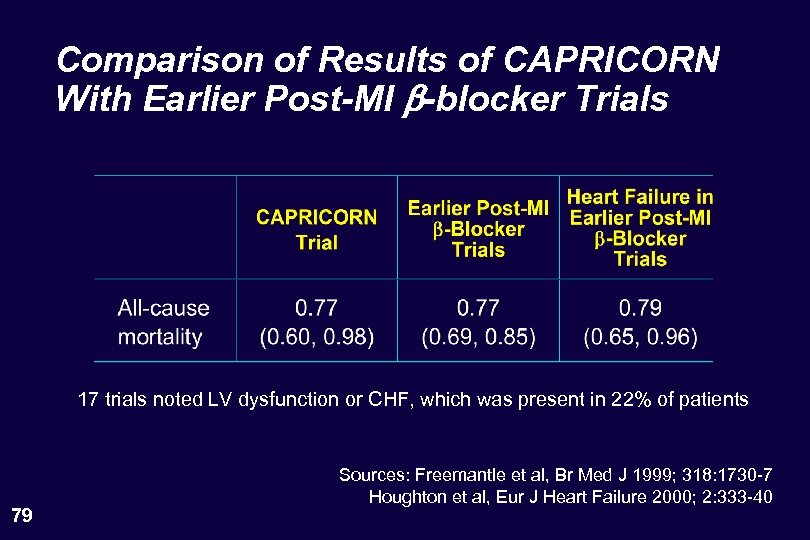 Comparison of Results of CAPRICORN With Earlier Post-MI b-blocker Trials 17 trials noted LV dysfunction or CHF, which was present in 22% of patients 79 Sources: Freemantle et al, Br Med J 1999; 318: 1730 -7 Houghton et al, Eur J Heart Failure 2000; 2: 333 -40
Comparison of Results of CAPRICORN With Earlier Post-MI b-blocker Trials 17 trials noted LV dysfunction or CHF, which was present in 22% of patients 79 Sources: Freemantle et al, Br Med J 1999; 318: 1730 -7 Houghton et al, Eur J Heart Failure 2000; 2: 333 -40
 Is It Appropriate To Consider the Results of Other Post-Infarction b-Blocker Trials? 80
Is It Appropriate To Consider the Results of Other Post-Infarction b-Blocker Trials? 80
 Is It Appropriate To Consider the Results of Other Post-Infarction b-Blocker Trials? Angiotensin Antagonists in Diabetic Nephropathy • 81 The Committee expressed skepticism about approvability of losartan based on a single trial — in the absence of other evidence.
Is It Appropriate To Consider the Results of Other Post-Infarction b-Blocker Trials? Angiotensin Antagonists in Diabetic Nephropathy • 81 The Committee expressed skepticism about approvability of losartan based on a single trial — in the absence of other evidence.
 Is It Appropriate To Consider the Results of Other Post-Infarction b-Blocker Trials? Angiotensin Antagonists in Diabetic Nephropathy • • 82 The Committee expressed skepticism about approvability of losartan based on a single trial — in the absence of other evidence. The Committee recommended approval when findings in the losartan trial were considered together with the highly concordant findings of a similar trial with irbesartan in the same disease — a trial which when considered alone did not lead the Committee to recommend approval of irbesartan.
Is It Appropriate To Consider the Results of Other Post-Infarction b-Blocker Trials? Angiotensin Antagonists in Diabetic Nephropathy • • 82 The Committee expressed skepticism about approvability of losartan based on a single trial — in the absence of other evidence. The Committee recommended approval when findings in the losartan trial were considered together with the highly concordant findings of a similar trial with irbesartan in the same disease — a trial which when considered alone did not lead the Committee to recommend approval of irbesartan.
 Is It Appropriate To Consider the Results of Other Post-Infarction b-Blocker Trials? Assumption Underlying Class Effect • • 83 The Committee believed that neither losartan nor irbesartan had effects that might detract from their ability as angiotensin antagonists to prevent the progression of renal disease. Does carvedilol have effects that might detract from its actions as a beta-blocker to reduce mortality in the postinfarction setting?
Is It Appropriate To Consider the Results of Other Post-Infarction b-Blocker Trials? Assumption Underlying Class Effect • • 83 The Committee believed that neither losartan nor irbesartan had effects that might detract from their ability as angiotensin antagonists to prevent the progression of renal disease. Does carvedilol have effects that might detract from its actions as a beta-blocker to reduce mortality in the postinfarction setting?
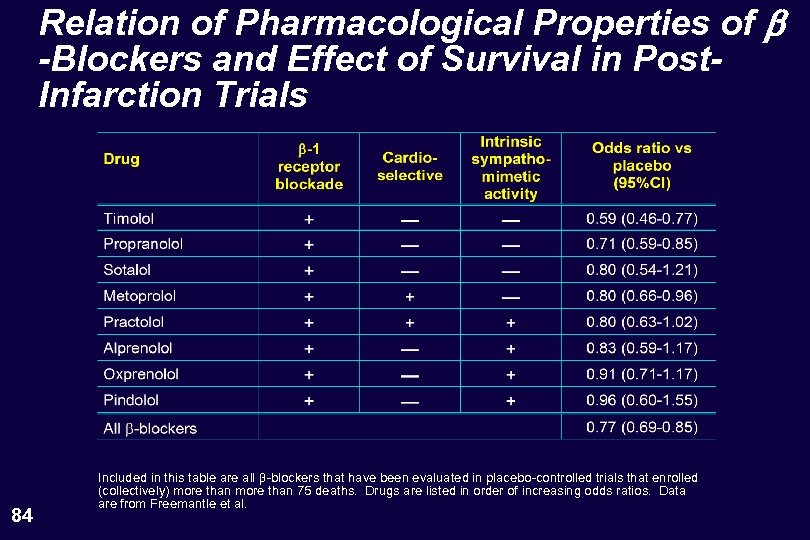 Relation of Pharmacological Properties of b -Blockers and Effect of Survival in Post. Infarction Trials 84 Included in this table are all -blockers that have been evaluated in placebo-controlled trials that enrolled (collectively) more than 75 deaths. Drugs are listed in order of increasing odds ratios. Data are from Freemantle et al.
Relation of Pharmacological Properties of b -Blockers and Effect of Survival in Post. Infarction Trials 84 Included in this table are all -blockers that have been evaluated in placebo-controlled trials that enrolled (collectively) more than 75 deaths. Drugs are listed in order of increasing odds ratios. Data are from Freemantle et al.
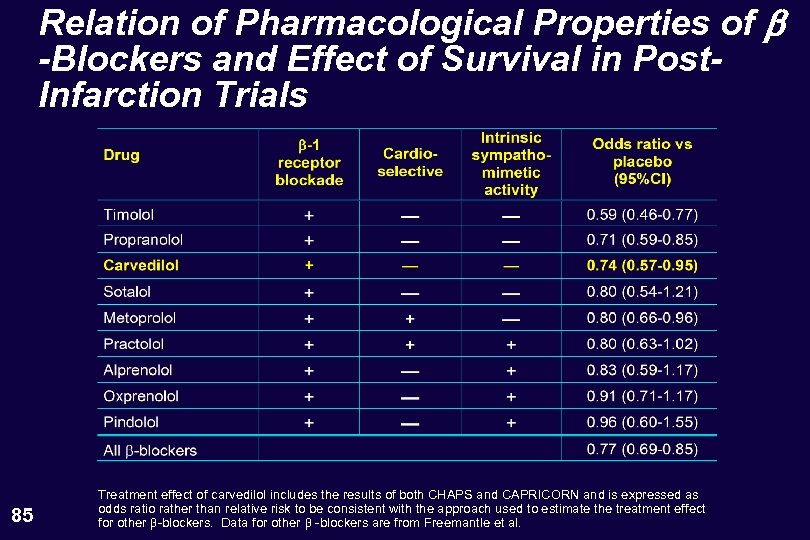 Relation of Pharmacological Properties of b -Blockers and Effect of Survival in Post. Infarction Trials 85 Treatment effect of carvedilol includes the results of both CHAPS and CAPRICORN and is expressed as odds ratio rather than relative risk to be consistent with the approach used to estimate the treatment effect for other -blockers. Data for other -blockers are from Freemantle et al.
Relation of Pharmacological Properties of b -Blockers and Effect of Survival in Post. Infarction Trials 85 Treatment effect of carvedilol includes the results of both CHAPS and CAPRICORN and is expressed as odds ratio rather than relative risk to be consistent with the approach used to estimate the treatment effect for other -blockers. Data for other -blockers are from Freemantle et al.
 Myocardial Injury Chronic Heart Failure LV dysfunction 86 Class III Class IV
Myocardial Injury Chronic Heart Failure LV dysfunction 86 Class III Class IV
 Myocardial Injury Chronic Heart Failure LV dysfunction 87 Class III Class IV
Myocardial Injury Chronic Heart Failure LV dysfunction 87 Class III Class IV
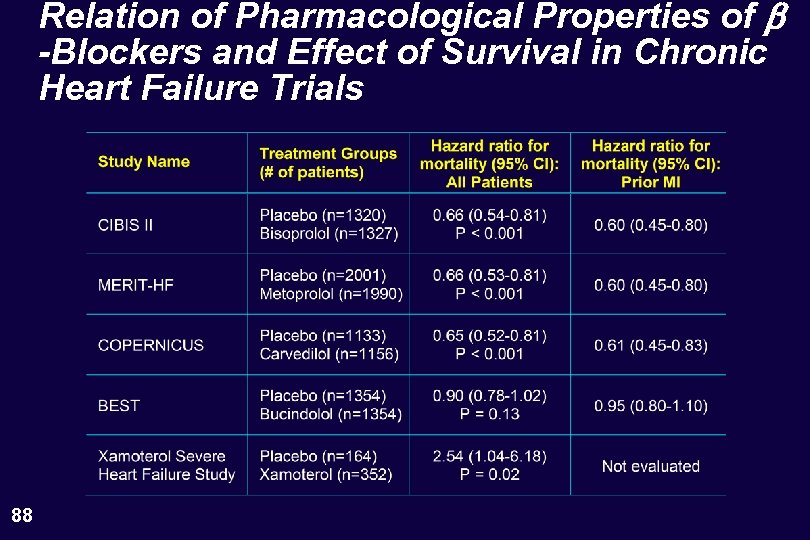 Relation of Pharmacological Properties of b -Blockers and Effect of Survival in Chronic Heart Failure Trials 88
Relation of Pharmacological Properties of b -Blockers and Effect of Survival in Chronic Heart Failure Trials 88
 Is It Appropriate To Consider the Results of Other Post-Infarction b-Blocker Trials? • • Drugs classified as -blockers can exert effects that may detract from their ability as -blockers to reduce mortality, and current approaches are able to detect such effects. • The pharmacological properties of -blockers that may diminish their survival effects appear to be similar in the postinfarction setting and in chronic heart failure. • 89 Long-term blockade of -adrenergic receptors can be expected to reduce mortality in post-infarction patients. The observed effects of carvedilol in both post-MI patients and in chronic CHF indicate that the drug does not exert effects that detract from its action as a -blocker to prolong life.
Is It Appropriate To Consider the Results of Other Post-Infarction b-Blocker Trials? • • Drugs classified as -blockers can exert effects that may detract from their ability as -blockers to reduce mortality, and current approaches are able to detect such effects. • The pharmacological properties of -blockers that may diminish their survival effects appear to be similar in the postinfarction setting and in chronic heart failure. • 89 Long-term blockade of -adrenergic receptors can be expected to reduce mortality in post-infarction patients. The observed effects of carvedilol in both post-MI patients and in chronic CHF indicate that the drug does not exert effects that detract from its action as a -blocker to prolong life.
 The Critical Question Is the totality of available data sufficiently credible and persuasive to conclude that carvedilol reduces mortality in the postinfarction patient with LV dysfunction? 90
The Critical Question Is the totality of available data sufficiently credible and persuasive to conclude that carvedilol reduces mortality in the postinfarction patient with LV dysfunction? 90
 CAPRICORN 91 Mortality reduction of anticipated magnitude seen in trial designed to find it
CAPRICORN 91 Mortality reduction of anticipated magnitude seen in trial designed to find it
 Betablocker studies in post MI pts Earlier post-MI -blocker trials CAPRICORN 92
Betablocker studies in post MI pts Earlier post-MI -blocker trials CAPRICORN 92
 Betablocker studies in post MI pts Earlier post-MI -blocker trials CAPRICORN US Trials COPERNICUS Carvedilol studies in LV dysfunction on top of ACE inhibitor 93
Betablocker studies in post MI pts Earlier post-MI -blocker trials CAPRICORN US Trials COPERNICUS Carvedilol studies in LV dysfunction on top of ACE inhibitor 93
 Class Betablocker studies in post MI pts Earlier post-MI -blocker trials CAPRICORN US Trials COPERNICUS Carvedilol studies in LV dysfunction 94 Time
Class Betablocker studies in post MI pts Earlier post-MI -blocker trials CAPRICORN US Trials COPERNICUS Carvedilol studies in LV dysfunction 94 Time
 What Rules Should Guide the Decision to Allow the Inclusion of a “Discovery” Into Labeling? • Finding should be be a reduction in all-cause in a trial designed to Finding should a reduction in all-cause mortality in a trial detect the finding designed to detect the finding • Magnitude of the benefit anticipated by the Magnitude of the benefit anticipated protocol • Observed magnitude of benefit both clinically relevant and Observed magnitude of benefitclinically relevant and realistic, with conclusions about benefit based on a meaningful number of events Substantial evidence of a similar benefit (both in nature and magnitude) Substantial evidence of a similar benefit (both in nature and in the same disease state with other members magnitude) in the same other members of the same class of drug of the same class of that the drug produces the same benefit later in the drug Substantial evidence that the drug produces the same benefit same in the same disease, magnitude of benefit to that with of later disease, with comparable magnitude other members of the class benefit to that with other members of the class Support within the trial by additional evidence of clinical benefits without Support within the trial by additional evidence of clinical overriding safety concerns benefits without overriding safety concerns • • • 95
What Rules Should Guide the Decision to Allow the Inclusion of a “Discovery” Into Labeling? • Finding should be be a reduction in all-cause in a trial designed to Finding should a reduction in all-cause mortality in a trial detect the finding designed to detect the finding • Magnitude of the benefit anticipated by the Magnitude of the benefit anticipated protocol • Observed magnitude of benefit both clinically relevant and Observed magnitude of benefitclinically relevant and realistic, with conclusions about benefit based on a meaningful number of events Substantial evidence of a similar benefit (both in nature and magnitude) Substantial evidence of a similar benefit (both in nature and in the same disease state with other members magnitude) in the same other members of the same class of drug of the same class of that the drug produces the same benefit later in the drug Substantial evidence that the drug produces the same benefit same in the same disease, magnitude of benefit to that with of later disease, with comparable magnitude other members of the class benefit to that with other members of the class Support within the trial by additional evidence of clinical benefits without Support within the trial by additional evidence of clinical overriding safety concerns benefits without overriding safety concerns • • • 95
 What Rules Should Guide the Decision to Allow the Inclusion of a “Discovery” Into Labeling? • Finding should be a reduction in all-cause mortality in a trial designed to detect the finding • Magnitude of the benefit anticipated by the protocol • Observed magnitude of benefit both clinically relevant and realistic, with conclusions about benefit based on a meaningful number of events • Substantial evidence of a similar benefit (both in nature and magnitude) in the same disease state with other members of the same class of drug • Substantial evidence that the drug produces the same benefit later in the same disease, with comparable magnitude of benefit to that with other members of the class • Support within the trial by additional evidence of clinical benefits without overriding safety concerns 96
What Rules Should Guide the Decision to Allow the Inclusion of a “Discovery” Into Labeling? • Finding should be a reduction in all-cause mortality in a trial designed to detect the finding • Magnitude of the benefit anticipated by the protocol • Observed magnitude of benefit both clinically relevant and realistic, with conclusions about benefit based on a meaningful number of events • Substantial evidence of a similar benefit (both in nature and magnitude) in the same disease state with other members of the same class of drug • Substantial evidence that the drug produces the same benefit later in the same disease, with comparable magnitude of benefit to that with other members of the class • Support within the trial by additional evidence of clinical benefits without overriding safety concerns 96
 Question to the Committee • 97 How much are you willing to allow an increase in the false positive rate by accepting data in a clinical trial that missed its primary endpoint?
Question to the Committee • 97 How much are you willing to allow an increase in the false positive rate by accepting data in a clinical trial that missed its primary endpoint?
 Question to the Committee • • 98 How much are you willing to allow an increase in the false positive rate by accepting data in a clinical trial that missed its primary endpoint? In making regulatory decisions based on trials that missed their primary endpoints ― can one reduce the false positive rate to acceptable levels, given the opportunity to consider not just the results of one trial but the totality of available data? If so, how?
Question to the Committee • • 98 How much are you willing to allow an increase in the false positive rate by accepting data in a clinical trial that missed its primary endpoint? In making regulatory decisions based on trials that missed their primary endpoints ― can one reduce the false positive rate to acceptable levels, given the opportunity to consider not just the results of one trial but the totality of available data? If so, how?
 Effect of Carvedilol on Non-Fatal Events in the CAPRICORN Trial Henry Dargie, MB. , Ch. B.
Effect of Carvedilol on Non-Fatal Events in the CAPRICORN Trial Henry Dargie, MB. , Ch. B.
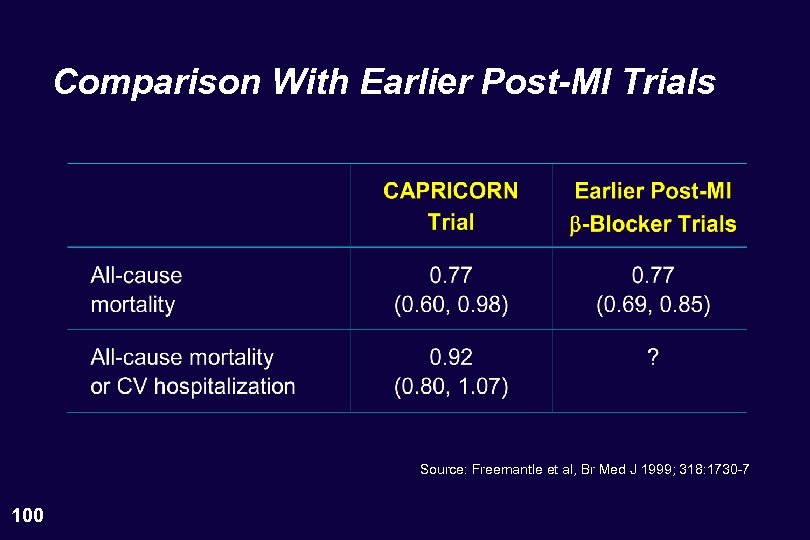 Comparison With Earlier Post-MI Trials Source: Freemantle et al, Br Med J 1999; 318: 1730 -7 100
Comparison With Earlier Post-MI Trials Source: Freemantle et al, Br Med J 1999; 318: 1730 -7 100
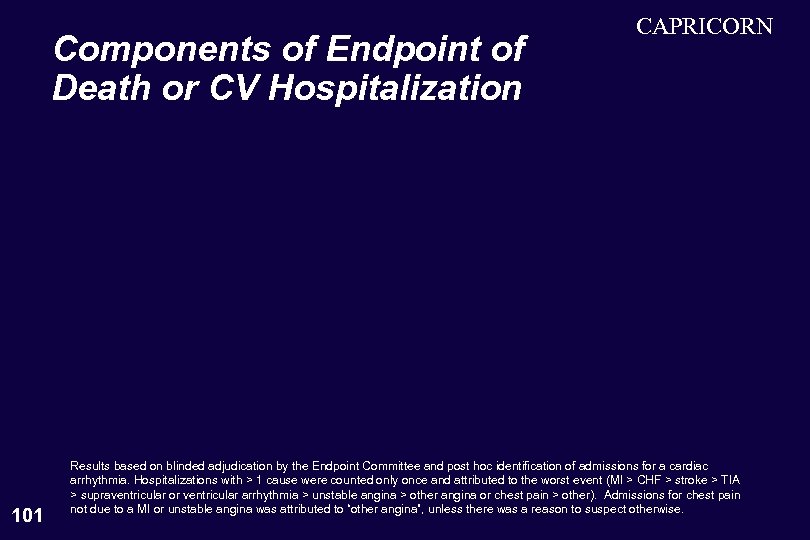 Components of Endpoint of Death or CV Hospitalization 101 CAPRICORN Results based on blinded adjudication by the Endpoint Committee and post hoc identification of admissions for a cardiac arrhythmia. Hospitalizations with > 1 cause were counted only once and attributed to the worst event (MI > CHF > stroke > TIA > supraventricular or ventricular arrhythmia > unstable angina > other angina or chest pain > other). Admissions for chest pain not due to a MI or unstable angina was attributed to “other angina”, unless there was a reason to suspect otherwise.
Components of Endpoint of Death or CV Hospitalization 101 CAPRICORN Results based on blinded adjudication by the Endpoint Committee and post hoc identification of admissions for a cardiac arrhythmia. Hospitalizations with > 1 cause were counted only once and attributed to the worst event (MI > CHF > stroke > TIA > supraventricular or ventricular arrhythmia > unstable angina > other angina or chest pain > other). Admissions for chest pain not due to a MI or unstable angina was attributed to “other angina”, unless there was a reason to suspect otherwise.
 Endpoints Used in Other Post-MI b-Blocker Trials 102
Endpoints Used in Other Post-MI b-Blocker Trials 102
 Effect of Propranolol on Cardiovascular Events Other Than Reinfarction in the BHAT Trial 103 Source: JAMA 183; 250: 2814 -2819
Effect of Propranolol on Cardiovascular Events Other Than Reinfarction in the BHAT Trial 103 Source: JAMA 183; 250: 2814 -2819
 Endpoints in Other LV Dysfunction Trials 104
Endpoints in Other LV Dysfunction Trials 104
 CAPRICORN Effect on Cardiovascular Endpoints 105
CAPRICORN Effect on Cardiovascular Endpoints 105
 CAPRICORN Other Analyses • • Mode of death • Recurrent myocardial infarction • 106 Mortality subgroups Cardiac arrhythmias
CAPRICORN Other Analyses • • Mode of death • Recurrent myocardial infarction • 106 Mortality subgroups Cardiac arrhythmias
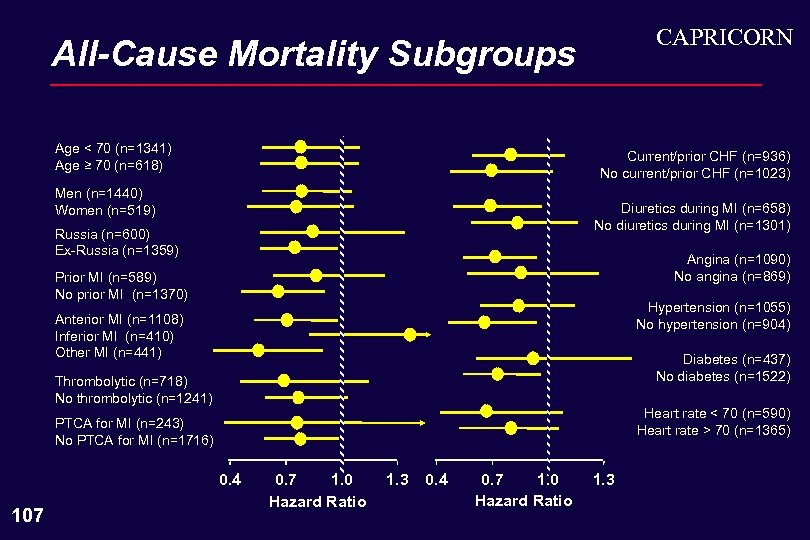 CAPRICORN All-Cause Mortality Subgroups Age < 70 (n=1341) Age ≥ 70 (n=618) Current/prior CHF (n=936) No current/prior CHF (n=1023) Men (n=1440) Women (n=519) Diuretics during MI (n=658) No diuretics during MI (n=1301) Russia (n=600) Ex-Russia (n=1359) Angina (n=1090) No angina (n=869) Prior MI (n=589) No prior MI (n=1370) Hypertension (n=1055) No hypertension (n=904) Anterior MI (n=1108) Inferior MI (n=410) Other MI (n=441) Diabetes (n=437) No diabetes (n=1522) Thrombolytic (n=718) No thrombolytic (n=1241) Heart rate < 70 (n=590) Heart rate > 70 (n=1365) PTCA for MI (n=243) No PTCA for MI (n=1716) 0. 4 107 0. 7 1. 0 Hazard Ratio 1. 3 0. 4 0. 7 1. 0 Hazard Ratio 1. 3
CAPRICORN All-Cause Mortality Subgroups Age < 70 (n=1341) Age ≥ 70 (n=618) Current/prior CHF (n=936) No current/prior CHF (n=1023) Men (n=1440) Women (n=519) Diuretics during MI (n=658) No diuretics during MI (n=1301) Russia (n=600) Ex-Russia (n=1359) Angina (n=1090) No angina (n=869) Prior MI (n=589) No prior MI (n=1370) Hypertension (n=1055) No hypertension (n=904) Anterior MI (n=1108) Inferior MI (n=410) Other MI (n=441) Diabetes (n=437) No diabetes (n=1522) Thrombolytic (n=718) No thrombolytic (n=1241) Heart rate < 70 (n=590) Heart rate > 70 (n=1365) PTCA for MI (n=243) No PTCA for MI (n=1716) 0. 4 107 0. 7 1. 0 Hazard Ratio 1. 3 0. 4 0. 7 1. 0 Hazard Ratio 1. 3
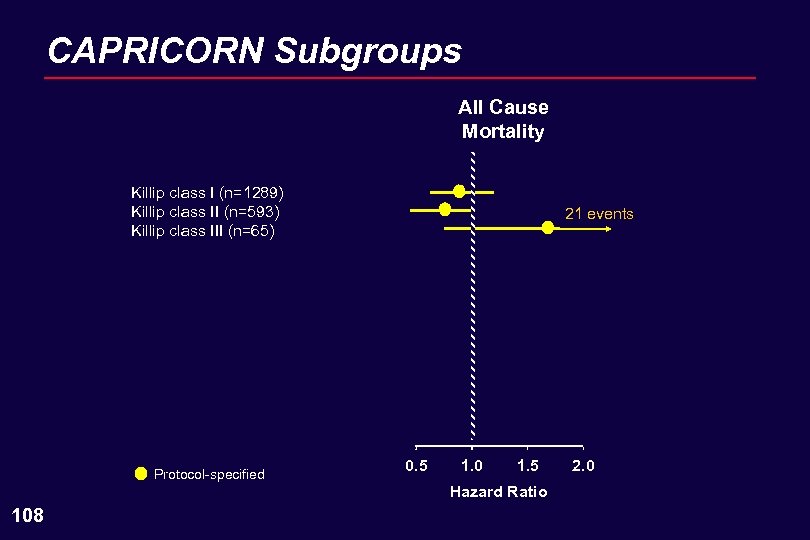 CAPRICORN Subgroups All Cause Mortality Killip class I (n=1289) Killip class II (n=593) Killip class III (n=65) Protocol-specified 21 events 0. 5 1. 0 1. 5 Hazard Ratio 108 2. 0
CAPRICORN Subgroups All Cause Mortality Killip class I (n=1289) Killip class II (n=593) Killip class III (n=65) Protocol-specified 21 events 0. 5 1. 0 1. 5 Hazard Ratio 108 2. 0
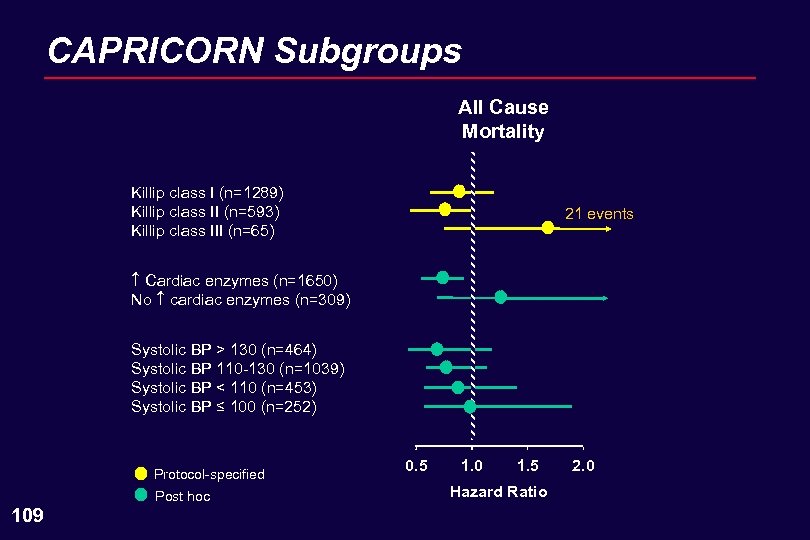 CAPRICORN Subgroups All Cause Mortality Killip class I (n=1289) Killip class II (n=593) Killip class III (n=65) 21 events Cardiac enzymes (n=1650) No cardiac enzymes (n=309) Systolic BP > 130 (n=464) Systolic BP 110 -130 (n=1039) Systolic BP < 110 (n=453) Systolic BP ≤ 100 (n=252) Protocol-specified Post hoc 109 0. 5 1. 0 1. 5 Hazard Ratio 2. 0
CAPRICORN Subgroups All Cause Mortality Killip class I (n=1289) Killip class II (n=593) Killip class III (n=65) 21 events Cardiac enzymes (n=1650) No cardiac enzymes (n=309) Systolic BP > 130 (n=464) Systolic BP 110 -130 (n=1039) Systolic BP < 110 (n=453) Systolic BP ≤ 100 (n=252) Protocol-specified Post hoc 109 0. 5 1. 0 1. 5 Hazard Ratio 2. 0
 Other Post-MI b-Blocker and Other Carvedilol Trials 110
Other Post-MI b-Blocker and Other Carvedilol Trials 110
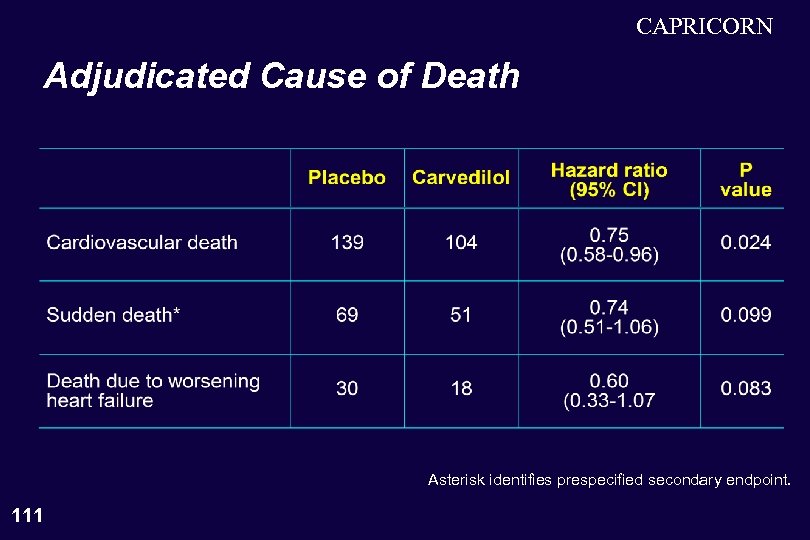 CAPRICORN Adjudicated Cause of Death Asterisk identifies prespecified secondary endpoint. 111
CAPRICORN Adjudicated Cause of Death Asterisk identifies prespecified secondary endpoint. 111
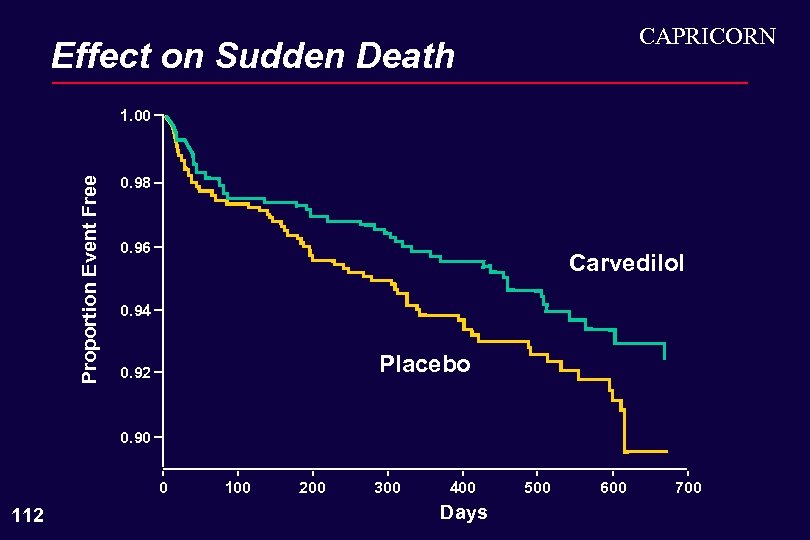 CAPRICORN Effect on Sudden Death Proportion Event Free 1. 00 0. 98 0. 96 Carvedilol 0. 94 Placebo 0. 92 0. 90 0 112 100 200 300 400 Days 500 600 700
CAPRICORN Effect on Sudden Death Proportion Event Free 1. 00 0. 98 0. 96 Carvedilol 0. 94 Placebo 0. 92 0. 90 0 112 100 200 300 400 Days 500 600 700
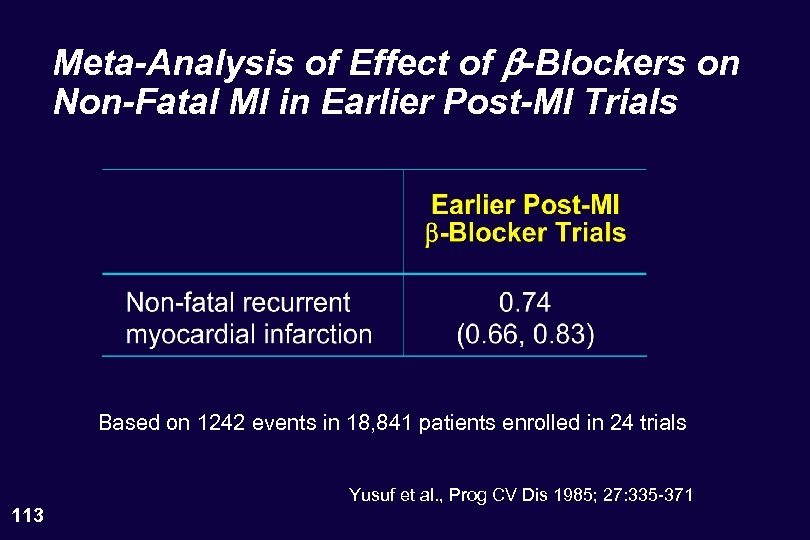 Meta-Analysis of Effect of b-Blockers on Non-Fatal MI in Earlier Post-MI Trials Based on 1242 events in 18, 841 patients enrolled in 24 trials 113 Yusuf et al. , Prog CV Dis 1985; 27: 335 -371
Meta-Analysis of Effect of b-Blockers on Non-Fatal MI in Earlier Post-MI Trials Based on 1242 events in 18, 841 patients enrolled in 24 trials 113 Yusuf et al. , Prog CV Dis 1985; 27: 335 -371
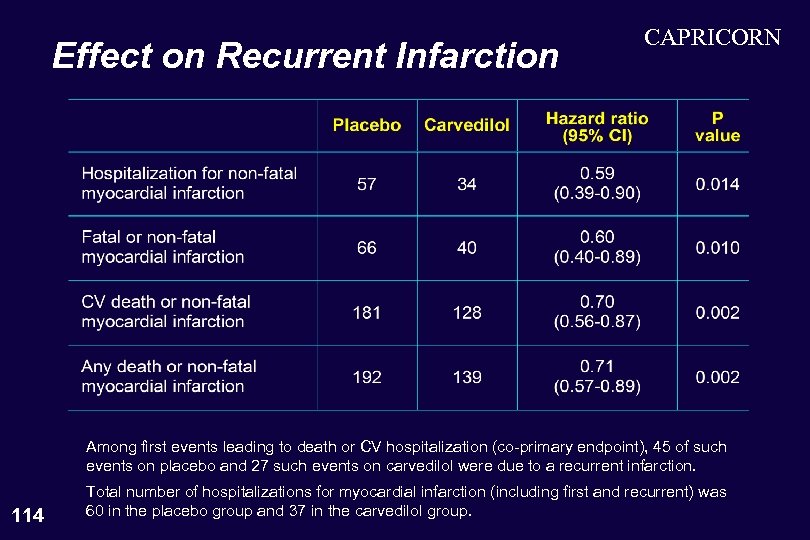 Effect on Recurrent Infarction CAPRICORN Among first events leading to death or CV hospitalization (co-primary endpoint), 45 of such events on placebo and 27 such events on carvedilol were due to a recurrent infarction. 114 Total number of hospitalizations for myocardial infarction (including first and recurrent) was 60 in the placebo group and 37 in the carvedilol group.
Effect on Recurrent Infarction CAPRICORN Among first events leading to death or CV hospitalization (co-primary endpoint), 45 of such events on placebo and 27 such events on carvedilol were due to a recurrent infarction. 114 Total number of hospitalizations for myocardial infarction (including first and recurrent) was 60 in the placebo group and 37 in the carvedilol group.
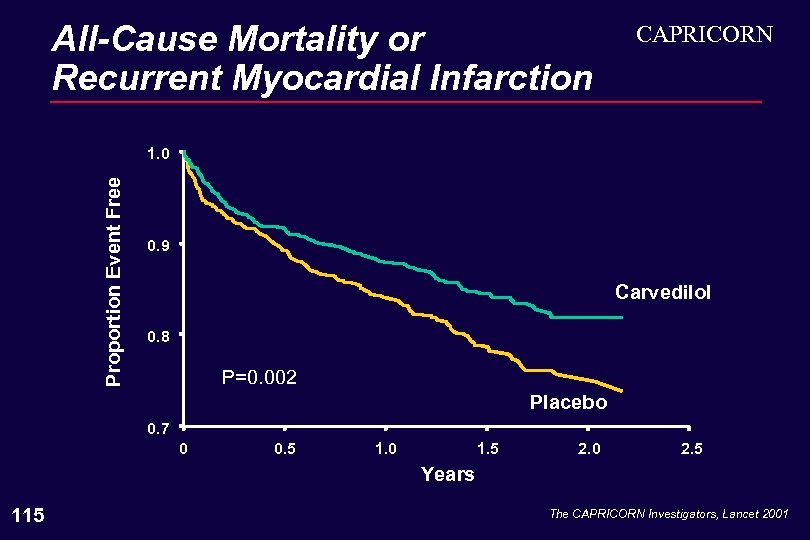 All-Cause Mortality or Recurrent Myocardial Infarction CAPRICORN Proportion Event Free 1. 0 0. 9 Carvedilol 0. 8 P=0. 002 Placebo 0. 7 0 0. 5 1. 0 1. 5 2. 0 2. 5 Years 115 The CAPRICORN Investigators, Lancet 2001
All-Cause Mortality or Recurrent Myocardial Infarction CAPRICORN Proportion Event Free 1. 0 0. 9 Carvedilol 0. 8 P=0. 002 Placebo 0. 7 0 0. 5 1. 0 1. 5 2. 0 2. 5 Years 115 The CAPRICORN Investigators, Lancet 2001
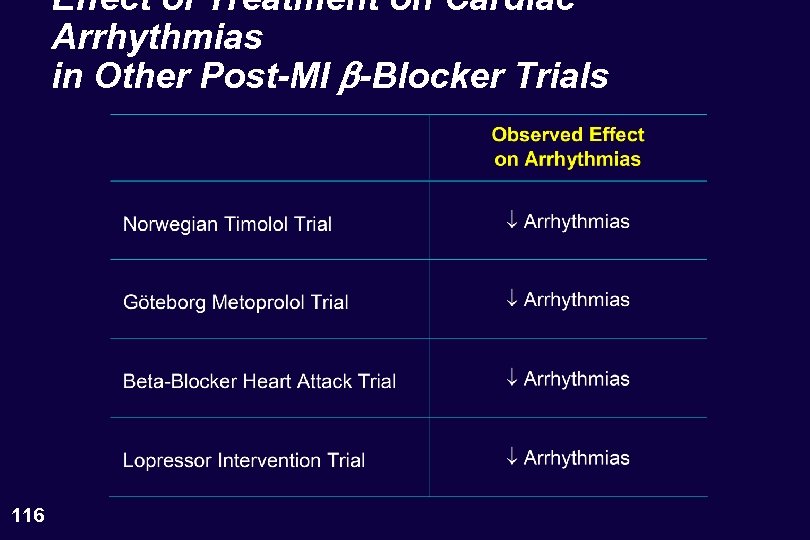 Effect of Treatment on Cardiac Arrhythmias in Other Post-MI b-Blocker Trials 116
Effect of Treatment on Cardiac Arrhythmias in Other Post-MI b-Blocker Trials 116
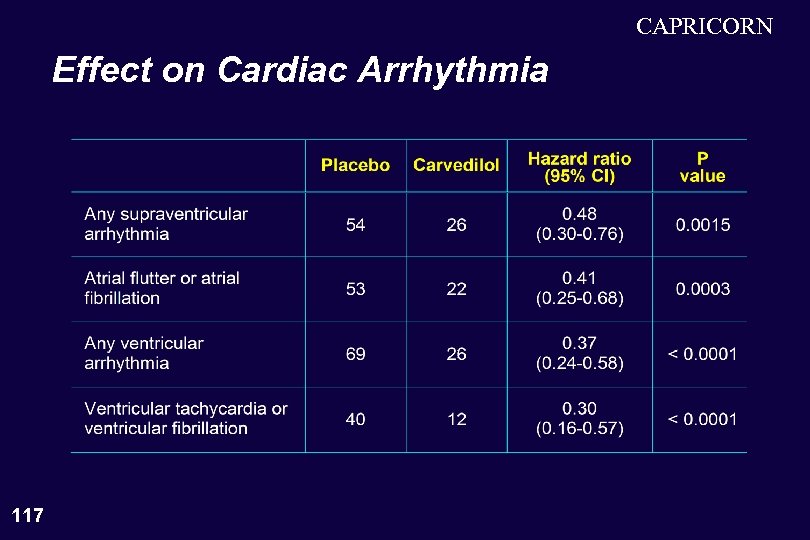 CAPRICORN Effect on Cardiac Arrhythmia 117
CAPRICORN Effect on Cardiac Arrhythmia 117
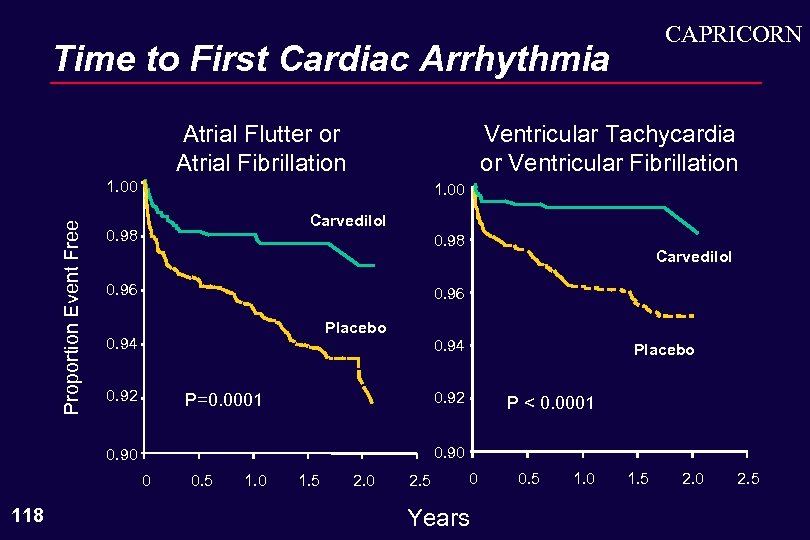 CAPRICORN Time to First Cardiac Arrhythmia Atrial Flutter or Atrial Fibrillation Ventricular Tachycardia or Ventricular Fibrillation Proportion Event Free 1. 00 Carvedilol 0. 98 0. 96 Placebo 0. 94 0. 92 0. 94 Placebo 0. 92 P=0. 0001 P < 0. 0001 0. 90 0 118 Carvedilol 0. 5 1. 0 1. 5 2. 0 2. 5 0 Years 0. 5 1. 0 1. 5 2. 0 2. 5
CAPRICORN Time to First Cardiac Arrhythmia Atrial Flutter or Atrial Fibrillation Ventricular Tachycardia or Ventricular Fibrillation Proportion Event Free 1. 00 Carvedilol 0. 98 0. 96 Placebo 0. 94 0. 92 0. 94 Placebo 0. 92 P=0. 0001 P < 0. 0001 0. 90 0 118 Carvedilol 0. 5 1. 0 1. 5 2. 0 2. 5 0 Years 0. 5 1. 0 1. 5 2. 0 2. 5
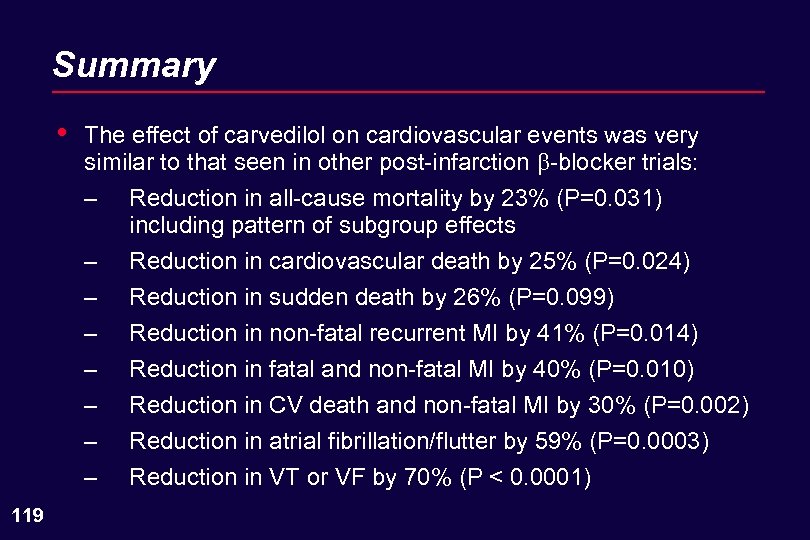 Summary • The effect of carvedilol on cardiovascular events was very similar to that seen in other post-infarction -blocker trials: – – – – 119 Reduction in all-cause mortality by 23% (P=0. 031) including pattern of subgroup effects Reduction in cardiovascular death by 25% (P=0. 024) Reduction in sudden death by 26% (P=0. 099) Reduction in non-fatal recurrent MI by 41% (P=0. 014) Reduction in fatal and non-fatal MI by 40% (P=0. 010) Reduction in CV death and non-fatal MI by 30% (P=0. 002) Reduction in atrial fibrillation/flutter by 59% (P=0. 0003) Reduction in VT or VF by 70% (P < 0. 0001)
Summary • The effect of carvedilol on cardiovascular events was very similar to that seen in other post-infarction -blocker trials: – – – – 119 Reduction in all-cause mortality by 23% (P=0. 031) including pattern of subgroup effects Reduction in cardiovascular death by 25% (P=0. 024) Reduction in sudden death by 26% (P=0. 099) Reduction in non-fatal recurrent MI by 41% (P=0. 014) Reduction in fatal and non-fatal MI by 40% (P=0. 010) Reduction in CV death and non-fatal MI by 30% (P=0. 002) Reduction in atrial fibrillation/flutter by 59% (P=0. 0003) Reduction in VT or VF by 70% (P < 0. 0001)
 Summary • All of these benefits were observed in patients – already taking an ACE inhibitor – receiving all appropriate treatments for the immediate and long term management of postinfarction patients. 120
Summary • All of these benefits were observed in patients – already taking an ACE inhibitor – receiving all appropriate treatments for the immediate and long term management of postinfarction patients. 120
 Safety of Carvedilol in the CAPRICORN Trial and Concluding Remarks Milton Packer, M. D
Safety of Carvedilol in the CAPRICORN Trial and Concluding Remarks Milton Packer, M. D
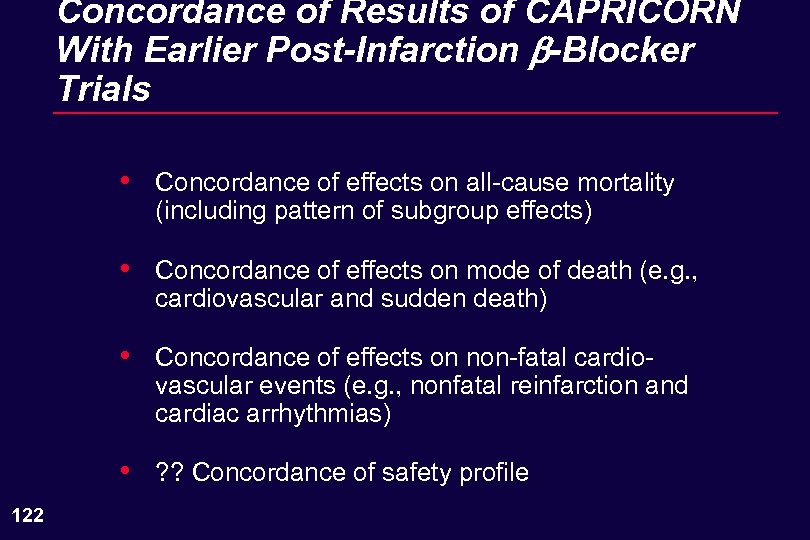 Concordance of Results of CAPRICORN With Earlier Post-Infarction b-Blocker Trials • • Concordance of effects on mode of death (e. g. , cardiovascular and sudden death) • Concordance of effects on non-fatal cardiovascular events (e. g. , nonfatal reinfarction and cardiac arrhythmias) • 122 Concordance of effects on all-cause mortality (including pattern of subgroup effects) ? ? Concordance of safety profile
Concordance of Results of CAPRICORN With Earlier Post-Infarction b-Blocker Trials • • Concordance of effects on mode of death (e. g. , cardiovascular and sudden death) • Concordance of effects on non-fatal cardiovascular events (e. g. , nonfatal reinfarction and cardiac arrhythmias) • 122 Concordance of effects on all-cause mortality (including pattern of subgroup effects) ? ? Concordance of safety profile
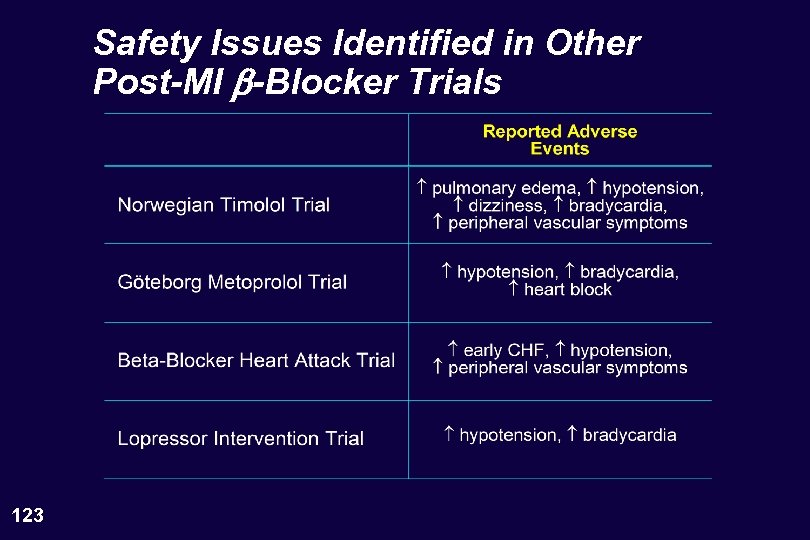 Safety Issues Identified in Other Post-MI b-Blocker Trials 123
Safety Issues Identified in Other Post-MI b-Blocker Trials 123
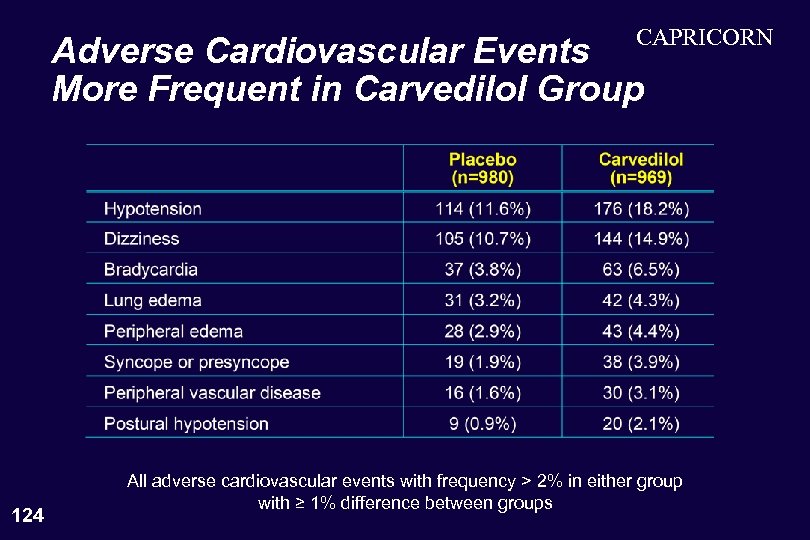 CAPRICORN Adverse Cardiovascular Events More Frequent in Carvedilol Group 124 All adverse cardiovascular events with frequency > 2% in either group with ≥ 1% difference between groups
CAPRICORN Adverse Cardiovascular Events More Frequent in Carvedilol Group 124 All adverse cardiovascular events with frequency > 2% in either group with ≥ 1% difference between groups
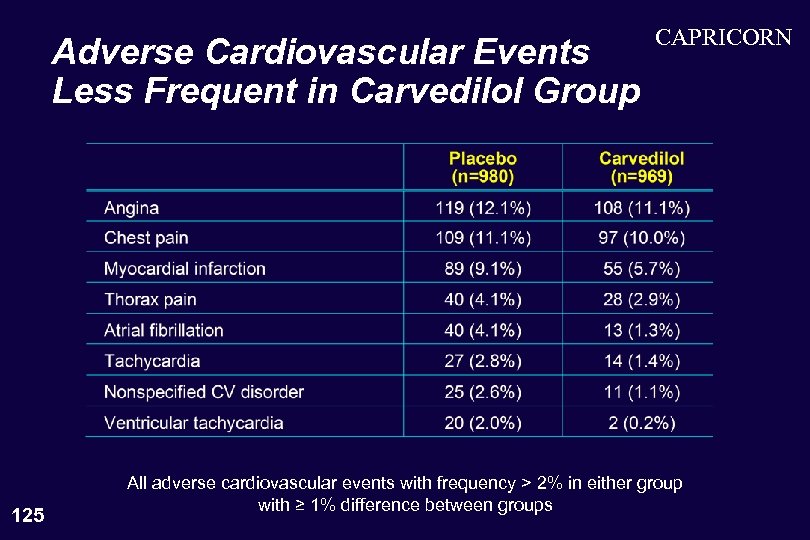 Adverse Cardiovascular Events Less Frequent in Carvedilol Group 125 CAPRICORN All adverse cardiovascular events with frequency > 2% in either group with ≥ 1% difference between groups
Adverse Cardiovascular Events Less Frequent in Carvedilol Group 125 CAPRICORN All adverse cardiovascular events with frequency > 2% in either group with ≥ 1% difference between groups
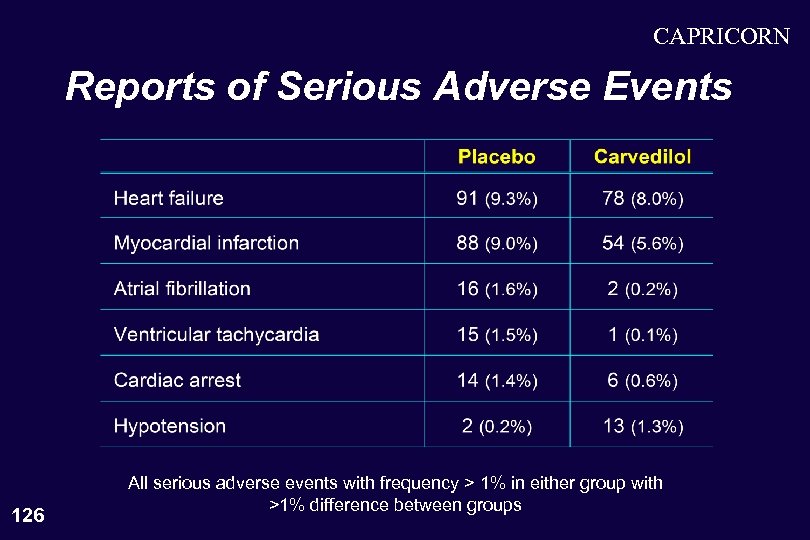 CAPRICORN Reports of Serious Adverse Events 126 All serious adverse events with frequency > 1% in either group with >1% difference between groups
CAPRICORN Reports of Serious Adverse Events 126 All serious adverse events with frequency > 1% in either group with >1% difference between groups
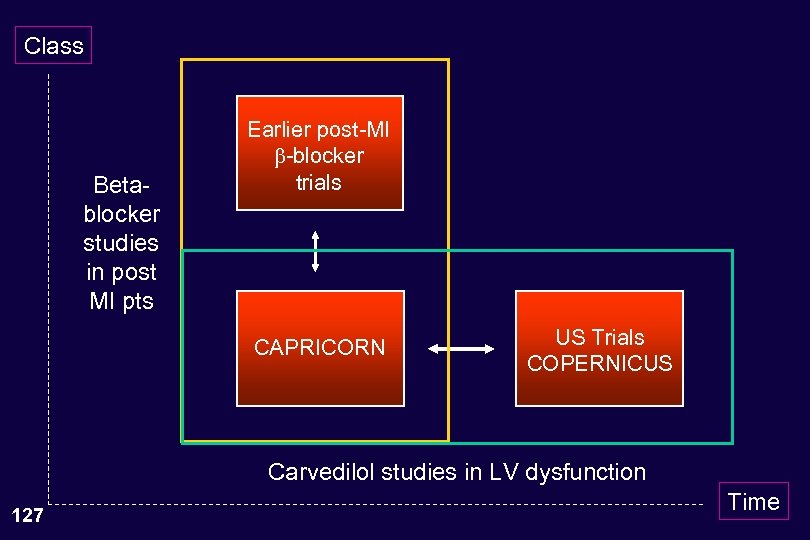 Class Betablocker studies in post MI pts Earlier post-MI -blocker trials CAPRICORN US Trials COPERNICUS Carvedilol studies in LV dysfunction 127 Time
Class Betablocker studies in post MI pts Earlier post-MI -blocker trials CAPRICORN US Trials COPERNICUS Carvedilol studies in LV dysfunction 127 Time
 One Last Question Even if the Committee were to agree that the mortality finding in the CAPRICORN trial is credible and persuasive, why should it recommend incorporation of the results of the CAPRICORN trial into current labeling for carvedilol? 128
One Last Question Even if the Committee were to agree that the mortality finding in the CAPRICORN trial is credible and persuasive, why should it recommend incorporation of the results of the CAPRICORN trial into current labeling for carvedilol? 128
 Is There a Need to Recommend the Approval of Carvedilol for the Post. Infarction Setting? • • All -blockers currently approved for use in infarct survivors carry a contraindication for use in patients with heart failure. • 129 No data to recommend the addition of any -blocker currently approved for use in infarct survivors to an ACE inhibitor (or post-infarction treatments) in patients who have LV dysfunction following their acute infarction. The frequency of use of any -blocker in post-infarction patients with LV dysfunction is low.
Is There a Need to Recommend the Approval of Carvedilol for the Post. Infarction Setting? • • All -blockers currently approved for use in infarct survivors carry a contraindication for use in patients with heart failure. • 129 No data to recommend the addition of any -blocker currently approved for use in infarct survivors to an ACE inhibitor (or post-infarction treatments) in patients who have LV dysfunction following their acute infarction. The frequency of use of any -blocker in post-infarction patients with LV dysfunction is low.
 Is There a Need to Recommend the Approval of Carvedilol for the Post. Infarction Setting? • • Best opportunity for intervention exists when patients are in the hospital after they have been stabilized following their acute infarction. • 130 Such use will remain low unless physicians are educated about the earlier administration of -blockers in patients likely to require them in the future. Among approved -blockers, the most persuasive data in post-infarction patients with LV dysfunction receiving an ACE inhibitor exist for carvedilol.
Is There a Need to Recommend the Approval of Carvedilol for the Post. Infarction Setting? • • Best opportunity for intervention exists when patients are in the hospital after they have been stabilized following their acute infarction. • 130 Such use will remain low unless physicians are educated about the earlier administration of -blockers in patients likely to require them in the future. Among approved -blockers, the most persuasive data in post-infarction patients with LV dysfunction receiving an ACE inhibitor exist for carvedilol.


