046d11fd0bc2a2b195d64f39811fe41c.ppt
- Количество слайдов: 15
 INTERNATIONAL CONFERENCE ON INTELLECTUAL PROPERTY IN THE PHARMACEUTICAL INDUSTRY Warsaw, April 24, 2009 Patents for Pharmaceuticals Products - Legal options and flexibilities WIPO Secretariat
INTERNATIONAL CONFERENCE ON INTELLECTUAL PROPERTY IN THE PHARMACEUTICAL INDUSTRY Warsaw, April 24, 2009 Patents for Pharmaceuticals Products - Legal options and flexibilities WIPO Secretariat
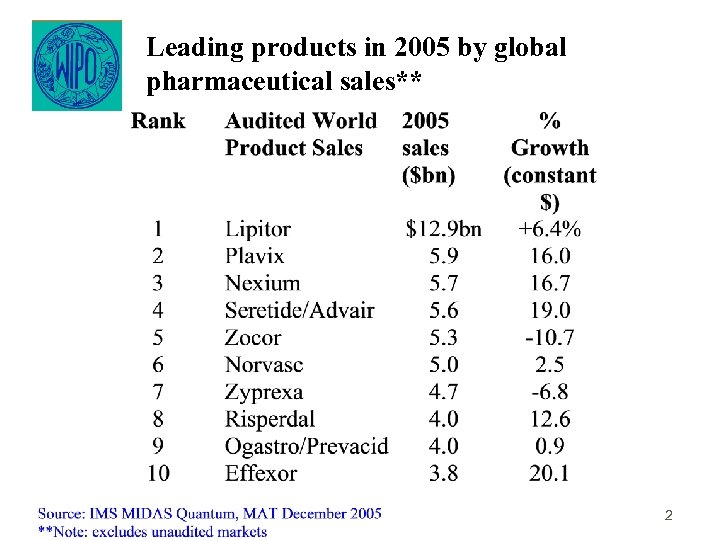 Leading products in 2005 by global pharmaceutical sales** 2
Leading products in 2005 by global pharmaceutical sales** 2
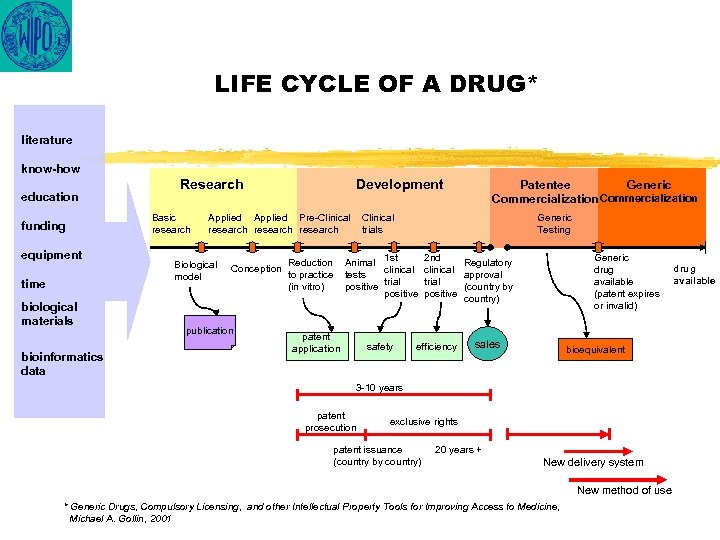 LIFE CYCLE OF A DRUG* literature know-how education funding equipment time biological materials bioinformatics data Research Basic research Development Applied Pre-Clinical research Biological model Conception publication Reduction to practice (in vitro) Patentee Generic Commercialization Clinical trials Generic Testing 1 st 2 nd Animal Regulatory clinical tests approval trial positive (country by positive country) patent application safety efficiency Generic drug available (patent expires or invalid) sales bioequivalent 3 -10 years patent prosecution exclusive rights patent issuance (country by country) 20 years + New delivery system New method of use * Generic Drugs, Compulsory Licensing, and other Intellectual Property Tools for Improving Access to Medicine, Michael A. Gollin, 2001 drug available
LIFE CYCLE OF A DRUG* literature know-how education funding equipment time biological materials bioinformatics data Research Basic research Development Applied Pre-Clinical research Biological model Conception publication Reduction to practice (in vitro) Patentee Generic Commercialization Clinical trials Generic Testing 1 st 2 nd Animal Regulatory clinical tests approval trial positive (country by positive country) patent application safety efficiency Generic drug available (patent expires or invalid) sales bioequivalent 3 -10 years patent prosecution exclusive rights patent issuance (country by country) 20 years + New delivery system New method of use * Generic Drugs, Compulsory Licensing, and other Intellectual Property Tools for Improving Access to Medicine, Michael A. Gollin, 2001 drug available
 Patents to Pharmaceuticals in the international context y The Multilateral Context: 1. Paris Convention 2. Trips Agreement; and y The Bilateral Context 4
Patents to Pharmaceuticals in the international context y The Multilateral Context: 1. Paris Convention 2. Trips Agreement; and y The Bilateral Context 4
 The Paris Convention and the asymmetries Some developing countries had excluded from patentability pharmaceutical products, without any challenge to the Paris Convention (Art. 4 bis, 4 ter, 5, 5 ter). This situation that has been gradually changing due multilateral commitments that were adopted in the framework of the TRIPS Agreement or because unilaterally some countries decided to do so. 5
The Paris Convention and the asymmetries Some developing countries had excluded from patentability pharmaceutical products, without any challenge to the Paris Convention (Art. 4 bis, 4 ter, 5, 5 ter). This situation that has been gradually changing due multilateral commitments that were adopted in the framework of the TRIPS Agreement or because unilaterally some countries decided to do so. 5
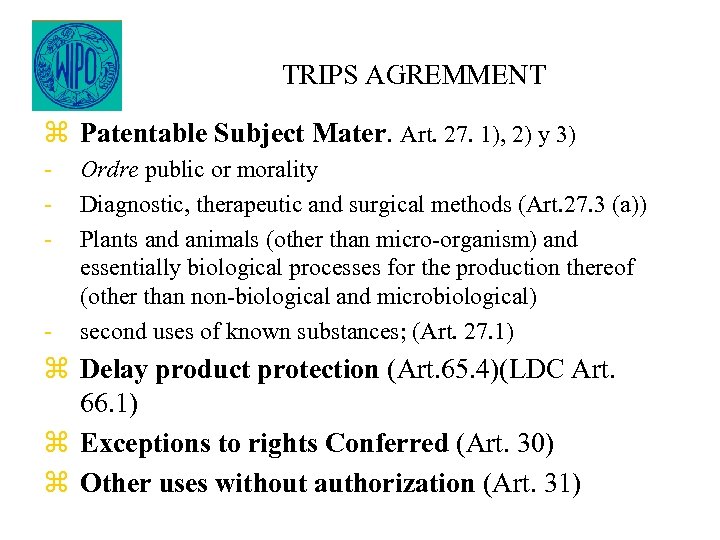 TRIPS AGREMMENT z Patentable Subject Mater. Art. 27. 1), 2) y 3) - - Ordre public or morality Diagnostic, therapeutic and surgical methods (Art. 27. 3 (a)) Plants and animals (other than micro-organism) and essentially biological processes for the production thereof (other than non-biological and microbiological) second uses of known substances; (Art. 27. 1) z Delay product protection (Art. 65. 4)(LDC Art. 66. 1) z Exceptions to rights Conferred (Art. 30) z Other uses without authorization (Art. 31)
TRIPS AGREMMENT z Patentable Subject Mater. Art. 27. 1), 2) y 3) - - Ordre public or morality Diagnostic, therapeutic and surgical methods (Art. 27. 3 (a)) Plants and animals (other than micro-organism) and essentially biological processes for the production thereof (other than non-biological and microbiological) second uses of known substances; (Art. 27. 1) z Delay product protection (Art. 65. 4)(LDC Art. 66. 1) z Exceptions to rights Conferred (Art. 30) z Other uses without authorization (Art. 31)
 Doha Declaration on TRIPS and Public Health z “We recognize that intellectual property protection is important for the development of new medicines. We also recognize the concerns about its effects on prices” z “We agree that the TRIPS Agreement does not and should not prevent Members from taking measures to protect public health. Accordingly, while reiterating our commitment to the TRIPS Agreement, we affirm that the Agreement can and should be interpreted and implemented in a manner supportive of WTO Members' right to protect public health and, in particular, to promote access to medicines for all”. 7
Doha Declaration on TRIPS and Public Health z “We recognize that intellectual property protection is important for the development of new medicines. We also recognize the concerns about its effects on prices” z “We agree that the TRIPS Agreement does not and should not prevent Members from taking measures to protect public health. Accordingly, while reiterating our commitment to the TRIPS Agreement, we affirm that the Agreement can and should be interpreted and implemented in a manner supportive of WTO Members' right to protect public health and, in particular, to promote access to medicines for all”. 7
 Doha and Public Health. Decisions of the WTO Bodies z Decision of the Trips Council of 1 July 2002 for the extension of the transition period (Art. 66. 1) for Least Develop Countries respect to pharmaceuticals products (2016) z Decision of the Trips Council of 8 July 2002, which waive for least Developed Countries the obligation under paragraph 9 of article 70 (2016) z Decision of the General Council of 30 August 2003 for the implementation of the paragraph 6 of the Doha Declaration 8
Doha and Public Health. Decisions of the WTO Bodies z Decision of the Trips Council of 1 July 2002 for the extension of the transition period (Art. 66. 1) for Least Develop Countries respect to pharmaceuticals products (2016) z Decision of the Trips Council of 8 July 2002, which waive for least Developed Countries the obligation under paragraph 9 of article 70 (2016) z Decision of the General Council of 30 August 2003 for the implementation of the paragraph 6 of the Doha Declaration 8
 Patents to Pharmaceuticals in the bilateral context (USA bilateral Agreements) y. Pending Congressional Approval Colombia Panama Republic of Korea y. In Force Israel NAFTA Jordan Chile Singapore Australia Morocco Bahrain y. Pending Implementation Peru Oman y. Other FTA Negotiations Malaysia Thailand SACU UAE 9
Patents to Pharmaceuticals in the bilateral context (USA bilateral Agreements) y. Pending Congressional Approval Colombia Panama Republic of Korea y. In Force Israel NAFTA Jordan Chile Singapore Australia Morocco Bahrain y. Pending Implementation Peru Oman y. Other FTA Negotiations Malaysia Thailand SACU UAE 9
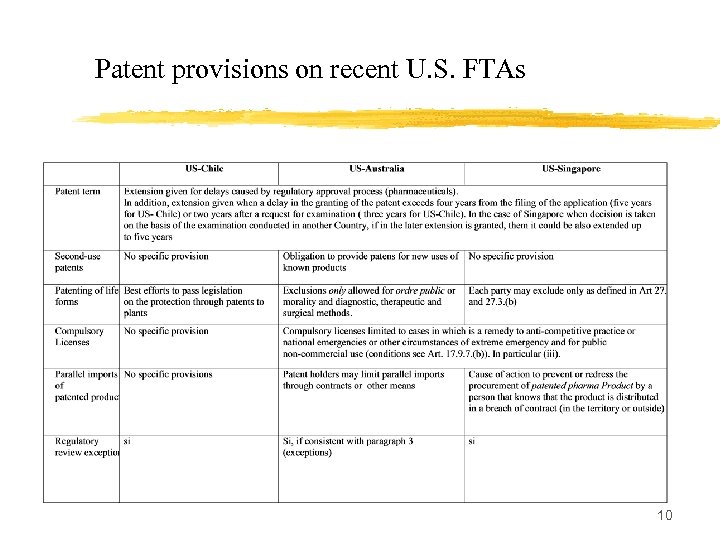 Patent provisions on recent U. S. FTAs 10
Patent provisions on recent U. S. FTAs 10
 Democrat's wish to incorporate key priorities on IP in FTAs (letter March 12 to USTR) The proposal y. Data protection. The inclusion of caps in the periods available; measures to facilitate the approval of generics to stimulate competition. y. Patent extension. To limit the total duration permitted. y. Linkage. To mitigate the burden on the regulatory authorities, like the obligation to withhold the approval if a patent could be violated. y. Compulsory licensing. Recalled each country freedom to determine the grounds upon which such licenses are granted. Avoid the use of side letters which are “non binding to the parties”. y. Consumer safeguards. Bolar provision; the applicant indications of the best mode to reproduce the invention and measures to avoid evergreening patents. 11
Democrat's wish to incorporate key priorities on IP in FTAs (letter March 12 to USTR) The proposal y. Data protection. The inclusion of caps in the periods available; measures to facilitate the approval of generics to stimulate competition. y. Patent extension. To limit the total duration permitted. y. Linkage. To mitigate the burden on the regulatory authorities, like the obligation to withhold the approval if a patent could be violated. y. Compulsory licensing. Recalled each country freedom to determine the grounds upon which such licenses are granted. Avoid the use of side letters which are “non binding to the parties”. y. Consumer safeguards. Bolar provision; the applicant indications of the best mode to reproduce the invention and measures to avoid evergreening patents. 11
 The Agreement (just for pharmaceutical products). y. DATA EXCLUSIVITY. Five years of data exclusivity for NCEs, considering the nature of the data and efforts and expenditure. If the parties grants the approval within six months when relying in FDA approval, the terms will count from them (concurrent period). y. PATENT EXTENSIONS. The obligation “shall” would be changed to “May”. Cooperation and assistance to avoid “unreasonable delays” is envisaged. 12
The Agreement (just for pharmaceutical products). y. DATA EXCLUSIVITY. Five years of data exclusivity for NCEs, considering the nature of the data and efforts and expenditure. If the parties grants the approval within six months when relying in FDA approval, the terms will count from them (concurrent period). y. PATENT EXTENSIONS. The obligation “shall” would be changed to “May”. Cooperation and assistance to avoid “unreasonable delays” is envisaged. 12
 The Agreement (just for pharmaceutical products). Cont… y THE LINKAGE. There is no obligation to establish a linkage between drug regulatory agencies and patent issues, particularly, no requirement that the agency withhold approval of the generic until it can certify that no patent would be violated. y NEW KING OF LINKAGE. The party would be required: 1. to provide procedures and remedies (judicial or administrative) and preliminary injunctions (equivalent) do dealt with patent infringement and validity disputes; and 2. A transparent system to give patent holders sufficient time and opportunity to enforce their rights (notifications, website info, etc). y LINKAGE OPTION. A party can be free to chose to fulfill this obligations (procedures and remedies) through a linkage system, if at the same time: adopt an expeditious system to challenge the validity or the infringement of a patent and a system to reward to those who successfully challenge a patent. 13
The Agreement (just for pharmaceutical products). Cont… y THE LINKAGE. There is no obligation to establish a linkage between drug regulatory agencies and patent issues, particularly, no requirement that the agency withhold approval of the generic until it can certify that no patent would be violated. y NEW KING OF LINKAGE. The party would be required: 1. to provide procedures and remedies (judicial or administrative) and preliminary injunctions (equivalent) do dealt with patent infringement and validity disputes; and 2. A transparent system to give patent holders sufficient time and opportunity to enforce their rights (notifications, website info, etc). y LINKAGE OPTION. A party can be free to chose to fulfill this obligations (procedures and remedies) through a linkage system, if at the same time: adopt an expeditious system to challenge the validity or the infringement of a patent and a system to reward to those who successfully challenge a patent. 13
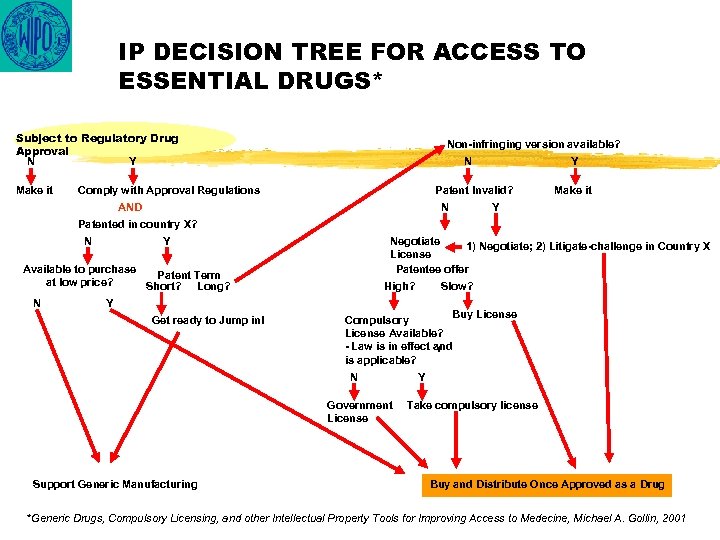 IP DECISION TREE FOR ACCESS TO ESSENTIAL DRUGS* Subject to Regulatory Drug Approval N Y Make it Non-infringing version available? N Comply with Approval Regulations Y Patent Invalid? AND N Make it Y Patented in country X? N Y Negotiate 1) Negotiate; 2) Litigate-challenge in Country X License Patentee offer Available to purchase Patent Term at low price? Short? Long? N Y Get ready to Jump in! High? Buy License Compulsory License Available? - Law is in effect and Y is applicable? N Government License Support Generic Manufacturing Slow? Y Take compulsory license Buy and Distribute Once Approved as a Drug *Generic Drugs, Compulsory Licensing, and other Intellectual Property Tools for Improving Access to Medecine, Michael A. Gollin, 2001
IP DECISION TREE FOR ACCESS TO ESSENTIAL DRUGS* Subject to Regulatory Drug Approval N Y Make it Non-infringing version available? N Comply with Approval Regulations Y Patent Invalid? AND N Make it Y Patented in country X? N Y Negotiate 1) Negotiate; 2) Litigate-challenge in Country X License Patentee offer Available to purchase Patent Term at low price? Short? Long? N Y Get ready to Jump in! High? Buy License Compulsory License Available? - Law is in effect and Y is applicable? N Government License Support Generic Manufacturing Slow? Y Take compulsory license Buy and Distribute Once Approved as a Drug *Generic Drugs, Compulsory Licensing, and other Intellectual Property Tools for Improving Access to Medecine, Michael A. Gollin, 2001
 Thank you! Marco M. Aleman Deputy Director, Division for Public Policy and Development marco. aleman@wipo. int 15
Thank you! Marco M. Aleman Deputy Director, Division for Public Policy and Development marco. aleman@wipo. int 15


