3 лекция.ppt
- Количество слайдов: 43
Intermolecular interaction

Covalent chemical bond are formed by one or several common electron pairs. If the density of electron is located symmetrically between the atoms, it’s called non-polar covalent bond. If the density of electron is shifted to the side of one of the atoms, it’s called a polar covalent bond.
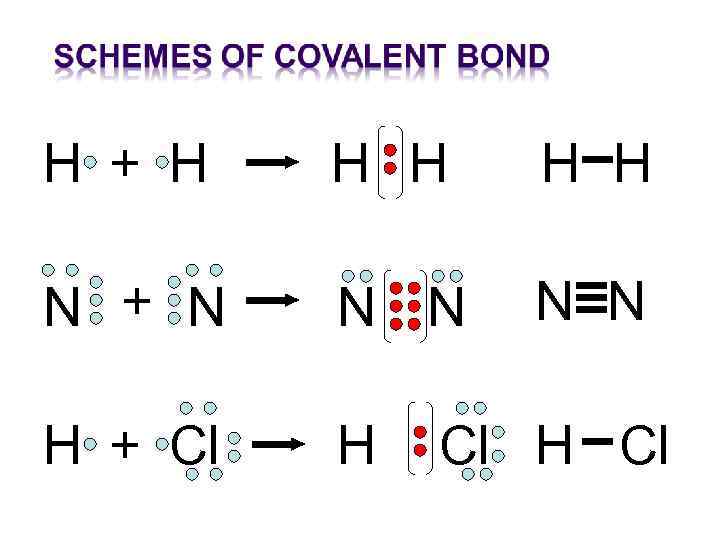
Н + Н Н Н N + N N N H + Cl H Cl
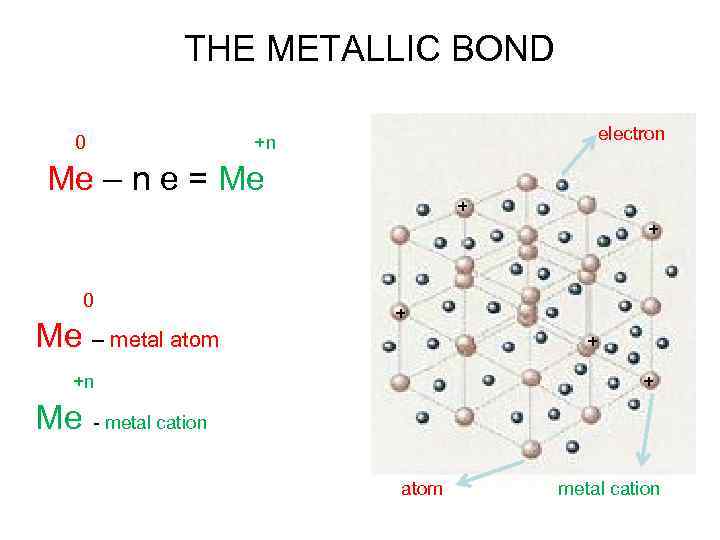
THE METALLIC BOND 0 +n Ме – n e = Ме electron + + 0 Ме – metal atom +n Ме - metal cation + + + atom metal cation
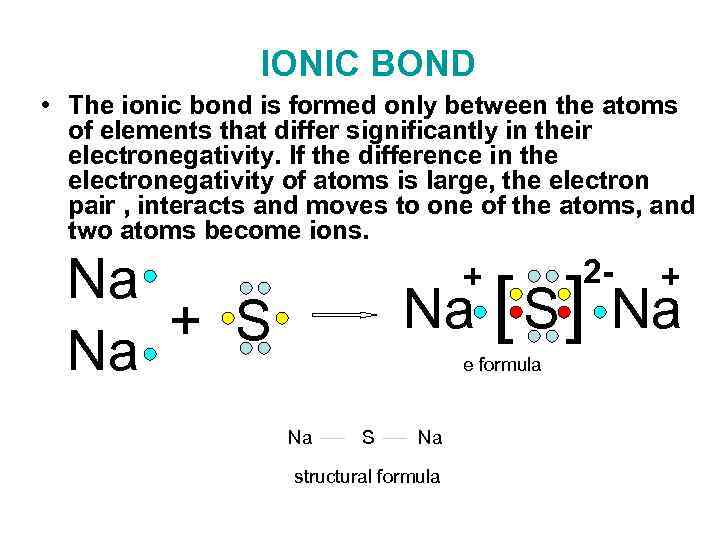
IONIC BOND • The ionic bond is formed only between the atoms of elements that differ significantly in their electronegativity. If the difference in the electronegativity of atoms is large, the electron pair , interacts and moves to one of the atoms, and two atoms become ions. Na + S Na + 2 - + Na [ S ] Na e formula Na S Na structural formula

• Intermolecular interaction of nonvalent nature is a phenomena of Van der Waals forces, caused mainly by electrostatic effects. • Van der Waals force is manifested in the interaction of charge-balanced systems (net charge is zero) - atoms, molecules, radicals • All these forces are the weak, about few k. J / mol (in the tens or even hundreds of times weaker than covalent chemical), but long-range, and therefore are largely in gases and liquids at low temperatures. The formation of crystals of inert gases is explained by the influence of these forces. They are also used in the sensitive sensors for scanning probe microscopes (SPM)
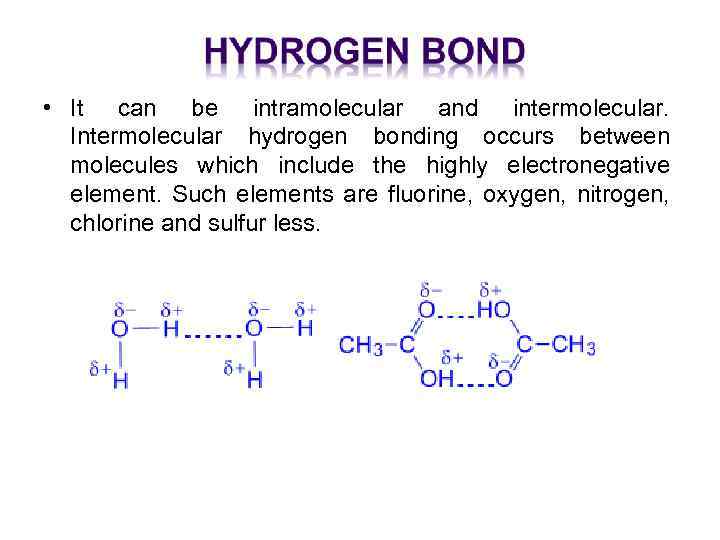
• It can be intramolecular and intermolecular. Intermolecular hydrogen bonding occurs between molecules which include the highly electronegative element. Such elements are fluorine, oxygen, nitrogen, chlorine and sulfur less.
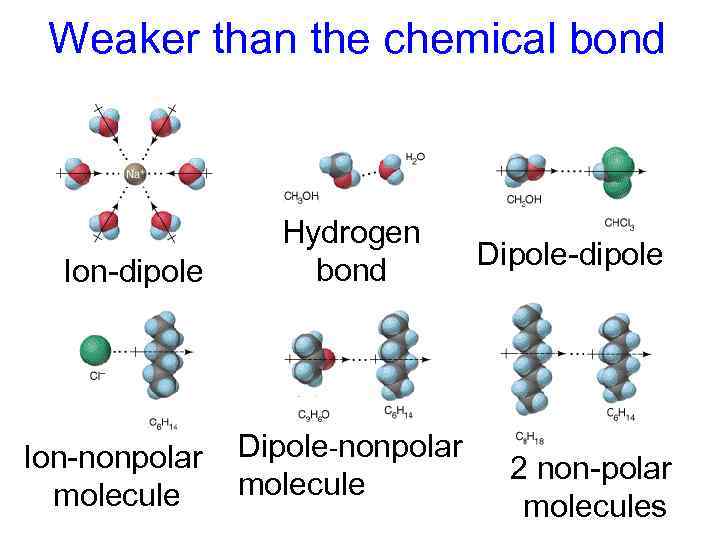
Weaker than the chemical bond Ion-dipole Hydrogen bond Ion-nonpolar Dipole-nonpolar molecule Dipole-dipole 2 non-polar molecules
• Very weak attractive forces between neutral atoms or molecules that appear at distances exceeding the dimensions of the particles are called intermolecular attraction or by Van der Waals forces • They operate in substances in the gaseous and liquid states, between molecules in molecular crystals. They play an important role in adsorption processes, catalysis, and in the processes of dissolution and solvation.

• Van der Waals attraction is electrical in nature and is seen as the result of three effects - orientation, induction, dispersion • E = Eor. + Eind. + Edisp. • The energy of all three terms is associated with the dipole interaction of different origin.
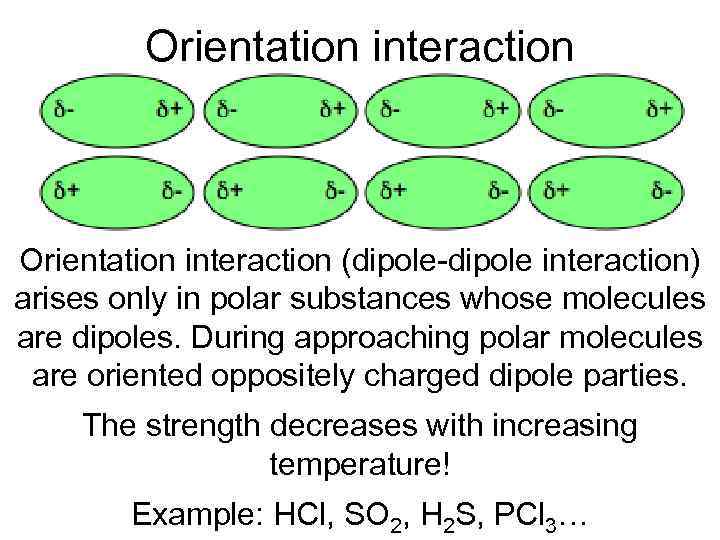
Orientation interaction (dipole-dipole interaction) arises only in polar substances whose molecules are dipoles. During approaching polar molecules are oriented oppositely charged dipole parties. The strength decreases with increasing temperature! Example: HCl, SO 2, H 2 S, PCl 3…
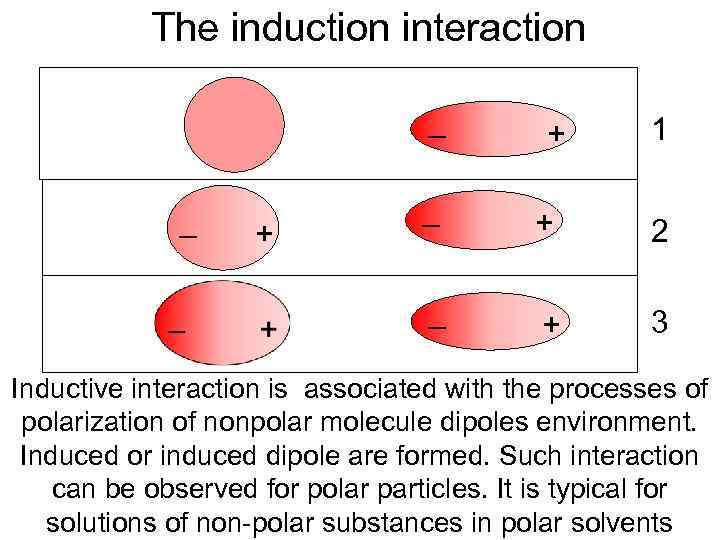
The induction interaction – + 1 + – + 2 – – + 3 Inductive interaction is associated with the processes of polarization of nonpolar molecule dipoles environment. Induced or induced dipole are formed. Such interaction can be observed for polar particles. It is typical for solutions of non-polar substances in polar solvents
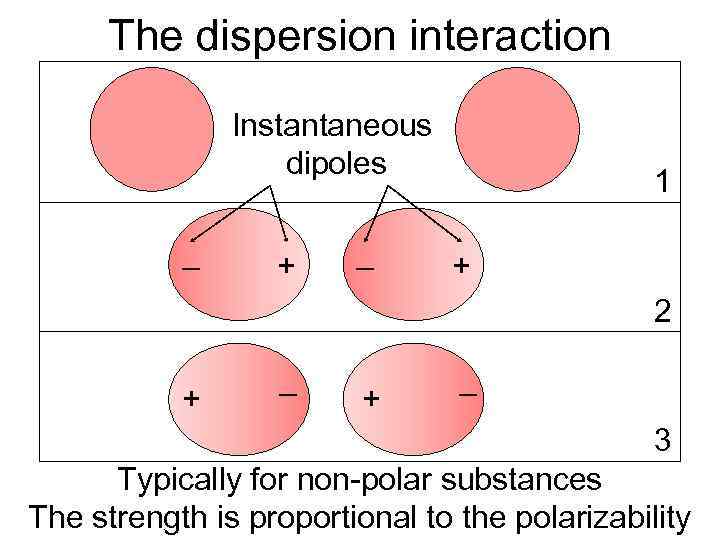
The dispersion interaction Instantaneous dipoles – + – 1 + 2 – + 3 Typically for non-polar substances The strength is proportional to the polarizability
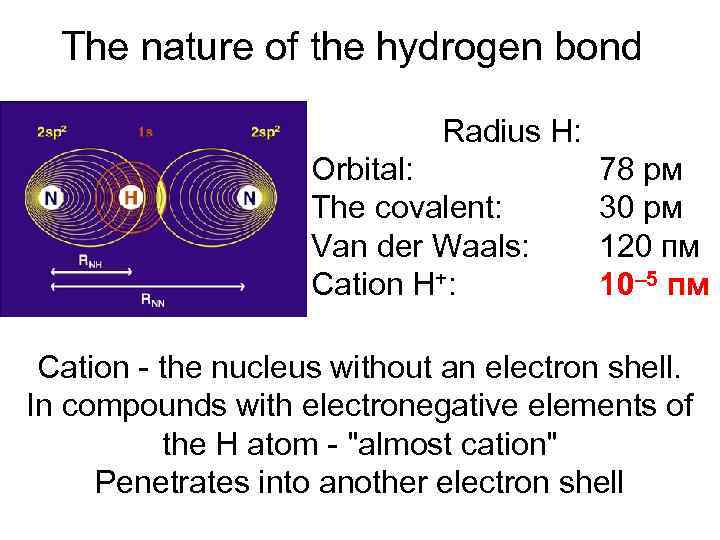
The nature of the hydrogen bond Radius Н: Orbital: The covalent: Van der Waals: Cation H+: 78 pм 30 pм 120 пм 10– 5 пм Cation - the nucleus without an electron shell. In compounds with electronegative elements of the H atom - "almost cation" Penetrates into another electron shell

• The hydrogen bond is some extent nature of donor -acceptor bond and is characterized by saturability and direction. The energy of the hydrogen bond is between 8 -40 k. J. There are strong and weak hydrogen bonds. Weak hydrogen bonds have energies of formation less than 15 k. J / mol. • The hydrogen bond makes a significant impact on the structure of matter and its physical and chemical properties. Many physical properties of materials with hydrogen bonding outliers among similar laws
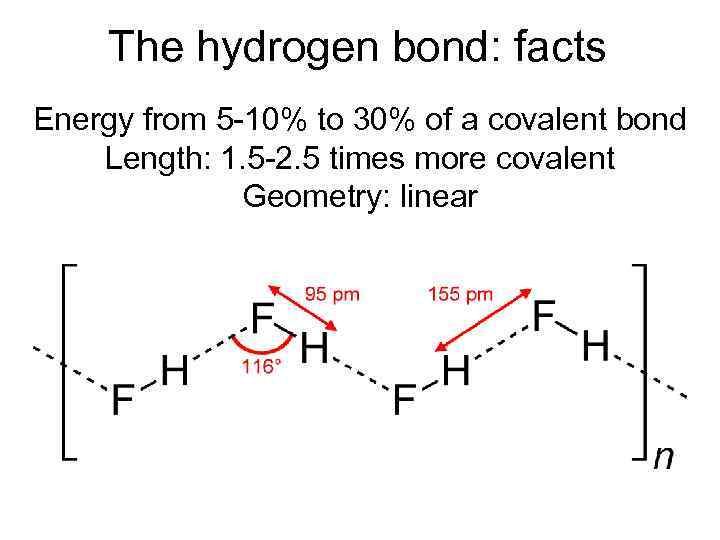
The hydrogen bond: facts Energy from 5 -10% to 30% of a covalent bond Length: 1. 5 -2. 5 times more covalent Geometry: linear
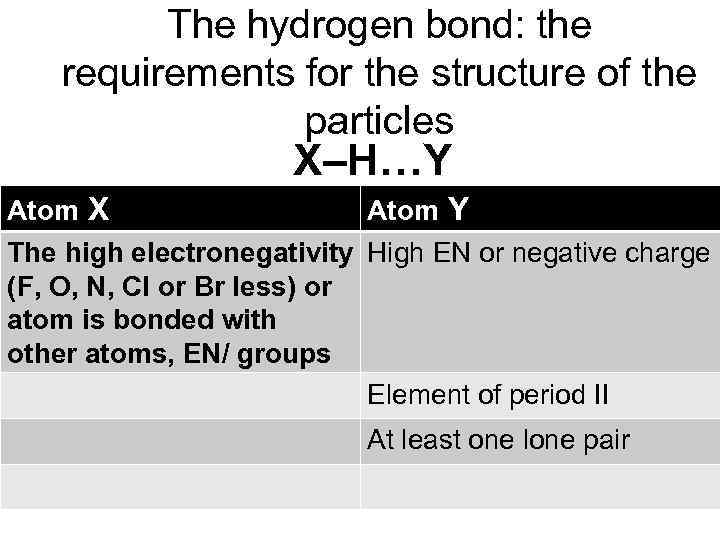
The hydrogen bond: the requirements for the structure of the particles X–H…Y Atom Х Atom Y The high electronegativity High EN or negative charge (F, O, N, Cl or Br less) or atom is bonded with other atoms, EN/ groups Element of period II At least one lone pair
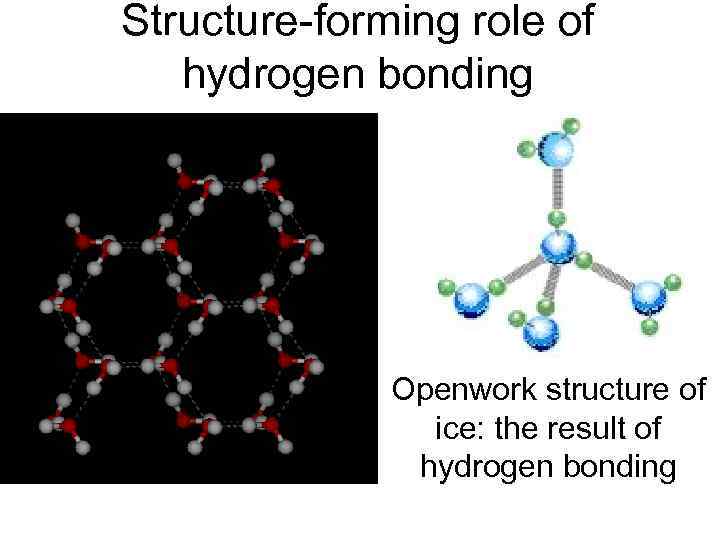
Structure-forming role of hydrogen bonding Openwork structure of ice: the result of hydrogen bonding
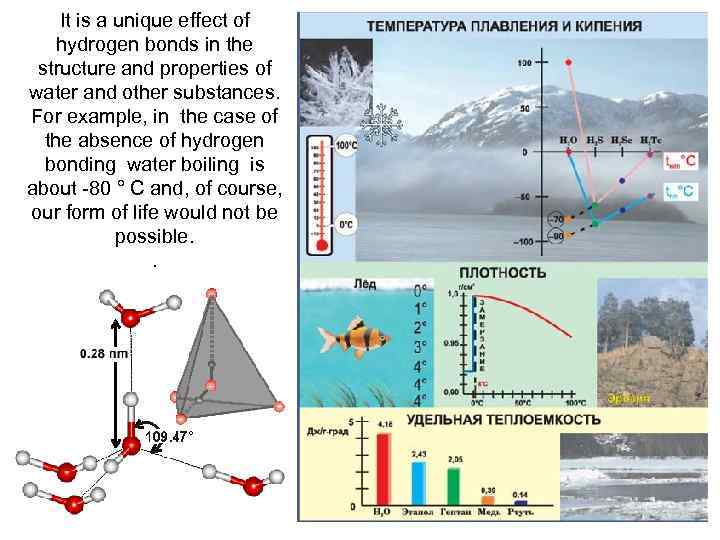
It is a unique effect of hydrogen bonds in the structure and properties of water and other substances. For example, in the case of the absence of hydrogen bonding water boiling is about -80 ° C and, of course, our form of life would not be possible. .
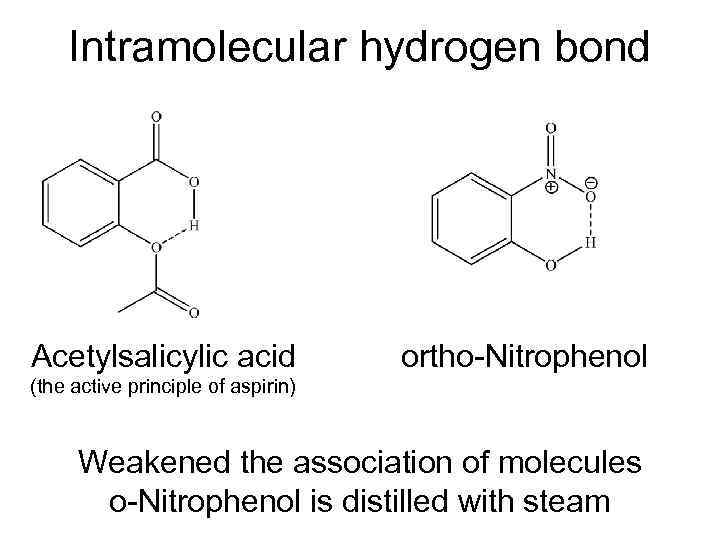
Intramolecular hydrogen bond Acetylsalicylic acid ortho-Nitrophenol (the active principle of aspirin) Weakened the association of molecules o-Nitrophenol is distilled with steam
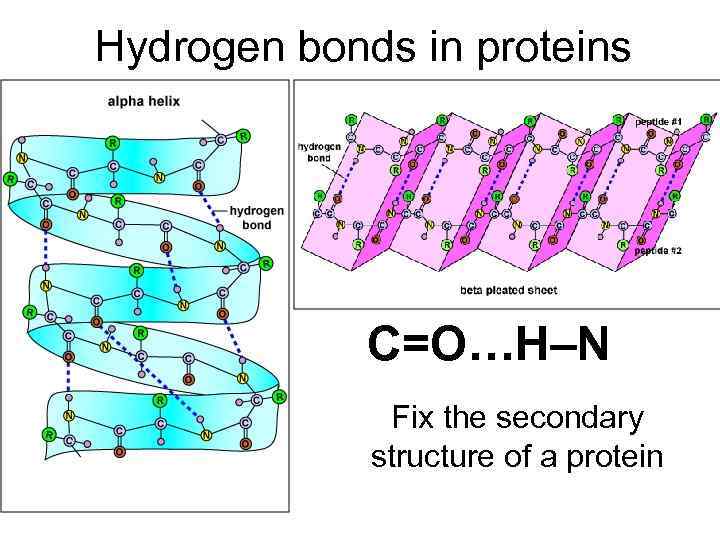
Hydrogen bonds in proteins C=O…H–N Fix the secondary structure of a protein
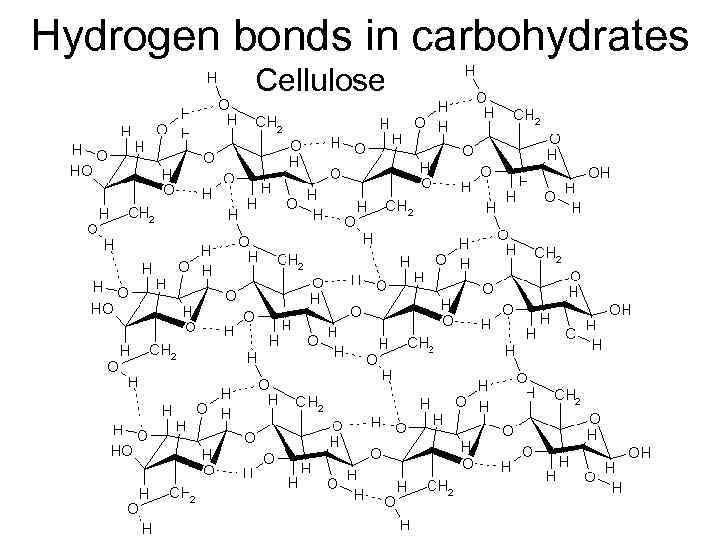
Hydrogen bonds in carbohydrates Cellulose
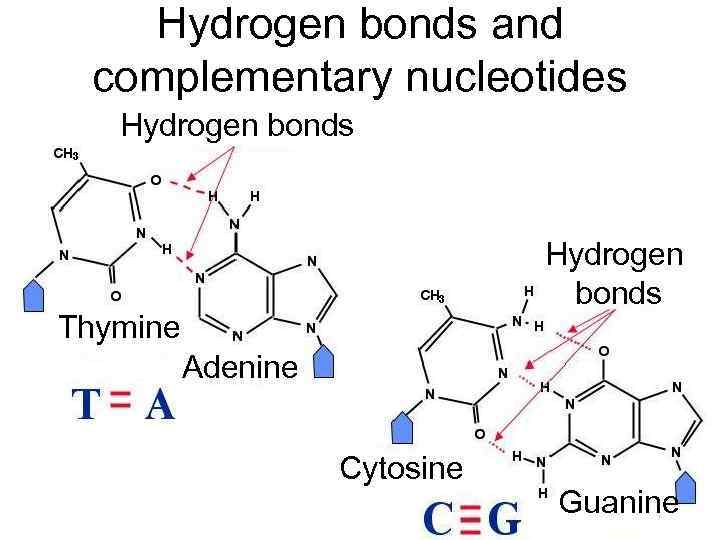
Hydrogen bonds and complementary nucleotides Hydrogen bonds Thymine Adenine Cytosine Guanine

The structure of condensed matter. Amorphous and crystalline state of matter • Phase is a collection of homogeneous parts of the system having the same composition and properties, and separated from other parts of the interface. • So, in a mixture of sugar and salt physically – there is a plurality of individual crystalline particles( grains). However, there are only three phases: two fused solid phase - sugar and salt, and gaseous one is the air between the individual grains. . • But the internal structure of condensed matter is clearly divided into two groups - amorphous and crystalline. • Amorphous bodies have a chaotic structure. They can be obtained by slowly cooling liquids (vitrification) and by rapid "freezing" and melting (amorphous metals). The main point of the crystals is the ordering of their structure.
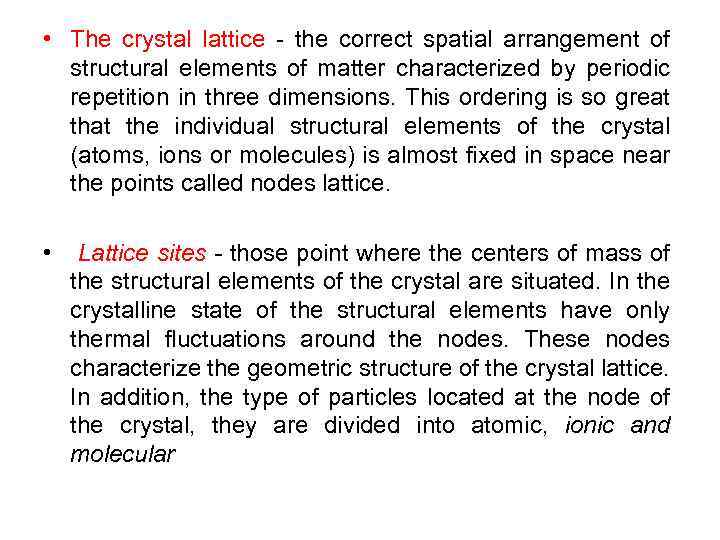
• The crystal lattice - the correct spatial arrangement of structural elements of matter characterized by periodic repetition in three dimensions. This ordering is so great the individual structural elements of the crystal (atoms, ions or molecules) is almost fixed in space near the points called nodes lattice. • Lattice sites - those point where the centers of mass of the structural elements of the crystal are situated. In the crystalline state of the structural elements have only thermal fluctuations around the nodes. These nodes characterize the geometric structure of the crystal lattice. In addition, the type of particles located at the node of the crystal, they are divided into atomic, ionic and molecular
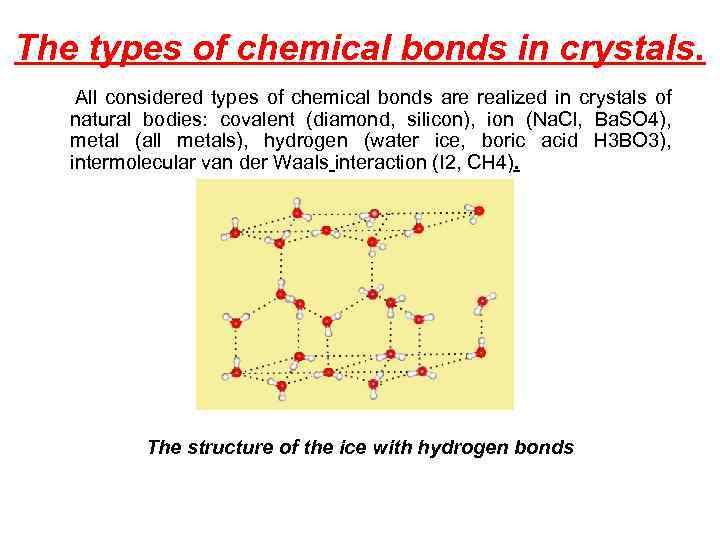
The types of chemical bonds in crystals. All considered types of chemical bonds are realized in crystals of natural bodies: covalent (diamond, silicon), ion (Na. Cl, Ba. SO 4), metal (all metals), hydrogen (water ice, boric acid H 3 BO 3), intermolecular van der Waals interaction (I 2, CH 4). The structure of the ice with hydrogen bonds

• The structure of the links is often mixed, for example, covalent and ionic polar (quartz, rutile). • Crystals with covalent bonds have, as a rule, high strength and melting point, but low electrical and thermal conductivity. • The crystals with ionic bonds are also strong enough and refractory but poor conductors of electricity and heat. In addition, they are fragile - shock deformation damage the balance of electrical forces and it causes electrostatic repulsion v which destroys the crystal. • The crystals with metallic bonds are strong, wellconductive and heat plastic. This is explained by the fact that a shift of atoms of the nodes do not have to break the directed covalent bonds, and the presence of free electrons does not destroy the balance of electrical forces. • Crystals with hydrogen and Van der Waals bonds have a low strength and a low melting point.
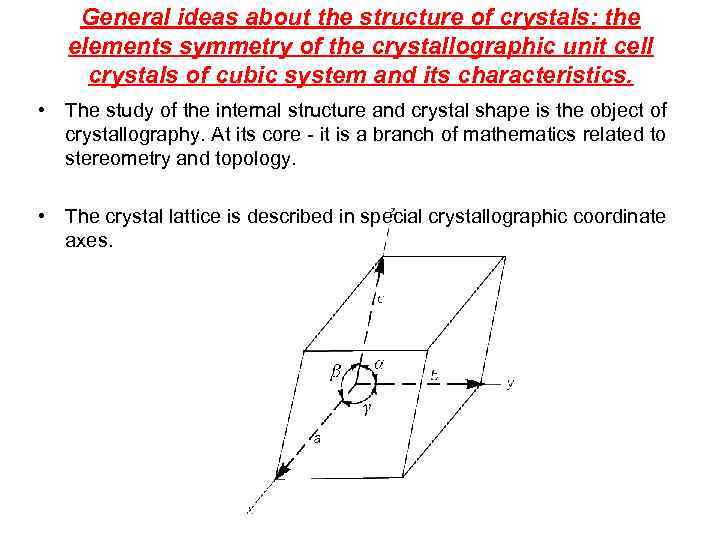
General ideas about the structure of crystals: the elements symmetry of the crystallographic unit cell crystals of cubic system and its characteristics. • The study of the internal structure and crystal shape is the object of crystallography. At its core - it is a branch of mathematics related to stereometry and topology. • The crystal lattice is described in special crystallographic coordinate axes.

• By setting the parameters 6 - segments of a, b, c and angles a b g get parallelepipeds, which are called a unit cell, and the parameters - lattice parameters. • The shift of the unit cell along the axis by an amount a, b, c, respectively, is called translation of unit cell. • Depending on the values of the lattice parameters of crystals appear at the various elements of symmetry - center axis plane.

Crystal - a part of the space occupied by parallel translations of the unit cell.
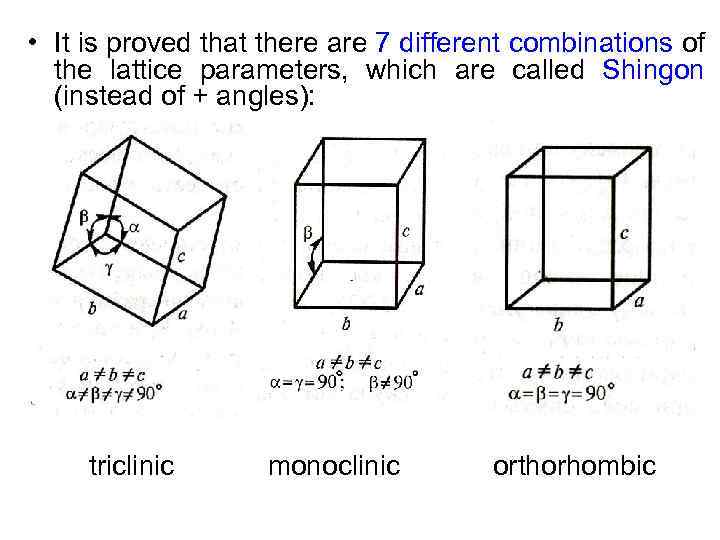
• It is proved that there are 7 different combinations of the lattice parameters, which are called Shingon (instead of + angles): triclinic monoclinic orthorhombic
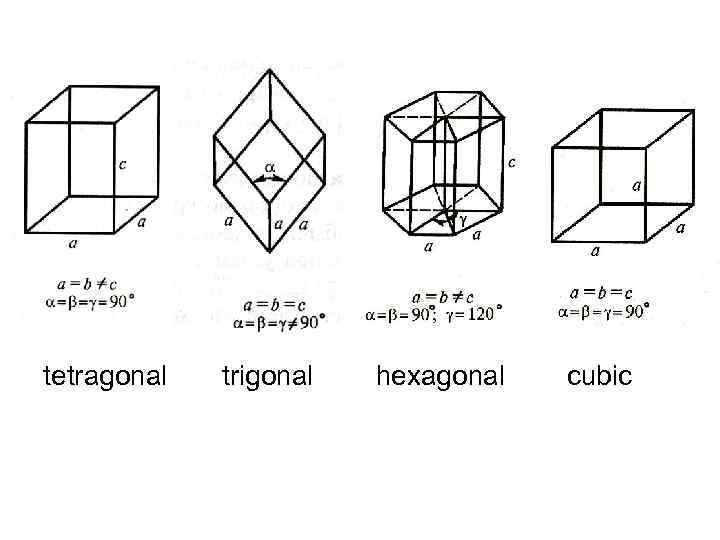
tetragonal trigonal hexagonal cubic
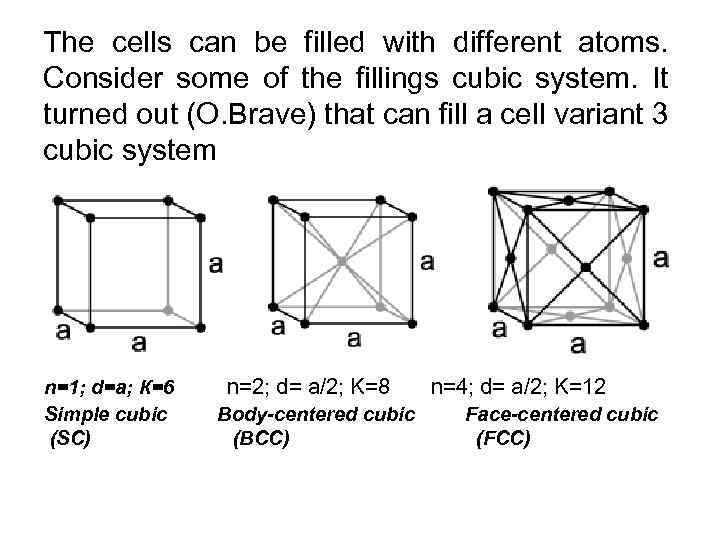
The cells can be filled with different atoms. Consider some of the fillings cubic system. It turned out (O. Brave) that can fill a cell variant 3 cubic system n=1; d=a; К=6 Simple cubic (SC) n=2; d= a/2; K=8 n=4; d= a/2; K=12 Body-centered cubic (BCC) Face-centered cubic (FCC)
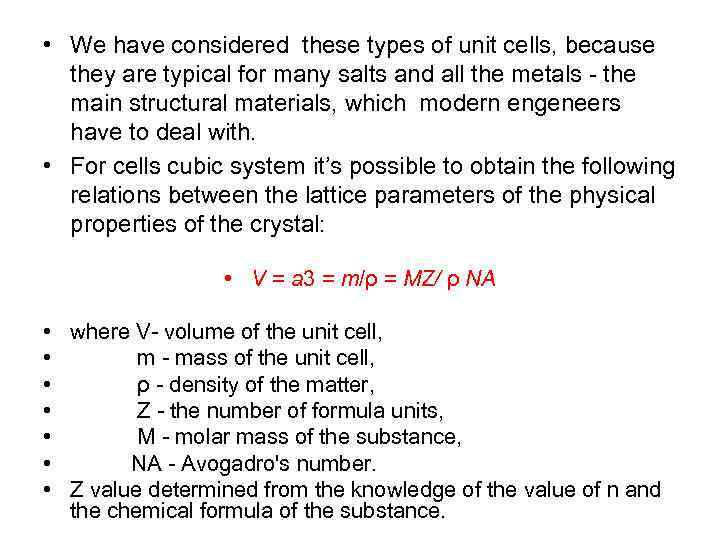
• We have considered these types of unit cells, because they are typical for many salts and all the metals - the main structural materials, which modern engeneers have to deal with. • For cells cubic system it’s possible to obtain the following relations between the lattice parameters of the physical properties of the crystal: • V = a 3 = m/ρ = MZ/ ρ NА • • where V- volume of the unit cell, m - mass of the unit cell, ρ - density of the matter, Z - the number of formula units, M - molar mass of the substance, NA - Avogadro's number. Z value determined from the knowledge of the value of n and the chemical formula of the substance.

• So, in the process of co-crystallization of several substances, the formation of mixed crystals. • When the total lattice sites will present structural units of several substances, such crystals are called solid solutions of substitution. For example, it is typical for aluminum-potassium alum – • KAl(SO 4)2 * 12 H 2 O, formed by joint crystallisation from solutions containing both potassium sulfate K 2 SO 4 and aluminum sulfate Al 2(SO 4)3. • This is - a manifestation of the properties of the isomorphism.

• When substances are presented in the crystallization of the structural units of other substances with significantly smaller dimensions than the lattice parameters of the crystallizing substance, the structural elements can be replaced in the interstices (solid solutions) • There may be instances when small structural units penetrate into the volume of the crystal lattice. Formed in this matter called clathrates.

• At the same time, the same substance can exist in various crystalline forms. For example, water, depending on external conditions, can form 10, and according to some, even the 12 crystalline forms! This phenomenon - the existence of the substance in several crystalline forms is called polymorphism

Chemical bond in complex compounds • Complex compound (or complex) is a complex particle consisting of metal atoms in a certain degree of oxidation associated with particles (molecules or ions) capable for independent existence.
![• For example, tetraamine copper (II) sulfate [Cu(NH 3)4] SO 4 contains in • For example, tetraamine copper (II) sulfate [Cu(NH 3)4] SO 4 contains in](https://present5.com/presentation/3/312893759_437079361.pdf-img/312893759_437079361.pdf-39.jpg)
• For example, tetraamine copper (II) sulfate [Cu(NH 3)4] SO 4 contains in its structure complex cation [Cu(NH 3)4] 2+, which can be regarded as one of the products of the interaction of copper sulfate and ammonia, the properties of the complex are distinct from properties of the starting materials. A complex [Fe(CO)5] (iron pentacarbonyl, a reaction product of metallic iron with carbon dioxide) - the neutral molecule.

• The formation of the metal-free hybrid orbitals can be an electron acceptor pairs of particles donor - is a physical basis for the emergence broad class of complex chemical compounds. • The formation of the complex ion or neutral complex, you can imagine in a general way as a certain reversible reaction: M + n. L[MLn] • where M - a neutral atom, positively or negatively charged ion, coordinating around him other atoms, ions or molecules. The M is called complexing agent or the central atom. Most often it is a metal with a positive oxidation state, and 8 particles L which are called ligands.

• Chemical bond in complex compounds in nature is a donor-acceptor bond center and complexing ligands. • The complexing agent serves as an acceptor, and ligands having electron pairs play the role of electron-pair donor. In this form s - due complexing ligand

• Quantitatively, the resulting complex is characterized by a coordination number of complexing - CN and dentate ligand. • The coordination number (CN) - the number of -bonds formed by the central atom with ligands. • Dentate ligand -bonds number generated data to the central atom of a ligand (chelator).

Homework: • Learn the complex compounds in detail; • Prepare for the boundary control on 3 lectures; • The questions about the IWS (2 and 3 lecture) must be written down in your copybooks.
3 лекция.ppt