05ea297431b51ba6b32481e240e52dc3.ppt
- Количество слайдов: 55
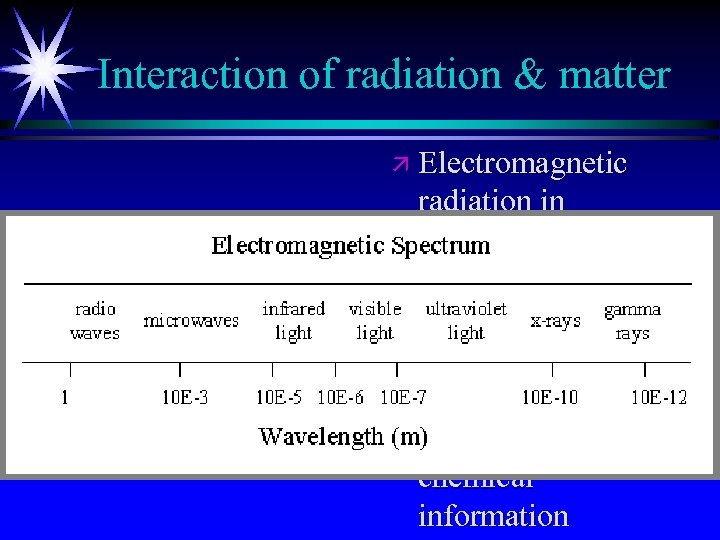 Interaction of radiation & matter ä Electromagnetic radiation in different regions of spectrum can be used for qualitative and quantitative information ä Different types of chemical information
Interaction of radiation & matter ä Electromagnetic radiation in different regions of spectrum can be used for qualitative and quantitative information ä Different types of chemical information
 Energy transfer from photon to molecule or atom At room temperature most molecules are at lowest electronic & vibrational state IR radiation can excite vibrational levels that then lose energy quickly in collisions with surroundings
Energy transfer from photon to molecule or atom At room temperature most molecules are at lowest electronic & vibrational state IR radiation can excite vibrational levels that then lose energy quickly in collisions with surroundings
 UV Visible Spectrometry äabsorption - specific energy äemission - excited molecule emits äfluorescence äphosphorescence
UV Visible Spectrometry äabsorption - specific energy äemission - excited molecule emits äfluorescence äphosphorescence
 What happens to molecule after excitation ä collisions deactivate vibrational levels (heat) ä emission of photon (fluorescence) ä intersystem crossover (phosphorescence)
What happens to molecule after excitation ä collisions deactivate vibrational levels (heat) ä emission of photon (fluorescence) ä intersystem crossover (phosphorescence)
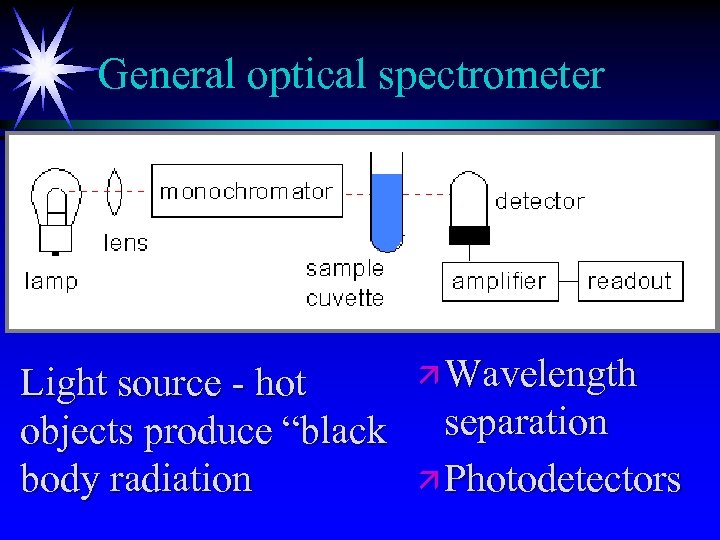 General optical spectrometer Light source - hot objects produce “black body radiation ä Wavelength separation ä Photodetectors
General optical spectrometer Light source - hot objects produce “black body radiation ä Wavelength separation ä Photodetectors
 Black body radiation ä Tungsten lamp, Globar, Nernst glower ä Intensity and peak emission wavelength are a function of Temperature ä As T increases the total intensity increases and there is shift to higher energies (toward visible and UV)
Black body radiation ä Tungsten lamp, Globar, Nernst glower ä Intensity and peak emission wavelength are a function of Temperature ä As T increases the total intensity increases and there is shift to higher energies (toward visible and UV)
 UV sources ä Arc discharge lamps with electrical discharge maintained in appropriate gases ä Low pressure hydrogen and deuterium lamps ä Lasers - narrow spectral widths, very high intensity, spatial beam, time resolution, problem with range of wavelengths ä Discrete spectroscopic- metal vapor & hollow cathode lamps
UV sources ä Arc discharge lamps with electrical discharge maintained in appropriate gases ä Low pressure hydrogen and deuterium lamps ä Lasers - narrow spectral widths, very high intensity, spatial beam, time resolution, problem with range of wavelengths ä Discrete spectroscopic- metal vapor & hollow cathode lamps
 Why separate wavelengths? ä Each compound absorbs different colors (energies) with different probabilities (absorbtivity) ä Selectivity ä Quantitative adherence to Beer’s Law A = abc ä Improves sensitivity
Why separate wavelengths? ä Each compound absorbs different colors (energies) with different probabilities (absorbtivity) ä Selectivity ä Quantitative adherence to Beer’s Law A = abc ä Improves sensitivity
 Why are UV-Vis bands broad? ä Electronic energy states give band with no vibrational structure ä Solvent interactions (microenvironments) averaged ä Low temperature gas phase molecules give structure if instrumental resolution is adequate
Why are UV-Vis bands broad? ä Electronic energy states give band with no vibrational structure ä Solvent interactions (microenvironments) averaged ä Low temperature gas phase molecules give structure if instrumental resolution is adequate
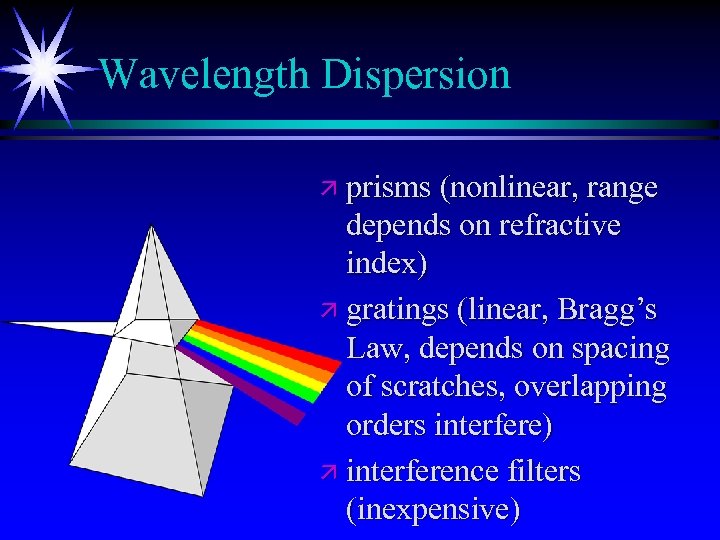 Wavelength Dispersion ä prisms (nonlinear, range depends on refractive index) ä gratings (linear, Bragg’s Law, depends on spacing of scratches, overlapping orders interfere) ä interference filters (inexpensive)
Wavelength Dispersion ä prisms (nonlinear, range depends on refractive index) ä gratings (linear, Bragg’s Law, depends on spacing of scratches, overlapping orders interfere) ä interference filters (inexpensive)
 Monochromator ä Entrance slit - provides narrow optical image ä Collimator - makes light hit dispersive element at same angle ä Dispersing element - directional ä Focusing element - image on slit ä Exit slit - isolates desired color to exit
Monochromator ä Entrance slit - provides narrow optical image ä Collimator - makes light hit dispersive element at same angle ä Dispersing element - directional ä Focusing element - image on slit ä Exit slit - isolates desired color to exit
 Resolution ä The ability to distinguish different wavelengths of light - R=l/Dl R= ä Linear dispersion - range of wavelengths spread over unit distance at exit slit ä Spectral bandwidth - range of wavelengths included in output of exit slit (FWHM) ä Resolution depends on how widely light is dispersed & how narrow a slice chosen
Resolution ä The ability to distinguish different wavelengths of light - R=l/Dl R= ä Linear dispersion - range of wavelengths spread over unit distance at exit slit ä Spectral bandwidth - range of wavelengths included in output of exit slit (FWHM) ä Resolution depends on how widely light is dispersed & how narrow a slice chosen
 Filters - inexpensive alternative ä Adsorption type - glass with dyes to adsorb chosen colors ä Interference filters - multiple reflections between 2 parallel reflective surfaces - only certain wavelengths have positive interferences temperature effects spacing between surfaces
Filters - inexpensive alternative ä Adsorption type - glass with dyes to adsorb chosen colors ä Interference filters - multiple reflections between 2 parallel reflective surfaces - only certain wavelengths have positive interferences temperature effects spacing between surfaces
 Wavelength dependence in spectrometer ä Source ä Monochromator ä Detector ä Sample - We hope so!
Wavelength dependence in spectrometer ä Source ä Monochromator ä Detector ä Sample - We hope so!
 Photodetectors - photoelectric effect E(e)=hn - w ä For sensitive detector we need a small work function - alkali metals are best ä Phototube - electrons attracted to anode giving a current flow proportional to light intensity ä Photomultiplier - amplification to improve sensitivity (10 million)
Photodetectors - photoelectric effect E(e)=hn - w ä For sensitive detector we need a small work function - alkali metals are best ä Phototube - electrons attracted to anode giving a current flow proportional to light intensity ä Photomultiplier - amplification to improve sensitivity (10 million)
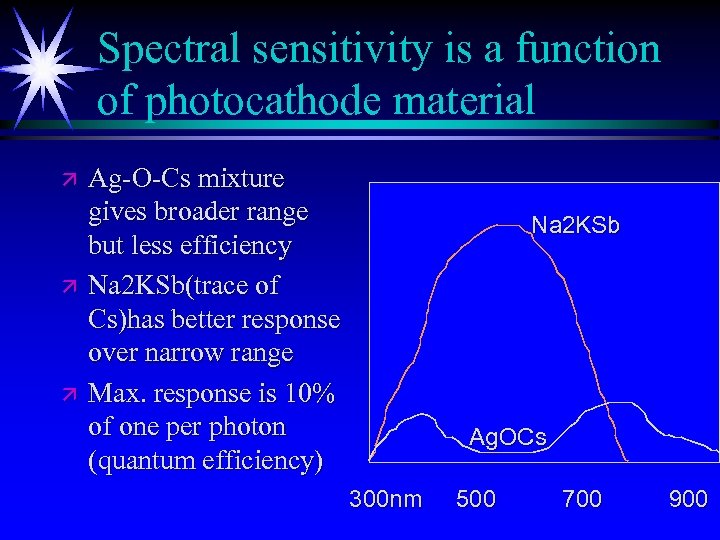 Spectral sensitivity is a function of photocathode material ä ä ä Ag-O-Cs mixture gives broader range but less efficiency Na 2 KSb(trace of Cs)has better response over narrow range Max. response is 10% of one per photon (quantum efficiency) Na 2 KSb Ag. OCs 300 nm 500 700 900
Spectral sensitivity is a function of photocathode material ä ä ä Ag-O-Cs mixture gives broader range but less efficiency Na 2 KSb(trace of Cs)has better response over narrow range Max. response is 10% of one per photon (quantum efficiency) Na 2 KSb Ag. OCs 300 nm 500 700 900
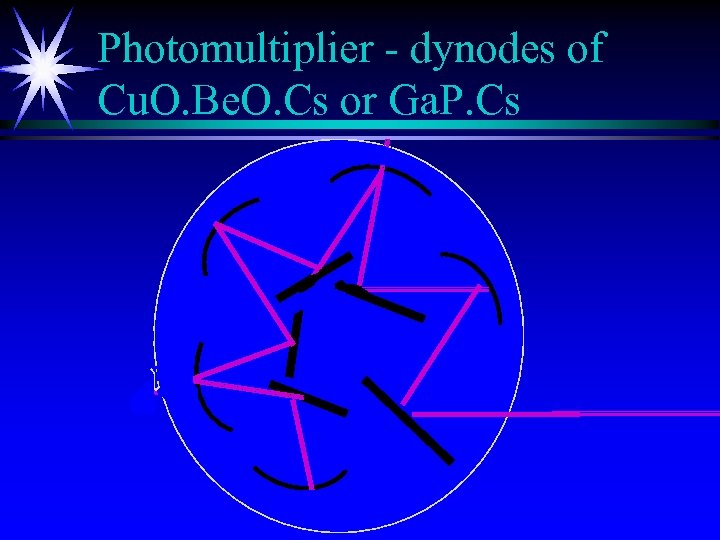 Photomultiplier - dynodes of Cu. O. Be. O. Cs or Ga. P. Cs
Photomultiplier - dynodes of Cu. O. Be. O. Cs or Ga. P. Cs
 Cooled Photomultiplier Tube
Cooled Photomultiplier Tube
 Dynode array
Dynode array
 Photodiodes - semiconductor that conducts in one direction only when light is present ä Rugged and small ä Photodiode arrays - allows observation of a number of different locations (wavelengths) simultaneously ä Somewhat less sensitive than PMT
Photodiodes - semiconductor that conducts in one direction only when light is present ä Rugged and small ä Photodiode arrays - allows observation of a number of different locations (wavelengths) simultaneously ä Somewhat less sensitive than PMT
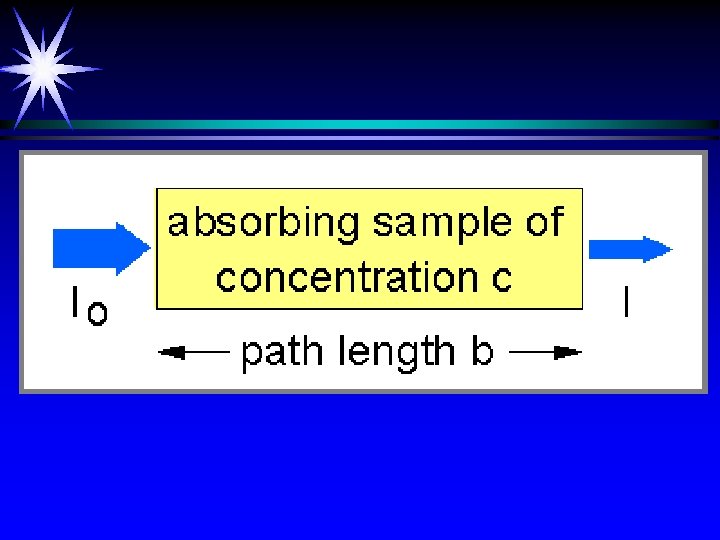
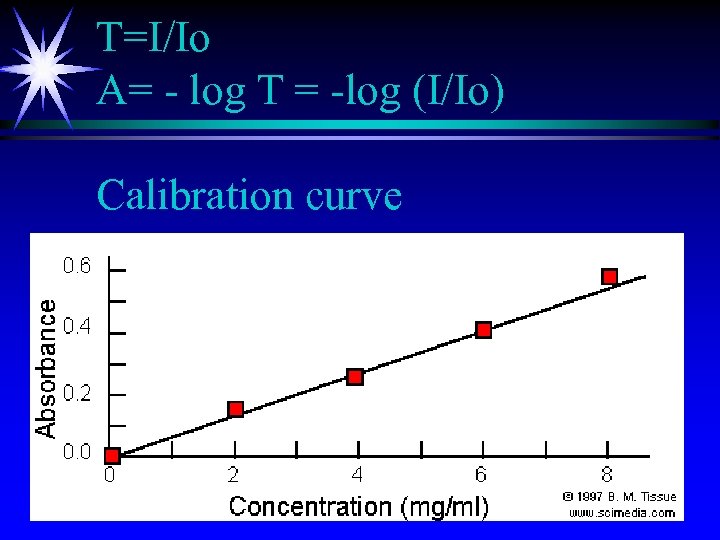 T=I/Io A= - log T = -log (I/Io) Calibration curve
T=I/Io A= - log T = -log (I/Io) Calibration curve

 Deviations from Beer’s Law ä High concentrations (0. 01 M) distort each molecules electronic structure & spectra ä Chemical equilibrium ä Stray light ä Polychromatic light ä Interferences
Deviations from Beer’s Law ä High concentrations (0. 01 M) distort each molecules electronic structure & spectra ä Chemical equilibrium ä Stray light ä Polychromatic light ä Interferences
 Interpretation - quantitative ä Broad adsorption bands - considerable overlap ä Specral dependence upon solvents ä Resolving mixtures as linear combinations - need to measure as many wavelengths as components ä Beer’s Law. html
Interpretation - quantitative ä Broad adsorption bands - considerable overlap ä Specral dependence upon solvents ä Resolving mixtures as linear combinations - need to measure as many wavelengths as components ä Beer’s Law. html
 Resolving mixtures ä Measure at different wavelengths and solve mathematically ä Use standard additions (measure A and then add known amounts of standard) ä Chemical methods to separate or shift spectrum ä Use time resolution (fluorescence and phosphorescence)
Resolving mixtures ä Measure at different wavelengths and solve mathematically ä Use standard additions (measure A and then add known amounts of standard) ä Chemical methods to separate or shift spectrum ä Use time resolution (fluorescence and phosphorescence)
 Improving resolution in mixtures ä Instrumental (resolution) ä Mathematical (derivatives) ä Use second parameter (fluorescence) ä Use third parameter (time for phosphorescence) ä Chemical separations (chromatography)
Improving resolution in mixtures ä Instrumental (resolution) ä Mathematical (derivatives) ä Use second parameter (fluorescence) ä Use third parameter (time for phosphorescence) ä Chemical separations (chromatography)
 Fluorescence ä Emission at lower energy than absorption ä Greater selectivity but fluorescent yields vary for different molecules ä Detection at right angles to excitation ä S/N is improved so sensitivity is better ä Fluorescent tags
Fluorescence ä Emission at lower energy than absorption ä Greater selectivity but fluorescent yields vary for different molecules ä Detection at right angles to excitation ä S/N is improved so sensitivity is better ä Fluorescent tags
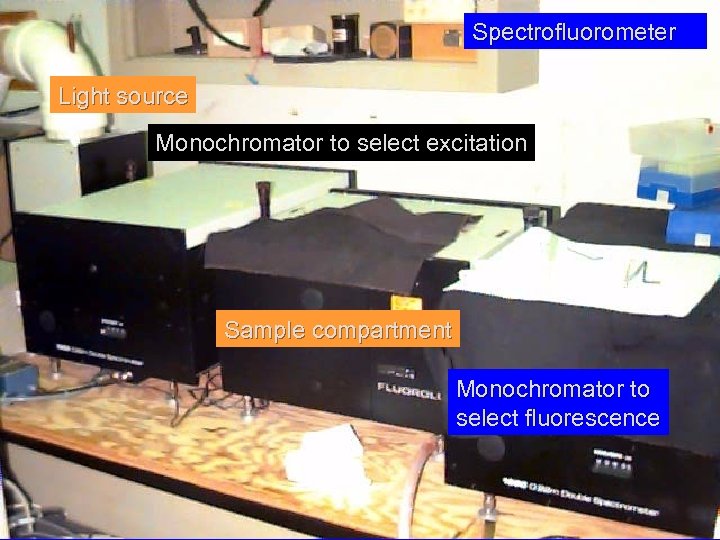 Spectrofluorometer Light source Monochromator to select excitation Sample compartment Monochromator to select fluorescence
Spectrofluorometer Light source Monochromator to select excitation Sample compartment Monochromator to select fluorescence
 Photoacoustic spectroscopy ä Edison’s observations ä If light is pulsed then as gas is excited it can expand (sound)
Photoacoustic spectroscopy ä Edison’s observations ä If light is pulsed then as gas is excited it can expand (sound)
 Principles of IR ä Absorption of energy at various frequencies is detected by IR ä plots the amount of radiation transmitted through the sample as a function of frequency ä compounds have “fingerprint” region of identity
Principles of IR ä Absorption of energy at various frequencies is detected by IR ä plots the amount of radiation transmitted through the sample as a function of frequency ä compounds have “fingerprint” region of identity
 Infrared Spectrometry ä Is especially useful for qualitative analysis ä functional groups ä other structural features ä establishing purity ä monitoring rates ä measuring concentrations ä theoretical studies
Infrared Spectrometry ä Is especially useful for qualitative analysis ä functional groups ä other structural features ä establishing purity ä monitoring rates ä measuring concentrations ä theoretical studies
 How does it work? ä Continuous beam of radiation ä Frequencies display different absorbances ä Beam comes to focus at entrance slit ä molecule absorbs radiation of the energy to excite it to the vibrational state
How does it work? ä Continuous beam of radiation ä Frequencies display different absorbances ä Beam comes to focus at entrance slit ä molecule absorbs radiation of the energy to excite it to the vibrational state
 How Does It Work? ä Monochromator disperses radiation into spectrum ä one frequency appears at exit slit ä radiation passed to detector ä detector converts energy to signal ä signal amplified and recorded
How Does It Work? ä Monochromator disperses radiation into spectrum ä one frequency appears at exit slit ä radiation passed to detector ä detector converts energy to signal ä signal amplified and recorded
 Instrumentation II ä Optical-null double-beam instruments ä Radiation is directed through both cells by mirrors ä sample beam and reference beam ä chopper ä diffraction grating
Instrumentation II ä Optical-null double-beam instruments ä Radiation is directed through both cells by mirrors ä sample beam and reference beam ä chopper ä diffraction grating
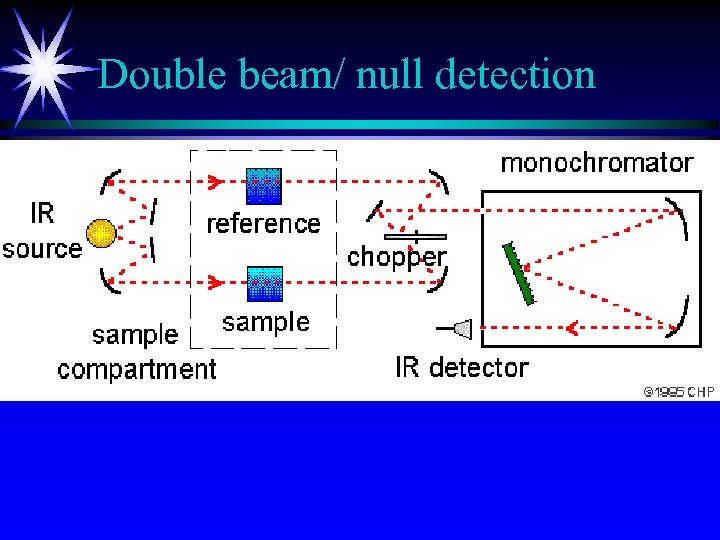 Double beam/ null detection
Double beam/ null detection
 Instrumentation III ä Exit slit ä detector ä servo motor ä Resulting spectrum is a plot of the intensity of the transmitted radiation versus the wavelength
Instrumentation III ä Exit slit ä detector ä servo motor ä Resulting spectrum is a plot of the intensity of the transmitted radiation versus the wavelength
 Detection of IR radiation ä Insufficient energy to excite electrons & hence photodetectors won’t work ä Sense heat - not very sensitive and must be protected from sources of heat ä Thermocouple - dissimilar metals characterized by voltage across gap proportional to temperature
Detection of IR radiation ä Insufficient energy to excite electrons & hence photodetectors won’t work ä Sense heat - not very sensitive and must be protected from sources of heat ä Thermocouple - dissimilar metals characterized by voltage across gap proportional to temperature
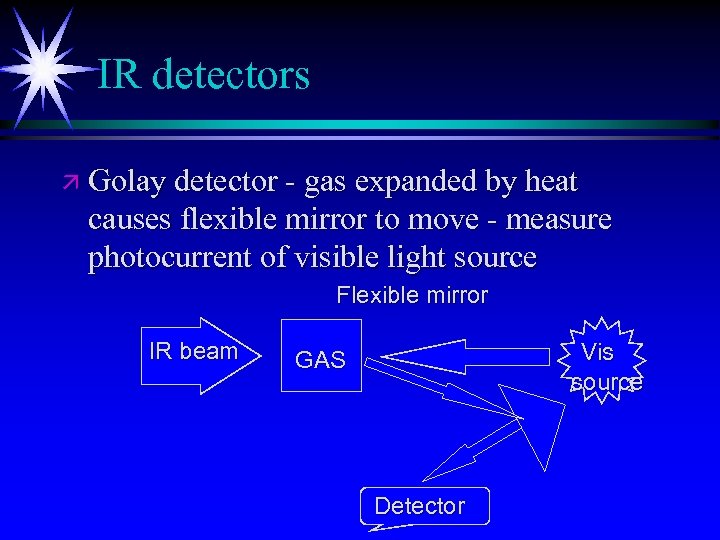 IR detectors ä Golay detector - gas expanded by heat causes flexible mirror to move - measure photocurrent of visible light source Flexible mirror IR beam Vis source GAS Detector
IR detectors ä Golay detector - gas expanded by heat causes flexible mirror to move - measure photocurrent of visible light source Flexible mirror IR beam Vis source GAS Detector
 Carbon analyzer - simple IR ä Sample flushed of carbon dioxide (inorganic) ä Organic carbon oxidized by persulfate & UV ä Carbon dioxide measured in gas cell (water interferences)
Carbon analyzer - simple IR ä Sample flushed of carbon dioxide (inorganic) ä Organic carbon oxidized by persulfate & UV ä Carbon dioxide measured in gas cell (water interferences)
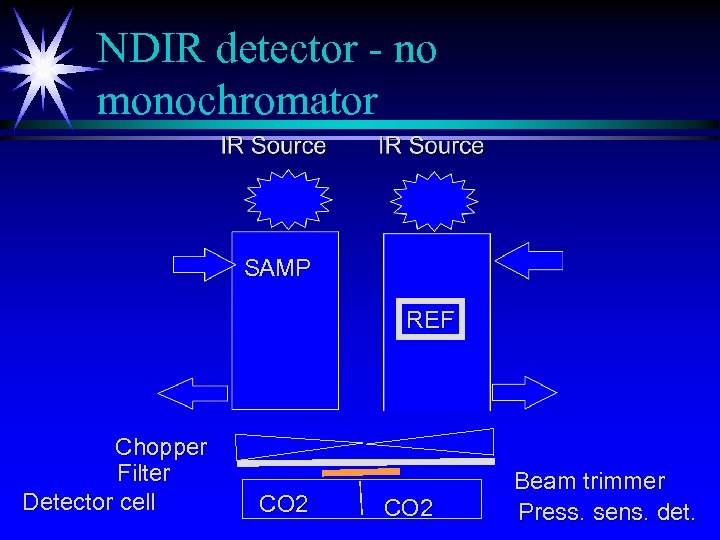 NDIR detector - no monochromator SAMP REF Chopper Filter Detector cell CO 2 Beam trimmer Press. sens. det.
NDIR detector - no monochromator SAMP REF Chopper Filter Detector cell CO 2 Beam trimmer Press. sens. det.
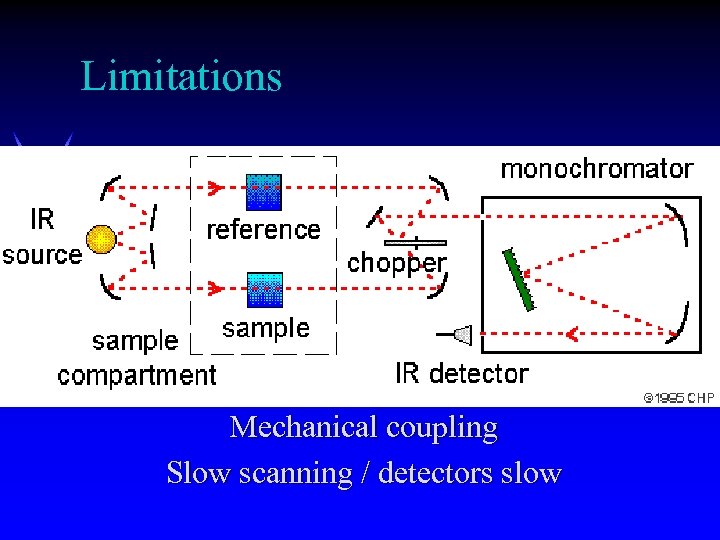 Limitations Mechanical coupling Slow scanning / detectors slow
Limitations Mechanical coupling Slow scanning / detectors slow
 Limitations of Dispersive IR ä Mechanically complex ä Sensitivity limited ä Requires external calibration ä Tracking errors limit resolution (scanning fast broadens peak, decreases absorbance, shifts peak
Limitations of Dispersive IR ä Mechanically complex ä Sensitivity limited ä Requires external calibration ä Tracking errors limit resolution (scanning fast broadens peak, decreases absorbance, shifts peak
 Problems with IR ä c no quantitative äH limited resolution ä D not reproducible ä A limited dynamic range ä I limited sensitivity ä E long analysis time ä B functional groups
Problems with IR ä c no quantitative äH limited resolution ä D not reproducible ä A limited dynamic range ä I limited sensitivity ä E long analysis time ä B functional groups
 Limitations ä ä ä Most equipment can measure one wavelength at a time Potentially timeconsuming A solution?
Limitations ä ä ä Most equipment can measure one wavelength at a time Potentially timeconsuming A solution?
 Fourier-Transform Infrared Spectroscopy (FTIR) A Solution!
Fourier-Transform Infrared Spectroscopy (FTIR) A Solution!
 FTIR ä Analyze all wavelengths simultaneously ä signal decoded to generate complete spectrum ä can be done quickly ä better resolution ä more resolution ä However, . . .
FTIR ä Analyze all wavelengths simultaneously ä signal decoded to generate complete spectrum ä can be done quickly ä better resolution ä more resolution ä However, . . .
 FTIR ä ä A solution, yet an expensive one! FTIR uses sophisticated machinery more complex than generic GCIR
FTIR ä ä A solution, yet an expensive one! FTIR uses sophisticated machinery more complex than generic GCIR
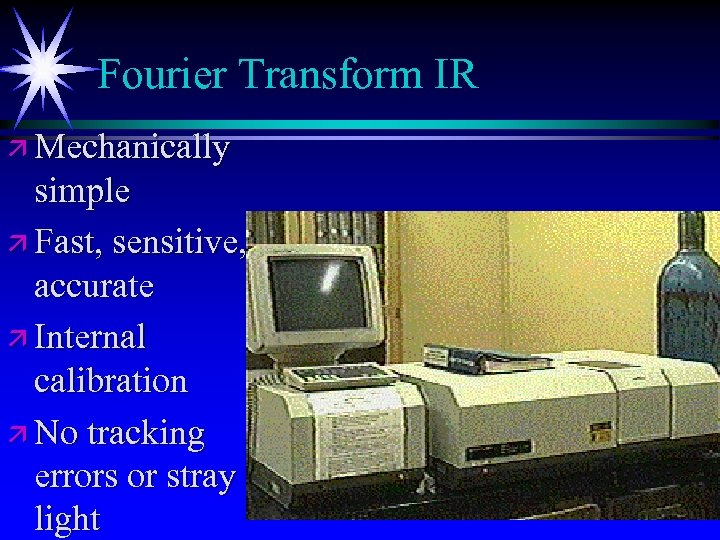 Fourier Transform IR ä Mechanically simple ä Fast, sensitive, accurate ä Internal calibration ä No tracking errors or stray light
Fourier Transform IR ä Mechanically simple ä Fast, sensitive, accurate ä Internal calibration ä No tracking errors or stray light
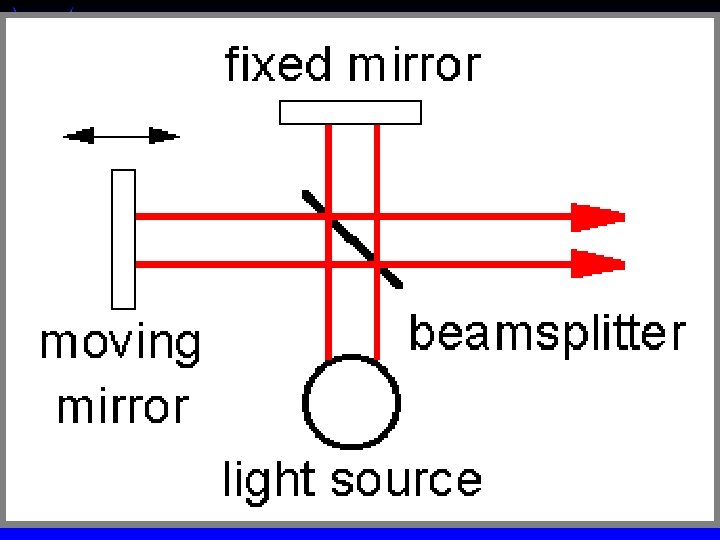 IR Spectroscopy - qualitative Double beam required to correct for blank at each wavelength ä Scan time (sensitivity) Vs resolution ä Michelson interferometer & FTIR
IR Spectroscopy - qualitative Double beam required to correct for blank at each wavelength ä Scan time (sensitivity) Vs resolution ä Michelson interferometer & FTIR
 Advantages of FTIR ä Multiplex--speed, sensitivity (Felgett) ä Throughput--greater energy, S/N (Jacquinot) ä Laser reference--accurate wavelength, reproducible (Connes) ä No stray light--quantitative accuracy ä No tracking errors--wavelength and photometric accuracy
Advantages of FTIR ä Multiplex--speed, sensitivity (Felgett) ä Throughput--greater energy, S/N (Jacquinot) ä Laser reference--accurate wavelength, reproducible (Connes) ä No stray light--quantitative accuracy ä No tracking errors--wavelength and photometric accuracy
 New FTIR Applications ä Quality control--speed, accuracy ä Micro, trace analysis--nanogram levels, small samples ä Kinetic studies--milliseconds ä Internal reflection ä Telescopic
New FTIR Applications ä Quality control--speed, accuracy ä Micro, trace analysis--nanogram levels, small samples ä Kinetic studies--milliseconds ä Internal reflection ä Telescopic
 Attenuated Internal Reflection ä ä Surface analysis Limited by 75% energy loss
Attenuated Internal Reflection ä ä Surface analysis Limited by 75% energy loss
 New FTIR Applications ä Quality control--speed, accuracy ä Micro, trace analysis--nanogram levels, small samples ä Kinetic studies--milliseconds ä Internal reflection ä Telescopic
New FTIR Applications ä Quality control--speed, accuracy ä Micro, trace analysis--nanogram levels, small samples ä Kinetic studies--milliseconds ä Internal reflection ä Telescopic


