321557b80224004b6ccf29164e58b14a.ppt
- Количество слайдов: 34

Intra-abdominal Infections Resident’s Lecture Edward L. Goodman, MD May 1, 2006

Outline • • Pathogenesis of IAI Magnitude of problem Questions and Controversy Antimicrobial Regimens
Complicated Intra-Abdominal Infections Definition Extends beyond the hollow viscus of origin into the peritoneal space Associated either with abscess formation or peritonitis Requires either operative or percutaneous intervention to resolve Medical Illustration Copyright © 2005 Nucleus Medical Art, All rights reserved. www. nucleusinc. com Solomkin J et al. Clin Infect Dis. 2003 Oct 15; 37(8): 997 -1005. Mazuski J et al. Surgical Infections. 2002. 3(3): 161 -173.
Complicated Intra-Abdominal Infection Types • Wide variety of conditions – Perforated gastroduodenal ulcers – Biliary tract infections – Small bowel perforations – Complicated Medical Illustration Copyright © 2005 Nucleus Medical Art, All rights reserved. www. nucleusinc. com appendicitis (with abscess or perforation)Goldstein E. Clin Infect Dis 2002 Sep 1; 35(Suppl 1): S 106 -11.
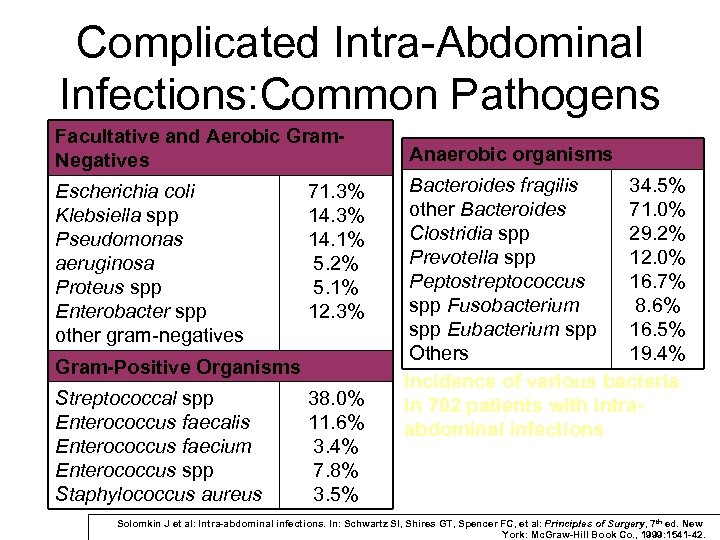
Complicated Intra-Abdominal Infections: Common Pathogens Facultative and Aerobic Gram. Negatives Escherichia coli Klebsiella spp Pseudomonas aeruginosa Proteus spp Enterobacter spp other gram-negatives 71. 3% 14. 1% 5. 2% 5. 1% 12. 3% Gram-Positive Organisms Streptococcal spp Enterococcus faecalis Enterococcus faecium Enterococcus spp Staphylococcus aureus 38. 0% 11. 6% 3. 4% 7. 8% 3. 5% Anaerobic organisms Bacteroides fragilis 34. 5% other Bacteroides 71. 0% Clostridia spp 29. 2% Prevotella spp 12. 0% Peptostreptococcus 16. 7% spp Fusobacterium 8. 6% spp Eubacterium spp 16. 5% Others 19. 4% Incidence of various bacteria in 702 patients with intraabdominal infections Solomkin J et al: Intra-abdominal infections. In: Schwartz SI, Shires GT, Spencer FC, et al: Principles of Surgery, 7 th ed. New York: Mc. Graw-Hill Book Co. , 1999: 1541 -42.
Pathogenesis Weinstein, Onderdonk et al. JID 1975; 132: 282 -286 • Animal models mimic clinical condition – Gelatin capsules with rat feces implanted in peritoneal cavity of rat – Early peritonitis: 37% mortality – Late abscesses in survivors: 100% incidence • Antimicrobial Probes – Gentamicin: acute mortality 4%; abscess in 98% of survivors – Clindamycin: acute mortality 35%; abscess in 5% of survivors – Combination: 7% mortality; 6% abscess
Magnitude of Problem Barie et al. Surg Infect 2004; 5(4): 365 -73 • 465 patients 1991 -2002 Major NYC Hosp – Viscus perforation – Peritonitis (78%) or abscess (22%) – Community acquired 72%, Hospital Acquired 28% • 74% organ dysfunction • 23% mortality
Which Patients Require Therapeutic Administration of ABX? • Considered prophylactic and given <=24 hours – Bowel injuries that are repaired within 12 hours – Acute perforation of stomach, duodenum and proximal jejunum in absence of antacid therapy or malignancy (is there anyone not on Protonix®? ) – Acute appendicitis without gangrene, perforation, abscess or peritonitis

Require ABX? • Acute cholecystitis often not infected – If infection strongly suspected • Empiric therapy directed against enteric GNR – Not necessary to cover enterococcus – Not necessary to cover anaerobes unless biliary-bowel anastamosis • Infected pancreatic necrosis = colonic flora – Prophylactic antibiotics for non infected pancreatic necrosis are “controversial” (i. e. , GI vs ID)
Identification of High Risk Patients (who need broader spectrum Rx) • High risk of death/complications – – – High APACHE II score Poor nutritional state Significant cardiovascular disease Inability to obtain source control Immunosuppressive therapy or condition • Certain acute and chronic diseases – e. , g, acute leukemia, dialysis – Prolonged preop hospital stay – Prolonged preop (>2 days) antimicrobials

Duration of Therapy • Until resolution of clinical signs – Normal temp and WBC (? CRP) – Return of GI tract function • If persisting clinical evidence of infection at 5 -7 days – Sono/CT • If diagnostic, obtain source control by draining and continue ABX and modify based on abscess culture • If negative for abscess, consider D/C ABX

When are Cultures Indicated? • Uncomplicated, perforated or gangrenous appendix without abscess: no impact on outcome when cultures obtained • Abscesses, peri-colonic infections: failure rates higher if empiric ABX don’t cover aerobic flora • Community epidemiology differs • Anaerobic susceptibility: – Unnecessary if predictably potent coverage with metronidazole, carbapenems, beta lactam inhibitors used • Resistance a concern with clindamycin, cefamycins, piperacillin alone, most quinolones – Indicated if persisting anaerobic isolates, bacteremias or prolonged therapy indicated

Health Care Associated (HCA) Infections (Nosocomial) • Infections occurring after initial surgery are HCA and may harbor resistant flora • If empiric therapy does not include coverage against subsequently recovered resistant flora, morbidity higher • Often require empiric combination therapy – To cover MRSA, (VRE), MDR GNR
Complicated IA Infections Infecting Flora by Onset Location • Community-acquired infections • Enteric GNB, facultative bacilli, and β-lactam-susceptible GPC, obligate anaerobic bacilli (distal small-bowel and colon-derived infections and for more proximal perforations when obstruction is present) – E coli, B fragilis • Healthcare-associated infections (postop/nosocomial) • Prolonged pre-op LOS or > 2 days pre-op antibiotics • Usually more resistant flora – Pseudomonas, Enterobacter and Proteus spp, MRSA, Enterococci, and Candida spp • Knowledge of local susceptibility patterns critical GNB=gram-negative bacilli GPC=gram-positive cocci LOS=length of stay Solomkin J et al. IDSA Guidelines Clin Infect Dis. 2003 Oct 15; 37(8): 997 -1005.

What Should be Cultured? • Blood cultures often no benefit in community acquired IAI (CA-IAI) • Intra-abdominal specimens – Should be representative of the process – Rarely need more than one or two – Should always be sent for anaerobic as well as routine • Anaerobic transport system • SWABS ARE NEVER APPROPRIATE

When Should Gram Stain be Done? • CA-IAI: not indicated • HCA-IAI: indicated to help guide empiric coverage – If GPC clusters seen, cover for MRSA
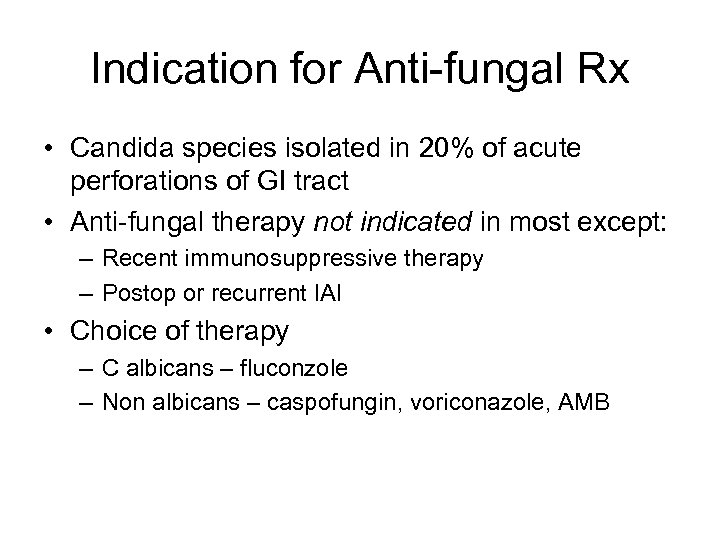
Indication for Anti-fungal Rx • Candida species isolated in 20% of acute perforations of GI tract • Anti-fungal therapy not indicated in most except: – Recent immunosuppressive therapy – Postop or recurrent IAI • Choice of therapy – C albicans – fluconzole – Non albicans – caspofungin, voriconazole, AMB
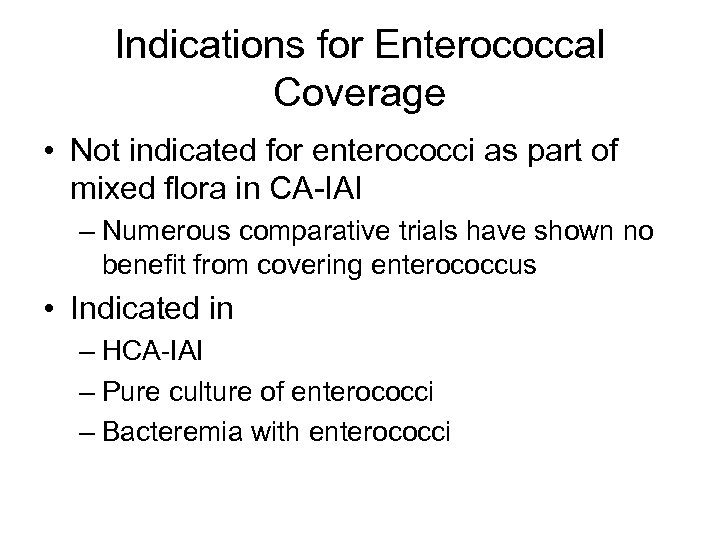
Indications for Enterococcal Coverage • Not indicated for enterococci as part of mixed flora in CA-IAI – Numerous comparative trials have shown no benefit from covering enterococcus • Indicated in – HCA-IAI – Pure culture of enterococci – Bacteremia with enterococci

Antimicrobial Regimens • IDSA Practice Guidelines 2003 • Newer regimens – Tigecycline CID 2006 – Moxifloxacin (FDA) 2005
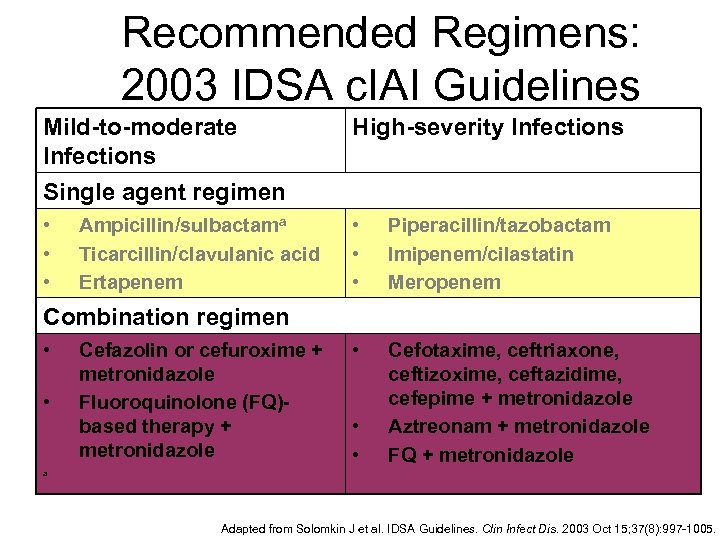
Recommended Regimens: 2003 IDSA c. IAI Guidelines Mild-to-moderate Infections Single agent regimen High-severity Infections • • • Piperacillin/tazobactam Imipenem/cilastatin Meropenem • Cefotaxime, ceftriaxone, ceftizoxime, ceftazidime, cefepime + metronidazole Aztreonam + metronidazole FQ + metronidazole Ampicillin/sulbactama Ticarcillin/clavulanic acid Ertapenem Combination regimen • • Cefazolin or cefuroxime + metronidazole Fluoroquinolone (FQ)based therapy + metronidazole • • a Adapted from Solomkin J et al. IDSA Guidelines. Clin Infect Dis. 2003 Oct 15; 37(8): 997 -1005.
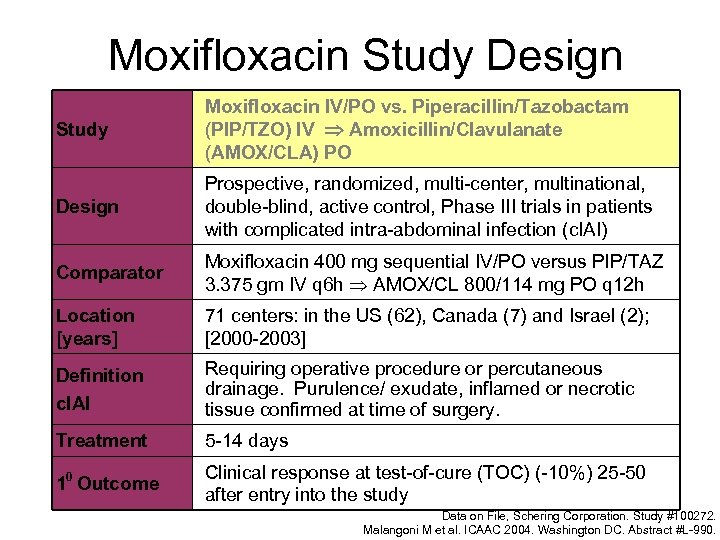
Moxifloxacin Study Design Study Moxifloxacin IV/PO vs. Piperacillin/Tazobactam (PIP/TZO) IV Amoxicillin/Clavulanate (AMOX/CLA) PO Design Prospective, randomized, multi-center, multinational, double-blind, active control, Phase III trials in patients with complicated intra-abdominal infection (c. IAI) Comparator Moxifloxacin 400 mg sequential IV/PO versus PIP/TAZ 3. 375 gm IV q 6 h AMOX/CL 800/114 mg PO q 12 h Location [years] 71 centers: in the US (62), Canada (7) and Israel (2); [2000 -2003] Definition c. IAI Requiring operative procedure or percutaneous drainage. Purulence/ exudate, inflamed or necrotic tissue confirmed at time of surgery. Treatment 5 -14 days 10 Outcome Clinical response at test-of-cure (TOC) (-10%) 25 -50 after entry into the study Data on File, Schering Corporation. Study #100272. Malangoni M et al. ICAAC 2004. Washington DC. Abstract #L-990.
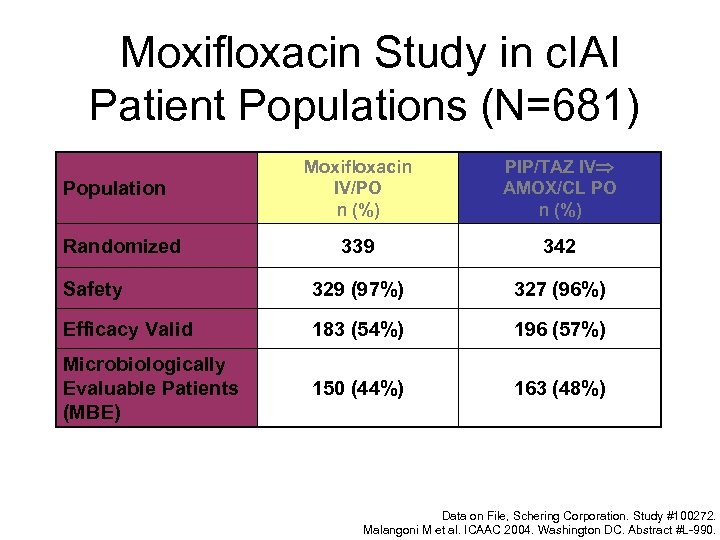
Moxifloxacin Study in c. IAI Patient Populations (N=681) Moxifloxacin IV/PO n (%) PIP/TAZ IV AMOX/CL PO n (%) 339 342 Safety 329 (97%) 327 (96%) Efficacy Valid 183 (54%) 196 (57%) Microbiologically Evaluable Patients (MBE) 150 (44%) 163 (48%) Population Randomized Data on File, Schering Corporation. Study #100272. Malangoni M et al. ICAAC 2004. Washington DC. Abstract #L-990.
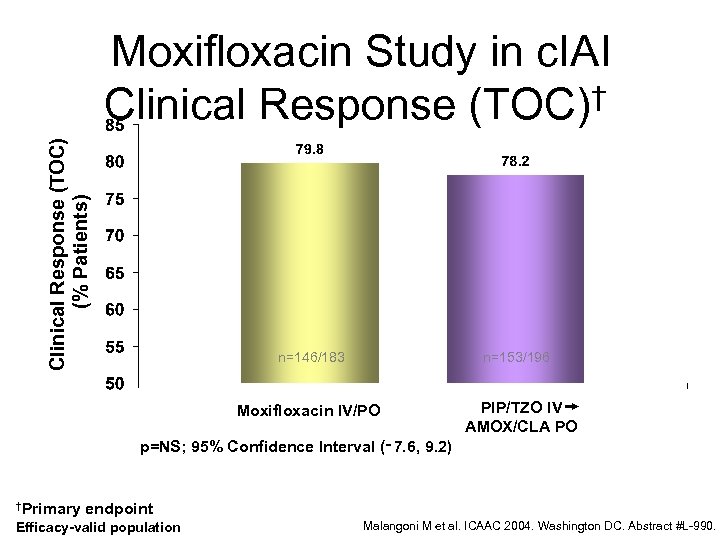
Clinical Response (TOC) (% Patients) Moxifloxacin Study in c. IAI Clinical Response (TOC)† n=146/183 n=153/196 Moxifloxacin IV/PO PIP/TZO IV AMOX/CLA PO p=NS; 95% Confidence Interval (‑ 7. 6, 9. 2) †Primary endpoint Efficacy-valid population Malangoni M et al. ICAAC 2004. Washington DC. Abstract #L-990.
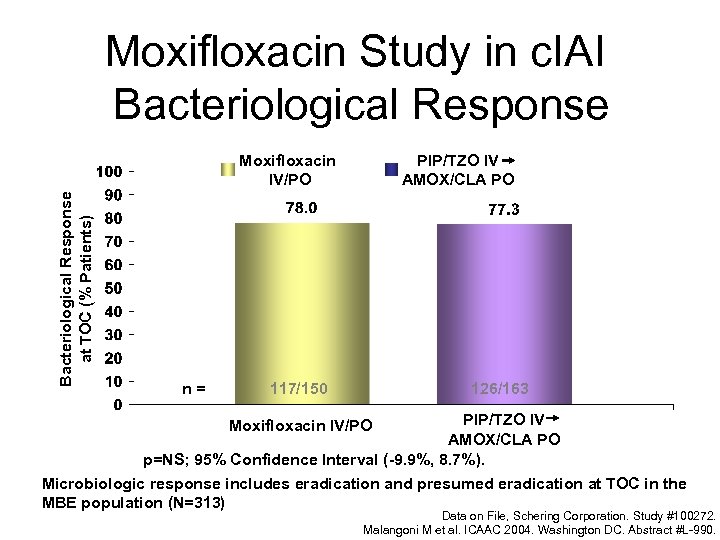
Moxifloxacin Study in c. IAI Bacteriological Response at TOC (% Patients) Moxifloxacin IV/PO n= PIP/TZO IV AMOX/CLA PO 126/163 117/150 PIP/TZO IV AMOX/CLA PO p=NS; 95% Confidence Interval (-9. 9%, 8. 7%). Microbiologic response includes eradication and presumed eradication at TOC in the MBE population (N=313) Moxifloxacin IV/PO Data on File, Schering Corporation. Study #100272. Malangoni M et al. ICAAC 2004. Washington DC. Abstract #L-990.
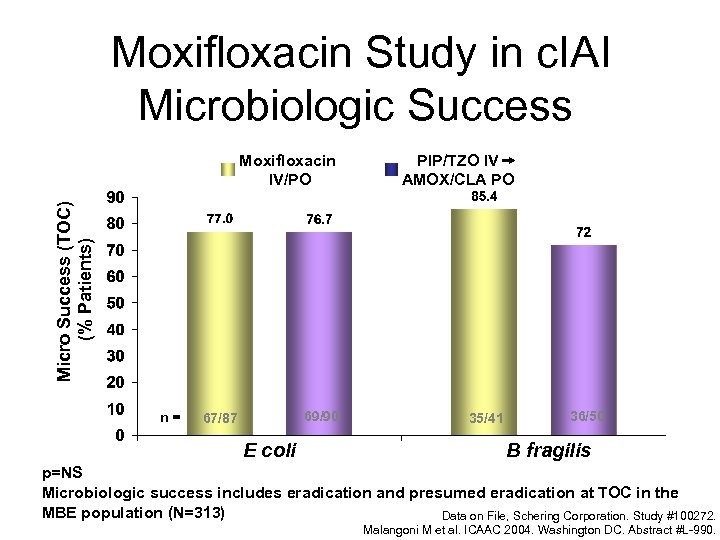
Moxifloxacin Study in c. IAI Microbiologic Success PIP/TZO IV AMOX/CLA PO Micro Success (TOC) (% Patients) Moxifloxacin IV/PO n= 69/90 67/87 E coli 35/41 36/50 B fragilis p=NS Microbiologic success includes eradication and presumed eradication at TOC in the MBE population (N=313) Data on File, Schering Corporation. Study #100272. Malangoni M et al. ICAAC 2004. Washington DC. Abstract #L-990.
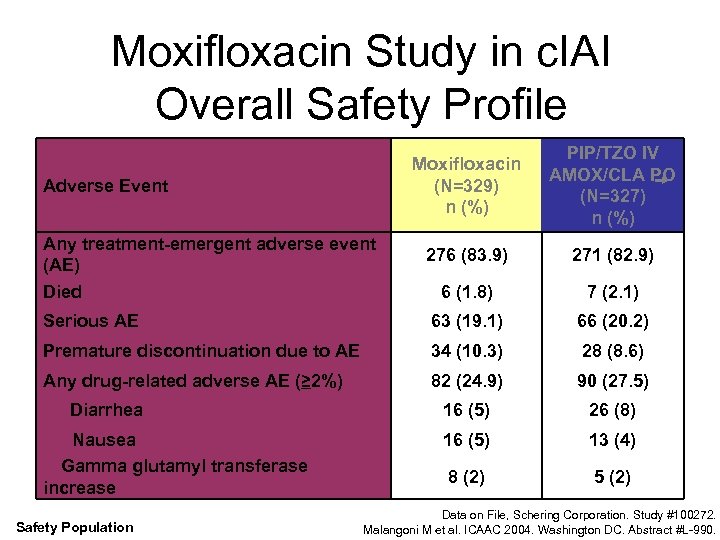
Moxifloxacin Study in c. IAI Overall Safety Profile Moxifloxacin (N=329) n (%) PIP/TZO IV AMOX/CLA PO (N=327) n (%) 276 (83. 9) 271 (82. 9) 6 (1. 8) 7 (2. 1) Serious AE 63 (19. 1) 66 (20. 2) Premature discontinuation due to AE 34 (10. 3) 28 (8. 6) Any drug-related adverse AE (≥ 2%) 82 (24. 9) 90 (27. 5) 16 (5) 26 (8) 16 (5) 13 (4) 8 (2) 5 (2) Adverse Event Any treatment-emergent adverse event (AE) Died Diarrhea Nausea Gamma glutamyl transferase increase Safety Population Data on File, Schering Corporation. Study #100272. Malangoni M et al. ICAAC 2004. Washington DC. Abstract #L-990.

Tigecycline for Complicated IAI • Pooled date from 2 phase 3 studies comparing Tigecycline to Imipenemcilastatin in 1642 adults




Caveats on Newer Regimens • Moxifloxacin – Anaerobic resistance to FQ may emerge – Limited experience – Nothing published yet • Tigecycline – Nausea/vomiting limiting factor in our experience – Literature: 44%

2003 IDSA c. IAI Guidelines Overall Antimicrobial Management • Fluid resuscitation required prior to initiating antibiotic to restore adequate visceral perfusion and ensure drug distribution • Empirical coverage initiated upon suspicion of c. IAI • Duration of therapy should be continued until resolution of clinical signs of infection: – Afebrile – Normalization of WBC count – Return of gastrointestinal function • If infection persists beyond 5 -7 days: – Diagnostic investigation required (CT or ultrasound) and/or additional intervention for source control – Ensure treatment regimen provides appropriate coverage Solomkin J et al. IDSA Guidelines Clin Infect Dis. 2003 Oct 15; 37(8): 997 -1005.

Bibliography • Babinchak T, Ellis-Grosse E et al. The Efficacy and Safety of Tigecycline for the Treatment of Complicated Intra-Abdominal Infections: Analysis of Pooled Clinical Trial Data. Clin Inf Dis 2005; 41 (Suppl 5): S 354 -67 • Barie PS, Hydo LJ, Eachempati. Longitudinal Outcomes of Intra-Abdominal Infection Complicated by Critical Illness. Surg Infect 2004; 5(4): 365 -73 • Goldstein EJC. Intra-Abdominal Anaerobic Infections. Clin Inf Dis 2002; 35(Suppl ): S 106 -11

Bibliography • Schering-Plough Company Data on File (personal communication) • Solomkin JS, Mazuski JE, Baron EJ et al. Guidelines for the Selection of Anti-Infective Agents for Complicated Intra-abdominal Infections. Clin Infect Dis 2003; 37: 997 -1005 Access on ID Society. org. Practice Guidelines • Weinstein WM, Onderdonk AB, Bartlett JG, Louie TJ, Gorbach SL. Antimicrobial therapy of experimental intraabdominal sepsis. J Infect Dis 1975; 132: 282 -6
321557b80224004b6ccf29164e58b14a.ppt