0efb4d5b0a517aa1bfa66a6c7e526538.ppt
- Количество слайдов: 47
 International study of caesarean section surgical techniques: a randomised factorial trial
International study of caesarean section surgical techniques: a randomised factorial trial
 Rationale for CORONIS • Caesarean section common around the world • By improving surgical techniques, we have potential to improve the health of very many women • Many existing trials have drawbacks (small sample size, poor methodology, less relevant outcomes)
Rationale for CORONIS • Caesarean section common around the world • By improving surgical techniques, we have potential to improve the health of very many women • Many existing trials have drawbacks (small sample size, poor methodology, less relevant outcomes)
 UK Caesar Trial • Trial of 3, 000 women, completed in UK (& Italy) in 2007 • Interventions single vs double layer closure of the uterus non-closure vs closure of pelvic peritoneum liberal vs restricted use of a sub-sheath drain
UK Caesar Trial • Trial of 3, 000 women, completed in UK (& Italy) in 2007 • Interventions single vs double layer closure of the uterus non-closure vs closure of pelvic peritoneum liberal vs restricted use of a sub-sheath drain
 WHO sponsored meeting 2002 • Delegates from 9 countries met in Oxford for 5 days • Discussed and agreed list of interventions to explore in international trial • NPEU team drew up draft protocol and draft data collection instrument leading to MRC application
WHO sponsored meeting 2002 • Delegates from 9 countries met in Oxford for 5 days • Discussed and agreed list of interventions to explore in international trial • NPEU team drew up draft protocol and draft data collection instrument leading to MRC application
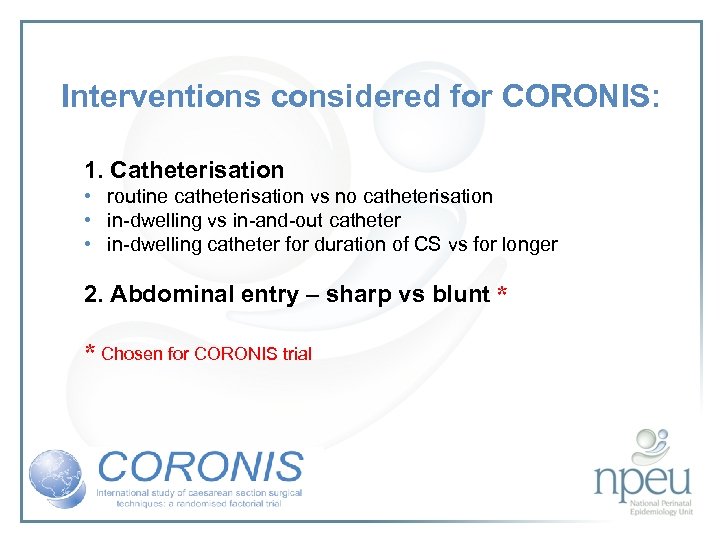 Interventions considered for CORONIS: 1. Catheterisation • routine catheterisation vs no catheterisation • in-dwelling vs in-and-out catheter • in-dwelling catheter for duration of CS vs for longer 2. Abdominal entry – sharp vs blunt * * Chosen for CORONIS trial
Interventions considered for CORONIS: 1. Catheterisation • routine catheterisation vs no catheterisation • in-dwelling vs in-and-out catheter • in-dwelling catheter for duration of CS vs for longer 2. Abdominal entry – sharp vs blunt * * Chosen for CORONIS trial
 Interventions considered for CORONIS: 3. Uterus a) Sharp vs blunt uterine entry b) Exteriorisation of uterus for repair vs intra-abdominal repair * c) Uterine swabbing vs no swabbing prior to uterine closure d) Single vs double layer uterine closure * e) Uterine repair: • chromic catgut vs vicryl (polyglactin-910) * • locking vs non-locking suture • continuous vs interrupted sutures * Chosen for CORONIS trial
Interventions considered for CORONIS: 3. Uterus a) Sharp vs blunt uterine entry b) Exteriorisation of uterus for repair vs intra-abdominal repair * c) Uterine swabbing vs no swabbing prior to uterine closure d) Single vs double layer uterine closure * e) Uterine repair: • chromic catgut vs vicryl (polyglactin-910) * • locking vs non-locking suture • continuous vs interrupted sutures * Chosen for CORONIS trial
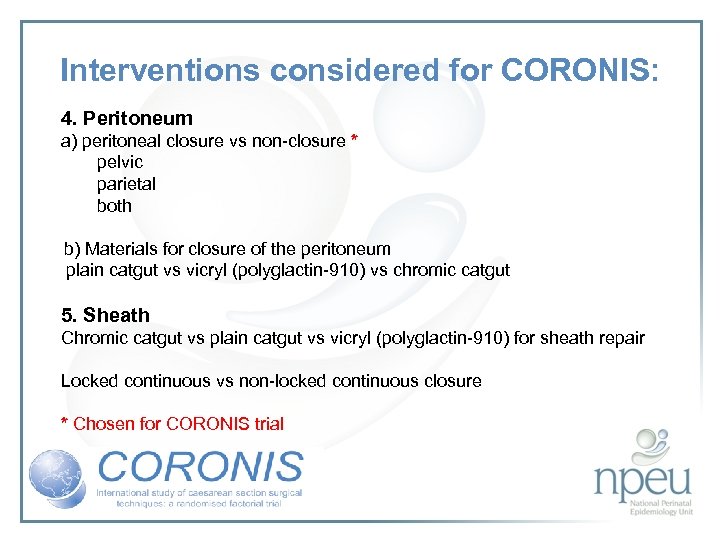 Interventions considered for CORONIS: 4. Peritoneum a) peritoneal closure vs non-closure * pelvic parietal both b) Materials for closure of the peritoneum plain catgut vs vicryl (polyglactin-910) vs chromic catgut 5. Sheath Chromic catgut vs plain catgut vs vicryl (polyglactin-910) for sheath repair Locked continuous vs non-locked continuous closure * Chosen for CORONIS trial
Interventions considered for CORONIS: 4. Peritoneum a) peritoneal closure vs non-closure * pelvic parietal both b) Materials for closure of the peritoneum plain catgut vs vicryl (polyglactin-910) vs chromic catgut 5. Sheath Chromic catgut vs plain catgut vs vicryl (polyglactin-910) for sheath repair Locked continuous vs non-locked continuous closure * Chosen for CORONIS trial
 Interventions considered for CORONIS: 6. Fat Subcutaneous fat closure vs no closure 7. Skin closure Subcutaneous absorbable suture vs interrupted absorbable suture, staples etc
Interventions considered for CORONIS: 6. Fat Subcutaneous fat closure vs no closure 7. Skin closure Subcutaneous absorbable suture vs interrupted absorbable suture, staples etc
 Meeting of co-investigators 2006 • Delegates from 6 countries met in Oxford for 3 days • Reviewed and agreed final list of interventions
Meeting of co-investigators 2006 • Delegates from 6 countries met in Oxford for 3 days • Reviewed and agreed final list of interventions
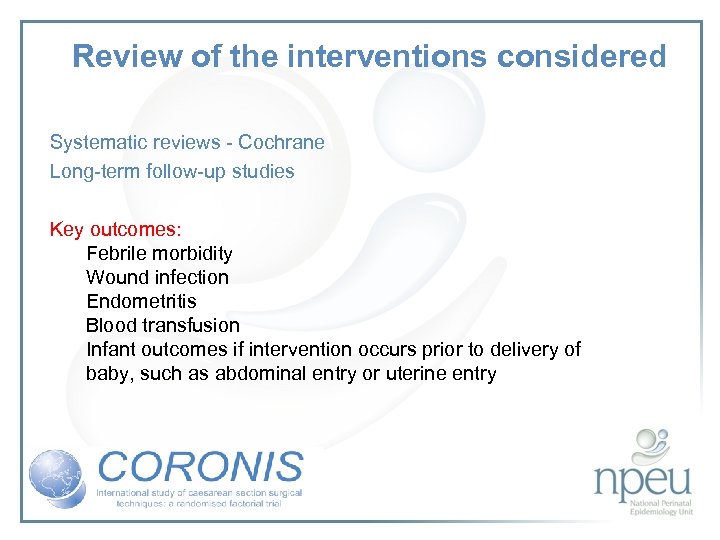 Review of the interventions considered Systematic reviews - Cochrane Long-term follow-up studies Key outcomes: Febrile morbidity Wound infection Endometritis Blood transfusion Infant outcomes if intervention occurs prior to delivery of baby, such as abdominal entry or uterine entry
Review of the interventions considered Systematic reviews - Cochrane Long-term follow-up studies Key outcomes: Febrile morbidity Wound infection Endometritis Blood transfusion Infant outcomes if intervention occurs prior to delivery of baby, such as abdominal entry or uterine entry
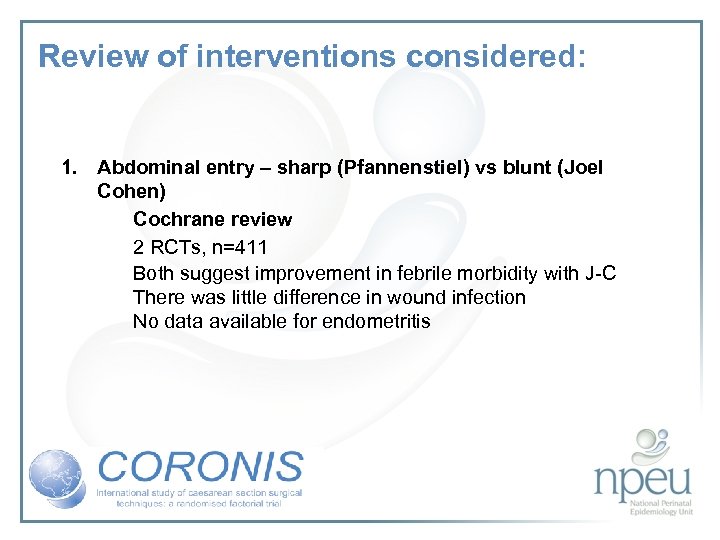 Review of interventions considered: 1. Abdominal entry – sharp (Pfannenstiel) vs blunt (Joel Cohen) Cochrane review 2 RCTs, n=411 Both suggest improvement in febrile morbidity with J-C There was little difference in wound infection No data available for endometritis
Review of interventions considered: 1. Abdominal entry – sharp (Pfannenstiel) vs blunt (Joel Cohen) Cochrane review 2 RCTs, n=411 Both suggest improvement in febrile morbidity with J-C There was little difference in wound infection No data available for endometritis
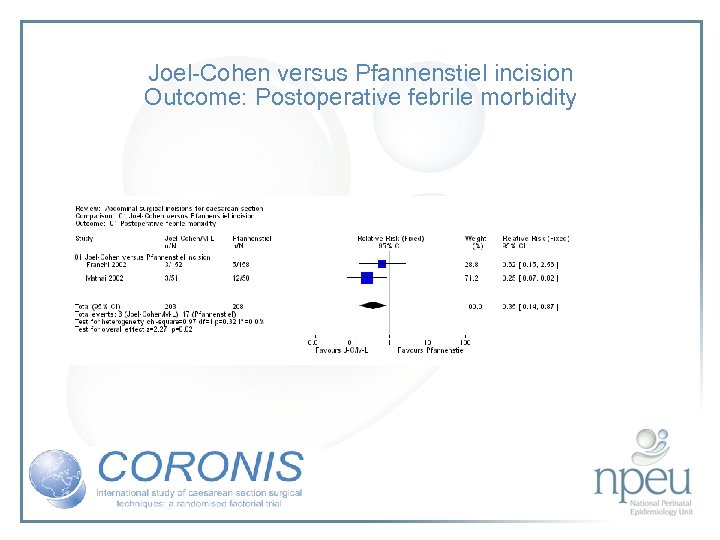 Joel-Cohen versus Pfannenstiel incision Outcome: Postoperative febrile morbidity
Joel-Cohen versus Pfannenstiel incision Outcome: Postoperative febrile morbidity
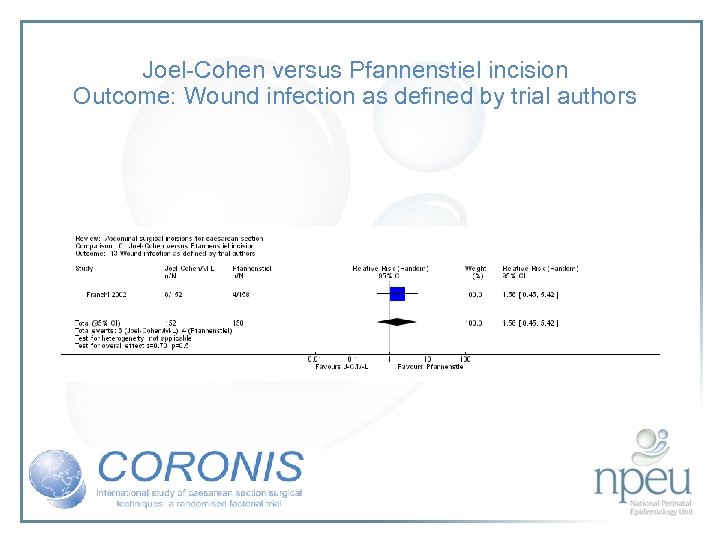 Joel-Cohen versus Pfannenstiel incision Outcome: Wound infection as defined by trial authors
Joel-Cohen versus Pfannenstiel incision Outcome: Wound infection as defined by trial authors
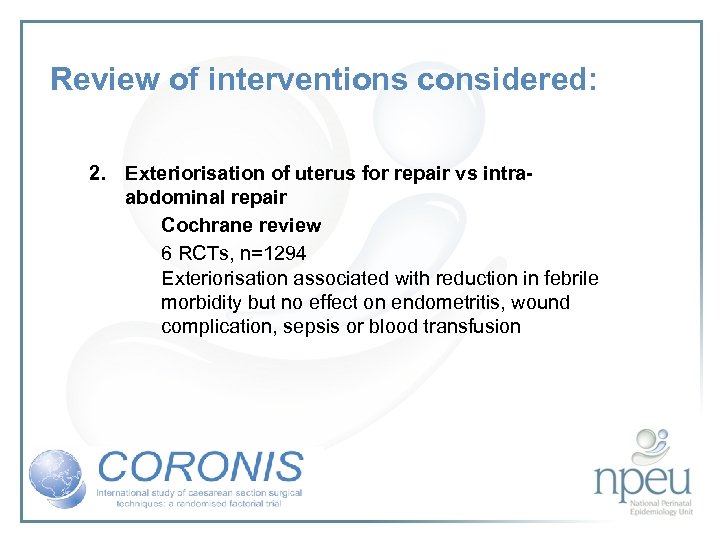 Review of interventions considered: 2. Exteriorisation of uterus for repair vs intraabdominal repair Cochrane review 6 RCTs, n=1294 Exteriorisation associated with reduction in febrile morbidity but no effect on endometritis, wound complication, sepsis or blood transfusion
Review of interventions considered: 2. Exteriorisation of uterus for repair vs intraabdominal repair Cochrane review 6 RCTs, n=1294 Exteriorisation associated with reduction in febrile morbidity but no effect on endometritis, wound complication, sepsis or blood transfusion
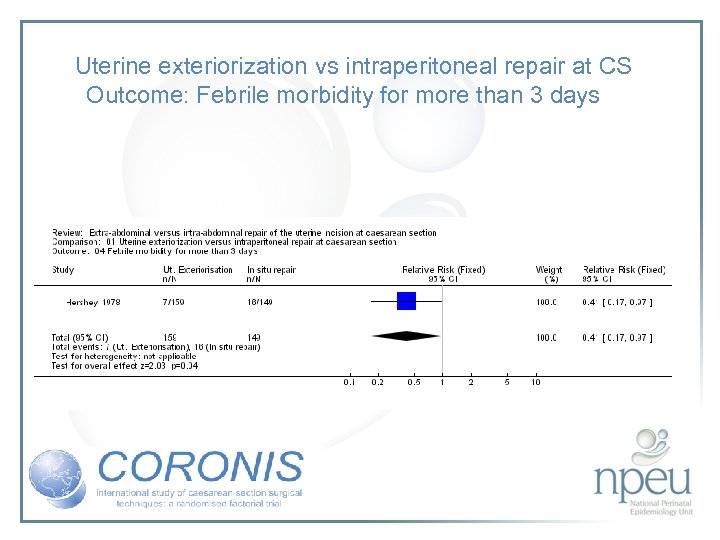 Uterine exteriorization vs intraperitoneal repair at CS Outcome: Febrile morbidity for more than 3 days
Uterine exteriorization vs intraperitoneal repair at CS Outcome: Febrile morbidity for more than 3 days
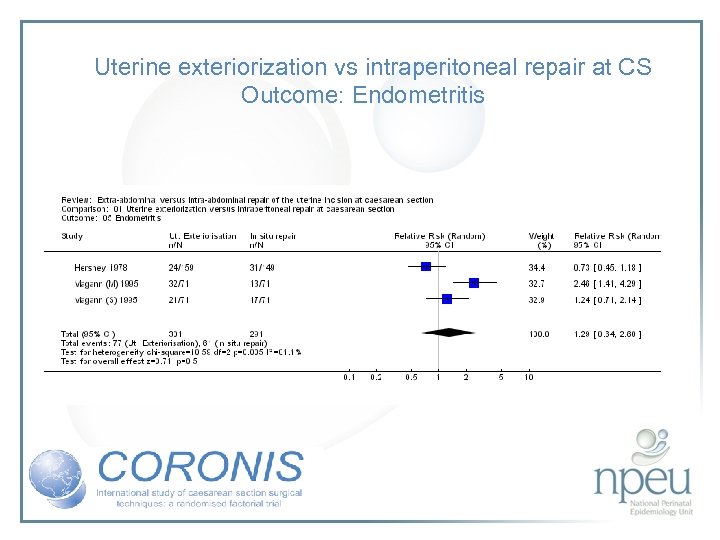 Uterine exteriorization vs intraperitoneal repair at CS Outcome: Endometritis
Uterine exteriorization vs intraperitoneal repair at CS Outcome: Endometritis
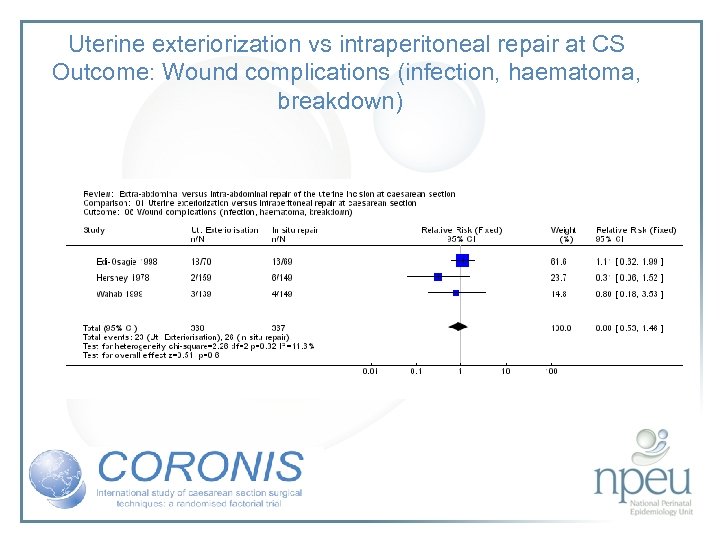 Uterine exteriorization vs intraperitoneal repair at CS Outcome: Wound complications (infection, haematoma, breakdown)
Uterine exteriorization vs intraperitoneal repair at CS Outcome: Wound complications (infection, haematoma, breakdown)
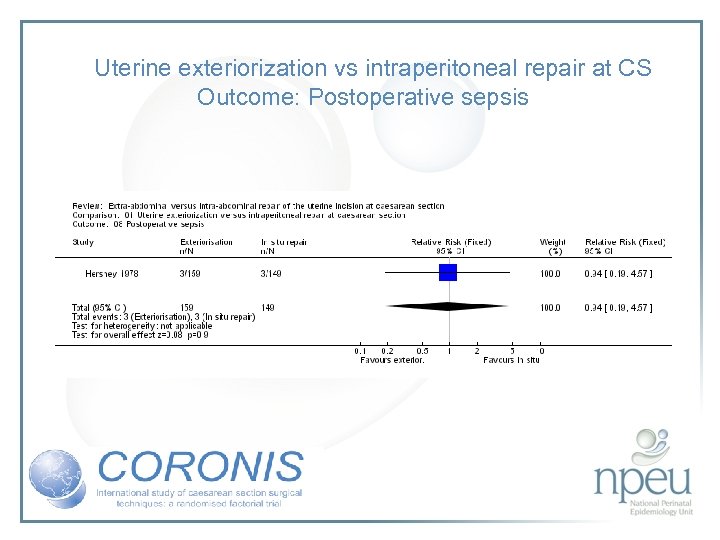 Uterine exteriorization vs intraperitoneal repair at CS Outcome: Postoperative sepsis
Uterine exteriorization vs intraperitoneal repair at CS Outcome: Postoperative sepsis
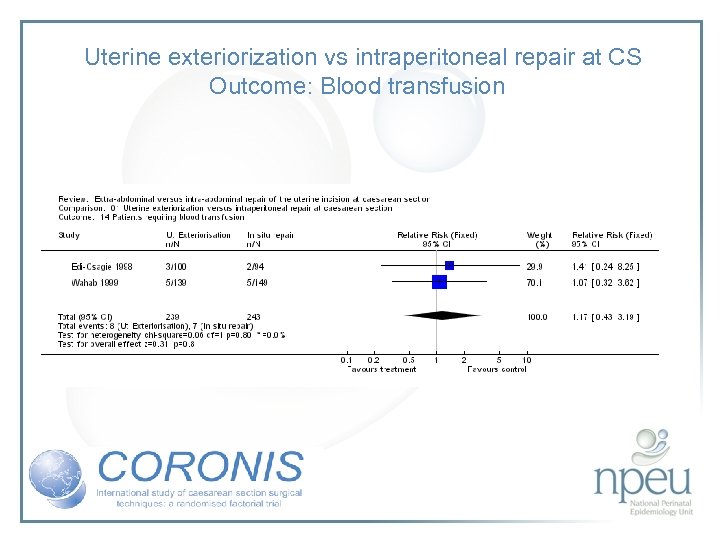 Uterine exteriorization vs intraperitoneal repair at CS Outcome: Blood transfusion
Uterine exteriorization vs intraperitoneal repair at CS Outcome: Blood transfusion
 Review of interventions considered: 3. Single vs double layer uterine closure Cochrane review 2 RCTs, n=1006 No effect on endometritis or blood transfusions
Review of interventions considered: 3. Single vs double layer uterine closure Cochrane review 2 RCTs, n=1006 No effect on endometritis or blood transfusions
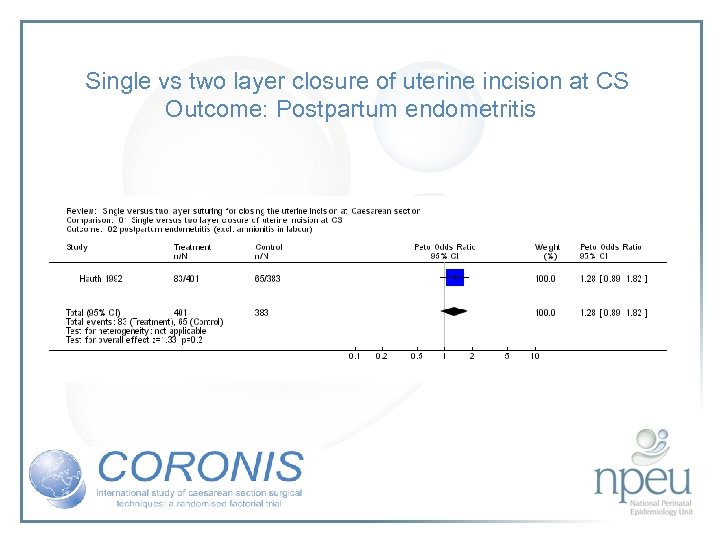 Single vs two layer closure of uterine incision at CS Outcome: Postpartum endometritis
Single vs two layer closure of uterine incision at CS Outcome: Postpartum endometritis
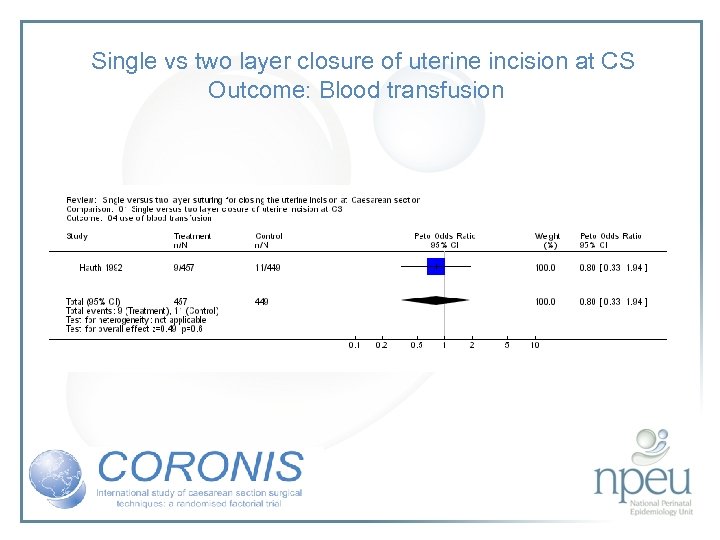 Single vs two layer closure of uterine incision at CS Outcome: Blood transfusion
Single vs two layer closure of uterine incision at CS Outcome: Blood transfusion
 Review of interventions considered: 4. Uterine repair • chromic catgut vs vicryl (polyglactin-910) Cochrane review No studies found
Review of interventions considered: 4. Uterine repair • chromic catgut vs vicryl (polyglactin-910) Cochrane review No studies found
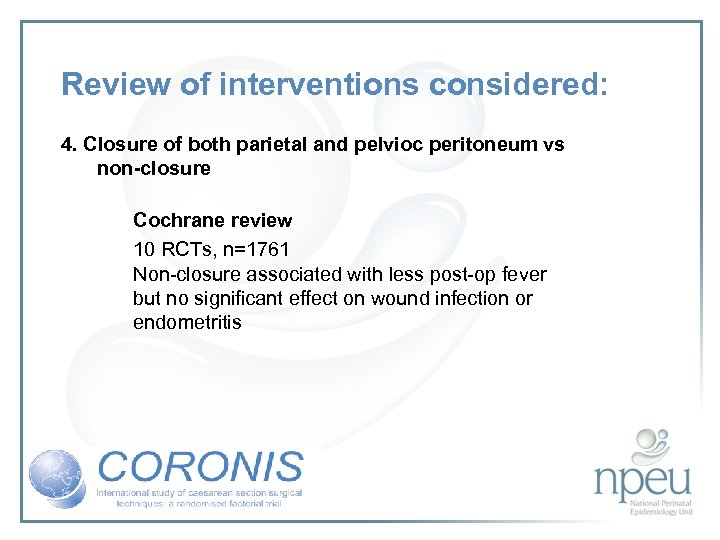 Review of interventions considered: 4. Closure of both parietal and pelvioc peritoneum vs non-closure Cochrane review 10 RCTs, n=1761 Non-closure associated with less post-op fever but no significant effect on wound infection or endometritis
Review of interventions considered: 4. Closure of both parietal and pelvioc peritoneum vs non-closure Cochrane review 10 RCTs, n=1761 Non-closure associated with less post-op fever but no significant effect on wound infection or endometritis
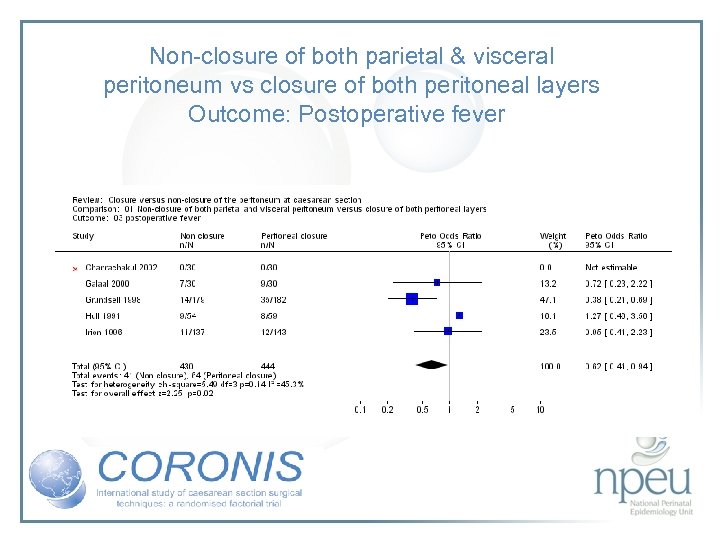 Non-closure of both parietal & visceral peritoneum vs closure of both peritoneal layers Outcome: Postoperative fever
Non-closure of both parietal & visceral peritoneum vs closure of both peritoneal layers Outcome: Postoperative fever
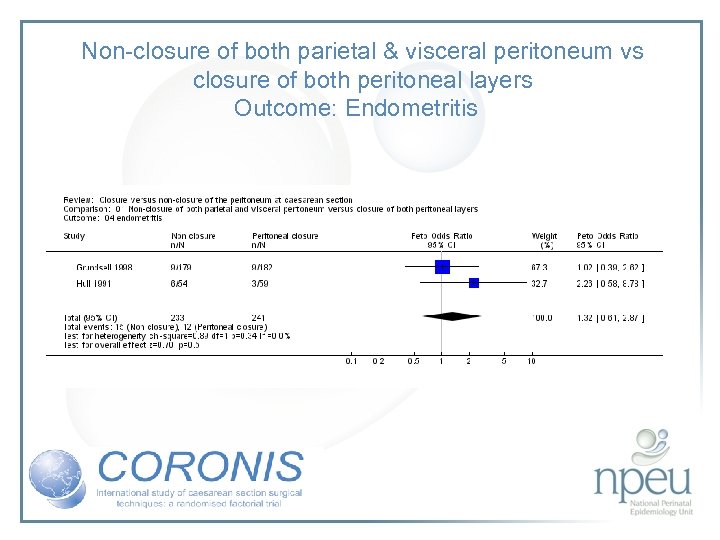 Non-closure of both parietal & visceral peritoneum vs closure of both peritoneal layers Outcome: Endometritis
Non-closure of both parietal & visceral peritoneum vs closure of both peritoneal layers Outcome: Endometritis
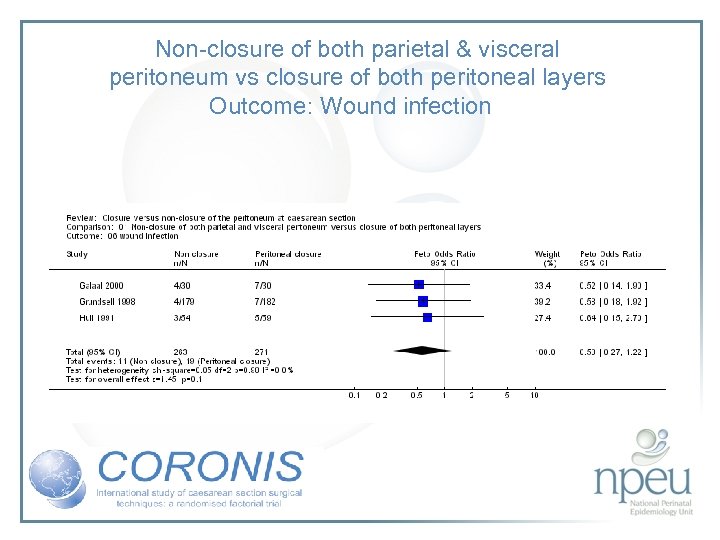 Non-closure of both parietal & visceral peritoneum vs closure of both peritoneal layers Outcome: Wound infection
Non-closure of both parietal & visceral peritoneum vs closure of both peritoneal layers Outcome: Wound infection
 2 studies of long-term follow-up Single vs double layer uterine closure • 145 women out of 906 randomised • followed up at time of next pregnancy • no difference found between the groups (not surprisingly) Chapman SJ, Owen J, Hauth JC. One versus two-layer closure of a low transverse cesarean: the next pregnancy. Obstet Gynecol 1997; 89: 16 -18. Non-closure vs closure of peritoneum • 144 women out of 280 randomised • no differences found between the groups Bahmanyar E, Boulvain M, Irion O. Non-closure of the peritoneum during cesarean section: long-term follow-up of a randomized controlled trial. Am J Obstet Gynecol 2001; 185: S 125.
2 studies of long-term follow-up Single vs double layer uterine closure • 145 women out of 906 randomised • followed up at time of next pregnancy • no difference found between the groups (not surprisingly) Chapman SJ, Owen J, Hauth JC. One versus two-layer closure of a low transverse cesarean: the next pregnancy. Obstet Gynecol 1997; 89: 16 -18. Non-closure vs closure of peritoneum • 144 women out of 280 randomised • no differences found between the groups Bahmanyar E, Boulvain M, Irion O. Non-closure of the peritoneum during cesarean section: long-term follow-up of a randomized controlled trial. Am J Obstet Gynecol 2001; 185: S 125.
 Conclusions for 5 interventions for CORONIS Uterine exteriorisation vs intra-abdominal repair - no diff for wound infection, sepsis, endometritis, blood transfusion (less fever in exterioriorisation) Single vs two layer uterine closure - no difference for substantive outcomes Chromic catgut vs polyglactin-910 (Vicryl) for uterine repair - no studies identified Closure vs non-closure of peritoneum - no diff for wound infection or endometritis (less post-op fever in non- closure) Blunt vs sharp abdominal entry - not enough evidence
Conclusions for 5 interventions for CORONIS Uterine exteriorisation vs intra-abdominal repair - no diff for wound infection, sepsis, endometritis, blood transfusion (less fever in exterioriorisation) Single vs two layer uterine closure - no difference for substantive outcomes Chromic catgut vs polyglactin-910 (Vicryl) for uterine repair - no studies identified Closure vs non-closure of peritoneum - no diff for wound infection or endometritis (less post-op fever in non- closure) Blunt vs sharp abdominal entry - not enough evidence
 The CORONIS Trial The CORONIS trial is funded by the UK Medical Research Council in collaboration with the World Health Organisation
The CORONIS Trial The CORONIS trial is funded by the UK Medical Research Council in collaboration with the World Health Organisation
 The Trial Collaborating countries • Argentina • Ghana • India: two regions; Delhi and Vellore • Kenya • Pakistan • Sudan • 18 participating hospitals
The Trial Collaborating countries • Argentina • Ghana • India: two regions; Delhi and Vellore • Kenya • Pakistan • Sudan • 18 participating hospitals
 Investigator Group Chief Investigator Professor Peter Brocklehurst Principal Investigators Argentina Dr Edgardo Abalos Ghana Dr Victor Addo India: Delhi Dr Jai Sharma India: Vellore Dr Jiji Mathews Kenya Professor James Oyieke Pakistan Dr Shabeen Masood Sudan Professor Mohamed El. Shiekh
Investigator Group Chief Investigator Professor Peter Brocklehurst Principal Investigators Argentina Dr Edgardo Abalos Ghana Dr Victor Addo India: Delhi Dr Jai Sharma India: Vellore Dr Jiji Mathews Kenya Professor James Oyieke Pakistan Dr Shabeen Masood Sudan Professor Mohamed El. Shiekh
 Sample size 15, 000 women world-wide • At least 2000 women from each country • All women followed-up 6 weeks after discharge from hospital • 3 year recruitment period: 2007 -2010 • Plans for 3 year follow-up of all women recruited are underway
Sample size 15, 000 women world-wide • At least 2000 women from each country • All women followed-up 6 weeks after discharge from hospital • 3 year recruitment period: 2007 -2010 • Plans for 3 year follow-up of all women recruited are underway
 Study design • The study is a multicentre, fractional factorial randomised controlled trial. • The collaborating institutions are centres with experience in conducting trials. • These centres also have experience in detailed follow-up of large numbers of women.
Study design • The study is a multicentre, fractional factorial randomised controlled trial. • The collaborating institutions are centres with experience in conducting trials. • These centres also have experience in detailed follow-up of large numbers of women.
 Fractional, factorial design In the CORONIS Trial five comparisons will be carried out in one trial, using a 2 x 2 x 2 factorial design. Such a design has rarely been used, but is appropriate for the evaluation of several procedures which will be used together in clinical practice. In this trial of different caesarean section techniques, using five pairs of possible allocated interventions (1 versus “not 1”, 2 versus “not 2”, 3 versus “not 3”, 4 versus “not 4”, 5 versus “not 5”), participants can receive one of 32 possible alternatives.
Fractional, factorial design In the CORONIS Trial five comparisons will be carried out in one trial, using a 2 x 2 x 2 factorial design. Such a design has rarely been used, but is appropriate for the evaluation of several procedures which will be used together in clinical practice. In this trial of different caesarean section techniques, using five pairs of possible allocated interventions (1 versus “not 1”, 2 versus “not 2”, 3 versus “not 3”, 4 versus “not 4”, 5 versus “not 5”), participants can receive one of 32 possible alternatives.
 Interventions • • • Blunt versus sharp abdominal entry Exteriorisation of the uterus for repair versus intra-abdominal repair Single versus double layer closure of the uterus Closure versus non-closure of the peritoneum (pelvic and parietal) Chromic catgut versus Polyglactin-910 for uterine repair
Interventions • • • Blunt versus sharp abdominal entry Exteriorisation of the uterus for repair versus intra-abdominal repair Single versus double layer closure of the uterus Closure versus non-closure of the peritoneum (pelvic and parietal) Chromic catgut versus Polyglactin-910 for uterine repair
 Training in surgical techniques • Training will vary between countries according to the national standards of training in new surgical techniques employed by each participating country. For example, if the accepted standard for surgical training in a country is that operators must perform a certain number of procedures before they are judged to be competent in that procedure, then this process should be followed. • If, however, the national standard is that operators are judged to be competent when a senior surgeon judges them to be competent, then this process should be followed. The accepted standard of surgical training in each centre will be determined at the start of the trial.
Training in surgical techniques • Training will vary between countries according to the national standards of training in new surgical techniques employed by each participating country. For example, if the accepted standard for surgical training in a country is that operators must perform a certain number of procedures before they are judged to be competent in that procedure, then this process should be followed. • If, however, the national standard is that operators are judged to be competent when a senior surgeon judges them to be competent, then this process should be followed. The accepted standard of surgical training in each centre will be determined at the start of the trial.
 Training in surgical techniques • To facilitate training, a film of all the interventions being tested in CORONIS will be provided to participating centres. In individual countries, visits by the Regional Co-ordinator to participating hospitals to teach specific surgical techniques may be required so that experience in the participating hospitals can be disseminated rapidly. • Participating centres will appoint a senior obstetrician to ensure that only clinical staff competent in the various surgical techniques to be used in the trial are ‘authorised’ to operate. A list of these personnel will be kept by the local centre with a copy at the Regional Trial Office.
Training in surgical techniques • To facilitate training, a film of all the interventions being tested in CORONIS will be provided to participating centres. In individual countries, visits by the Regional Co-ordinator to participating hospitals to teach specific surgical techniques may be required so that experience in the participating hospitals can be disseminated rapidly. • Participating centres will appoint a senior obstetrician to ensure that only clinical staff competent in the various surgical techniques to be used in the trial are ‘authorised’ to operate. A list of these personnel will be kept by the local centre with a copy at the Regional Trial Office.
 Primary outcome Composite outcome of: Death or maternal infectious morbidity (one or more of the following: antibiotic use for maternal febrile morbidity during postnatal hospital stay, antibiotic use for endometritis, wound infection or peritonitis); or further operative procedures; or blood transfusion.
Primary outcome Composite outcome of: Death or maternal infectious morbidity (one or more of the following: antibiotic use for maternal febrile morbidity during postnatal hospital stay, antibiotic use for endometritis, wound infection or peritonitis); or further operative procedures; or blood transfusion.
 Secondary outcomes Clinical • All components of the primary composite outcome as secondary outcomes • Pain • Interventions used for severe primary post-partum haemorrhage (PPH) • Stillbirth after trial entry • Apgar score < 3 at five minutes • Laceration of baby at time of caesarean section • Death of the baby by six weeks of age • Other severe maternal morbidity Health Service Utilisation • • Duration of operation (from incision to closure) Duration of hospital stay post-caesarean section Duration of stay in Intensive Care Unit post-caesarean section Number and duration of re-admissions to hospital within 6 weeks of the caesarean section
Secondary outcomes Clinical • All components of the primary composite outcome as secondary outcomes • Pain • Interventions used for severe primary post-partum haemorrhage (PPH) • Stillbirth after trial entry • Apgar score < 3 at five minutes • Laceration of baby at time of caesarean section • Death of the baby by six weeks of age • Other severe maternal morbidity Health Service Utilisation • • Duration of operation (from incision to closure) Duration of hospital stay post-caesarean section Duration of stay in Intensive Care Unit post-caesarean section Number and duration of re-admissions to hospital within 6 weeks of the caesarean section
 Eligibility criteria Women ARE eligible for trial entry if: • they are undergoing delivery by lower segment caesarean section through a transverse abdominal incision, irrespective of fever in labour, gestational age or whether they have a multiple pregnancy.
Eligibility criteria Women ARE eligible for trial entry if: • they are undergoing delivery by lower segment caesarean section through a transverse abdominal incision, irrespective of fever in labour, gestational age or whether they have a multiple pregnancy.
 Exclusion criteria Women are NOT eligible if: • there is a clear indication for a particular surgical technique or material to be used that prevents any of the allocated interventions being used, e. g. for a woman with a previous vertical abdominal incision it maybe considered inappropriate to do a transverse abdominal incision for this caesarean section. However, if a transverse incision is going to be performed the woman is eligible.
Exclusion criteria Women are NOT eligible if: • there is a clear indication for a particular surgical technique or material to be used that prevents any of the allocated interventions being used, e. g. for a woman with a previous vertical abdominal incision it maybe considered inappropriate to do a transverse abdominal incision for this caesarean section. However, if a transverse incision is going to be performed the woman is eligible.
 Exclusion criteria (cont. ) Women are NOT eligible if: • they have had more than one previous • caesarean section. they have already been recruited into the trial during a previous pregnancy
Exclusion criteria (cont. ) Women are NOT eligible if: • they have had more than one previous • caesarean section. they have already been recruited into the trial during a previous pregnancy
 Informed consent • Information leaflets will be made available to local centres, in appropriate languages, which explain the justification for the trial, the process of trial entry and follow up. • Once a woman becomes eligible, the trial should be discussed with her (and her partner as appropriate). • A signed, or marked, consent form must be provided before the woman is entered into the trial.
Informed consent • Information leaflets will be made available to local centres, in appropriate languages, which explain the justification for the trial, the process of trial entry and follow up. • Once a woman becomes eligible, the trial should be discussed with her (and her partner as appropriate). • A signed, or marked, consent form must be provided before the woman is entered into the trial.
 Data collection • • • At study entry Immediately following delivery During the postpartum stay in hospital At 6 weeks after discharge from hospital Data Collection Booklets contain all the necessary data collection forms
Data collection • • • At study entry Immediately following delivery During the postpartum stay in hospital At 6 weeks after discharge from hospital Data Collection Booklets contain all the necessary data collection forms
 International Co-ordinating Team Chief Investigator Trial Statistician Trial Director IT Co-ordinator Study Administrator Peter Brocklehurst Ed Juszczak Barbara Farrell Patsy Spark Shan Rich
International Co-ordinating Team Chief Investigator Trial Statistician Trial Director IT Co-ordinator Study Administrator Peter Brocklehurst Ed Juszczak Barbara Farrell Patsy Spark Shan Rich
 International Co-ordinating Centre National Perinatal Epidemiology Unit University of Oxford www. npeu. ox. ac. uk/CORONIS
International Co-ordinating Centre National Perinatal Epidemiology Unit University of Oxford www. npeu. ox. ac. uk/CORONIS


