fac712a92079b81000ff820fc926ed71.ppt
- Количество слайдов: 22
 INTERGROUP COALITION AGAINST SARCOMAS (ICAS) A COMMITTEE FOCUSED ON INTERGROUP DEVELOPMENT OF NEW SARCOMA THERAPEUTICS CALGB ECOG NCIC SWOG Collaborations with COG and ACOSOG Communication with SARC and RTOG • SCIENTIFIC STEERING COMMITTEE: EC Borden, G Demetri, M von Mehren, K Albritton, P Pisters • BIOSTATISTICS: C Rankin, J Crowley • OPERATIONS: G Goetz (SWOG)
INTERGROUP COALITION AGAINST SARCOMAS (ICAS) A COMMITTEE FOCUSED ON INTERGROUP DEVELOPMENT OF NEW SARCOMA THERAPEUTICS CALGB ECOG NCIC SWOG Collaborations with COG and ACOSOG Communication with SARC and RTOG • SCIENTIFIC STEERING COMMITTEE: EC Borden, G Demetri, M von Mehren, K Albritton, P Pisters • BIOSTATISTICS: C Rankin, J Crowley • OPERATIONS: G Goetz (SWOG)
 2001 -2006 INTERGROUP COALITION AGAINST SARCOMAS Goals and Hypotheses • Sharpen definition of histologic subtypes to improve therapeutic outcomes and prognosis – Hypothesis: Molecular pathology will better define subtypes and thus improve clinical management and outcomes • Emphasize targeted therapeutics – Hypothesis: Signal transduction modulators will be effective and histology specific • Facilitate intergroup collaborations – Hypothesis: Increased intergroup collaboration will stimulate sarcoma research State of Science Clin Cancer Research 2003
2001 -2006 INTERGROUP COALITION AGAINST SARCOMAS Goals and Hypotheses • Sharpen definition of histologic subtypes to improve therapeutic outcomes and prognosis – Hypothesis: Molecular pathology will better define subtypes and thus improve clinical management and outcomes • Emphasize targeted therapeutics – Hypothesis: Signal transduction modulators will be effective and histology specific • Facilitate intergroup collaborations – Hypothesis: Increased intergroup collaboration will stimulate sarcoma research State of Science Clin Cancer Research 2003
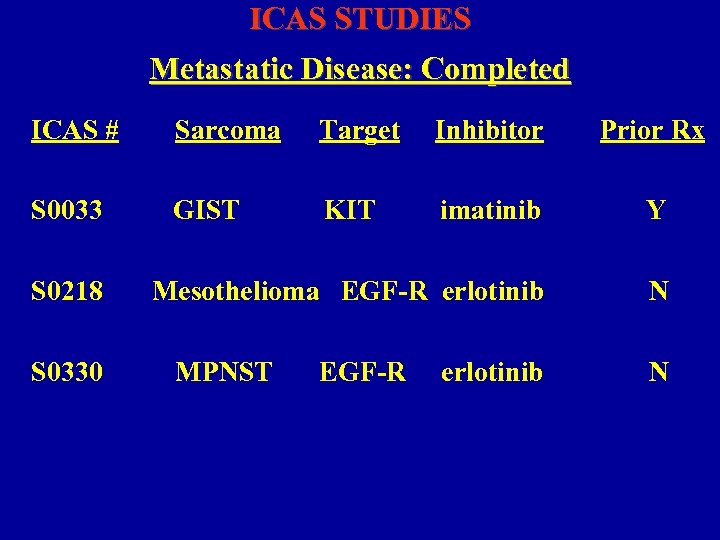 ICAS STUDIES Metastatic Disease: Completed ICAS # Sarcoma Target Inhibitor Prior Rx S 0033 GIST KIT imatinib Y Mesothelioma EGF-R erlotinib N S 0218 S 0330 MPNST EGF-R erlotinib N
ICAS STUDIES Metastatic Disease: Completed ICAS # Sarcoma Target Inhibitor Prior Rx S 0033 GIST KIT imatinib Y Mesothelioma EGF-R erlotinib N S 0218 S 0330 MPNST EGF-R erlotinib N
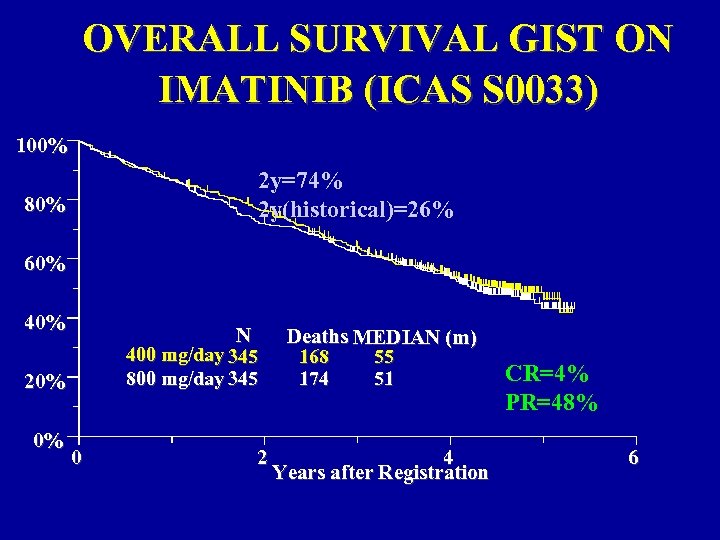 OVERALL SURVIVAL GIST ON IMATINIB (ICAS S 0033) 100% 2 y=74% 2 y(historical)=26% 80% 60% 40% N 400 mg/day 345 800 mg/day 345 20% 0% 0 2 Deaths MEDIAN (m) 168 55 174 51 4 Years after Registration CR=4% PR=48% 6
OVERALL SURVIVAL GIST ON IMATINIB (ICAS S 0033) 100% 2 y=74% 2 y(historical)=26% 80% 60% 40% N 400 mg/day 345 800 mg/day 345 20% 0% 0 2 Deaths MEDIAN (m) 168 55 174 51 4 Years after Registration CR=4% PR=48% 6
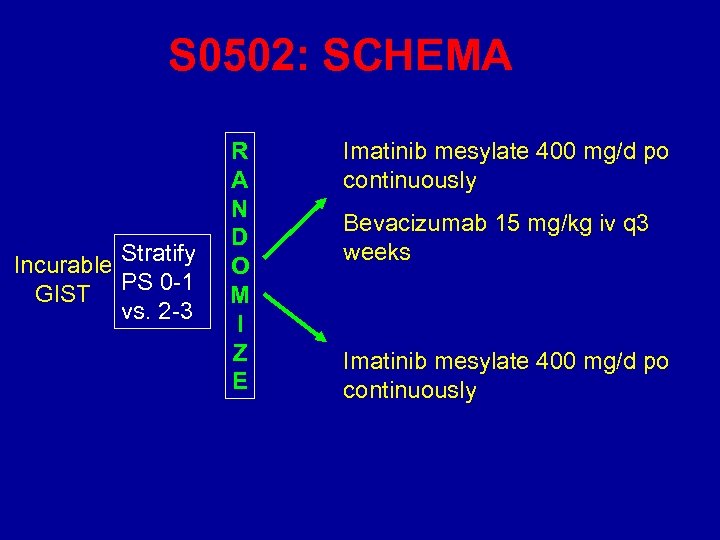 S 0502: SCHEMA Incurable Stratify PS 0 -1 GIST vs. 2 -3 R A N D O M I Z E Imatinib mesylate 400 mg/d po continuously Bevacizumab 15 mg/kg iv q 3 weeks Imatinib mesylate 400 mg/d po continuously
S 0502: SCHEMA Incurable Stratify PS 0 -1 GIST vs. 2 -3 R A N D O M I Z E Imatinib mesylate 400 mg/d po continuously Bevacizumab 15 mg/kg iv q 3 weeks Imatinib mesylate 400 mg/d po continuously
 S 0502: Objectives • Primary: PFS of imatinib + bevacizumab vs imatinib alone • Secondary: – Compare response between arms – Compare frequency and severity of toxicities – Assess survival differences – Correlative • Mutations • PET VEGF and Receptors PK
S 0502: Objectives • Primary: PFS of imatinib + bevacizumab vs imatinib alone • Secondary: – Compare response between arms – Compare frequency and severity of toxicities – Assess survival differences – Correlative • Mutations • PET VEGF and Receptors PK
 Study Coordinators • • OVERALL CHAIR: Charles Blanke SWOG Translational Medicine: Michael Heinrich SWOG Pathology: Christopher Corless SWOG ECOG: Meg von Mehren CALGB: George Demetri NCIC: Vivien Bramwell Imaging: Janet Eary
Study Coordinators • • OVERALL CHAIR: Charles Blanke SWOG Translational Medicine: Michael Heinrich SWOG Pathology: Christopher Corless SWOG ECOG: Meg von Mehren CALGB: George Demetri NCIC: Vivien Bramwell Imaging: Janet Eary
 ICAS STUDIES CTSU!!!!!! Metastatic Disease: Active ICAS # Sarcoma Target Inhibitor Prior Rx S 0345 DFSP PDGF-R imatinib Y Accrual 7 S 0346 Synovial HER 2* trastuzumab Y 0 S 0505 STS sorafenib Y 22 S 0423 Chondro MTAP pemetrexed Y 11 S 0344 Chondro N 16 VEGF ---- Surgery
ICAS STUDIES CTSU!!!!!! Metastatic Disease: Active ICAS # Sarcoma Target Inhibitor Prior Rx S 0345 DFSP PDGF-R imatinib Y Accrual 7 S 0346 Synovial HER 2* trastuzumab Y 0 S 0505 STS sorafenib Y 22 S 0423 Chondro MTAP pemetrexed Y 11 S 0344 Chondro N 16 VEGF ---- Surgery
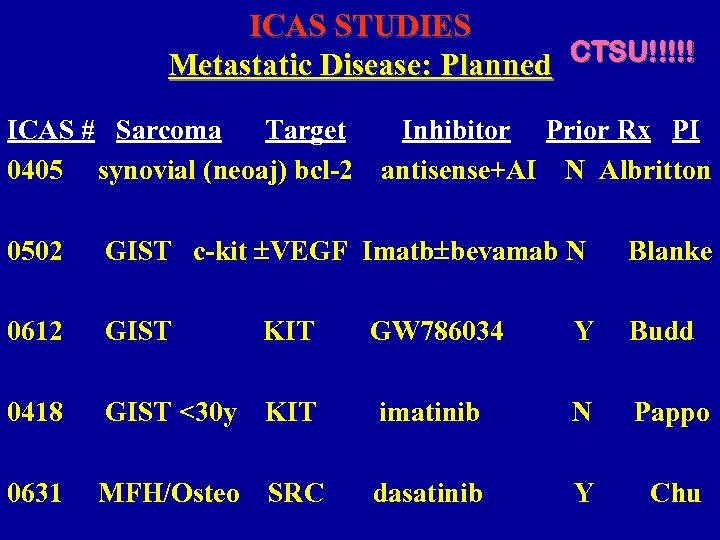 ICAS STUDIES Metastatic Disease: Planned CTSU!!!!! ICAS # Sarcoma Target 0405 synovial (neoaj) bcl-2 Inhibitor Prior Rx PI antisense+AI N Albritton 0502 GIST c-kit VEGF Imatb bevamab N Blanke 0612 GIST KIT GW 786034 Y Budd 0418 GIST <30 y KIT imatinib N Pappo 0631 MFH/Osteo SRC dasatinib Y Chu
ICAS STUDIES Metastatic Disease: Planned CTSU!!!!! ICAS # Sarcoma Target 0405 synovial (neoaj) bcl-2 Inhibitor Prior Rx PI antisense+AI N Albritton 0502 GIST c-kit VEGF Imatb bevamab N Blanke 0612 GIST KIT GW 786034 Y Budd 0418 GIST <30 y KIT imatinib N Pappo 0631 MFH/Osteo SRC dasatinib Y Chu
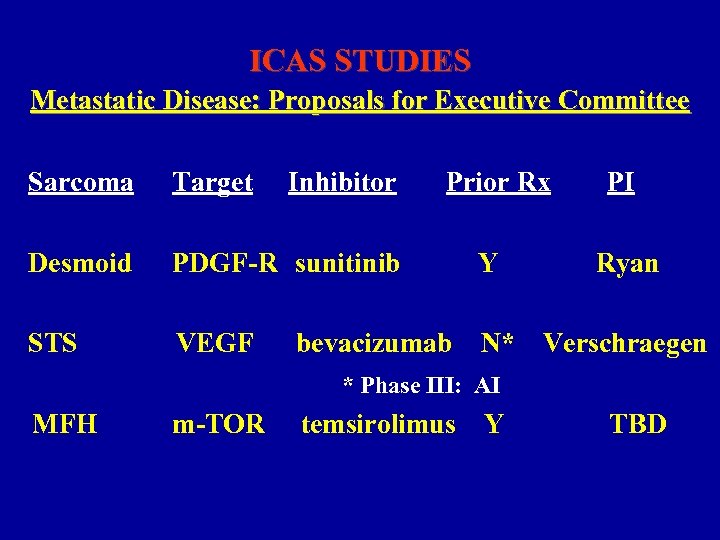 ICAS STUDIES Metastatic Disease: Proposals for Executive Committee Sarcoma Target Inhibitor Prior Rx Desmoid PDGF-R sunitinib Y STS VEGF N* bevacizumab PI Ryan Verschraegen * Phase III: AI MFH m-TOR temsirolimus Y TBD
ICAS STUDIES Metastatic Disease: Proposals for Executive Committee Sarcoma Target Inhibitor Prior Rx Desmoid PDGF-R sunitinib Y STS VEGF N* bevacizumab PI Ryan Verschraegen * Phase III: AI MFH m-TOR temsirolimus Y TBD
 CHALLENGES SARCOMAS: 2006 • CLINICAL BIOLOGY AND OUTCOMES • CURRENT MANAGEMENT AND STANDARDS OF CARE • EMPHASIZE CLINICAL PROGRESS
CHALLENGES SARCOMAS: 2006 • CLINICAL BIOLOGY AND OUTCOMES • CURRENT MANAGEMENT AND STANDARDS OF CARE • EMPHASIZE CLINICAL PROGRESS
 CHALLENGES SARCOMAS: 2006 • CLINICAL BIOLOGY AND OUTCOMES – What do we know? • CURRENT MANAGEMENT AND STANDARDS OF CARE – How are we doing? • FUTURE RESEARCH CHALLENGES – Where should we be going?
CHALLENGES SARCOMAS: 2006 • CLINICAL BIOLOGY AND OUTCOMES – What do we know? • CURRENT MANAGEMENT AND STANDARDS OF CARE – How are we doing? • FUTURE RESEARCH CHALLENGES – Where should we be going?
 CHALLENGES SARCOMAS: 2006
CHALLENGES SARCOMAS: 2006
 Euramos 1 Phase 3 Trial European and American Osteosarcoma Study Group • Collaborators: COG German Austrian Swiss (COSS) European (EOI) Scandinavian (SSG) Trial Mgmt Group Chair M Bernstein Montreal COG • Objectives: Improve EFS – Will IE when added to MAP for poor histological responders? – Will PEG-IFN when added to MAP for good histological responders? • Eligibility – Resectable axial or extremity osteosarcoma – <40 • Registration 2 cycles MAP surgery path reveiw Random MAPx 2 MPx 2 PEG-IFNx 2 y or IEx 3 – n=1400 – 10% improvement in EFS
Euramos 1 Phase 3 Trial European and American Osteosarcoma Study Group • Collaborators: COG German Austrian Swiss (COSS) European (EOI) Scandinavian (SSG) Trial Mgmt Group Chair M Bernstein Montreal COG • Objectives: Improve EFS – Will IE when added to MAP for poor histological responders? – Will PEG-IFN when added to MAP for good histological responders? • Eligibility – Resectable axial or extremity osteosarcoma – <40 • Registration 2 cycles MAP surgery path reveiw Random MAPx 2 MPx 2 PEG-IFNx 2 y or IEx 3 – n=1400 – 10% improvement in EFS
 INTERGROUP COALITION AGAINST SARCOMAS (ICAS) Ernest C. Borden Chair • • Catherine Rankin Biostatistics SWOG • John Crowley Biostatistics SWOG • Gretchen Goetz Operations SWOG – SWOG ECOG CALGB NCIC – collaboration with COG ACOSOG • M von Mehren G Demetri B Redman V Bramwell • K Albritton P Pisters – Communication with SARC A COMMITTEE FOCUSED ON INTERGROUP DEVELOPMENT OF NEW SARCOMA THERAPEUTICS
INTERGROUP COALITION AGAINST SARCOMAS (ICAS) Ernest C. Borden Chair • • Catherine Rankin Biostatistics SWOG • John Crowley Biostatistics SWOG • Gretchen Goetz Operations SWOG – SWOG ECOG CALGB NCIC – collaboration with COG ACOSOG • M von Mehren G Demetri B Redman V Bramwell • K Albritton P Pisters – Communication with SARC A COMMITTEE FOCUSED ON INTERGROUP DEVELOPMENT OF NEW SARCOMA THERAPEUTICS
 SARCOMAS NEW ERA 2000 • MOLECULAR REDEFINITION • IMPROVE PRIMARY TREATMENT • TARGETED THERAPIES
SARCOMAS NEW ERA 2000 • MOLECULAR REDEFINITION • IMPROVE PRIMARY TREATMENT • TARGETED THERAPIES
 IMPROVING GIST PATIENT OUTCOMES • Eliminating GIST stem cell – Few residual clones: ACOSOG #Z 9001 – Imatinib with other KIT/PDGF-R tyrosine kinase inhibitors • S 0612 • Targeting of specific mutations – S 0033 and EORTC combined analysis – new inhibitors: S 0612 • Inhibition of alternate pathways – Angiogenesis: Bevacizumab S 0502 • Improved resonse assessement
IMPROVING GIST PATIENT OUTCOMES • Eliminating GIST stem cell – Few residual clones: ACOSOG #Z 9001 – Imatinib with other KIT/PDGF-R tyrosine kinase inhibitors • S 0612 • Targeting of specific mutations – S 0033 and EORTC combined analysis – new inhibitors: S 0612 • Inhibition of alternate pathways – Angiogenesis: Bevacizumab S 0502 • Improved resonse assessement
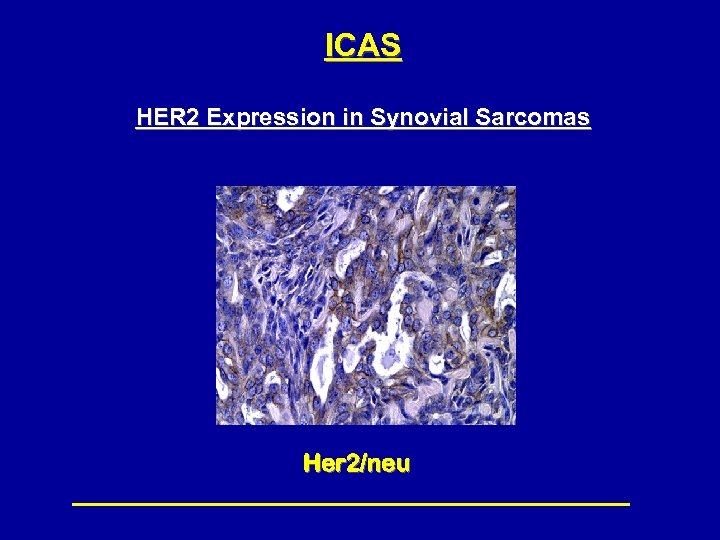 ICAS HER 2 Expression in Synovial Sarcomas Her 2/neu
ICAS HER 2 Expression in Synovial Sarcomas Her 2/neu
 S 0346 Trastuzumab for Synovial Sarcomas • Eligibility – Metastatic Synovial Sarcomas – Central confirmation of her 2 expression 2+ – 1 prior treatment • Dose/Schedule – IV weekly 4 mg/kg loading, then 2 mg/kg
S 0346 Trastuzumab for Synovial Sarcomas • Eligibility – Metastatic Synovial Sarcomas – Central confirmation of her 2 expression 2+ – 1 prior treatment • Dose/Schedule – IV weekly 4 mg/kg loading, then 2 mg/kg
 SARCOMAS NEW ERA 2000 • MOLECULAR REDEFINITION • IMPROVED PRIMARY OUTCOMES Case Comprehensive Cancer Center CCF Taussig Cancer Center • TARGETED THERAPIES
SARCOMAS NEW ERA 2000 • MOLECULAR REDEFINITION • IMPROVED PRIMARY OUTCOMES Case Comprehensive Cancer Center CCF Taussig Cancer Center • TARGETED THERAPIES
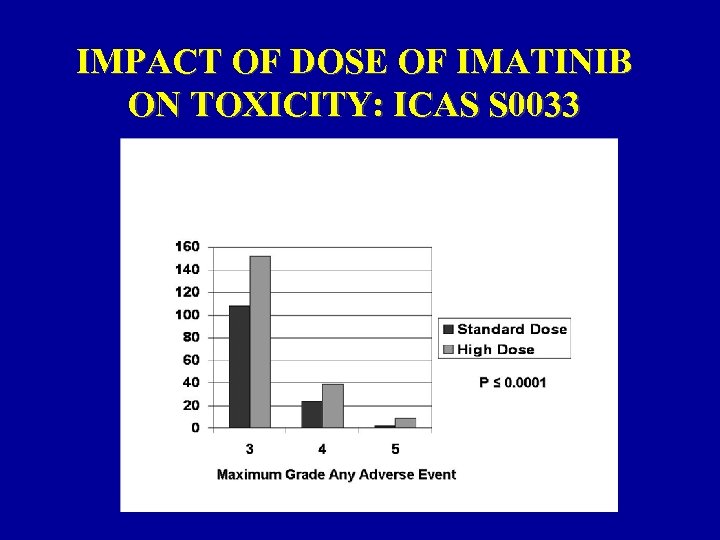 IMPACT OF DOSE OF IMATINIB ON TOXICITY: ICAS S 0033
IMPACT OF DOSE OF IMATINIB ON TOXICITY: ICAS S 0033
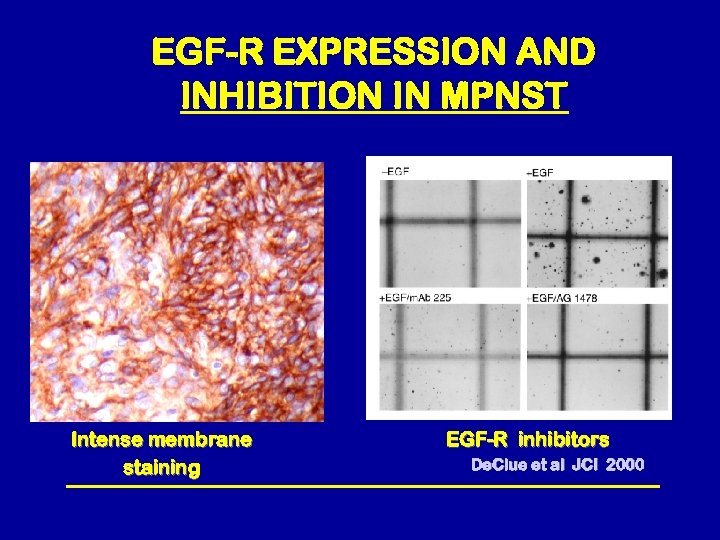 EGF-R EXPRESSION AND INHIBITION IN MPNST Intense membrane staining EGF-R inhibitors De. Clue et al JCI 2000
EGF-R EXPRESSION AND INHIBITION IN MPNST Intense membrane staining EGF-R inhibitors De. Clue et al JCI 2000


