67290e650f4414dbcf26bd822907a672.ppt
- Количество слайдов: 41
 International consensus on serum free light chain analysis Alex Legg Ph. D Scientific Affairs Manager The Binding Site Distributor in Poland BIOKOM beata. olsz@biokom. com. pl
International consensus on serum free light chain analysis Alex Legg Ph. D Scientific Affairs Manager The Binding Site Distributor in Poland BIOKOM beata. olsz@biokom. com. pl
 Serum free light chain immunoassay Heavy chain Light chain Kappa Hidden surface Exposed surface Lambda
Serum free light chain immunoassay Heavy chain Light chain Kappa Hidden surface Exposed surface Lambda
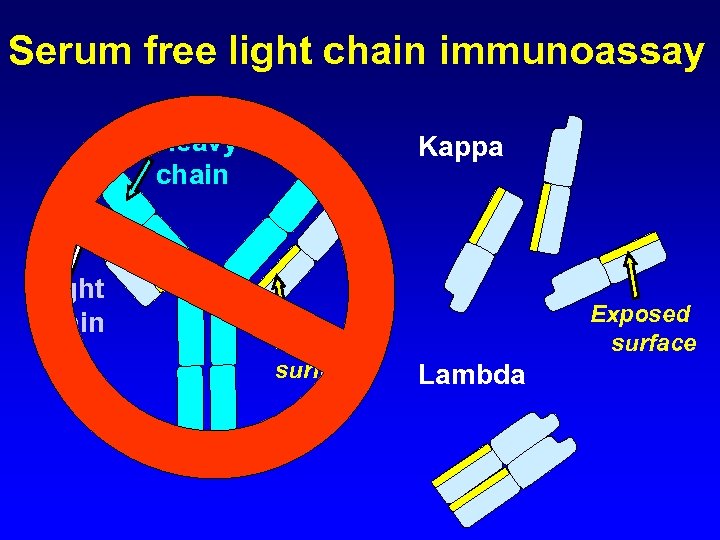 Serum free light chain immunoassay Heavy chain Light chain Kappa Hidden surface Exposed surface Lambda
Serum free light chain immunoassay Heavy chain Light chain Kappa Hidden surface Exposed surface Lambda
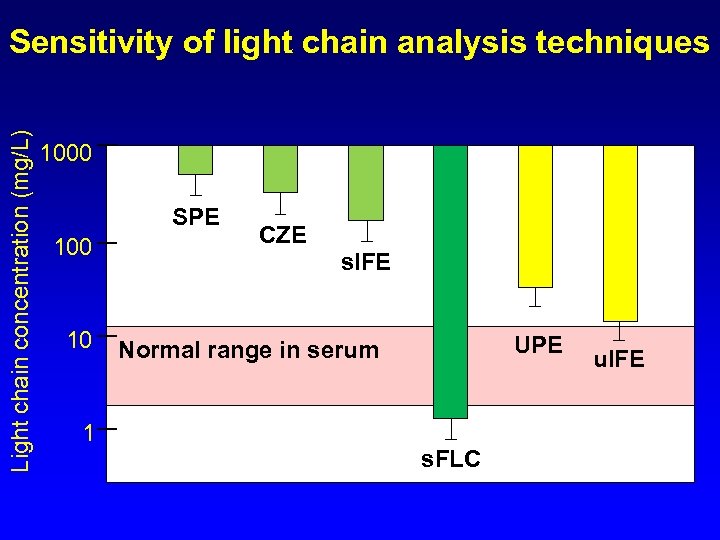 Light chain concentration (mg/L) Sensitivity of light chain analysis techniques 1000 SPE 100 10 1 CZE s. IFE UPE Normal range in serum s. FLC u. IFE
Light chain concentration (mg/L) Sensitivity of light chain analysis techniques 1000 SPE 100 10 1 CZE s. IFE UPE Normal range in serum s. FLC u. IFE
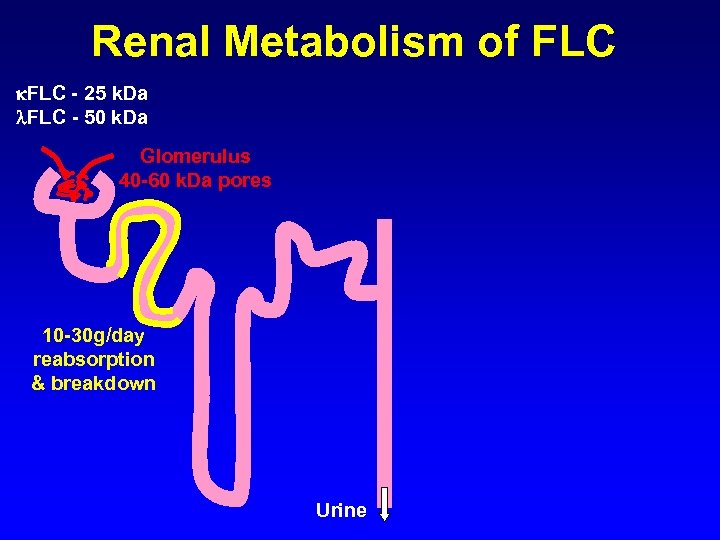 Renal Metabolism of FLC - 25 k. Da FLC - 50 k. Da Glomerulus 40 -60 k. Da pores 10 -30 g/day reabsorption & breakdown Urine
Renal Metabolism of FLC - 25 k. Da FLC - 50 k. Da Glomerulus 40 -60 k. Da pores 10 -30 g/day reabsorption & breakdown Urine
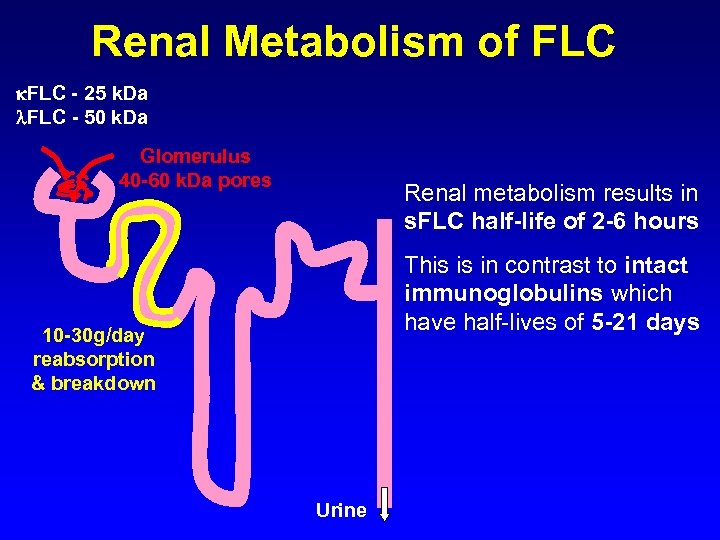 Renal Metabolism of FLC - 25 k. Da FLC - 50 k. Da Glomerulus 40 -60 k. Da pores Renal metabolism results in s. FLC half-life of 2 -6 hours This is in contrast to intact immunoglobulins which have half-lives of 5 -21 days 10 -30 g/day reabsorption & breakdown Urine
Renal Metabolism of FLC - 25 k. Da FLC - 50 k. Da Glomerulus 40 -60 k. Da pores Renal metabolism results in s. FLC half-life of 2 -6 hours This is in contrast to intact immunoglobulins which have half-lives of 5 -21 days 10 -30 g/day reabsorption & breakdown Urine
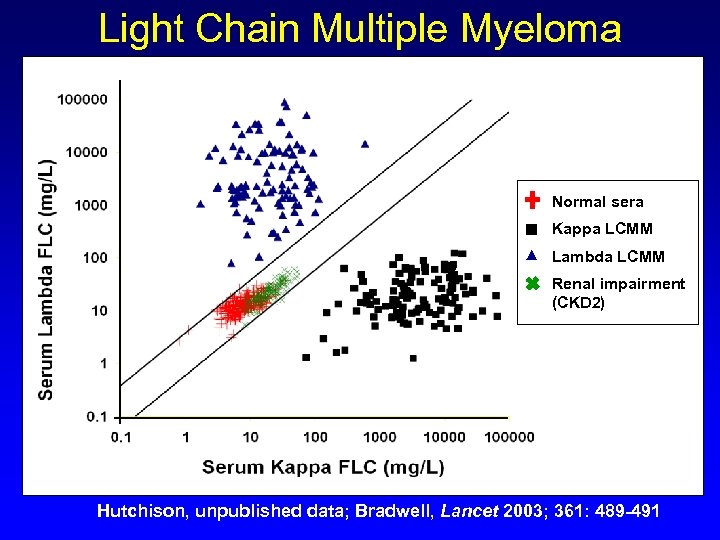 Light Chain Multiple Myeloma Normal sera Kappa LCMM Lambda LCMM Renal impairment (CKD 2) Hutchison, unpublished data; Bradwell, Lancet 2003; 361: 489 -491
Light Chain Multiple Myeloma Normal sera Kappa LCMM Lambda LCMM Renal impairment (CKD 2) Hutchison, unpublished data; Bradwell, Lancet 2003; 361: 489 -491
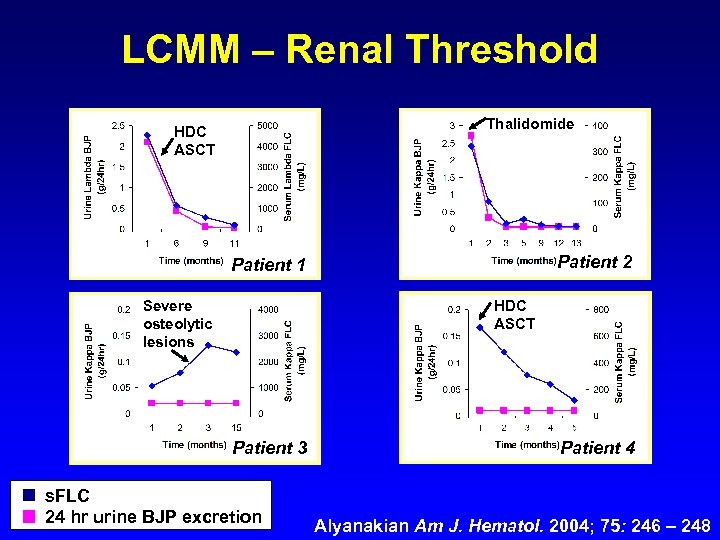 LCMM – Renal Threshold Thalidomide HDC ASCT Patient 2 Patient 1 Severe osteolytic lesions HDC ASCT Patient 3 s. FLC 24 hr urine BJP excretion Patient 4 Alyanakian Am J. Hematol. 2004; 75: 246 – 248
LCMM – Renal Threshold Thalidomide HDC ASCT Patient 2 Patient 1 Severe osteolytic lesions HDC ASCT Patient 3 s. FLC 24 hr urine BJP excretion Patient 4 Alyanakian Am J. Hematol. 2004; 75: 246 – 248
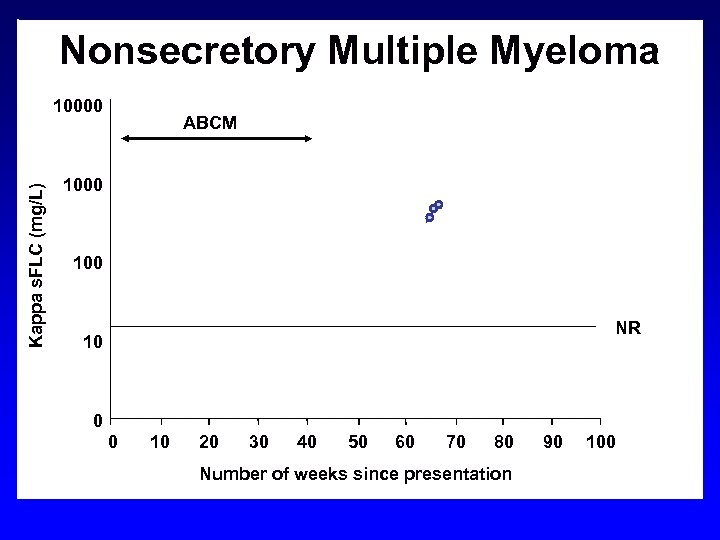 Nonsecretory Multiple Myeloma Kappa s. FLC (mg/L) 10000 VAD ABCM HDM/ PBSCT 1000 100 NR 10 0 0 10 20 30 40 50 60 70 80 Number of weeks since presentation 90 100
Nonsecretory Multiple Myeloma Kappa s. FLC (mg/L) 10000 VAD ABCM HDM/ PBSCT 1000 100 NR 10 0 0 10 20 30 40 50 60 70 80 Number of weeks since presentation 90 100
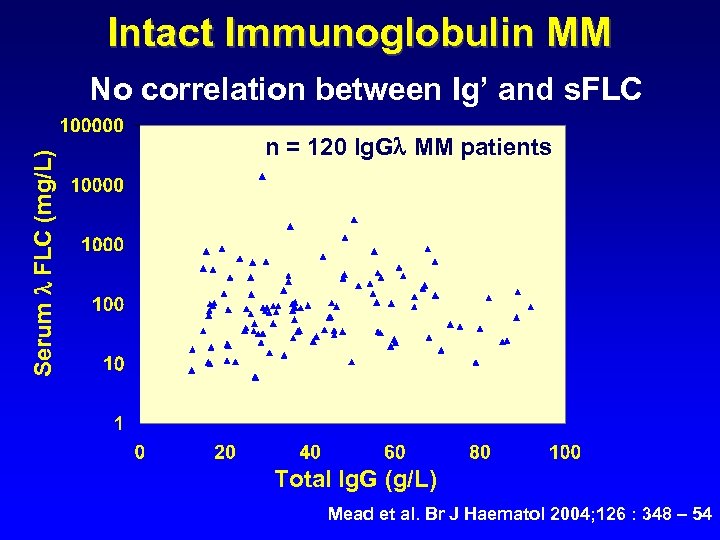 Intact Immunoglobulin MM Serum FLC (mg/L) No correlation between Ig’ and s. FLC n = 120 Ig. G MM patients Total Ig. G (g/L) Mead et al. Br J Haematol 2004; 126 : 348 – 54
Intact Immunoglobulin MM Serum FLC (mg/L) No correlation between Ig’ and s. FLC n = 120 Ig. G MM patients Total Ig. G (g/L) Mead et al. Br J Haematol 2004; 126 : 348 – 54
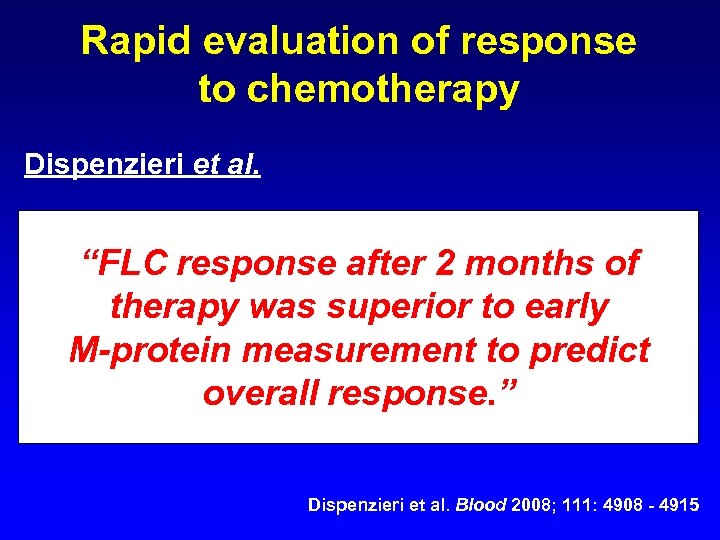 Rapid evaluation of response to chemotherapy Dispenzieri et al. • Retrospective analysis of ECOG trial E 9486 “FLC response after 2 months of • VBMCP IFN or superior to early cyclophosphamide therapy was • 399 patients measurement to predict M-protein • Assessed overall response. ” s. FLC and M-protein responses to therapy Dispenzieri et al. Blood 2008; 111: 4908 - 4915
Rapid evaluation of response to chemotherapy Dispenzieri et al. • Retrospective analysis of ECOG trial E 9486 “FLC response after 2 months of • VBMCP IFN or superior to early cyclophosphamide therapy was • 399 patients measurement to predict M-protein • Assessed overall response. ” s. FLC and M-protein responses to therapy Dispenzieri et al. Blood 2008; 111: 4908 - 4915
 Light chain escape “Rising monoclonal free light chain production at relapse without increased monoclonal intact immunoglobulin” Drayson et al. • Myeloma IX trial • Estimate incidence: 5% for Ig. G MM 15% for Ig. A MM Drayson, M. T. , et al. , Clin Lymphoma Myeloma, 2009. February: 346 a.
Light chain escape “Rising monoclonal free light chain production at relapse without increased monoclonal intact immunoglobulin” Drayson et al. • Myeloma IX trial • Estimate incidence: 5% for Ig. G MM 15% for Ig. A MM Drayson, M. T. , et al. , Clin Lymphoma Myeloma, 2009. February: 346 a.
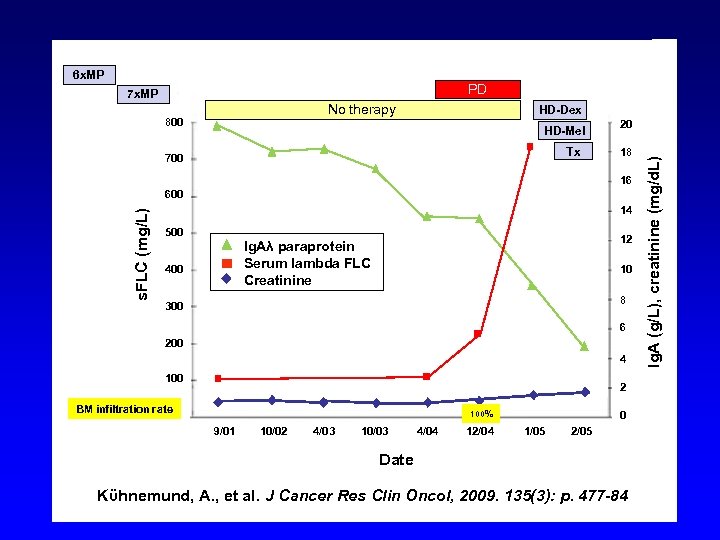 6 x. MP PD 7 x. MP No therapy HD-Dex HD-Mel Tx 700 20 18 16 s. FLC (mg/L) 600 14 500 12 Ig. Aλ paraprotein Serum lambda FLC Creatinine 400 10 8 300 6 200 4 100 2 BM infiltration rate 100% 9/01 10/02 4/03 10/03 4/04 12/04 0 1/05 2/05 Date Kϋhnemund, A. , et al. J Cancer Res Clin Oncol, 2009. 135(3): p. 477 -84 Ig. A (g/L), creatinine (mg/d. L) 800
6 x. MP PD 7 x. MP No therapy HD-Dex HD-Mel Tx 700 20 18 16 s. FLC (mg/L) 600 14 500 12 Ig. Aλ paraprotein Serum lambda FLC Creatinine 400 10 8 300 6 200 4 100 2 BM infiltration rate 100% 9/01 10/02 4/03 10/03 4/04 12/04 0 1/05 2/05 Date Kϋhnemund, A. , et al. J Cancer Res Clin Oncol, 2009. 135(3): p. 477 -84 Ig. A (g/L), creatinine (mg/d. L) 800
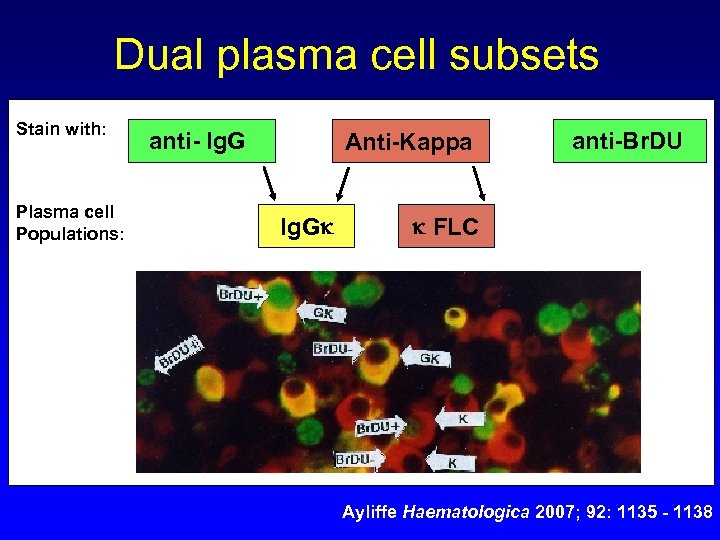 Dual plasma cell subsets Stain with: Plasma cell Populations: anti- Ig. G Anti-Kappa Ig. G anti-Br. DU FLC Ayliffe Haematologica 2007; 92: 1135 - 1138
Dual plasma cell subsets Stain with: Plasma cell Populations: anti- Ig. G Anti-Kappa Ig. G anti-Br. DU FLC Ayliffe Haematologica 2007; 92: 1135 - 1138
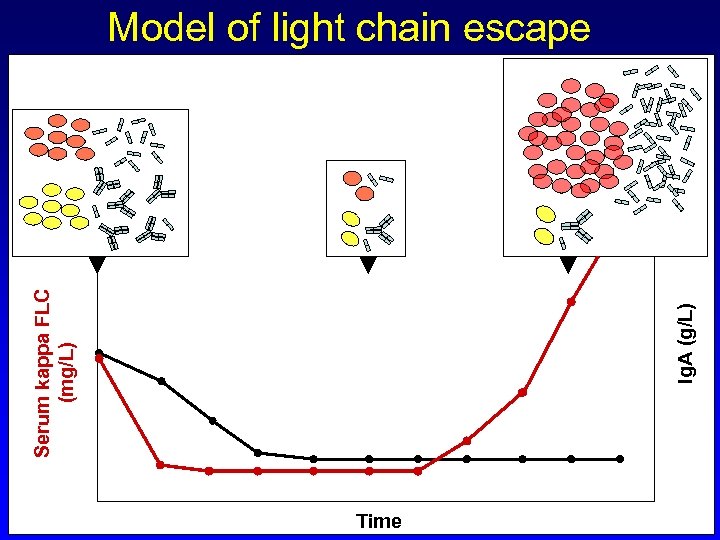 Ig. A (g/L) Serum kappa FLC (mg/L) Model of light chain escape Time
Ig. A (g/L) Serum kappa FLC (mg/L) Model of light chain escape Time
 International guidelines for s. FLC analysis in MM and related disorders • Assessment of response • Serial s. FLC should be routinely performed in: • Oligosecretory MM/ NSMM * • AL amyloidosis * • LCDD (personal experience of authors) • Periodic urine or s. FLC assessment for LCE Dispenzieri et al Leukemia (2009) 23, 215– 224
International guidelines for s. FLC analysis in MM and related disorders • Assessment of response • Serial s. FLC should be routinely performed in: • Oligosecretory MM/ NSMM * • AL amyloidosis * • LCDD (personal experience of authors) • Periodic urine or s. FLC assessment for LCE Dispenzieri et al Leukemia (2009) 23, 215– 224
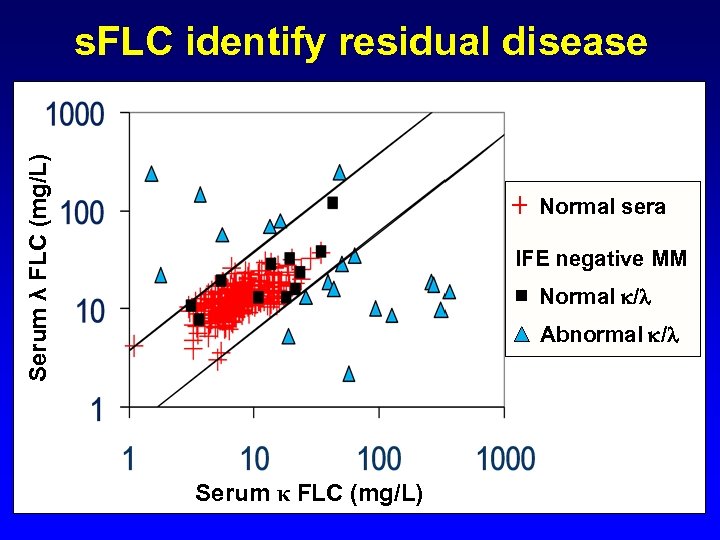 Serum λ FLC (mg/L) s. FLC identify residual disease Normal sera IFE negative MM Normal / Abnormal / Serum κ FLC (mg/L)
Serum λ FLC (mg/L) s. FLC identify residual disease Normal sera IFE negative MM Normal / Abnormal / Serum κ FLC (mg/L)
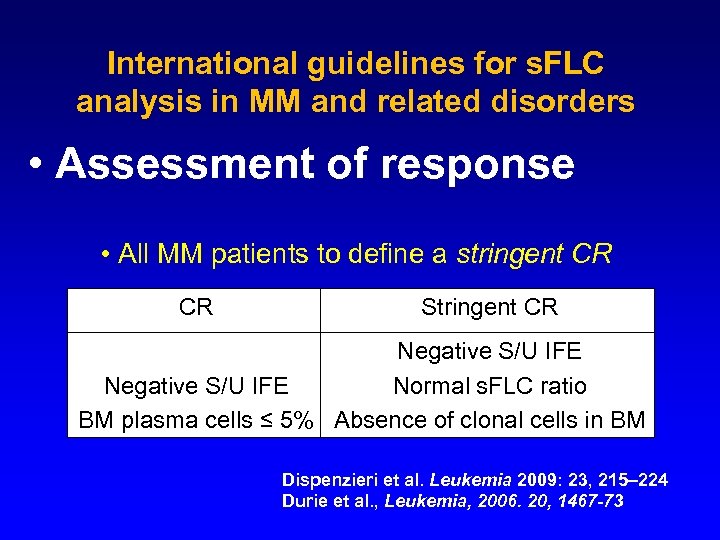 International guidelines for s. FLC analysis in MM and related disorders • Assessment of response • All MM patients to define a stringent CR CR Stringent CR Negative S/U IFE Normal s. FLC ratio BM plasma cells ≤ 5% Absence of clonal cells in BM Dispenzieri et al. Leukemia 2009: 23, 215– 224 Durie et al. , Leukemia, 2006. 20, 1467 -73
International guidelines for s. FLC analysis in MM and related disorders • Assessment of response • All MM patients to define a stringent CR CR Stringent CR Negative S/U IFE Normal s. FLC ratio BM plasma cells ≤ 5% Absence of clonal cells in BM Dispenzieri et al. Leukemia 2009: 23, 215– 224 Durie et al. , Leukemia, 2006. 20, 1467 -73
 Screening for Monoclonal Gammopathies • Serum electrophoresis → Monoclonal intact immunoglobulins • Urine electrophoresis → Monoclonal free light chains Can the serum FLC assay replace urine electrophoresis?
Screening for Monoclonal Gammopathies • Serum electrophoresis → Monoclonal intact immunoglobulins • Urine electrophoresis → Monoclonal free light chains Can the serum FLC assay replace urine electrophoresis?
 Screening for monoclonal gammopathy s. FLC + CZE • 9 additional B-cell disorders identified from 1003 consecutive unknown samples Bakshi et al. Am J Clin Path 2005; 124: 214 -218 Adding the serum FLC assay to screening protocol increased tumour detection rate by 56% s. FLC + SPE • 8 additional monoclonal gammopathies identified from 923 unknown samples Hill et al. Clin Chem 2006; 52: 1743 -1748
Screening for monoclonal gammopathy s. FLC + CZE • 9 additional B-cell disorders identified from 1003 consecutive unknown samples Bakshi et al. Am J Clin Path 2005; 124: 214 -218 Adding the serum FLC assay to screening protocol increased tumour detection rate by 56% s. FLC + SPE • 8 additional monoclonal gammopathies identified from 923 unknown samples Hill et al. Clin Chem 2006; 52: 1743 -1748
 Replacement of urine tests with s. FLC • 428 urine samples positive by u. IFE • On paired serum samples: Serum electrophoresis + s. FLC missed only 2 urine positive light chain MGUS patients No significant pathology was missed Katzmann et al. Mayo Clin Proc 2006; 81: 1575 - 1578
Replacement of urine tests with s. FLC • 428 urine samples positive by u. IFE • On paired serum samples: Serum electrophoresis + s. FLC missed only 2 urine positive light chain MGUS patients No significant pathology was missed Katzmann et al. Mayo Clin Proc 2006; 81: 1575 - 1578
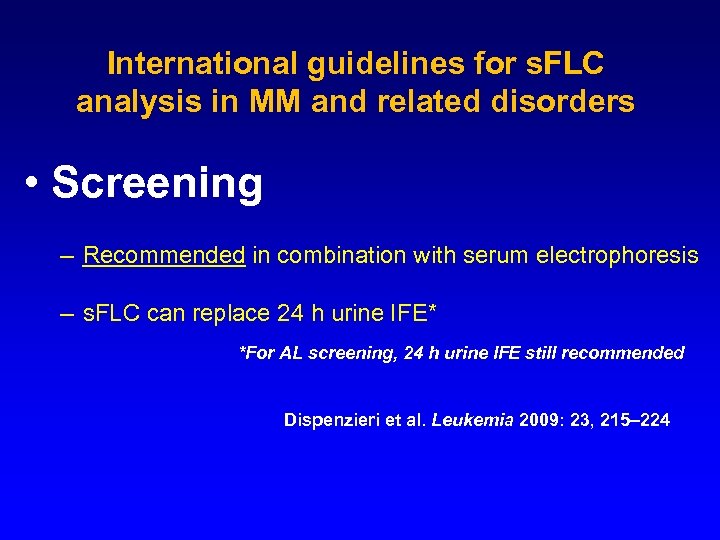 International guidelines for s. FLC analysis in MM and related disorders • Screening – Recommended in combination with serum electrophoresis – s. FLC can replace 24 h urine IFE* *For AL screening, 24 h urine IFE still recommended Dispenzieri et al. Leukemia 2009: 23, 215– 224
International guidelines for s. FLC analysis in MM and related disorders • Screening – Recommended in combination with serum electrophoresis – s. FLC can replace 24 h urine IFE* *For AL screening, 24 h urine IFE still recommended Dispenzieri et al. Leukemia 2009: 23, 215– 224
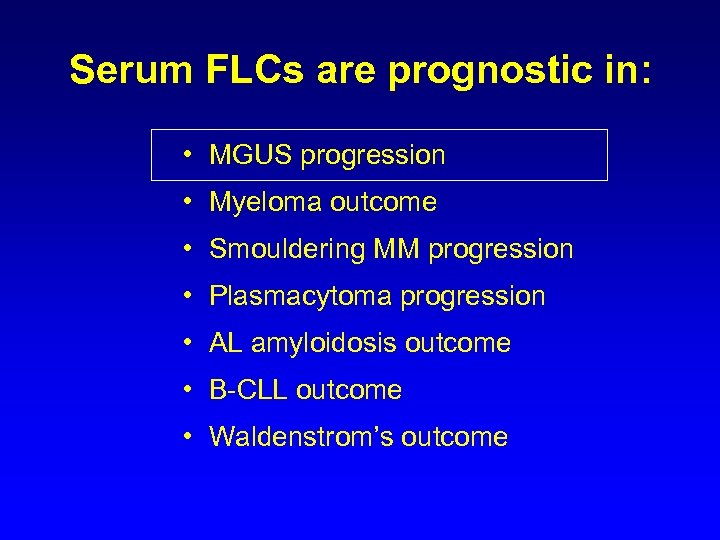 Serum FLCs are prognostic in: • MGUS progression • Myeloma outcome • Smouldering MM progression • Plasmacytoma progression • AL amyloidosis outcome • B-CLL outcome • Waldenstrom’s outcome
Serum FLCs are prognostic in: • MGUS progression • Myeloma outcome • Smouldering MM progression • Plasmacytoma progression • AL amyloidosis outcome • B-CLL outcome • Waldenstrom’s outcome
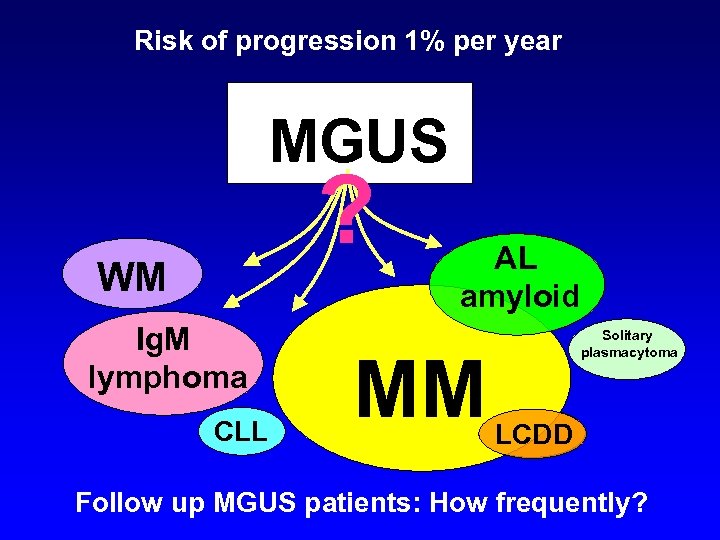 Risk of progression 1% per year MGUS ? WM Ig. M lymphoma CLL AL amyloid MM Solitary plasmacytoma LCDD Follow up MGUS patients: How frequently?
Risk of progression 1% per year MGUS ? WM Ig. M lymphoma CLL AL amyloid MM Solitary plasmacytoma LCDD Follow up MGUS patients: How frequently?
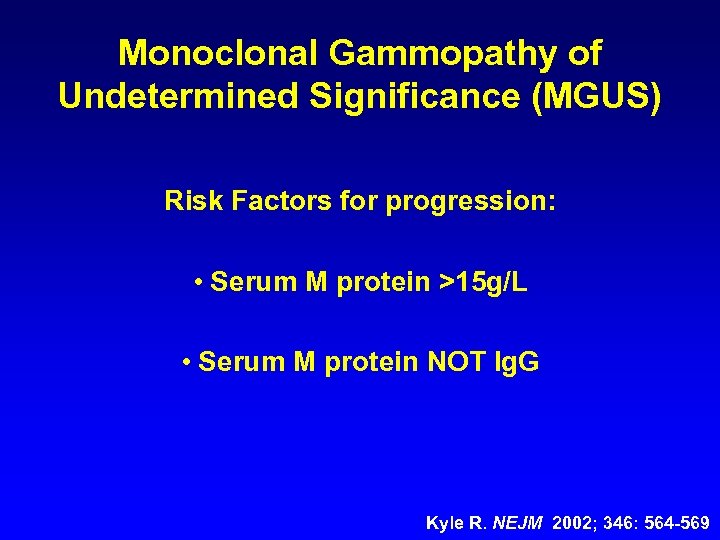 Monoclonal Gammopathy of Undetermined Significance (MGUS) Risk Factors for progression: • Serum M protein >15 g/L • Serum M protein NOT Ig. G Kyle R. NEJM 2002; 346: 564 -569
Monoclonal Gammopathy of Undetermined Significance (MGUS) Risk Factors for progression: • Serum M protein >15 g/L • Serum M protein NOT Ig. G Kyle R. NEJM 2002; 346: 564 -569
 Monoclonal Gammopathy of Undetermined Significance (MGUS) Risk Factors for progression: • Serum M protein >15 g/L • Serum M protein NOT Ig. G • s. FLC ratio Kyle R. NEJM 2002; 346: 564 -569 Rajkumar Blood 2005; 106: 812 -817
Monoclonal Gammopathy of Undetermined Significance (MGUS) Risk Factors for progression: • Serum M protein >15 g/L • Serum M protein NOT Ig. G • s. FLC ratio Kyle R. NEJM 2002; 346: 564 -569 Rajkumar Blood 2005; 106: 812 -817
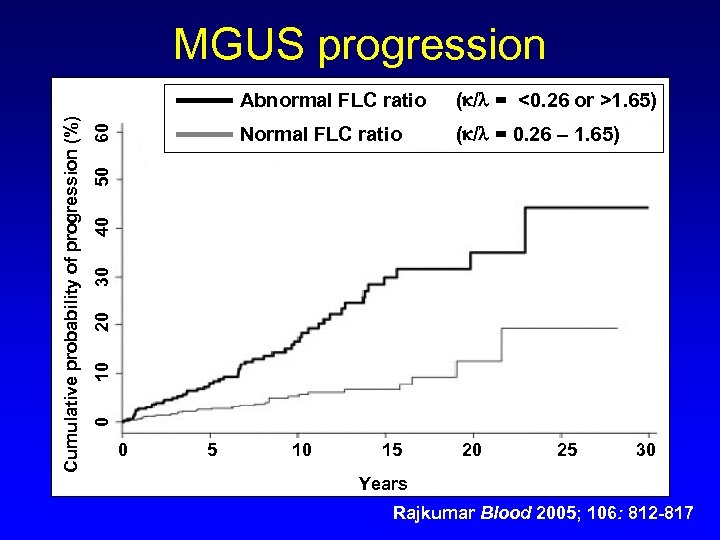 MGUS progression 60 Normal FLC ratio ( / = 0. 26 – 1. 65) 10 20 30 40 50 ( / = <0. 26 or >1. 65) 0 Cumulative probability of progression (%) Abnormal FLC ratio 0 5 10 15 20 25 30 Years Rajkumar Blood 2005; 106: 812 -817
MGUS progression 60 Normal FLC ratio ( / = 0. 26 – 1. 65) 10 20 30 40 50 ( / = <0. 26 or >1. 65) 0 Cumulative probability of progression (%) Abnormal FLC ratio 0 5 10 15 20 25 30 Years Rajkumar Blood 2005; 106: 812 -817
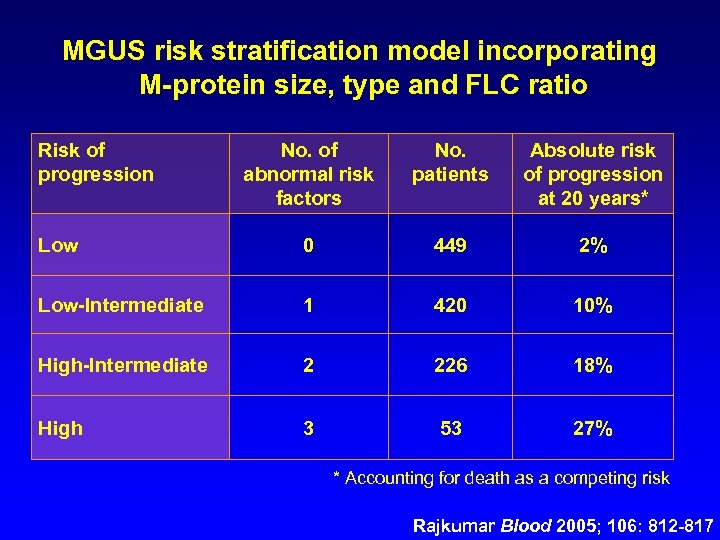 MGUS risk stratification model incorporating M-protein size, type and FLC ratio Risk of progression No. of abnormal risk factors No. patients Absolute risk of progression at 20 years* Low 0 449 2% Low-Intermediate 1 420 10% High-Intermediate 2 226 18% High 3 53 27% * Accounting for death as a competing risk Rajkumar Blood 2005; 106: 812 -817
MGUS risk stratification model incorporating M-protein size, type and FLC ratio Risk of progression No. of abnormal risk factors No. patients Absolute risk of progression at 20 years* Low 0 449 2% Low-Intermediate 1 420 10% High-Intermediate 2 226 18% High 3 53 27% * Accounting for death as a competing risk Rajkumar Blood 2005; 106: 812 -817
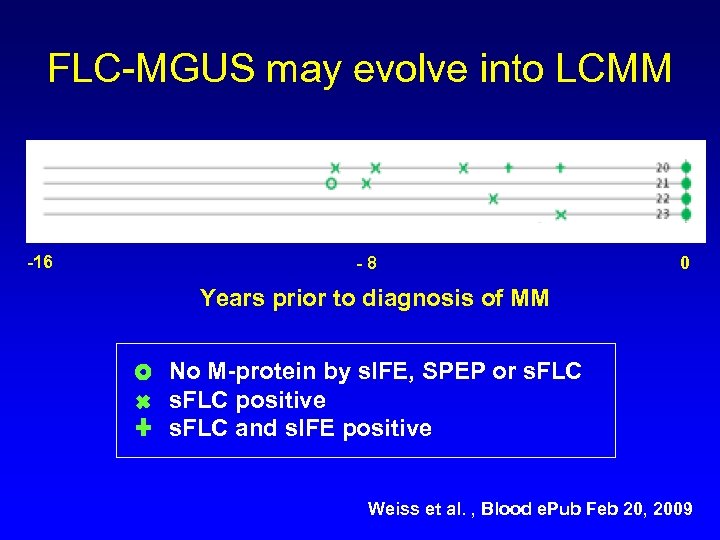 FLC-MGUS may evolve into LCMM -16 -8 0 Years prior to diagnosis of MM No M-protein by s. IFE, SPEP or s. FLC positive s. FLC and s. IFE positive Weiss et al. , Blood e. Pub Feb 20, 2009
FLC-MGUS may evolve into LCMM -16 -8 0 Years prior to diagnosis of MM No M-protein by s. IFE, SPEP or s. FLC positive s. FLC and s. IFE positive Weiss et al. , Blood e. Pub Feb 20, 2009
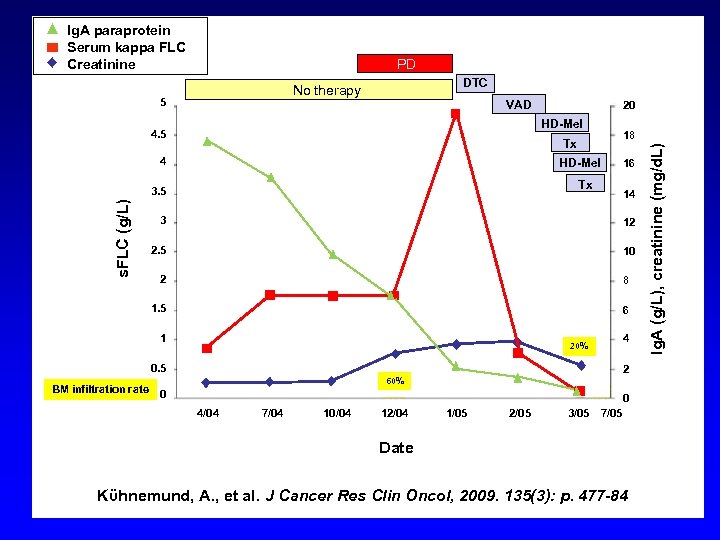 Ig. A paraprotein Serum kappa FLC Creatinine PD DTC 5 VAD HD-Mel 4. 5 18 Tx 4 HD-Mel Tx 3. 5 s. FLC (g/L) 20 16 14 3 12 2. 5 10 2 8 1. 5 6 1 4 20% 0. 5 BM infiltration rate 2 60% 0 0 4/04 7/04 10/04 12/04 1/05 2/05 3/05 7/05 Date Kϋhnemund, A. , et al. J Cancer Res Clin Oncol, 2009. 135(3): p. 477 -84 Ig. A (g/L), creatinine (mg/d. L) No therapy
Ig. A paraprotein Serum kappa FLC Creatinine PD DTC 5 VAD HD-Mel 4. 5 18 Tx 4 HD-Mel Tx 3. 5 s. FLC (g/L) 20 16 14 3 12 2. 5 10 2 8 1. 5 6 1 4 20% 0. 5 BM infiltration rate 2 60% 0 0 4/04 7/04 10/04 12/04 1/05 2/05 3/05 7/05 Date Kϋhnemund, A. , et al. J Cancer Res Clin Oncol, 2009. 135(3): p. 477 -84 Ig. A (g/L), creatinine (mg/d. L) No therapy
 International guidelines for s. FLC analysis in MM and related disorders • Prognosis – s. FLC should be measured at diagnosis for all patients with MGUS, SMM or MM, solitary plasmacytoma and AL amyloidosis Dispenzieri et al. Leukemia 2009: 23, 215– 224
International guidelines for s. FLC analysis in MM and related disorders • Prognosis – s. FLC should be measured at diagnosis for all patients with MGUS, SMM or MM, solitary plasmacytoma and AL amyloidosis Dispenzieri et al. Leukemia 2009: 23, 215– 224
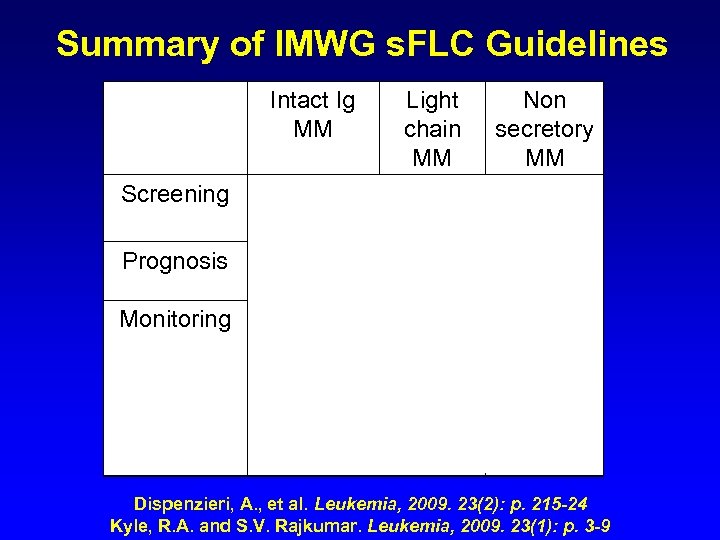 Summary of IMWG s. FLC Guidelines Intact Ig MM Screening Light Non chain secretory MM MM + serum electrophoresis Prognosis Monitoring (Oligosecretory) (LCE) (s. CR) Dispenzieri, A. , et al. Leukemia, 2009. 23(2): p. 215 -24 Kyle, R. A. and S. V. Rajkumar. Leukemia, 2009. 23(1): p. 3 -9
Summary of IMWG s. FLC Guidelines Intact Ig MM Screening Light Non chain secretory MM MM + serum electrophoresis Prognosis Monitoring (Oligosecretory) (LCE) (s. CR) Dispenzieri, A. , et al. Leukemia, 2009. 23(2): p. 215 -24 Kyle, R. A. and S. V. Rajkumar. Leukemia, 2009. 23(1): p. 3 -9
 Any Questions? • alex. legg@bindingsite. com Distributor in Poland BIOKOM beata. olsz@biokom. com. pl
Any Questions? • alex. legg@bindingsite. com Distributor in Poland BIOKOM beata. olsz@biokom. com. pl
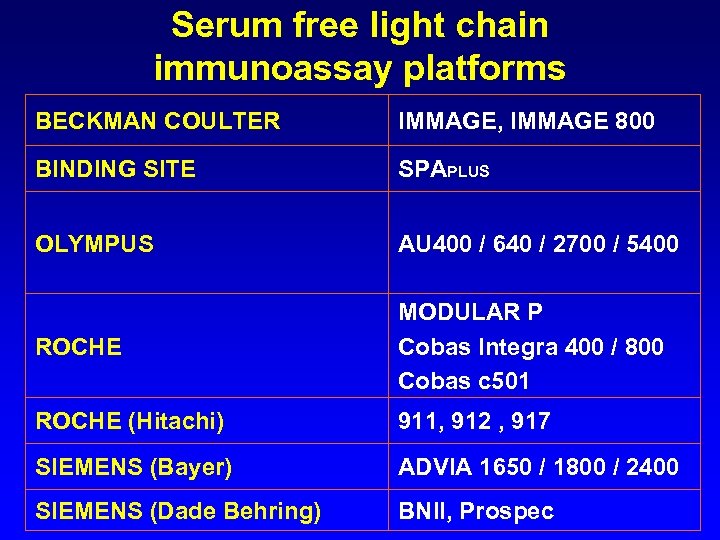 Serum free light chain immunoassay platforms BECKMAN COULTER IMMAGE, IMMAGE 800 BINDING SITE SPAPLUS OLYMPUS AU 400 / 640 / 2700 / 5400 ROCHE MODULAR P Cobas Integra 400 / 800 Cobas c 501 ROCHE (Hitachi) 911, 912 , 917 SIEMENS (Bayer) ADVIA 1650 / 1800 / 2400 SIEMENS (Dade Behring) BNII, Prospec
Serum free light chain immunoassay platforms BECKMAN COULTER IMMAGE, IMMAGE 800 BINDING SITE SPAPLUS OLYMPUS AU 400 / 640 / 2700 / 5400 ROCHE MODULAR P Cobas Integra 400 / 800 Cobas c 501 ROCHE (Hitachi) 911, 912 , 917 SIEMENS (Bayer) ADVIA 1650 / 1800 / 2400 SIEMENS (Dade Behring) BNII, Prospec
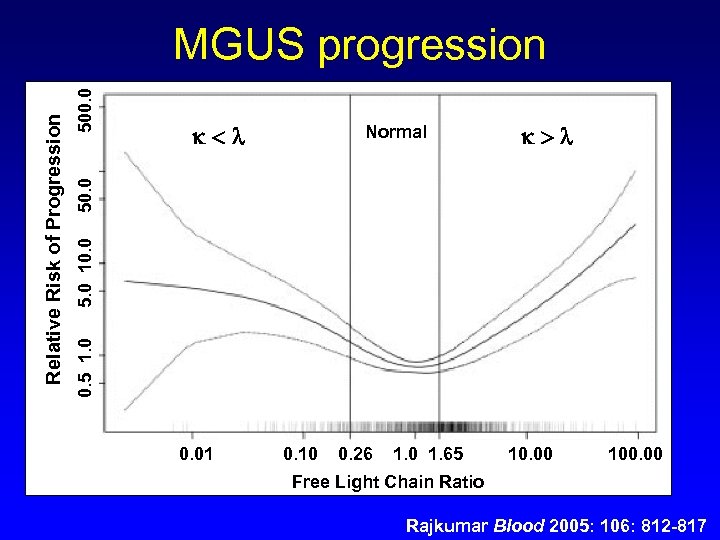 Normal 5. 0 10. 0 500. 0 0. 5 1. 0 Relative Risk of Progression MGUS progression 0. 01 0. 10 0. 26 1. 0 1. 65 10. 00 100. 00 Free Light Chain Ratio Rajkumar Blood 2005: 106: 812 -817
Normal 5. 0 10. 0 500. 0 0. 5 1. 0 Relative Risk of Progression MGUS progression 0. 01 0. 10 0. 26 1. 0 1. 65 10. 00 100. 00 Free Light Chain Ratio Rajkumar Blood 2005: 106: 812 -817
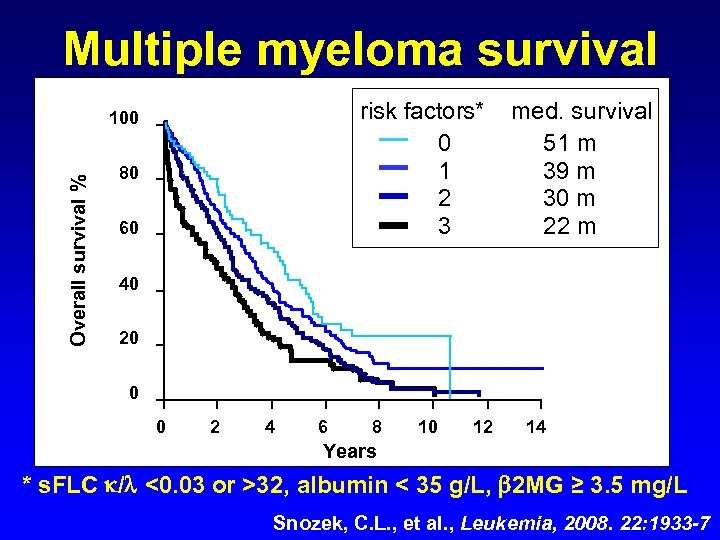 Multiple myeloma survival risk factors* 0 1 2 3 Overall survival % 100 80 60 med. survival 51 m 39 m 30 m 22 m 40 20 0 0 2 4 6 8 10 12 14 Years * s. FLC / <0. 03 or >32, albumin < 35 g/L, 2 MG ≥ 3. 5 mg/L Snozek, C. L. , et al. , Leukemia, 2008. 22: 1933 -7
Multiple myeloma survival risk factors* 0 1 2 3 Overall survival % 100 80 60 med. survival 51 m 39 m 30 m 22 m 40 20 0 0 2 4 6 8 10 12 14 Years * s. FLC / <0. 03 or >32, albumin < 35 g/L, 2 MG ≥ 3. 5 mg/L Snozek, C. L. , et al. , Leukemia, 2008. 22: 1933 -7
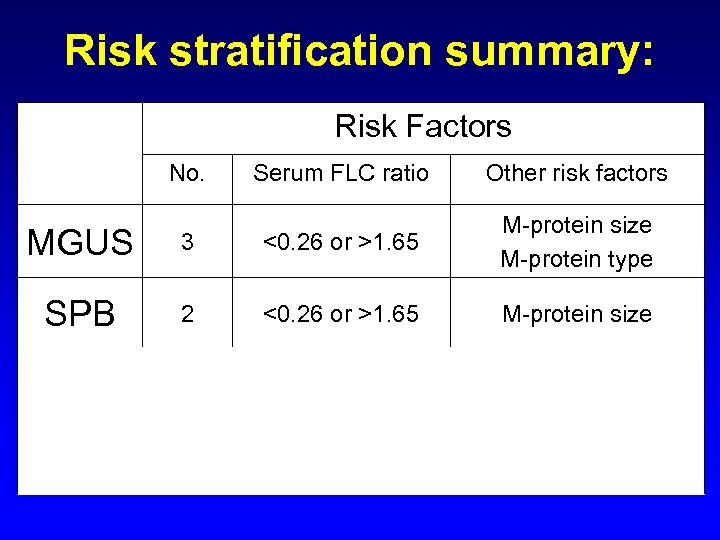 Risk stratification summary: Risk Factors No. Serum FLC ratio Other risk factors MGUS 3 <0. 26 or >1. 65 M-protein size M-protein type SPB 2 <0. 26 or >1. 65 M-protein size <0. 125 or >8 % BM plasma cells M-protein size <0. 03 or >32 Albumin 2 -microglobulin SMM MM 3 3
Risk stratification summary: Risk Factors No. Serum FLC ratio Other risk factors MGUS 3 <0. 26 or >1. 65 M-protein size M-protein type SPB 2 <0. 26 or >1. 65 M-protein size <0. 125 or >8 % BM plasma cells M-protein size <0. 03 or >32 Albumin 2 -microglobulin SMM MM 3 3
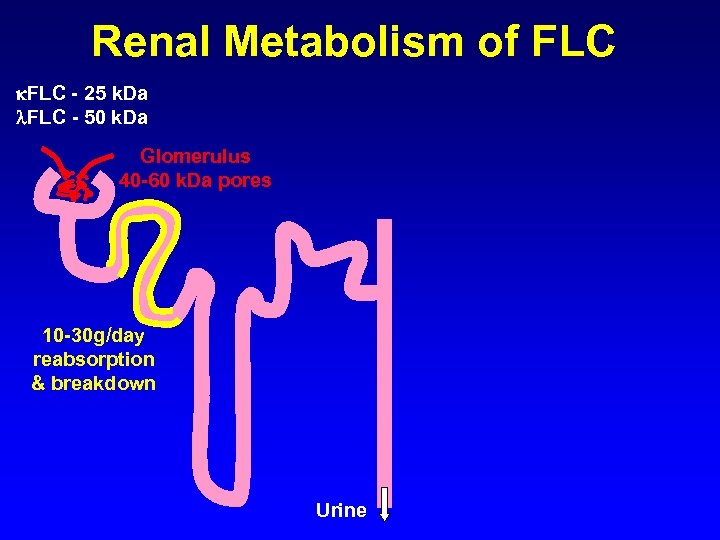 Renal Metabolism of FLC - 25 k. Da FLC - 50 k. Da Glomerulus 40 -60 k. Da pores 10 -30 g/day reabsorption & breakdown Urine
Renal Metabolism of FLC - 25 k. Da FLC - 50 k. Da Glomerulus 40 -60 k. Da pores 10 -30 g/day reabsorption & breakdown Urine
 Light chain escape “Rising monoclonal free light chain production at relapse without increased monoclonal intact immunoglobulin” Kühnemund et al: • Largest case series of LCE • Estimate incidence is 2. 5% MM at relapse • 5/10 cases of LCE had renal impairment Kuhnemund, A. , et al. , J Cancer Res Clin Oncol, 2009. 135, 477 -84
Light chain escape “Rising monoclonal free light chain production at relapse without increased monoclonal intact immunoglobulin” Kühnemund et al: • Largest case series of LCE • Estimate incidence is 2. 5% MM at relapse • 5/10 cases of LCE had renal impairment Kuhnemund, A. , et al. , J Cancer Res Clin Oncol, 2009. 135, 477 -84
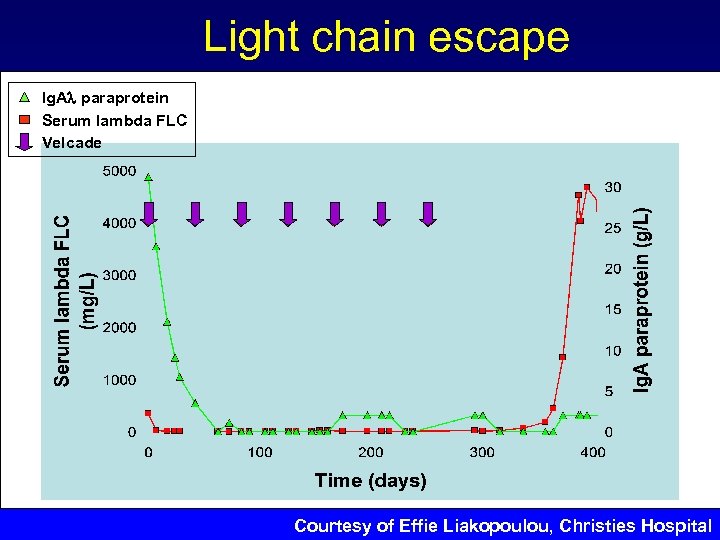 Light chain escape Ig. A paraprotein Serum lambda FLC Velcade Courtesy of Effie Liakopoulou, Christies Hospital
Light chain escape Ig. A paraprotein Serum lambda FLC Velcade Courtesy of Effie Liakopoulou, Christies Hospital
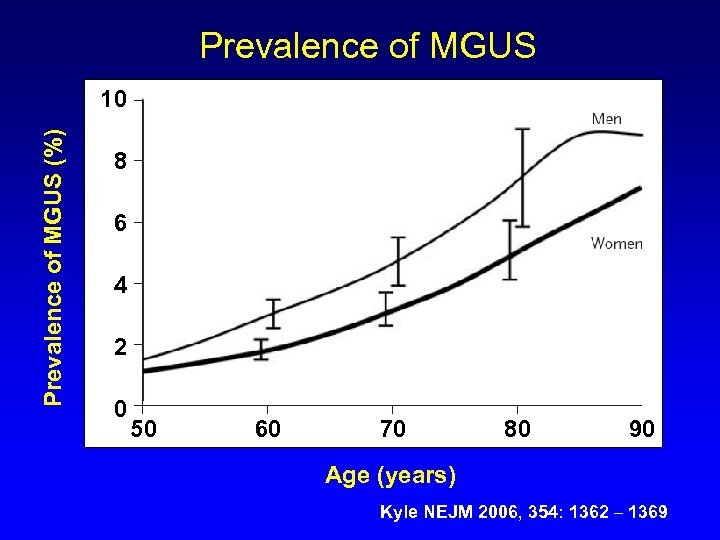 Prevalence of MGUS (%) 10 8 6 4 2 0 50 60 70 80 90 Age (years) Kyle NEJM 2006, 354: 1362 – 1369
Prevalence of MGUS (%) 10 8 6 4 2 0 50 60 70 80 90 Age (years) Kyle NEJM 2006, 354: 1362 – 1369


