80648f45a3eecf651106f149060a8fe7.ppt
- Количество слайдов: 67
 INTD 5000, Lectures C 7 & C 8 Professor Eileen M. Lafer Tel#: 7 -3764 Email: Lafer@biochem. uthscsa. edu Office: Room 415 B THE SECRETORY AND ENDOCYTIC PATHWAYS Reading: Chapters 12 and 13 from Alberts et al. , Molecular Biology of the Cell
INTD 5000, Lectures C 7 & C 8 Professor Eileen M. Lafer Tel#: 7 -3764 Email: Lafer@biochem. uthscsa. edu Office: Room 415 B THE SECRETORY AND ENDOCYTIC PATHWAYS Reading: Chapters 12 and 13 from Alberts et al. , Molecular Biology of the Cell
 CELL COMPARTMENTS (ANIMATION 12. 1 ALBERTS)
CELL COMPARTMENTS (ANIMATION 12. 1 ALBERTS)
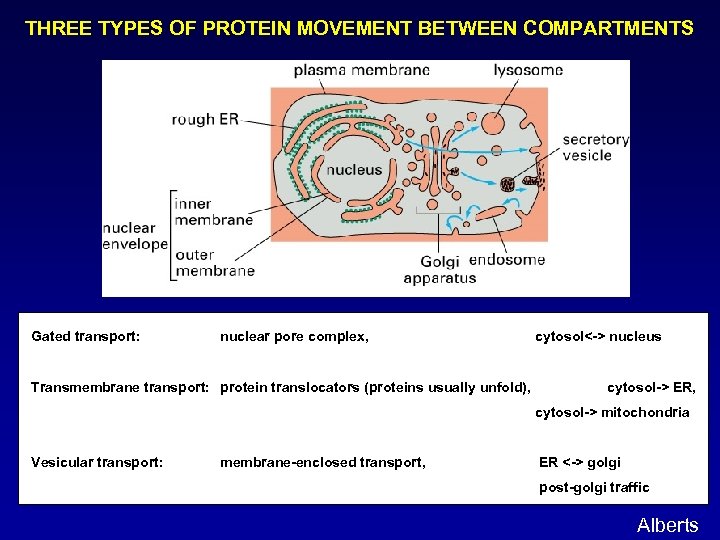 THREE TYPES OF PROTEIN MOVEMENT BETWEEN COMPARTMENTS Gated transport: nuclear pore complex, Transmembrane transport: protein translocators (proteins usually unfold), cytosol<-> nucleus cytosol-> ER, cytosol-> mitochondria Vesicular transport: membrane-enclosed transport, ER <-> golgi post-golgi traffic Alberts
THREE TYPES OF PROTEIN MOVEMENT BETWEEN COMPARTMENTS Gated transport: nuclear pore complex, Transmembrane transport: protein translocators (proteins usually unfold), cytosol<-> nucleus cytosol-> ER, cytosol-> mitochondria Vesicular transport: membrane-enclosed transport, ER <-> golgi post-golgi traffic Alberts
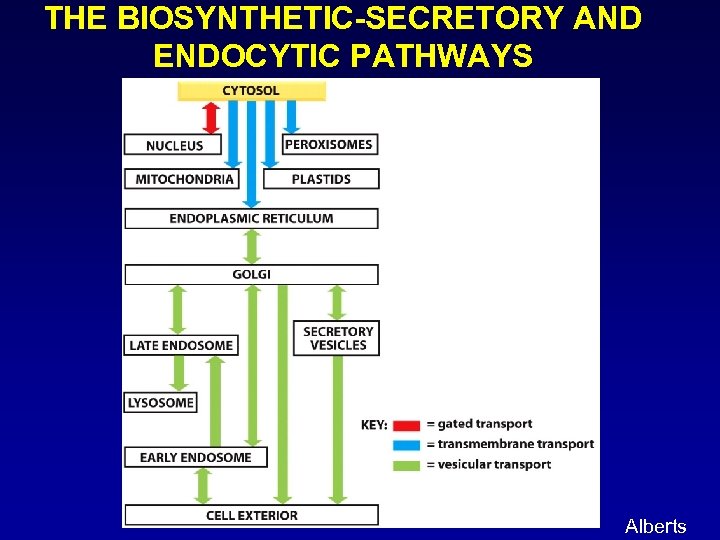 THE BIOSYNTHETIC-SECRETORY AND ENDOCYTIC PATHWAYS Alberts
THE BIOSYNTHETIC-SECRETORY AND ENDOCYTIC PATHWAYS Alberts
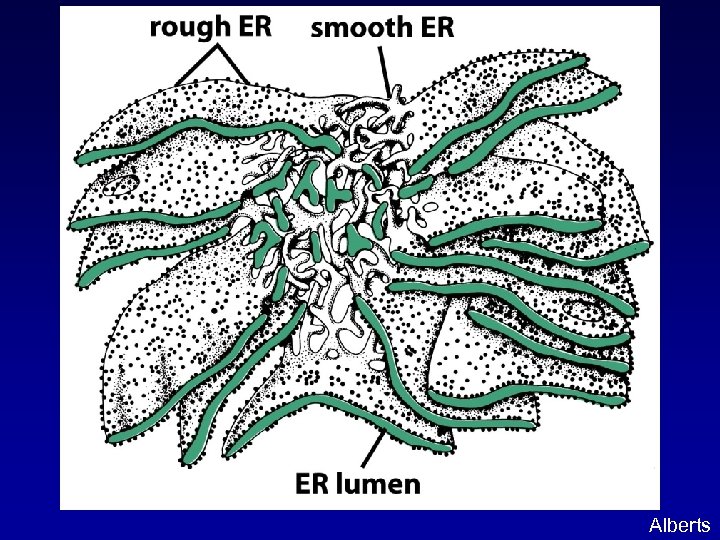
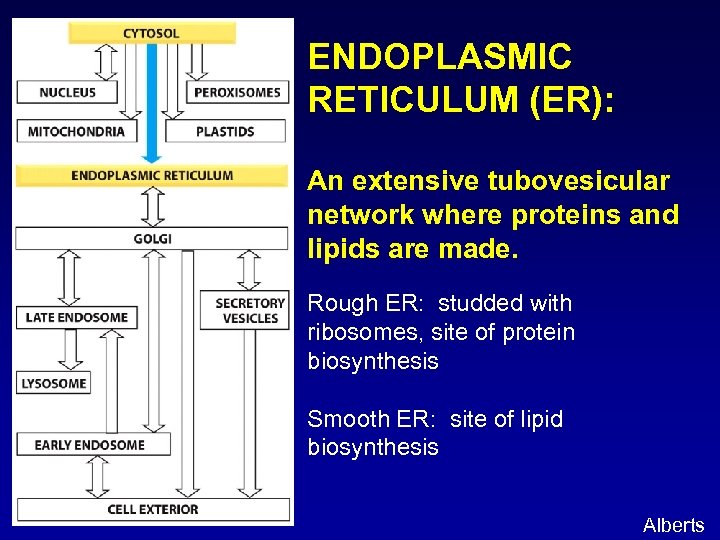 ENDOPLASMIC RETICULUM (ER): An extensive tubovesicular network where proteins and lipids are made. Rough ER: studded with ribosomes, site of protein biosynthesis Smooth ER: site of lipid biosynthesis Alberts
ENDOPLASMIC RETICULUM (ER): An extensive tubovesicular network where proteins and lipids are made. Rough ER: studded with ribosomes, site of protein biosynthesis Smooth ER: site of lipid biosynthesis Alberts
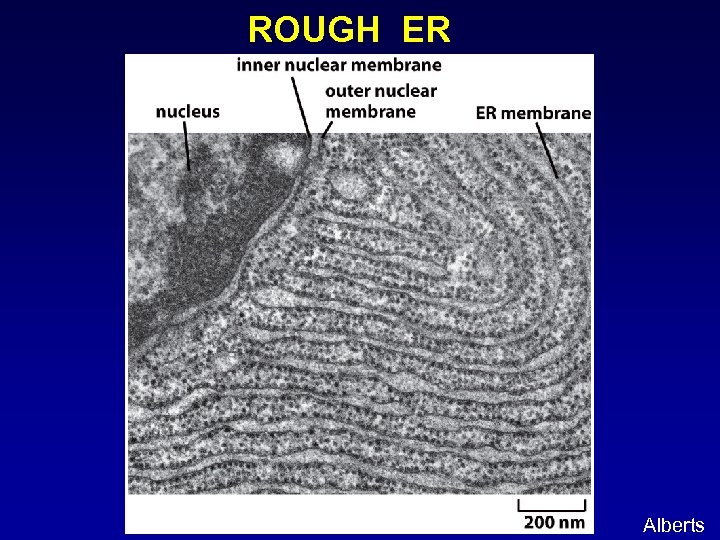 ROUGH ER Alberts
ROUGH ER Alberts
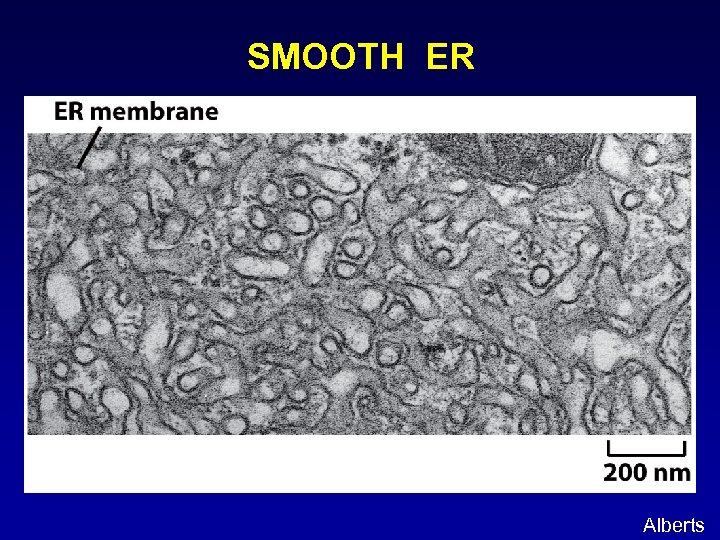 SMOOTH ER Alberts
SMOOTH ER Alberts
 Alberts
Alberts
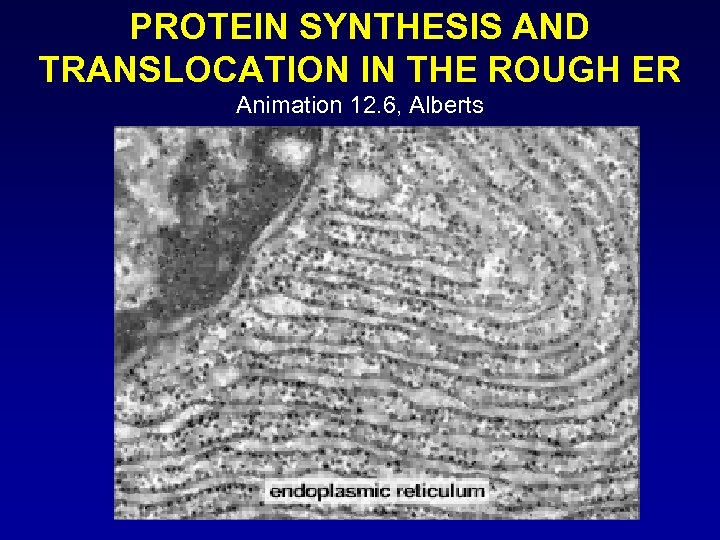 PROTEIN SYNTHESIS AND TRANSLOCATION IN THE ROUGH ER Animation 12. 6, Alberts
PROTEIN SYNTHESIS AND TRANSLOCATION IN THE ROUGH ER Animation 12. 6, Alberts
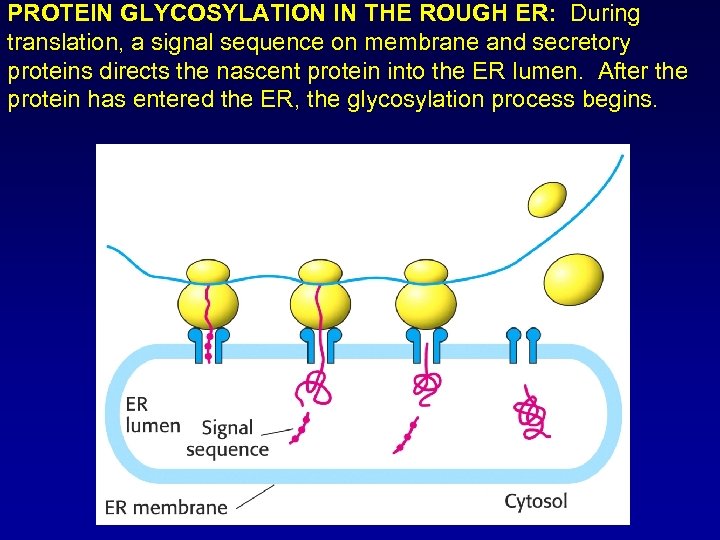 PROTEIN GLYCOSYLATION IN THE ROUGH ER: During translation, a signal sequence on membrane and secretory proteins directs the nascent protein into the ER lumen. After the protein has entered the ER, the glycosylation process begins.
PROTEIN GLYCOSYLATION IN THE ROUGH ER: During translation, a signal sequence on membrane and secretory proteins directs the nascent protein into the ER lumen. After the protein has entered the ER, the glycosylation process begins.
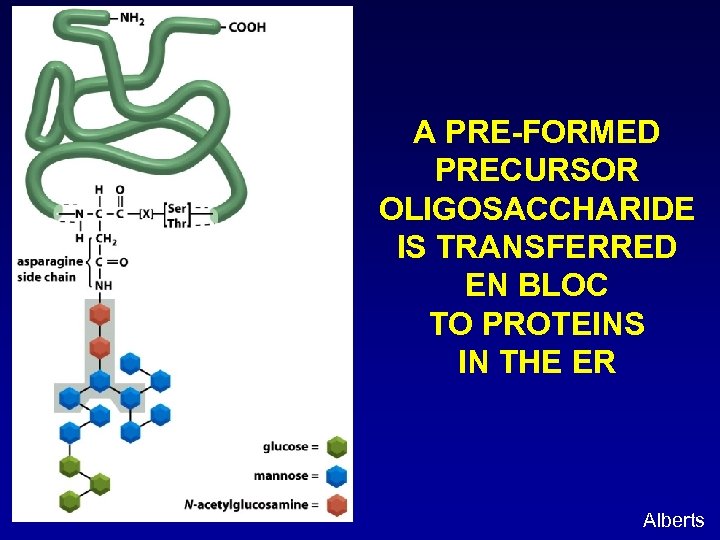 A PRE-FORMED PRECURSOR OLIGOSACCHARIDE IS TRANSFERRED EN BLOC TO PROTEINS IN THE ER Alberts
A PRE-FORMED PRECURSOR OLIGOSACCHARIDE IS TRANSFERRED EN BLOC TO PROTEINS IN THE ER Alberts
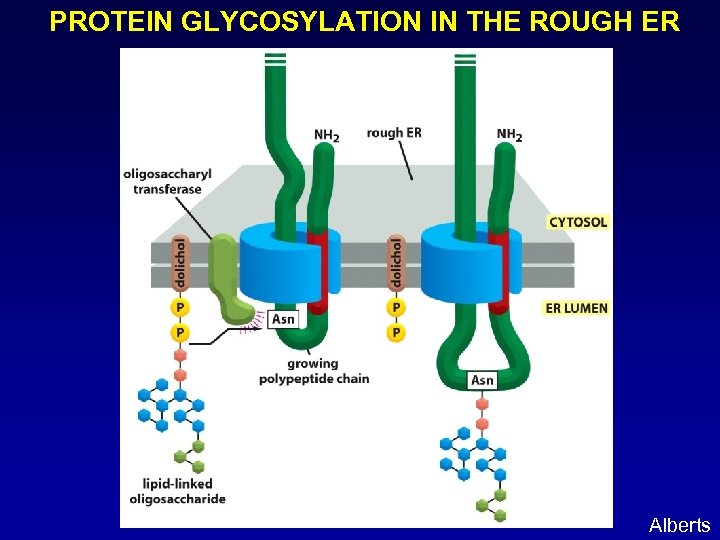 PROTEIN GLYCOSYLATION IN THE ROUGH ER Alberts
PROTEIN GLYCOSYLATION IN THE ROUGH ER Alberts
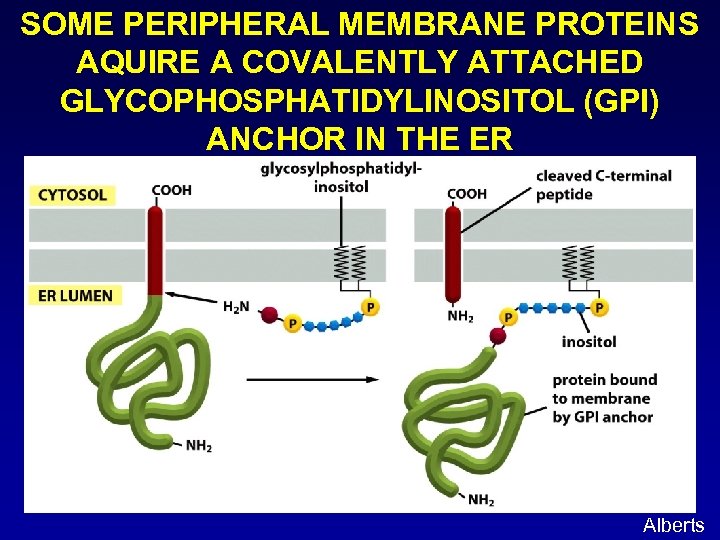 SOME PERIPHERAL MEMBRANE PROTEINS AQUIRE A COVALENTLY ATTACHED GLYCOPHOSPHATIDYLINOSITOL (GPI) ANCHOR IN THE ER Alberts
SOME PERIPHERAL MEMBRANE PROTEINS AQUIRE A COVALENTLY ATTACHED GLYCOPHOSPHATIDYLINOSITOL (GPI) ANCHOR IN THE ER Alberts
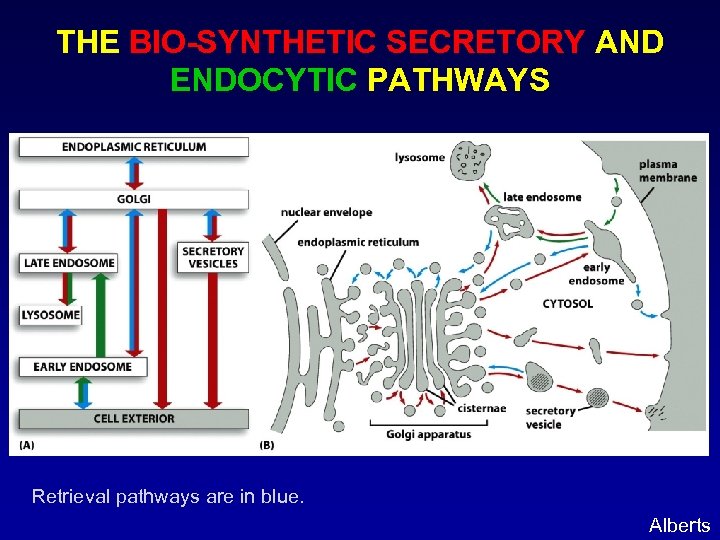 THE BIO-SYNTHETIC SECRETORY AND ENDOCYTIC PATHWAYS Retrieval pathways are in blue. Alberts
THE BIO-SYNTHETIC SECRETORY AND ENDOCYTIC PATHWAYS Retrieval pathways are in blue. Alberts
 MOVIE: MOVEMENT OF A FLUORESCENTLY TAGGED MEMBRANE PROTEIN THROUGH THE SECRETORY PATHWAY Alberts Video 13. 2
MOVIE: MOVEMENT OF A FLUORESCENTLY TAGGED MEMBRANE PROTEIN THROUGH THE SECRETORY PATHWAY Alberts Video 13. 2
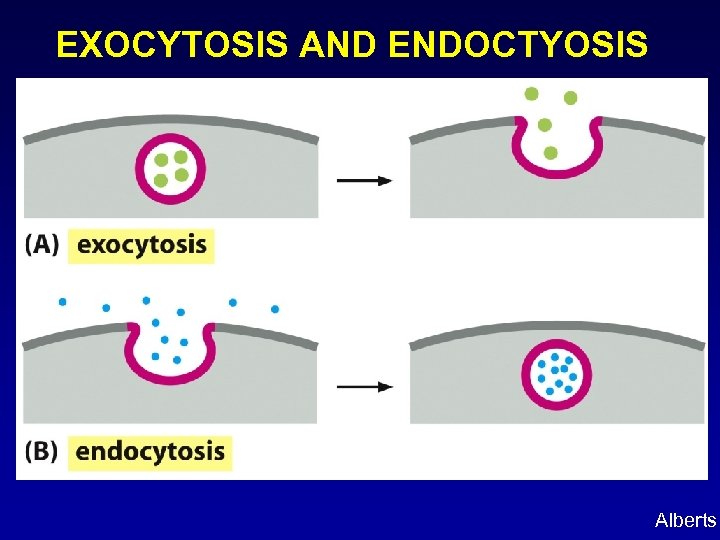 EXOCYTOSIS AND ENDOCTYOSIS Alberts
EXOCYTOSIS AND ENDOCTYOSIS Alberts
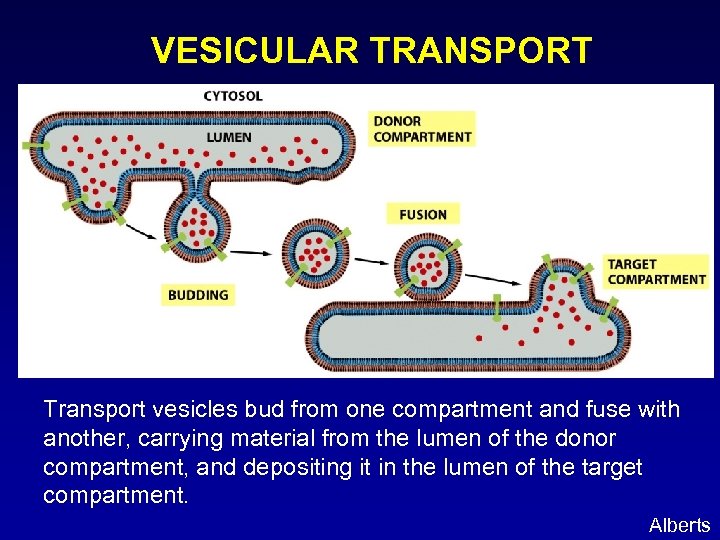 VESICULAR TRANSPORT Transport vesicles bud from one compartment and fuse with another, carrying material from the lumen of the donor compartment, and depositing it in the lumen of the target compartment. Alberts
VESICULAR TRANSPORT Transport vesicles bud from one compartment and fuse with another, carrying material from the lumen of the donor compartment, and depositing it in the lumen of the target compartment. Alberts
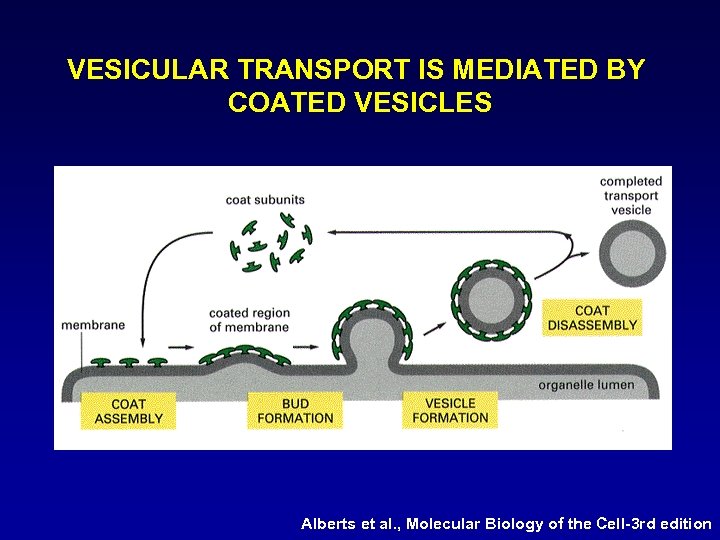 VESICULAR TRANSPORT IS MEDIATED BY COATED VESICLES Alberts et al. , Molecular Biology of the Cell-3 rd edition
VESICULAR TRANSPORT IS MEDIATED BY COATED VESICLES Alberts et al. , Molecular Biology of the Cell-3 rd edition
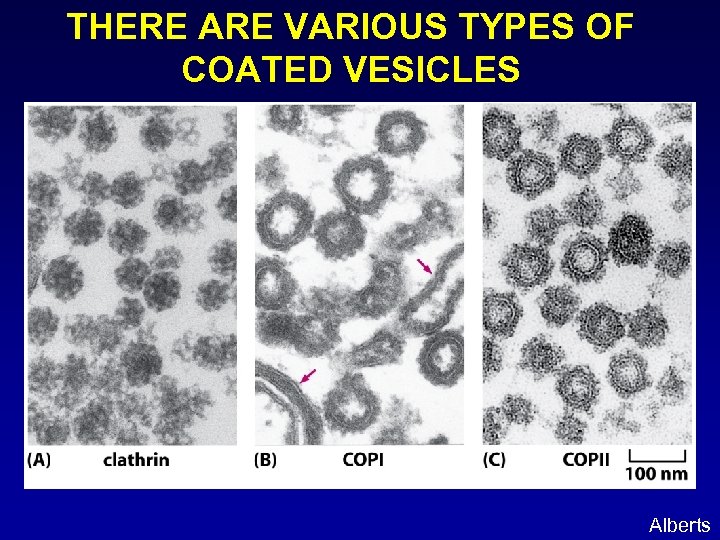 THERE ARE VARIOUS TYPES OF COATED VESICLES Alberts
THERE ARE VARIOUS TYPES OF COATED VESICLES Alberts
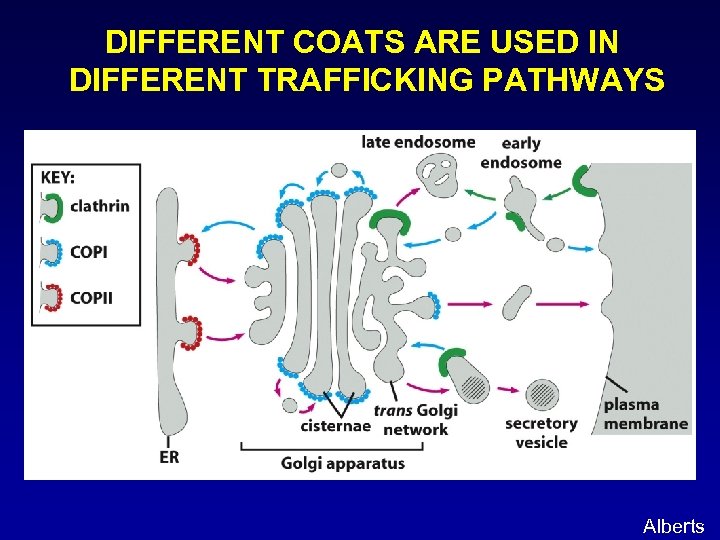 DIFFERENT COATS ARE USED IN DIFFERENT TRAFFICKING PATHWAYS Alberts
DIFFERENT COATS ARE USED IN DIFFERENT TRAFFICKING PATHWAYS Alberts
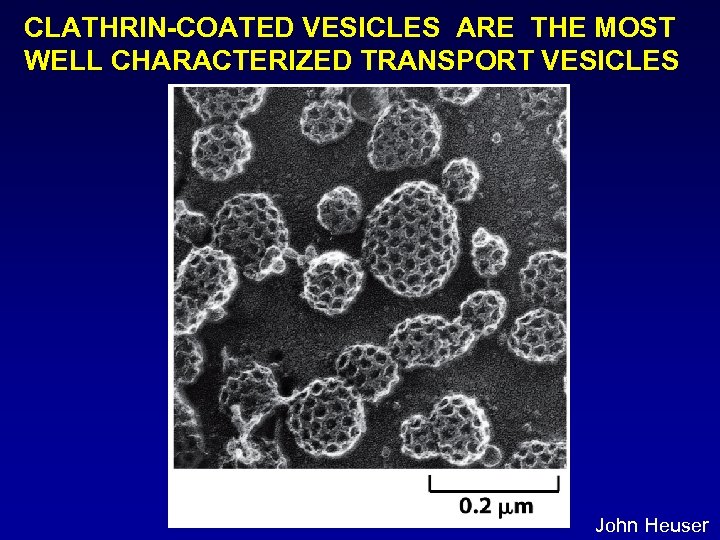 CLATHRIN-COATED VESICLES ARE THE MOST WELL CHARACTERIZED TRANSPORT VESICLES John Heuser
CLATHRIN-COATED VESICLES ARE THE MOST WELL CHARACTERIZED TRANSPORT VESICLES John Heuser
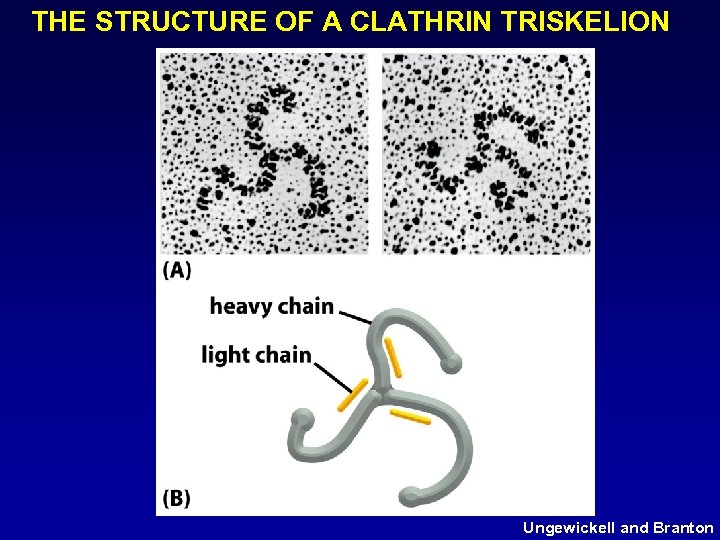 THE STRUCTURE OF A CLATHRIN TRISKELION Ungewickell and Branton
THE STRUCTURE OF A CLATHRIN TRISKELION Ungewickell and Branton
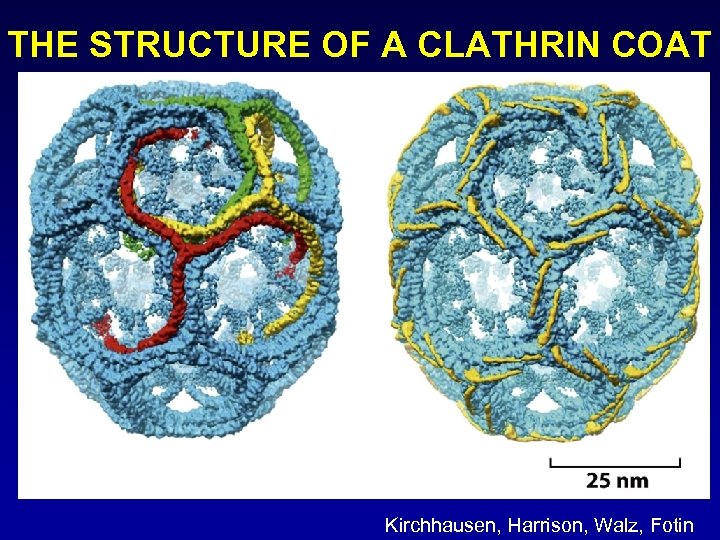 THE STRUCTURE OF A CLATHRIN COAT Kirchhausen, Harrison, Walz, Fotin
THE STRUCTURE OF A CLATHRIN COAT Kirchhausen, Harrison, Walz, Fotin
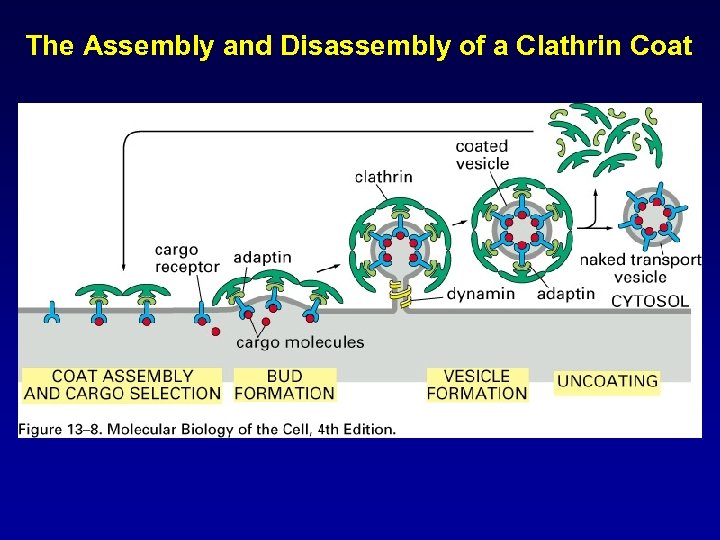 The Assembly and Disassembly of a Clathrin Coat
The Assembly and Disassembly of a Clathrin Coat
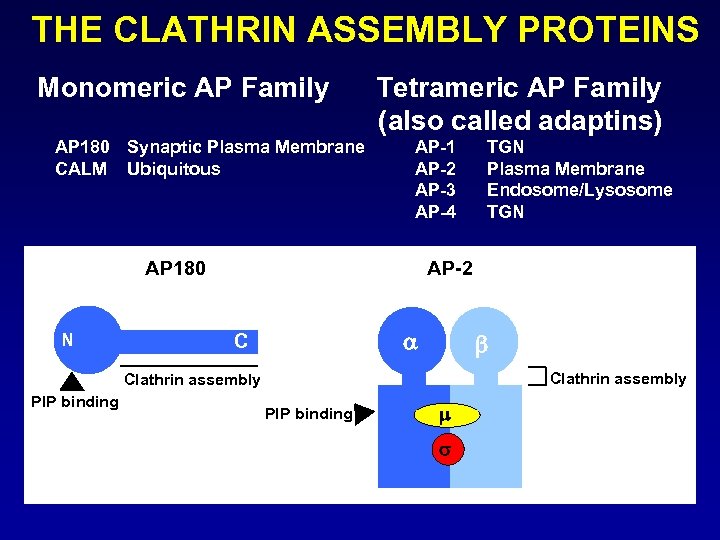 THE CLATHRIN ASSEMBLY PROTEINS Monomeric AP Family AP 180 Synaptic Plasma Membrane CALM Ubiquitous Tetrameric AP Family (also called adaptins) AP-1 AP-2 AP-3 AP-4 AP 180 N AP-2 C b Clathrin assembly PIP binding TGN Plasma Membrane Endosome/Lysosome TGN PIP binding m s
THE CLATHRIN ASSEMBLY PROTEINS Monomeric AP Family AP 180 Synaptic Plasma Membrane CALM Ubiquitous Tetrameric AP Family (also called adaptins) AP-1 AP-2 AP-3 AP-4 AP 180 N AP-2 C b Clathrin assembly PIP binding TGN Plasma Membrane Endosome/Lysosome TGN PIP binding m s
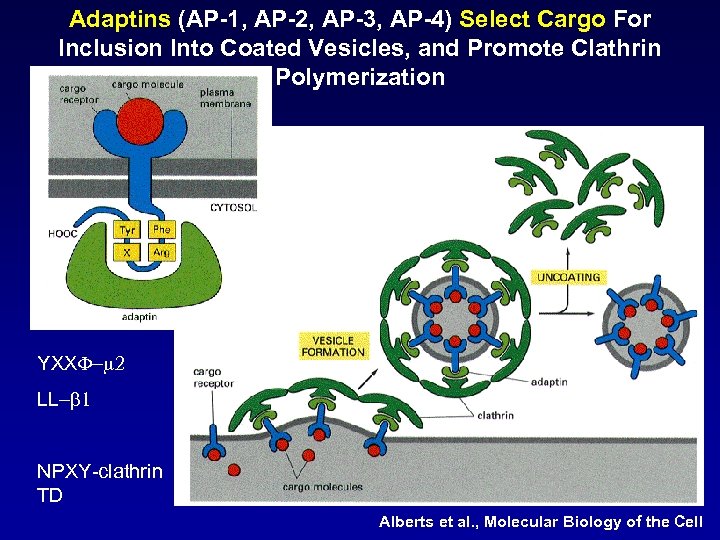 Adaptins (AP-1, AP-2, AP-3, AP-4) Select Cargo For Inclusion Into Coated Vesicles, and Promote Clathrin Polymerization YXXF-m 2 LL-b 1 NPXY-clathrin TD Alberts et al. , Molecular Biology of the Cell
Adaptins (AP-1, AP-2, AP-3, AP-4) Select Cargo For Inclusion Into Coated Vesicles, and Promote Clathrin Polymerization YXXF-m 2 LL-b 1 NPXY-clathrin TD Alberts et al. , Molecular Biology of the Cell
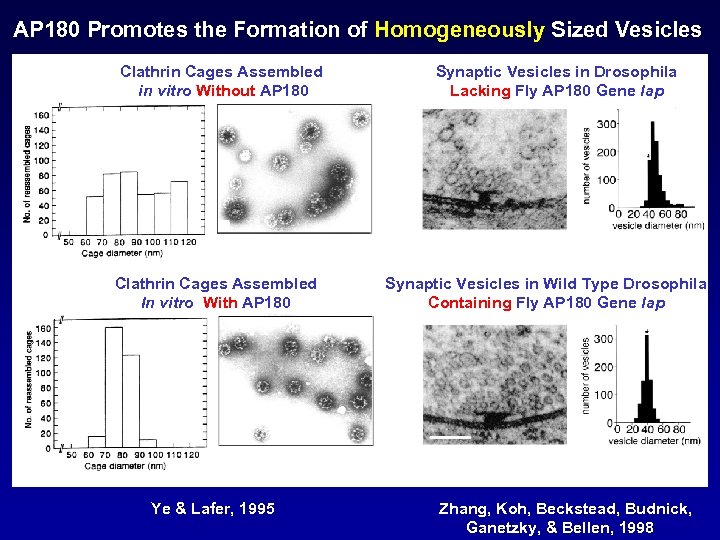 AP 180 Promotes the Formation of Homogeneously Sized Vesicles Clathrin Cages Assembled in vitro Without AP 180 Clathrin Cages Assembled In vitro With AP 180 Ye & Lafer, 1995 Synaptic Vesicles in Drosophila Lacking Fly AP 180 Gene lap Synaptic Vesicles in Wild Type Drosophila Containing Fly AP 180 Gene lap Zhang, Koh, Beckstead, Budnick, Ganetzky, & Bellen, 1998
AP 180 Promotes the Formation of Homogeneously Sized Vesicles Clathrin Cages Assembled in vitro Without AP 180 Clathrin Cages Assembled In vitro With AP 180 Ye & Lafer, 1995 Synaptic Vesicles in Drosophila Lacking Fly AP 180 Gene lap Synaptic Vesicles in Wild Type Drosophila Containing Fly AP 180 Gene lap Zhang, Koh, Beckstead, Budnick, Ganetzky, & Bellen, 1998
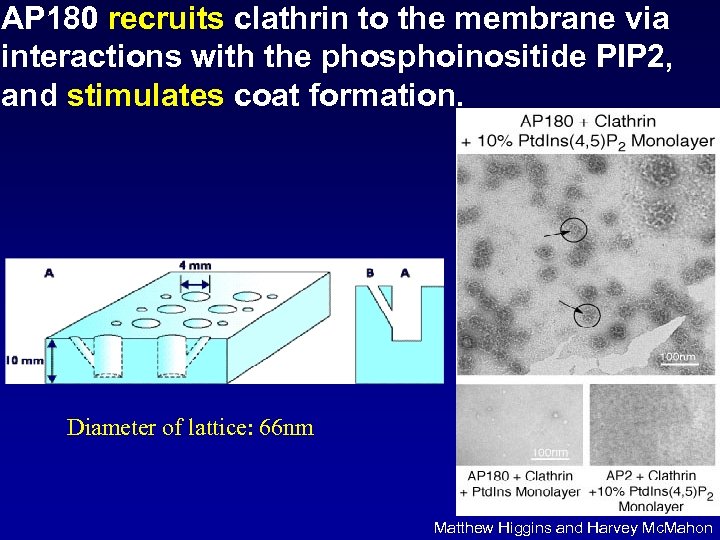 AP 180 recruits clathrin to the membrane via interactions with the phosphoinositide PIP 2, and stimulates coat formation. Diameter of lattice: 66 nm Matthew Higgins and Harvey Mc. Mahon
AP 180 recruits clathrin to the membrane via interactions with the phosphoinositide PIP 2, and stimulates coat formation. Diameter of lattice: 66 nm Matthew Higgins and Harvey Mc. Mahon
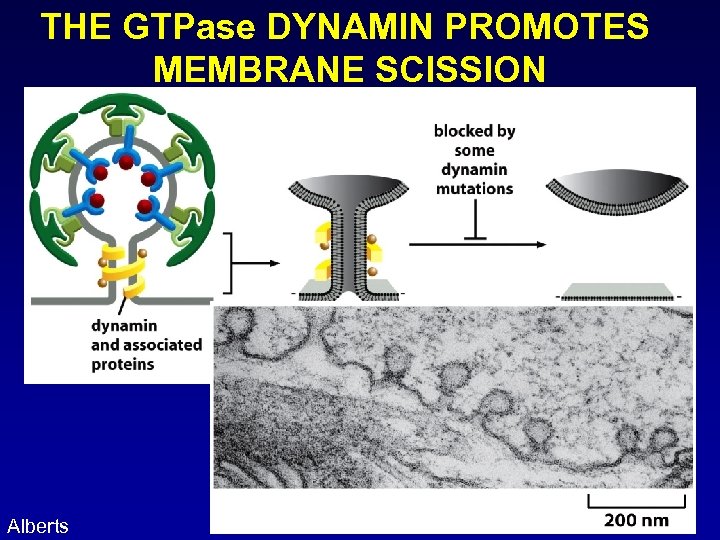 THE GTPase DYNAMIN PROMOTES MEMBRANE SCISSION Alberts
THE GTPase DYNAMIN PROMOTES MEMBRANE SCISSION Alberts
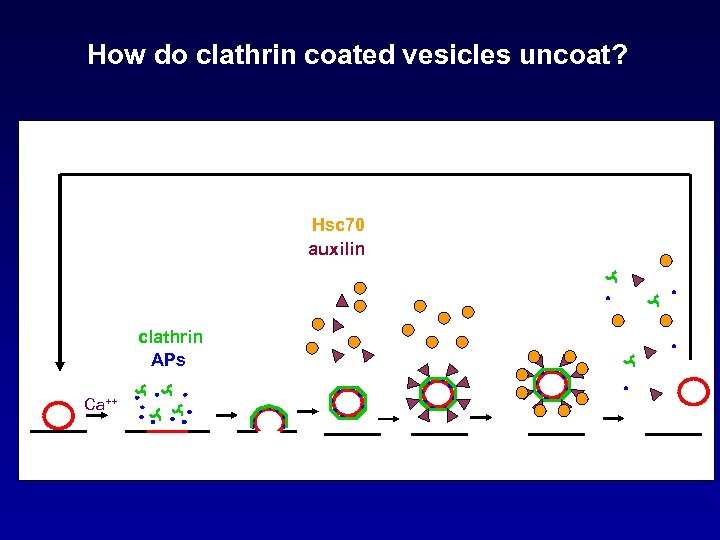 How do clathrin coated vesicles uncoat? Hsc 70 auxilin clathrin APs Ca++
How do clathrin coated vesicles uncoat? Hsc 70 auxilin clathrin APs Ca++
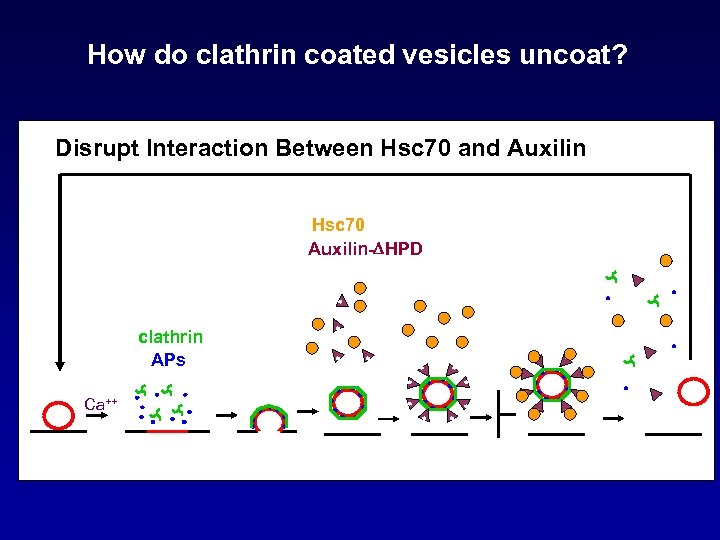 How do clathrin coated vesicles uncoat? Disrupt Interaction Between Hsc 70 and Auxilin Hsc 70 Auxilin-DHPD clathrin APs Ca++
How do clathrin coated vesicles uncoat? Disrupt Interaction Between Hsc 70 and Auxilin Hsc 70 Auxilin-DHPD clathrin APs Ca++
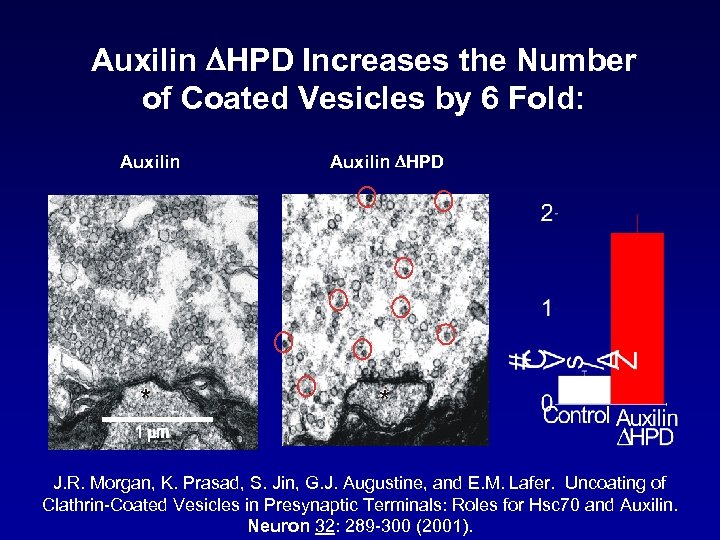 Auxilin DHPD Increases the Number of Coated Vesicles by 6 Fold: Auxilin DHPD * * J. R. Morgan, K. Prasad, S. Jin, G. J. Augustine, and E. M. Lafer. Uncoating of Clathrin-Coated Vesicles in Presynaptic Terminals: Roles for Hsc 70 and Auxilin. Neuron 32: 289 -300 (2001).
Auxilin DHPD Increases the Number of Coated Vesicles by 6 Fold: Auxilin DHPD * * J. R. Morgan, K. Prasad, S. Jin, G. J. Augustine, and E. M. Lafer. Uncoating of Clathrin-Coated Vesicles in Presynaptic Terminals: Roles for Hsc 70 and Auxilin. Neuron 32: 289 -300 (2001).
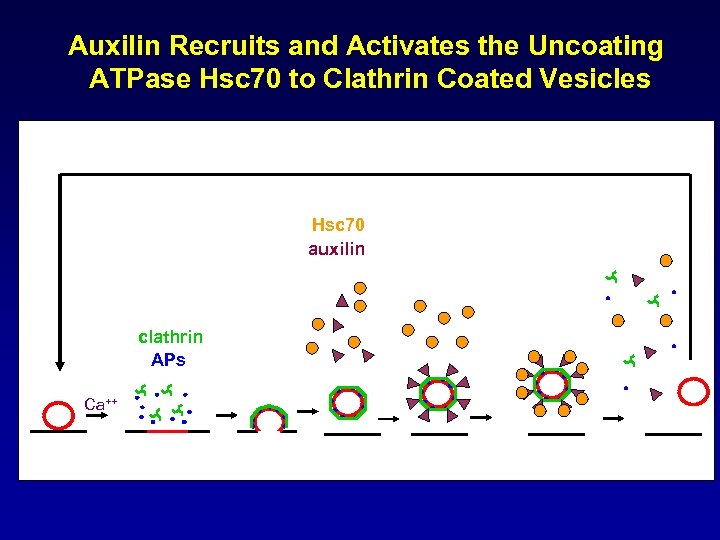 Auxilin Recruits and Activates the Uncoating ATPase Hsc 70 to Clathrin Coated Vesicles Hsc 70 auxilin clathrin APs Ca++
Auxilin Recruits and Activates the Uncoating ATPase Hsc 70 to Clathrin Coated Vesicles Hsc 70 auxilin clathrin APs Ca++
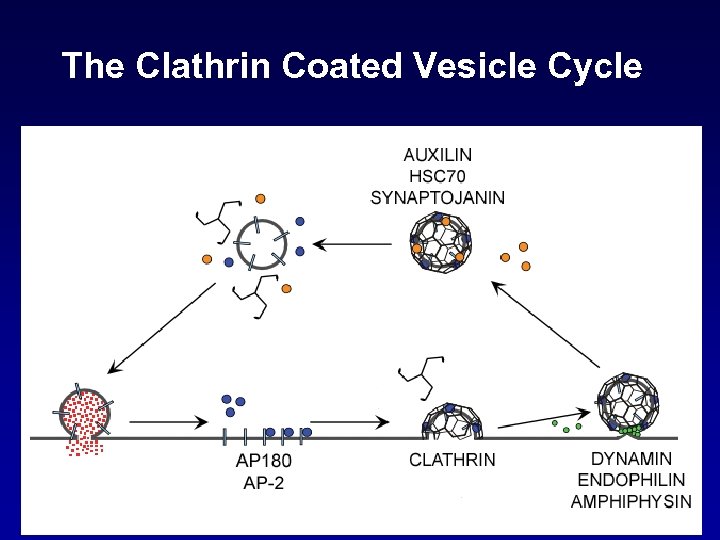 The Clathrin Coated Vesicle Cycle
The Clathrin Coated Vesicle Cycle
 CLATHRIN – THE MOVIE ANIMATION 13. 1 ALBERTS
CLATHRIN – THE MOVIE ANIMATION 13. 1 ALBERTS
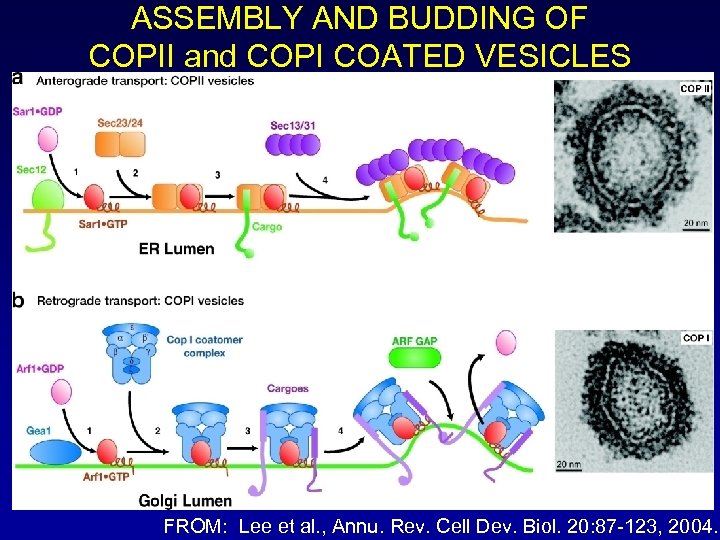 ASSEMBLY AND BUDDING OF COPII and COPI COATED VESICLES FROM: Lee et al. , Annu. Rev. Cell Dev. Biol. 20: 87 -123, 2004.
ASSEMBLY AND BUDDING OF COPII and COPI COATED VESICLES FROM: Lee et al. , Annu. Rev. Cell Dev. Biol. 20: 87 -123, 2004.
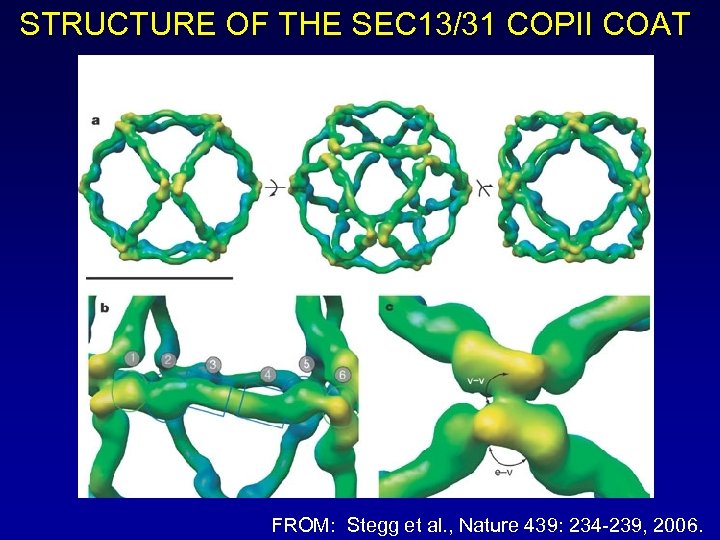 STRUCTURE OF THE SEC 13/31 COPII COAT FROM: Stegg et al. , Nature 439: 234 -239, 2006.
STRUCTURE OF THE SEC 13/31 COPII COAT FROM: Stegg et al. , Nature 439: 234 -239, 2006.
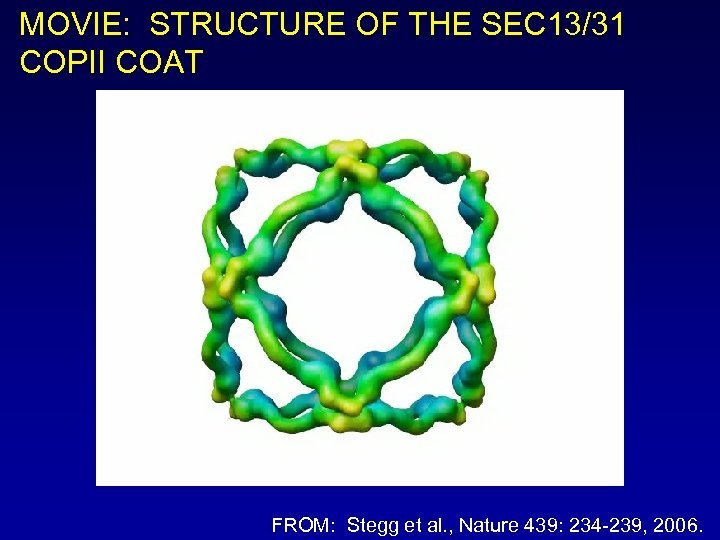 MOVIE: STRUCTURE OF THE SEC 13/31 COPII COAT FROM: Stegg et al. , Nature 439: 234 -239, 2006.
MOVIE: STRUCTURE OF THE SEC 13/31 COPII COAT FROM: Stegg et al. , Nature 439: 234 -239, 2006.
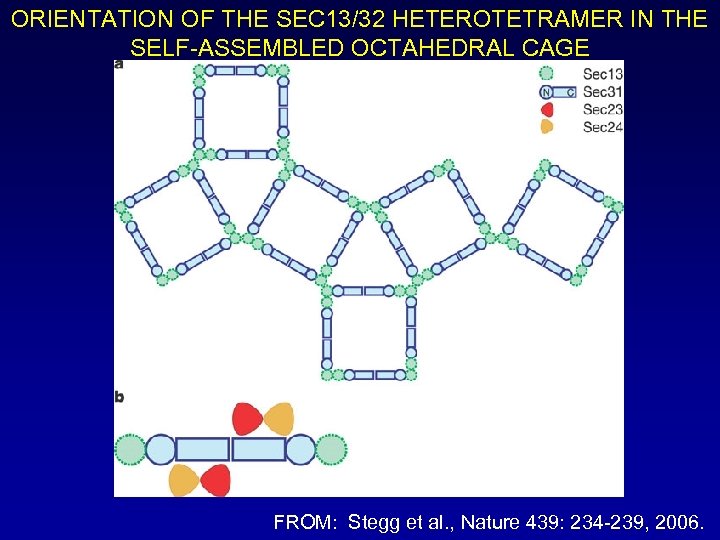 ORIENTATION OF THE SEC 13/32 HETEROTETRAMER IN THE SELF-ASSEMBLED OCTAHEDRAL CAGE FROM: Stegg et al. , Nature 439: 234 -239, 2006.
ORIENTATION OF THE SEC 13/32 HETEROTETRAMER IN THE SELF-ASSEMBLED OCTAHEDRAL CAGE FROM: Stegg et al. , Nature 439: 234 -239, 2006.
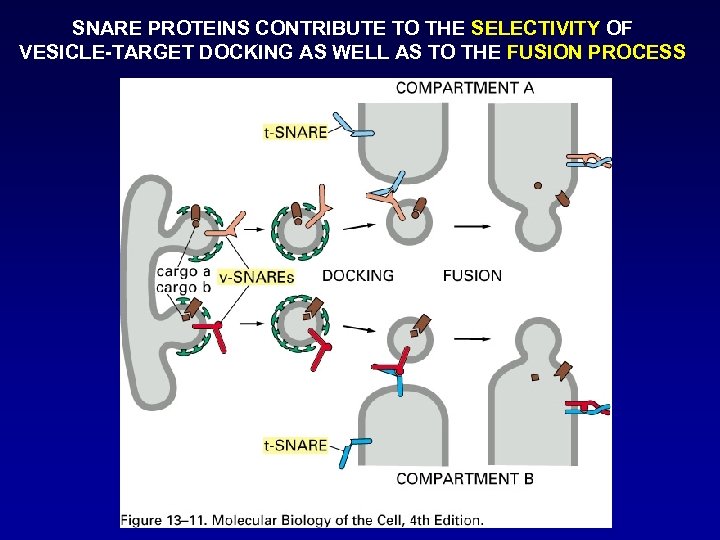 SNARE PROTEINS CONTRIBUTE TO THE SELECTIVITY OF VESICLE-TARGET DOCKING AS WELL AS TO THE FUSION PROCESS
SNARE PROTEINS CONTRIBUTE TO THE SELECTIVITY OF VESICLE-TARGET DOCKING AS WELL AS TO THE FUSION PROCESS
 SNARE PROTEINS: The group of proteins commonly referred to as SNARES for Soluble NSF Attachment REceptor. S were originally discovered as the synaptic proteins: VAMP/synaptobrevin syntaxin SNAP-25 They were later re-discovered as the receptors for the soluble Golgi trafficking protein SNAP – Soluble NSF Attachment Protein. NSF (NEM-Sensitive Fusion factor) The SNARE proteins were also identified as substrates for the clostridial neurotoxins, potent agents which inhibit neurotransmitter release.
SNARE PROTEINS: The group of proteins commonly referred to as SNARES for Soluble NSF Attachment REceptor. S were originally discovered as the synaptic proteins: VAMP/synaptobrevin syntaxin SNAP-25 They were later re-discovered as the receptors for the soluble Golgi trafficking protein SNAP – Soluble NSF Attachment Protein. NSF (NEM-Sensitive Fusion factor) The SNARE proteins were also identified as substrates for the clostridial neurotoxins, potent agents which inhibit neurotransmitter release.
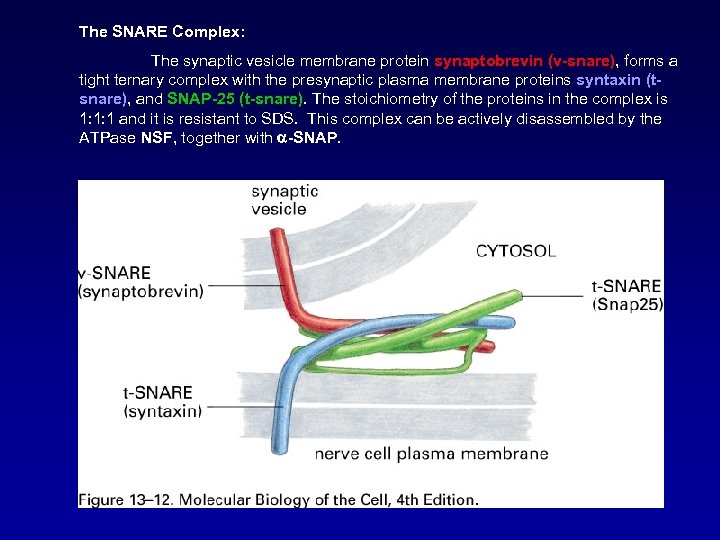 The SNARE Complex: The synaptic vesicle membrane protein synaptobrevin (v-snare), forms a tight ternary complex with the presynaptic plasma membrane proteins syntaxin (tsnare), and SNAP-25 (t-snare). The stoichiometry of the proteins in the complex is 1: 1: 1 and it is resistant to SDS. This complex can be actively disassembled by the ATPase NSF, together with -SNAP.
The SNARE Complex: The synaptic vesicle membrane protein synaptobrevin (v-snare), forms a tight ternary complex with the presynaptic plasma membrane proteins syntaxin (tsnare), and SNAP-25 (t-snare). The stoichiometry of the proteins in the complex is 1: 1: 1 and it is resistant to SDS. This complex can be actively disassembled by the ATPase NSF, together with -SNAP.
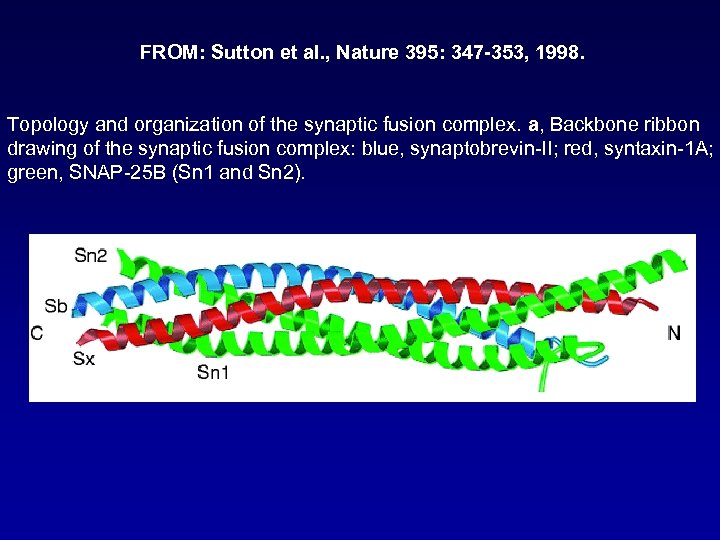 FROM: Sutton et al. , Nature 395: 347 -353, 1998. Topology and organization of the synaptic fusion complex. a, Backbone ribbon drawing of the synaptic fusion complex: blue, synaptobrevin-II; red, syntaxin-1 A; green, SNAP-25 B (Sn 1 and Sn 2).
FROM: Sutton et al. , Nature 395: 347 -353, 1998. Topology and organization of the synaptic fusion complex. a, Backbone ribbon drawing of the synaptic fusion complex: blue, synaptobrevin-II; red, syntaxin-1 A; green, SNAP-25 B (Sn 1 and Sn 2).
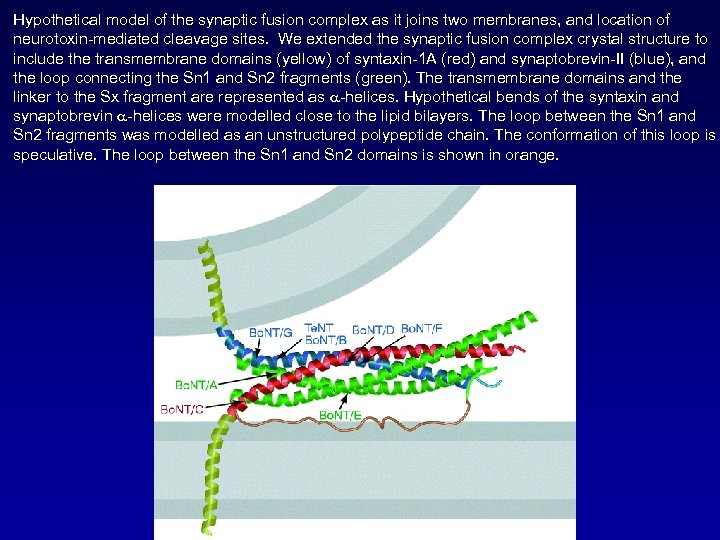 Hypothetical model of the synaptic fusion complex as it joins two membranes, and location of neurotoxin-mediated cleavage sites. We extended the synaptic fusion complex crystal structure to include the transmembrane domains (yellow) of syntaxin-1 A (red) and synaptobrevin-II (blue), and the loop connecting the Sn 1 and Sn 2 fragments (green). The transmembrane domains and the linker to the Sx fragment are represented as a-helices. Hypothetical bends of the syntaxin and synaptobrevin a-helices were modelled close to the lipid bilayers. The loop between the Sn 1 and Sn 2 fragments was modelled as an unstructured polypeptide chain. The conformation of this loop is speculative. The loop between the Sn 1 and Sn 2 domains is shown in orange.
Hypothetical model of the synaptic fusion complex as it joins two membranes, and location of neurotoxin-mediated cleavage sites. We extended the synaptic fusion complex crystal structure to include the transmembrane domains (yellow) of syntaxin-1 A (red) and synaptobrevin-II (blue), and the loop connecting the Sn 1 and Sn 2 fragments (green). The transmembrane domains and the linker to the Sx fragment are represented as a-helices. Hypothetical bends of the syntaxin and synaptobrevin a-helices were modelled close to the lipid bilayers. The loop between the Sn 1 and Sn 2 fragments was modelled as an unstructured polypeptide chain. The conformation of this loop is speculative. The loop between the Sn 1 and Sn 2 domains is shown in orange.
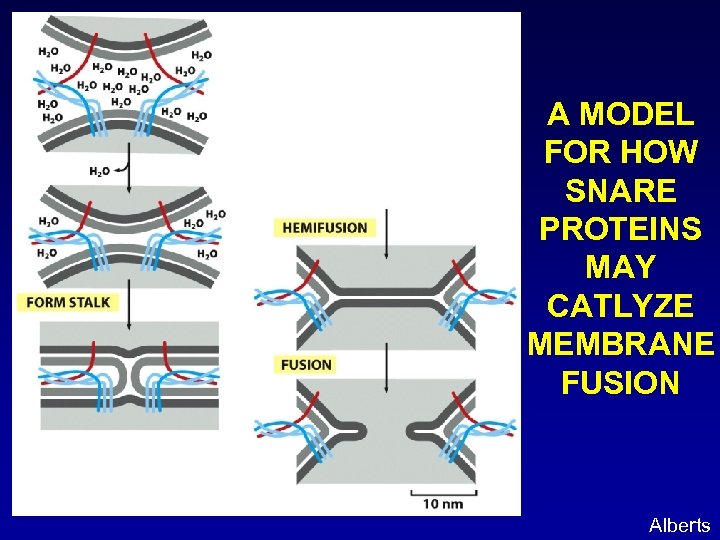 A MODEL FOR HOW SNARE PROTEINS MAY CATLYZE MEMBRANE FUSION Alberts
A MODEL FOR HOW SNARE PROTEINS MAY CATLYZE MEMBRANE FUSION Alberts
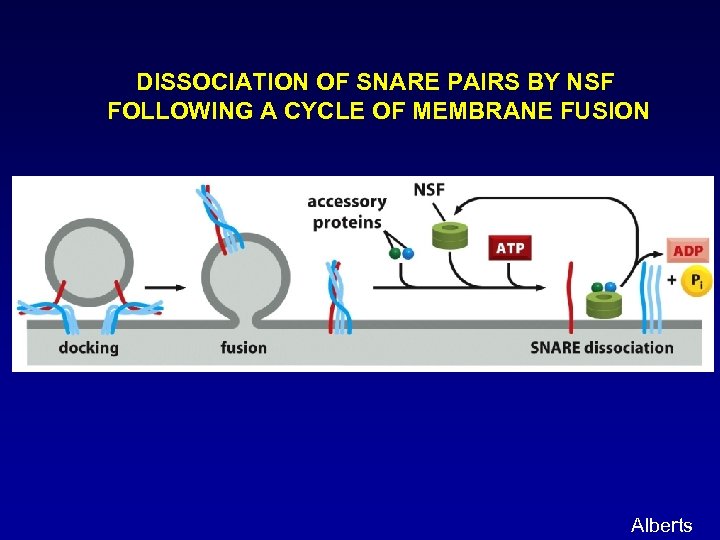 DISSOCIATION OF SNARE PAIRS BY NSF FOLLOWING A CYCLE OF MEMBRANE FUSION Alberts
DISSOCIATION OF SNARE PAIRS BY NSF FOLLOWING A CYCLE OF MEMBRANE FUSION Alberts
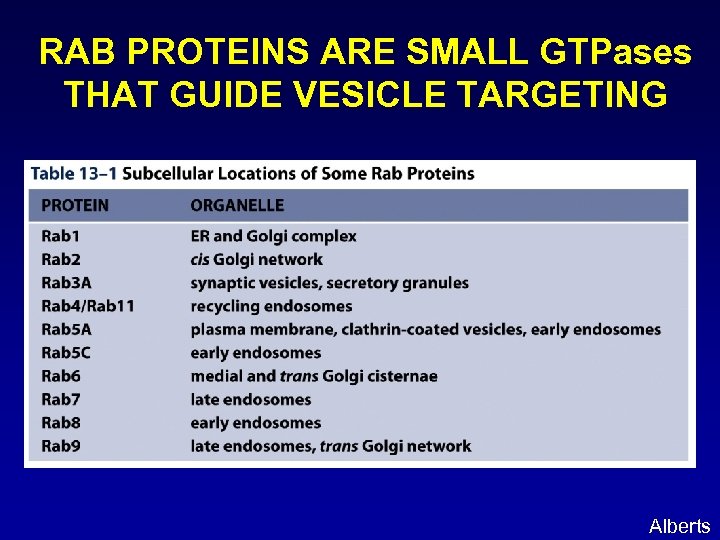 RAB PROTEINS ARE SMALL GTPases THAT GUIDE VESICLE TARGETING Alberts
RAB PROTEINS ARE SMALL GTPases THAT GUIDE VESICLE TARGETING Alberts
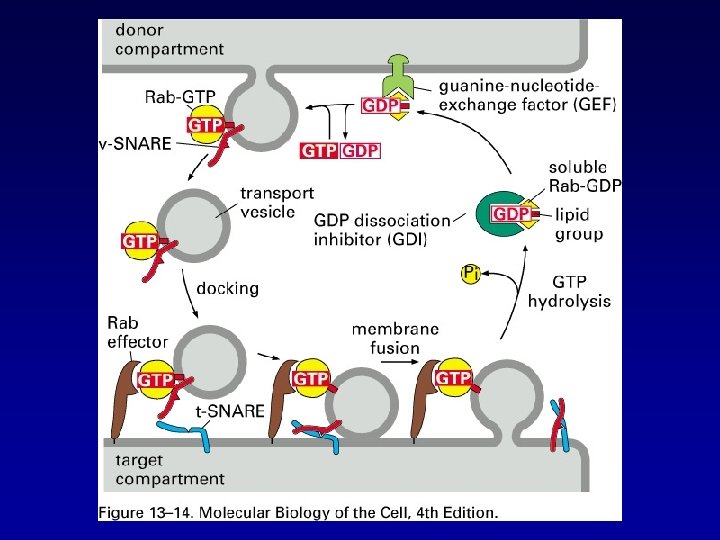
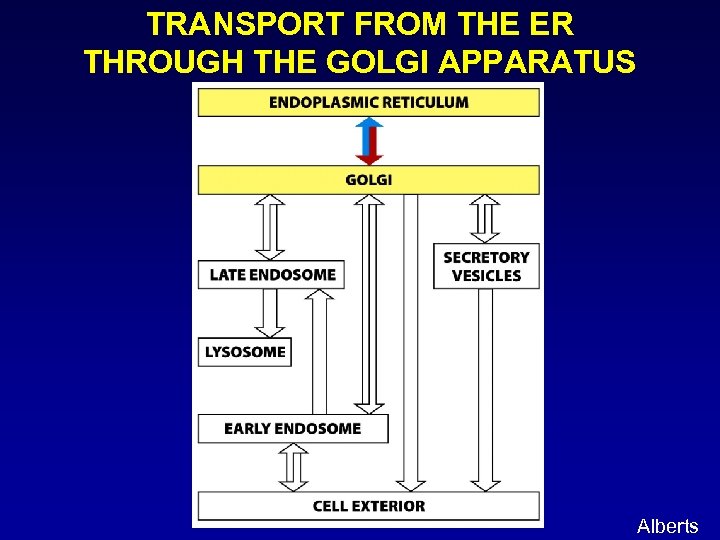 TRANSPORT FROM THE ER THROUGH THE GOLGI APPARATUS Alberts
TRANSPORT FROM THE ER THROUGH THE GOLGI APPARATUS Alberts
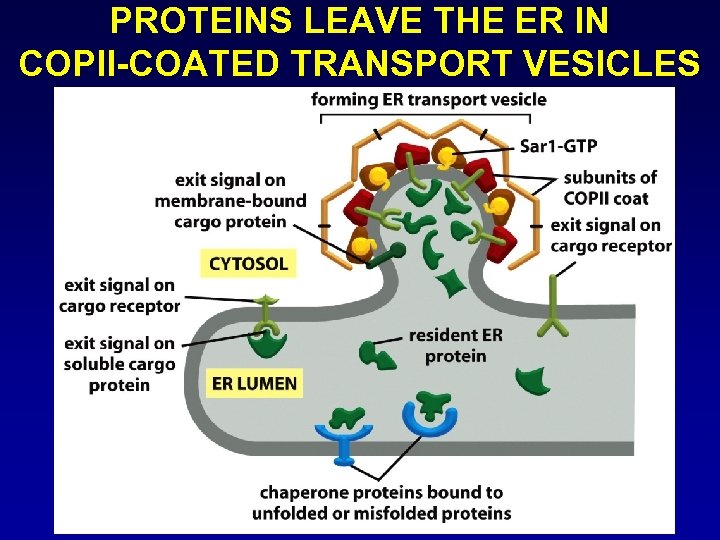 PROTEINS LEAVE THE ER IN COPII-COATED TRANSPORT VESICLES
PROTEINS LEAVE THE ER IN COPII-COATED TRANSPORT VESICLES
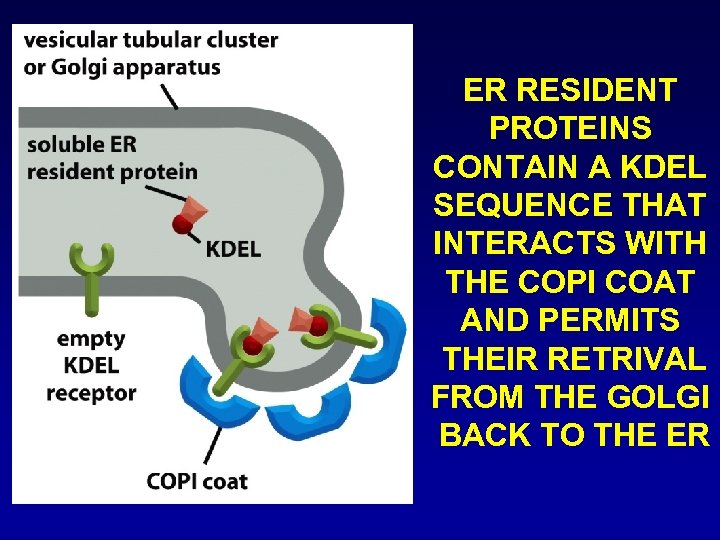 ER RESIDENT PROTEINS CONTAIN A KDEL SEQUENCE THAT INTERACTS WITH THE COPI COAT AND PERMITS THEIR RETRIVAL FROM THE GOLGI BACK TO THE ER
ER RESIDENT PROTEINS CONTAIN A KDEL SEQUENCE THAT INTERACTS WITH THE COPI COAT AND PERMITS THEIR RETRIVAL FROM THE GOLGI BACK TO THE ER
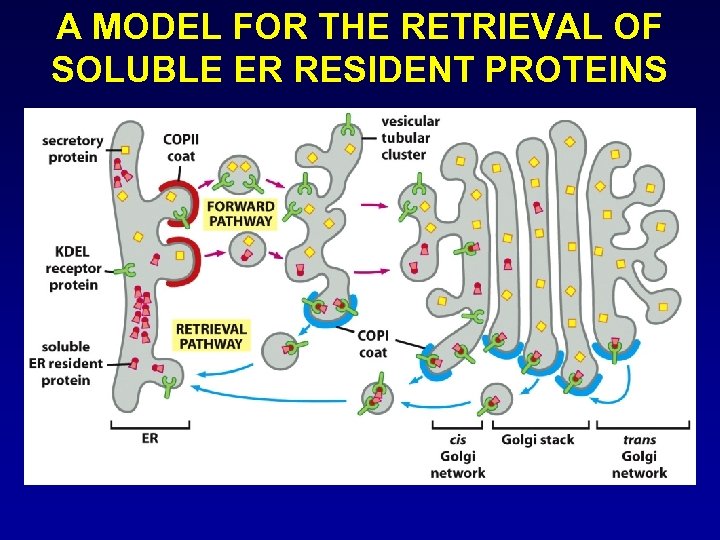 A MODEL FOR THE RETRIEVAL OF SOLUBLE ER RESIDENT PROTEINS
A MODEL FOR THE RETRIEVAL OF SOLUBLE ER RESIDENT PROTEINS
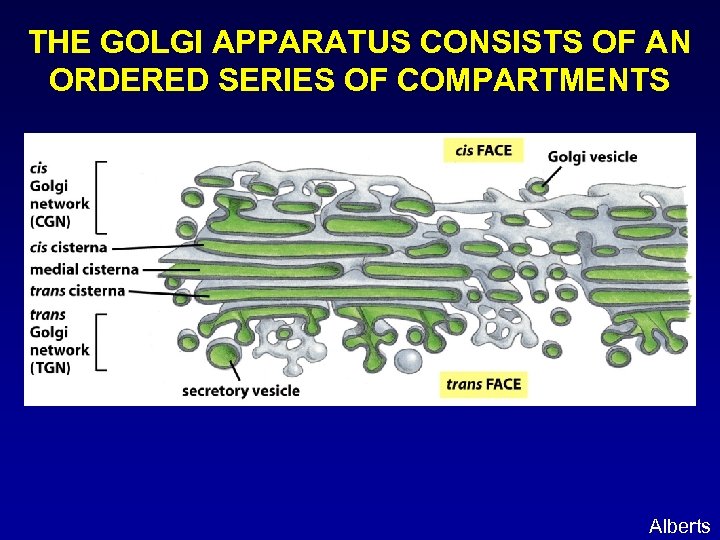 THE GOLGI APPARATUS CONSISTS OF AN ORDERED SERIES OF COMPARTMENTS Alberts
THE GOLGI APPARATUS CONSISTS OF AN ORDERED SERIES OF COMPARTMENTS Alberts
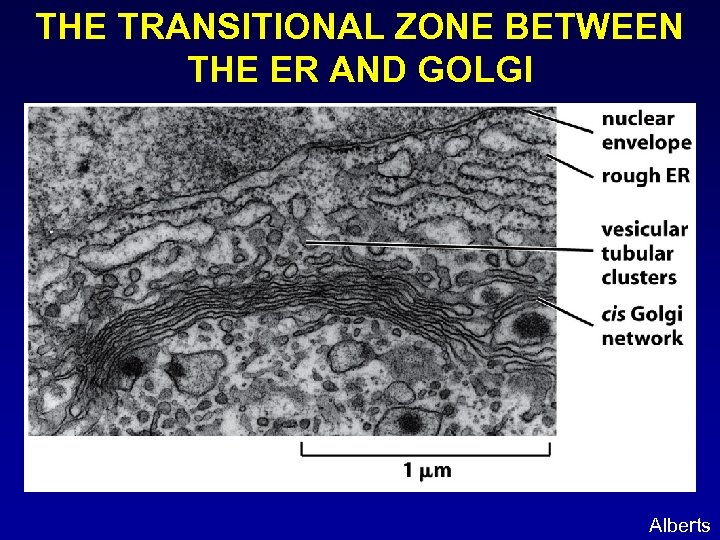 THE TRANSITIONAL ZONE BETWEEN THE ER AND GOLGI Alberts
THE TRANSITIONAL ZONE BETWEEN THE ER AND GOLGI Alberts
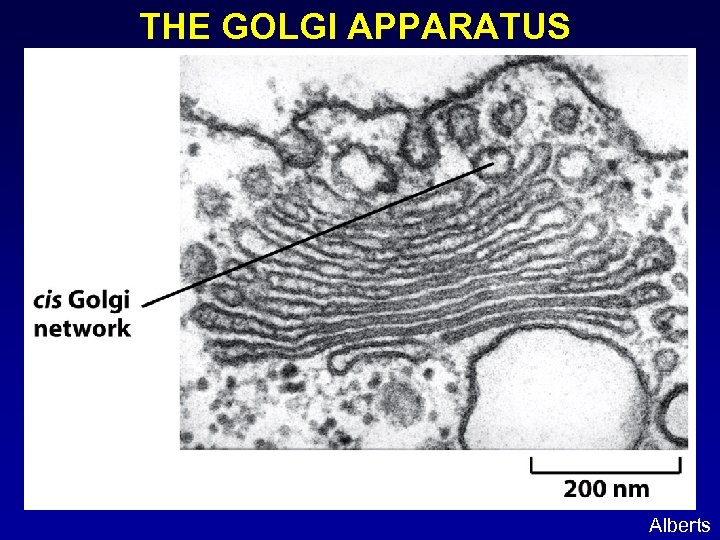 THE GOLGI APPARATUS Alberts
THE GOLGI APPARATUS Alberts
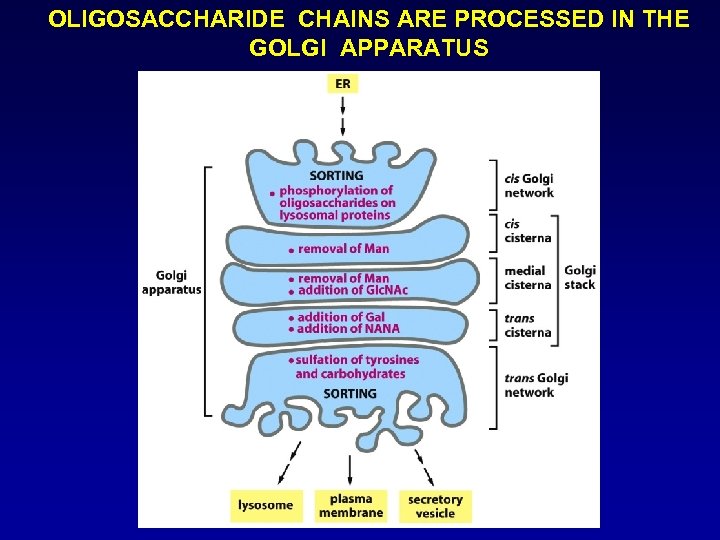 OLIGOSACCHARIDE CHAINS ARE PROCESSED IN THE GOLGI APPARATUS
OLIGOSACCHARIDE CHAINS ARE PROCESSED IN THE GOLGI APPARATUS
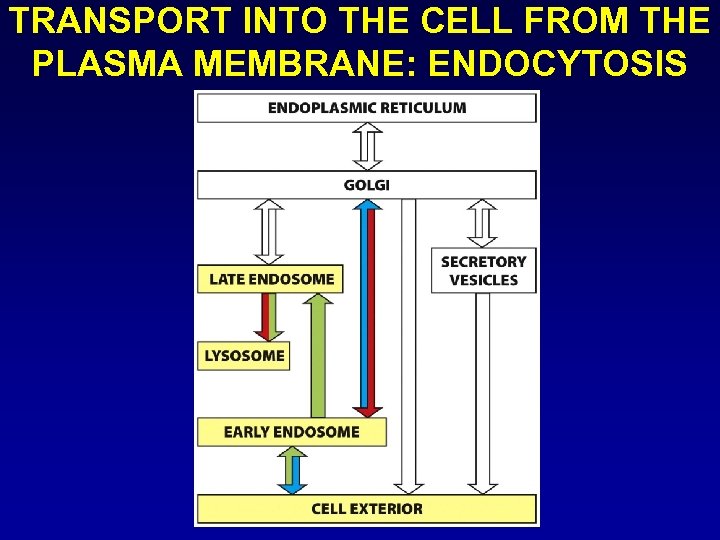 TRANSPORT INTO THE CELL FROM THE PLASMA MEMBRANE: ENDOCYTOSIS
TRANSPORT INTO THE CELL FROM THE PLASMA MEMBRANE: ENDOCYTOSIS
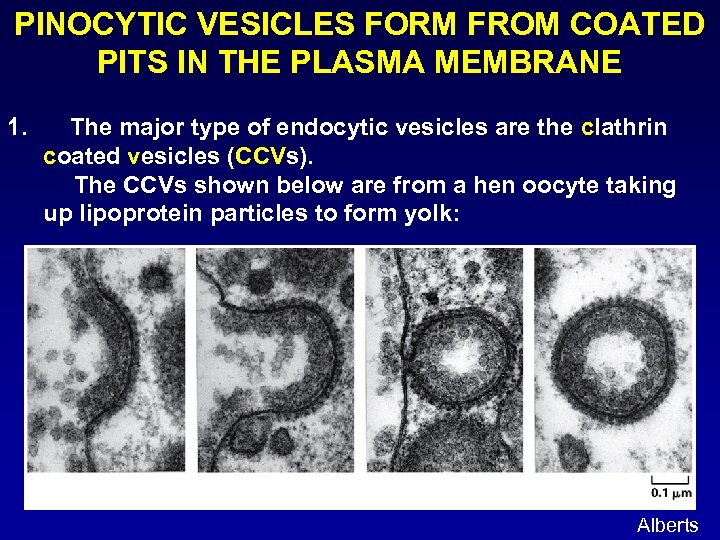 PINOCYTIC VESICLES FORM FROM COATED PITS IN THE PLASMA MEMBRANE 1. The major type of endocytic vesicles are the clathrin coated vesicles (CCVs). The CCVs shown below are from a hen oocyte taking up lipoprotein particles to form yolk: Alberts
PINOCYTIC VESICLES FORM FROM COATED PITS IN THE PLASMA MEMBRANE 1. The major type of endocytic vesicles are the clathrin coated vesicles (CCVs). The CCVs shown below are from a hen oocyte taking up lipoprotein particles to form yolk: Alberts
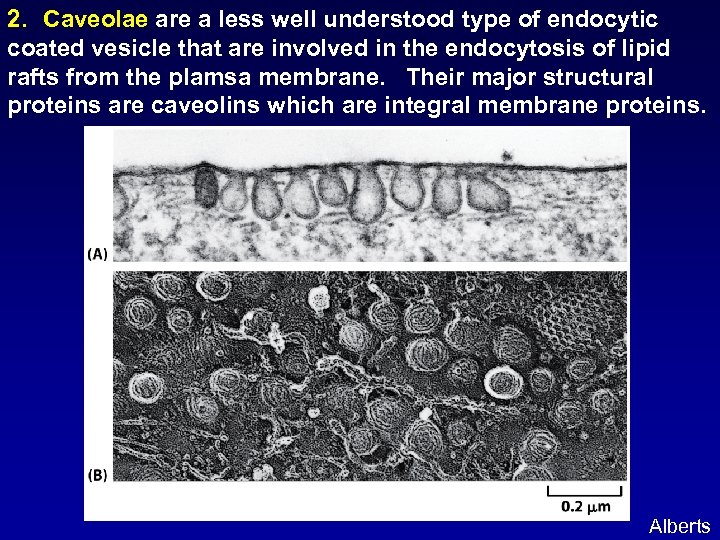 2. Caveolae are a less well understood type of endocytic coated vesicle that are involved in the endocytosis of lipid rafts from the plamsa membrane. Their major structural proteins are caveolins which are integral membrane proteins. Alberts
2. Caveolae are a less well understood type of endocytic coated vesicle that are involved in the endocytosis of lipid rafts from the plamsa membrane. Their major structural proteins are caveolins which are integral membrane proteins. Alberts
 ENDOCYTOSIS IS IMPORTANT FOR CELLS TO: 1. Import selected extracelluar molecules (i. e. receptor-mediated endocytosis). 2. Regulate levels of membrane proteins on the cell surface (i. e. receptor down-regulation). 3. Synaptic vesicle recycling and biogenesis.
ENDOCYTOSIS IS IMPORTANT FOR CELLS TO: 1. Import selected extracelluar molecules (i. e. receptor-mediated endocytosis). 2. Regulate levels of membrane proteins on the cell surface (i. e. receptor down-regulation). 3. Synaptic vesicle recycling and biogenesis.
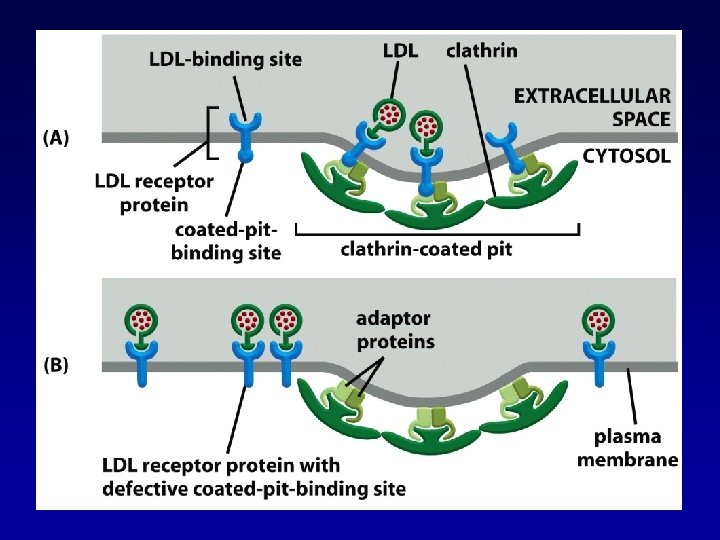
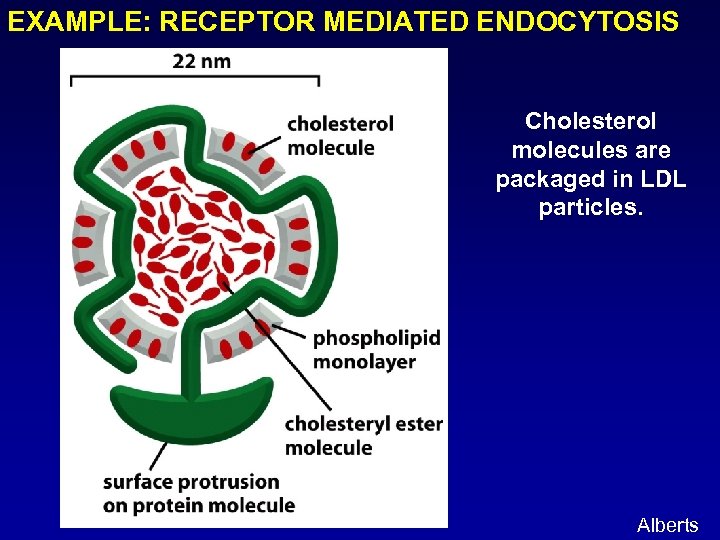 EXAMPLE: RECEPTOR MEDIATED ENDOCYTOSIS Cholesterol molecules are packaged in LDL particles. Alberts
EXAMPLE: RECEPTOR MEDIATED ENDOCYTOSIS Cholesterol molecules are packaged in LDL particles. Alberts
 RECEPTOR-MEDIATED ENDOCYTOSIS: THE MOVIE Animation 13. 3 from Alberts
RECEPTOR-MEDIATED ENDOCYTOSIS: THE MOVIE Animation 13. 3 from Alberts
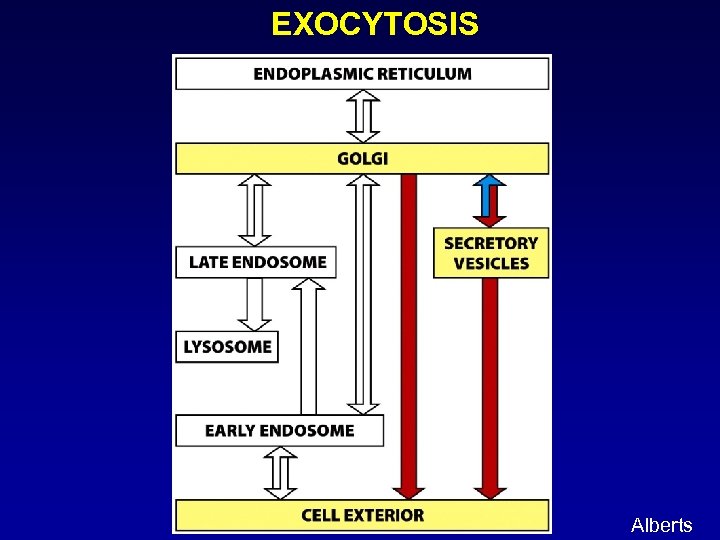 EXOCYTOSIS Alberts
EXOCYTOSIS Alberts
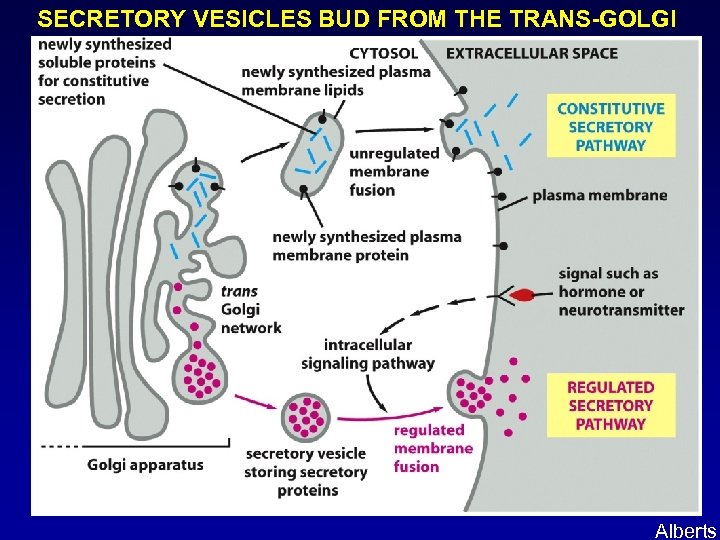 SECRETORY VESICLES BUD FROM THE TRANS-GOLGI Alberts
SECRETORY VESICLES BUD FROM THE TRANS-GOLGI Alberts
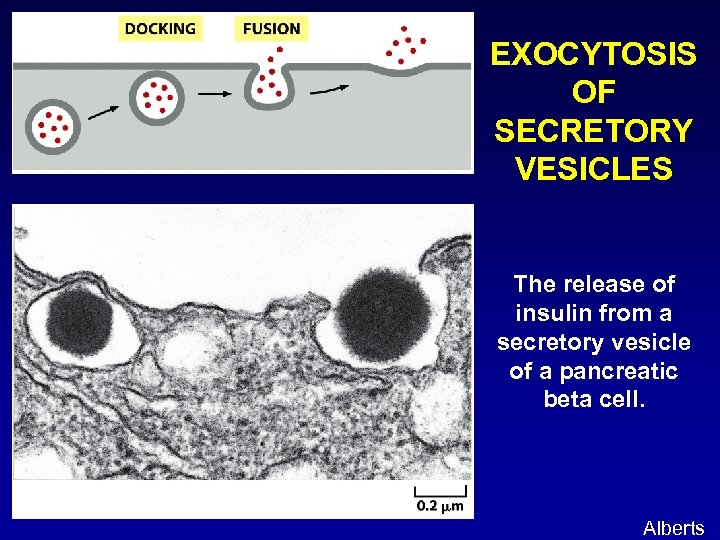 EXOCYTOSIS OF SECRETORY VESICLES The release of insulin from a secretory vesicle of a pancreatic beta cell. Alberts
EXOCYTOSIS OF SECRETORY VESICLES The release of insulin from a secretory vesicle of a pancreatic beta cell. Alberts
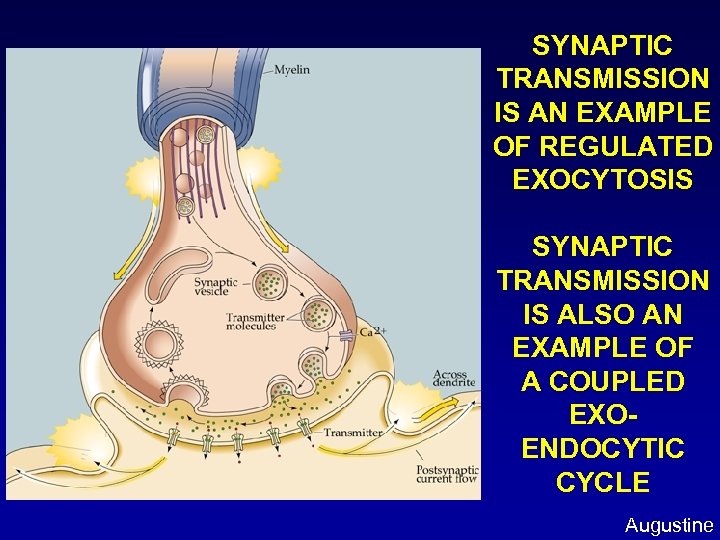 SYNAPTIC TRANSMISSION IS AN EXAMPLE OF REGULATED EXOCYTOSIS SYNAPTIC TRANSMISSION IS ALSO AN EXAMPLE OF A COUPLED EXOENDOCYTIC CYCLE Augustine
SYNAPTIC TRANSMISSION IS AN EXAMPLE OF REGULATED EXOCYTOSIS SYNAPTIC TRANSMISSION IS ALSO AN EXAMPLE OF A COUPLED EXOENDOCYTIC CYCLE Augustine


