be96d121025d50e0e20fd83526a030d4.ppt
- Количество слайдов: 107
 INSTRUMENTAL ANALYSIS CHEM 4811 CHAPTER 1 Dr. au. Gustine ofori a. Gyeman assistant professor of chemistry Department of natural sciences clayton state university
INSTRUMENTAL ANALYSIS CHEM 4811 CHAPTER 1 Dr. au. Gustine ofori a. Gyeman assistant professor of chemistry Department of natural sciences clayton state university
 CHAPTER 1 FUNDAMENTAL CONCEPTS
CHAPTER 1 FUNDAMENTAL CONCEPTS
 WHAT IS ANALYTICAL CHEMISTRY - The qualitative and quantitative characterization of matter - The scope is very wide and it is critical to our understanding of almost all scientific disciplines Characterization - The identification of chemical compounds or elements present in a sample (qualitative) - The determination of the amount of compound or element present in a sample (quantitative)
WHAT IS ANALYTICAL CHEMISTRY - The qualitative and quantitative characterization of matter - The scope is very wide and it is critical to our understanding of almost all scientific disciplines Characterization - The identification of chemical compounds or elements present in a sample (qualitative) - The determination of the amount of compound or element present in a sample (quantitative)
 CHATACTERIZATION Qualitative Analysis - The identification of one or more chemical species present in a sample Quantitative Analysis - The determination of the exact amount of a chemical species present in a sample Chemical Species - Could be an element, ion or compound (organic or inorgnic)
CHATACTERIZATION Qualitative Analysis - The identification of one or more chemical species present in a sample Quantitative Analysis - The determination of the exact amount of a chemical species present in a sample Chemical Species - Could be an element, ion or compound (organic or inorgnic)
 CHATACTERIZATION Bulk Analysis - Characterization of the entire sample Example: determination of the elemental composition of a mixture (alloys) Surface Analysis - Characterization of the surface of a sample Example: finding the thickness of a thin layer on the surface of a solid material - Characterization may also include Structural Analysis and measurement of physical properties of materials
CHATACTERIZATION Bulk Analysis - Characterization of the entire sample Example: determination of the elemental composition of a mixture (alloys) Surface Analysis - Characterization of the surface of a sample Example: finding the thickness of a thin layer on the surface of a solid material - Characterization may also include Structural Analysis and measurement of physical properties of materials
 WET CHEMICAL ANALYSIS Volumetric Analysis - Analysis by volume Gravimetric Analysis - Analysis by mass - Wet analysis is time consuming and demands attention to detail Examples Acid-base titrations, redox titrations, complexometric titrations, precipitation reactions
WET CHEMICAL ANALYSIS Volumetric Analysis - Analysis by volume Gravimetric Analysis - Analysis by mass - Wet analysis is time consuming and demands attention to detail Examples Acid-base titrations, redox titrations, complexometric titrations, precipitation reactions
 WET CHEMICAL ANALYSIS Nondestructive Analysis - Useful when evidence needs to be preserved - Used to analyze samples without destroying them Examples Forensic analysis Paintings
WET CHEMICAL ANALYSIS Nondestructive Analysis - Useful when evidence needs to be preserved - Used to analyze samples without destroying them Examples Forensic analysis Paintings
 INSTRUMENTAL ANALYSIS - Use of automated instruments in place of volumetric methods - Carried out by specially designed instruments which are controlled by computers - Samples are characterized by the interaction of electromagnetic radiation and matter - All the analytical steps (from sample preparation through data processing) are automated
INSTRUMENTAL ANALYSIS - Use of automated instruments in place of volumetric methods - Carried out by specially designed instruments which are controlled by computers - Samples are characterized by the interaction of electromagnetic radiation and matter - All the analytical steps (from sample preparation through data processing) are automated
 INSTRUMENTAL ANALYSIS This course covers - The fundamentals of common analytical instruments - Measurements with these instruments - Interpretation of data obtained from the measurements - Communication of the meaning of the results
INSTRUMENTAL ANALYSIS This course covers - The fundamentals of common analytical instruments - Measurements with these instruments - Interpretation of data obtained from the measurements - Communication of the meaning of the results
 THE ANALYTICAL APPROACH - Problems continuously occur around the world in - Manufacturing industries - The environment - The health sector (medicine) etc. - The analytical chemist is the solution to these problems -The analytical chemist must understand the analytical approach uses, capabilities, and limitations of analytical techniques
THE ANALYTICAL APPROACH - Problems continuously occur around the world in - Manufacturing industries - The environment - The health sector (medicine) etc. - The analytical chemist is the solution to these problems -The analytical chemist must understand the analytical approach uses, capabilities, and limitations of analytical techniques
 THE ANALYTICAL APPROACH Analyte - A substance to be measured in a given sample Matrix - Everything else in the sample Interferences - Other compounds in the sample matrix that interfere with the measurement of the analyte
THE ANALYTICAL APPROACH Analyte - A substance to be measured in a given sample Matrix - Everything else in the sample Interferences - Other compounds in the sample matrix that interfere with the measurement of the analyte
 THE ANALYTICAL APPROACH Homogeneous Sample - Same chemical composition throughout (steel, sugar water, juice with no pulp, alcoholic beverages) Heterogeneous Sample - Composition varies from region to region within the sample (pudding with raisins, granola bars with peanuts) - Differences in composition may be visible or invisible to the human eye (most real samples are invisible) - Variation of composition may be random or segregated
THE ANALYTICAL APPROACH Homogeneous Sample - Same chemical composition throughout (steel, sugar water, juice with no pulp, alcoholic beverages) Heterogeneous Sample - Composition varies from region to region within the sample (pudding with raisins, granola bars with peanuts) - Differences in composition may be visible or invisible to the human eye (most real samples are invisible) - Variation of composition may be random or segregated
 THE ANALYTICAL APPROACH Analyze/Analysis - Applied to the sample under study Determine/Determination - Applied to the measurement of the analyte in the sample Multiple Samples - Identically prepared from another source Replicate Samples - Splits of sample from the same source
THE ANALYTICAL APPROACH Analyze/Analysis - Applied to the sample under study Determine/Determination - Applied to the measurement of the analyte in the sample Multiple Samples - Identically prepared from another source Replicate Samples - Splits of sample from the same source
 THE ANALYTICAL APPROACH General Steps in Chemical Analysis 1. Formulating the question or defining the problem - To be answered through chemical measurements 2. Designing the analytical method (selecting techniques) - Find appropriate analytical procedures 3. Sampling and sample storage - Select representative material to be analyzed 4. Sample preparation - Convert representative material into a suitable form for analysis
THE ANALYTICAL APPROACH General Steps in Chemical Analysis 1. Formulating the question or defining the problem - To be answered through chemical measurements 2. Designing the analytical method (selecting techniques) - Find appropriate analytical procedures 3. Sampling and sample storage - Select representative material to be analyzed 4. Sample preparation - Convert representative material into a suitable form for analysis
 THE ANALYTICAL APPROACH General Steps in Chemical Analysis 5. Analysis (performing the measurement) - Measure the concentration of analyte in several identical portions 6. Assessing the data 7. Method validation 8. Documentation
THE ANALYTICAL APPROACH General Steps in Chemical Analysis 5. Analysis (performing the measurement) - Measure the concentration of analyte in several identical portions 6. Assessing the data 7. Method validation 8. Documentation
 DEFINING THE PROBLEM - Find out the information that needs to be known about a sample (or what procedure is being studied) - How accurate and precise the information must be - Whether qualitative or quantitative analysis or both is required - How much sample is available for study - Whether nondestructive analysis must be employed
DEFINING THE PROBLEM - Find out the information that needs to be known about a sample (or what procedure is being studied) - How accurate and precise the information must be - Whether qualitative or quantitative analysis or both is required - How much sample is available for study - Whether nondestructive analysis must be employed
 DEFINING THE PROBLEM - Bulk analysis or analysis of certain parts is required - Sample is organic or inorganic - Sample a pure substance or a mixture - Homogeneous or heterogeneous sample - Chemical information or elemental information needed
DEFINING THE PROBLEM - Bulk analysis or analysis of certain parts is required - Sample is organic or inorganic - Sample a pure substance or a mixture - Homogeneous or heterogeneous sample - Chemical information or elemental information needed
 DEFINING THE PROBLEM Qualitative Analysis - Provides information about what is present in the sample - If quantitative analysis is required, qualitative analysis is usually done first - Capabilities and limitations of analysis must be well understood
DEFINING THE PROBLEM Qualitative Analysis - Provides information about what is present in the sample - If quantitative analysis is required, qualitative analysis is usually done first - Capabilities and limitations of analysis must be well understood
 DEFINING THE PROBLEM Qualitative Analysis Qualitative Elemental Analysis - Used to identify elements present in a material - Can provide empirical formula of organic compounds (X-Ray Fluorescence, AAS) Qualitative Molecular Analysis - Used to identify molecules present in a material - Can be used to obtain molecular formula - Can be used to distinguish between isomers (NMR, IR, MS)
DEFINING THE PROBLEM Qualitative Analysis Qualitative Elemental Analysis - Used to identify elements present in a material - Can provide empirical formula of organic compounds (X-Ray Fluorescence, AAS) Qualitative Molecular Analysis - Used to identify molecules present in a material - Can be used to obtain molecular formula - Can be used to distinguish between isomers (NMR, IR, MS)
 DEFINING THE PROBLEM Qualitative Analysis Empirical Formula - The simplest whole number ratios of atoms of each element present in a molecule Molecular Formula - Contains the total number of atoms of each element in a single molecule of the compound Isomers - Different structures with the same molecular formula (n-butane and iso-butane)
DEFINING THE PROBLEM Qualitative Analysis Empirical Formula - The simplest whole number ratios of atoms of each element present in a molecule Molecular Formula - Contains the total number of atoms of each element in a single molecule of the compound Isomers - Different structures with the same molecular formula (n-butane and iso-butane)
 DEFINING THE PROBLEM Qualitative Analysis Enantiomers - Nonsuperimposable mirror-image isomers - Said to be chiral - Have the same IR, NMR, and MS - Mostly same physical properties (boiling-point, melting point, refractive index) - Chiral Chromatography can be used to distinguish between such optically active compounds (erythrose, glyceraldehyde)
DEFINING THE PROBLEM Qualitative Analysis Enantiomers - Nonsuperimposable mirror-image isomers - Said to be chiral - Have the same IR, NMR, and MS - Mostly same physical properties (boiling-point, melting point, refractive index) - Chiral Chromatography can be used to distinguish between such optically active compounds (erythrose, glyceraldehyde)
 DEFINING THE PROBLEM Qualitative Analysis Mixtures of Organic Compounds - Mixtures are usually separated before the individual components are identified - Separation techniques include GC LC HPLC CE
DEFINING THE PROBLEM Qualitative Analysis Mixtures of Organic Compounds - Mixtures are usually separated before the individual components are identified - Separation techniques include GC LC HPLC CE
 DEFINING THE PROBLEM Quantitative Analysis - The determination of the amount of analyte in a given sample - Often expressed in terms of concentrations Concentration - The quantity of analyte in a given volume or mass of sample Molarity = moles/liters, ppm = µg/g sample ppb = ng/g sample, ppt = pg/g sample Percent by mass [%(m/m)], Percent by volume [%(v/v)]
DEFINING THE PROBLEM Quantitative Analysis - The determination of the amount of analyte in a given sample - Often expressed in terms of concentrations Concentration - The quantity of analyte in a given volume or mass of sample Molarity = moles/liters, ppm = µg/g sample ppb = ng/g sample, ppt = pg/g sample Percent by mass [%(m/m)], Percent by volume [%(v/v)]
 DEFINING THE PROBLEM Quantitative Analysis - Early methods include volumetric, gravimetric, and combustion analysis - Automated and extremely sensitive methods are being used today (GC, IR, HPLC, CE, XRD) - Require micron amounts and a few minutes Hyphenated techniques are used for qualitative and quantitative measurements of the components mixtures (GC-MS, LC-MS)
DEFINING THE PROBLEM Quantitative Analysis - Early methods include volumetric, gravimetric, and combustion analysis - Automated and extremely sensitive methods are being used today (GC, IR, HPLC, CE, XRD) - Require micron amounts and a few minutes Hyphenated techniques are used for qualitative and quantitative measurements of the components mixtures (GC-MS, LC-MS)
 DESIGNING THE ANALYTICAL METHOD - Analytical procedure is designed after the problem has been defined Analyst must consider - Accuracy and precision - Amount of sample to be used - Cost analysis - Turnaround time (time between receipt of sample and delivery of results)
DESIGNING THE ANALYTICAL METHOD - Analytical procedure is designed after the problem has been defined Analyst must consider - Accuracy and precision - Amount of sample to be used - Cost analysis - Turnaround time (time between receipt of sample and delivery of results)
 DESIGNING THE ANALYTICAL METHOD Green chemistry processes preferred for modern analytical procedures - The goal is to minimize waste and pollution - Use of less toxic or biodegradable solvents - Use of chemicals that can be recycled - Standard methods are available in literature (reproducible with known accuracy and precision)
DESIGNING THE ANALYTICAL METHOD Green chemistry processes preferred for modern analytical procedures - The goal is to minimize waste and pollution - Use of less toxic or biodegradable solvents - Use of chemicals that can be recycled - Standard methods are available in literature (reproducible with known accuracy and precision)
 DESIGNING THE ANALYTICAL METHOD - Do not waste time developing a method that already exists - Method of choice must be reliable and robust - Interferences must be evaluated Interference - Element or compound that respond directly to measurement to give false analyte signal - Signal may be enhanced or suppressed
DESIGNING THE ANALYTICAL METHOD - Do not waste time developing a method that already exists - Method of choice must be reliable and robust - Interferences must be evaluated Interference - Element or compound that respond directly to measurement to give false analyte signal - Signal may be enhanced or suppressed
 DESIGNING THE ANALYTICAL METHOD Fundamental Features of Method - A blank must be analyzed - The blank is usually the pure solvent used for sample preparation - Used to identify and correct for interferences in the analysis - Analyst uses blank to set baseline Reagent blank: contains all the reagents used to prepare the sample Matrix blank: similar in chemical composition to the sample but without the analyte
DESIGNING THE ANALYTICAL METHOD Fundamental Features of Method - A blank must be analyzed - The blank is usually the pure solvent used for sample preparation - Used to identify and correct for interferences in the analysis - Analyst uses blank to set baseline Reagent blank: contains all the reagents used to prepare the sample Matrix blank: similar in chemical composition to the sample but without the analyte
 DESIGNING THE ANALYTICAL METHOD Fundamental Features of Method - Methods require calibration standards (except coulometry) - Used to establish relationship between analytical signal being measured and the concentration of analyte - This relationship (known as the calibration curve) is used to determine the concentration of unknown analyte in samples
DESIGNING THE ANALYTICAL METHOD Fundamental Features of Method - Methods require calibration standards (except coulometry) - Used to establish relationship between analytical signal being measured and the concentration of analyte - This relationship (known as the calibration curve) is used to determine the concentration of unknown analyte in samples
 DESIGNING THE ANALYTICAL METHOD Fundamental Features of Method - Reference (check) standards are required - Standards of known composition with known concentration of analyte - Run as a sample to confirm that the calibration is correct - Used to access the precision and accuracy of the analysis Government and private sources of reference standards are available (National Institute of Standards and Technology, NIST)
DESIGNING THE ANALYTICAL METHOD Fundamental Features of Method - Reference (check) standards are required - Standards of known composition with known concentration of analyte - Run as a sample to confirm that the calibration is correct - Used to access the precision and accuracy of the analysis Government and private sources of reference standards are available (National Institute of Standards and Technology, NIST)
 SAMPLING - The most important step is the collection of the sample of the material to be analyzed - Sample should be representative of the material - Sample should be properly taken to provide reliable characterization of the material - Sufficient amount must be taken for all analysis Representative Sample - Reflects the true value and distribution of analyte in the original material
SAMPLING - The most important step is the collection of the sample of the material to be analyzed - Sample should be representative of the material - Sample should be properly taken to provide reliable characterization of the material - Sufficient amount must be taken for all analysis Representative Sample - Reflects the true value and distribution of analyte in the original material
 SAMPLING Steps in Sampling Process - Gross representative sample is collected from the lot - Portions of gross sample is taken from various parts of material Sampling methods include - Long pile and alternate shovel (used for very large lots) - Cone and quarter Aliquot - Quantitative amount of a test portion of sample solution
SAMPLING Steps in Sampling Process - Gross representative sample is collected from the lot - Portions of gross sample is taken from various parts of material Sampling methods include - Long pile and alternate shovel (used for very large lots) - Cone and quarter Aliquot - Quantitative amount of a test portion of sample solution
 SAMPLING - Care must be taken since collection tools and storage containers can contaminate samples - Make room for multiple test portions of sample for replicate analysis or analysis by more than one technique Samples may undergo - grinding - chopping - milling - cutting
SAMPLING - Care must be taken since collection tools and storage containers can contaminate samples - Make room for multiple test portions of sample for replicate analysis or analysis by more than one technique Samples may undergo - grinding - chopping - milling - cutting
 SAMPLING Gas Samples - Generally considered homogeneous - Samples are stirred before portions are taken for analysis - Gas samples may be filtered if solid materials are present Grab samples - Samples taken at a single point in time Composite Samples - Samples taken over a period of time or from different locations
SAMPLING Gas Samples - Generally considered homogeneous - Samples are stirred before portions are taken for analysis - Gas samples may be filtered if solid materials are present Grab samples - Samples taken at a single point in time Composite Samples - Samples taken over a period of time or from different locations
 SAMPLING Gas Samples Scrubbing - Trapping an analyte out of the gas phase Examples - Passing air through activated charcoal to adsorb organic vapors - Bubbling gas samples through a solution to absorb the analyte Samples may be taken with - Gas-tight syringes - Ballons (volatile organic compounds may contaminate samples) - Plastic bags (volatile organic compounds may contaminate samples) - Glass containers (may adsorb gas components)
SAMPLING Gas Samples Scrubbing - Trapping an analyte out of the gas phase Examples - Passing air through activated charcoal to adsorb organic vapors - Bubbling gas samples through a solution to absorb the analyte Samples may be taken with - Gas-tight syringes - Ballons (volatile organic compounds may contaminate samples) - Plastic bags (volatile organic compounds may contaminate samples) - Glass containers (may adsorb gas components)
 SAMPLING Liquid Samples - May be collected as grab samples or composite samples - Adequate stirring is necessary to obtain representative sample - Stirring may not be desired under certain conditions (analysis of oily layer on water) - Undesired solid materials are removed by filtration or centrifugation - Layers of immiscible liquids may be separated with the separatory funnel
SAMPLING Liquid Samples - May be collected as grab samples or composite samples - Adequate stirring is necessary to obtain representative sample - Stirring may not be desired under certain conditions (analysis of oily layer on water) - Undesired solid materials are removed by filtration or centrifugation - Layers of immiscible liquids may be separated with the separatory funnel
 SAMPLING Solid Samples - The most difficult to sample since least homogeneous compared to gases and liquids - Large amounts are difficult to stir - Must undergo size reduction (milling, drilling, crushing, etc. ) to homogenize sample - Adsorbed water is often removed by oven drying
SAMPLING Solid Samples - The most difficult to sample since least homogeneous compared to gases and liquids - Large amounts are difficult to stir - Must undergo size reduction (milling, drilling, crushing, etc. ) to homogenize sample - Adsorbed water is often removed by oven drying
 SAMPLING Sample Storage - Samples are stored if cannot be analyzed immediately - Sample composition can be changed by interaction with container material, light, or air - Appropriate storage container and conditions must be chosen - Organic components must not be stored in plastic containers due to leaching - Glass containers may adsorb or release trace levels of ionic species
SAMPLING Sample Storage - Samples are stored if cannot be analyzed immediately - Sample composition can be changed by interaction with container material, light, or air - Appropriate storage container and conditions must be chosen - Organic components must not be stored in plastic containers due to leaching - Glass containers may adsorb or release trace levels of ionic species
 SAMPLING Sample Storage - Appropriate cleaning of containers is necessary - Containers for organic samples are washed in solvent - Containers for metal samples are soaked in acid and deionized water - Containers must be first filled with inert gas to displace air - Biological samples are usually kept in freezers - Samples that interact with light are stored in the dark
SAMPLING Sample Storage - Appropriate cleaning of containers is necessary - Containers for organic samples are washed in solvent - Containers for metal samples are soaked in acid and deionized water - Containers must be first filled with inert gas to displace air - Biological samples are usually kept in freezers - Samples that interact with light are stored in the dark
 SAMPLING Sample Storage - Some samples require p. H adjustment - Some samples require addition of preservatives (EDTA added to blood samples) - Appropriate labeling is necessary - Computer based Laboratory Information Management Systems (LIMS) are used to label and track samples
SAMPLING Sample Storage - Some samples require p. H adjustment - Some samples require addition of preservatives (EDTA added to blood samples) - Appropriate labeling is necessary - Computer based Laboratory Information Management Systems (LIMS) are used to label and track samples
 SAMPLE PREPARATION - Make samples in the physical form required by the instrument - Make concentrations in the range required by the instrument - Free analytes from interfering substances - Solvent is usually water or organic
SAMPLE PREPARATION - Make samples in the physical form required by the instrument - Make concentrations in the range required by the instrument - Free analytes from interfering substances - Solvent is usually water or organic
 SAMPLE PREPARATION Type of sample preparation depends on - nature of sample - technique chosen - analyte to be measured - the problem to be solved Samples may be - dissolved in water (or other solvents) - pressed into pellets - cast into thin films - etc.
SAMPLE PREPARATION Type of sample preparation depends on - nature of sample - technique chosen - analyte to be measured - the problem to be solved Samples may be - dissolved in water (or other solvents) - pressed into pellets - cast into thin films - etc.
 SAMPLE PREPARATION METHODS - Specific methods are discussed in later chapters Acid Dissolution and Digestion - Used for dissolving metals, alloys, ores, glass, ceramics - Used for dissolving trace elements in organic materials (food, plastics) - Concentrated acid is added to sample and then heated - Choice of acid depends on sample to be dissolved analyte Acids commonly used: HCl, HNO 3, H 2 SO 4 HF and HCl. O 4 require special care and supervision
SAMPLE PREPARATION METHODS - Specific methods are discussed in later chapters Acid Dissolution and Digestion - Used for dissolving metals, alloys, ores, glass, ceramics - Used for dissolving trace elements in organic materials (food, plastics) - Concentrated acid is added to sample and then heated - Choice of acid depends on sample to be dissolved analyte Acids commonly used: HCl, HNO 3, H 2 SO 4 HF and HCl. O 4 require special care and supervision
 SAMPLE PREPARATION METHODS Fusion (Molten Salt Fusion) - Heating a finely powdered solid sample with a finely powdered salt at high temperatures until mixture melts - Useful for the determination of silica-containing minerals, glass, ceramics, bones, carbides Salts (Fluxes) Usually Used Sodium carbonate, sodium tetraborate (borax), sodium peroxide, lithium metaborate
SAMPLE PREPARATION METHODS Fusion (Molten Salt Fusion) - Heating a finely powdered solid sample with a finely powdered salt at high temperatures until mixture melts - Useful for the determination of silica-containing minerals, glass, ceramics, bones, carbides Salts (Fluxes) Usually Used Sodium carbonate, sodium tetraborate (borax), sodium peroxide, lithium metaborate
 SAMPLE PREPARATION METHODS Dry Ashing and Combustion - Burning an organic material in air or oxygen - Organic components form CO 2 and H 2 O vapor leaving inorganic components behind as solid oxides - Cannot be used for the determination of mercury, arsenic, and cadmium
SAMPLE PREPARATION METHODS Dry Ashing and Combustion - Burning an organic material in air or oxygen - Organic components form CO 2 and H 2 O vapor leaving inorganic components behind as solid oxides - Cannot be used for the determination of mercury, arsenic, and cadmium
 SAMPLE PREPARATION METHODS Extraction - Used for determining organic analytes - Makes use of solvents - Solvents are chosen based on polarity of analyte (like dissolves like) Common Solvents Hexane, xylene, methylene chloride
SAMPLE PREPARATION METHODS Extraction - Used for determining organic analytes - Makes use of solvents - Solvents are chosen based on polarity of analyte (like dissolves like) Common Solvents Hexane, xylene, methylene chloride
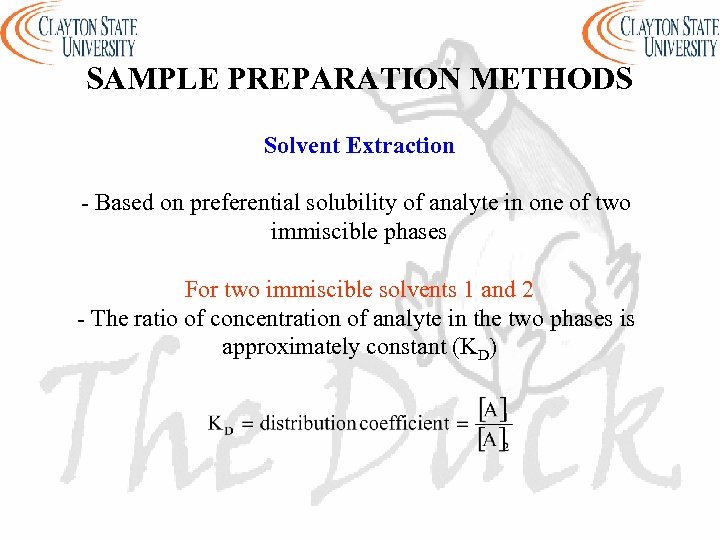 SAMPLE PREPARATION METHODS Solvent Extraction - Based on preferential solubility of analyte in one of two immiscible phases For two immiscible solvents 1 and 2 - The ratio of concentration of analyte in the two phases is approximately constant (KD)
SAMPLE PREPARATION METHODS Solvent Extraction - Based on preferential solubility of analyte in one of two immiscible phases For two immiscible solvents 1 and 2 - The ratio of concentration of analyte in the two phases is approximately constant (KD)
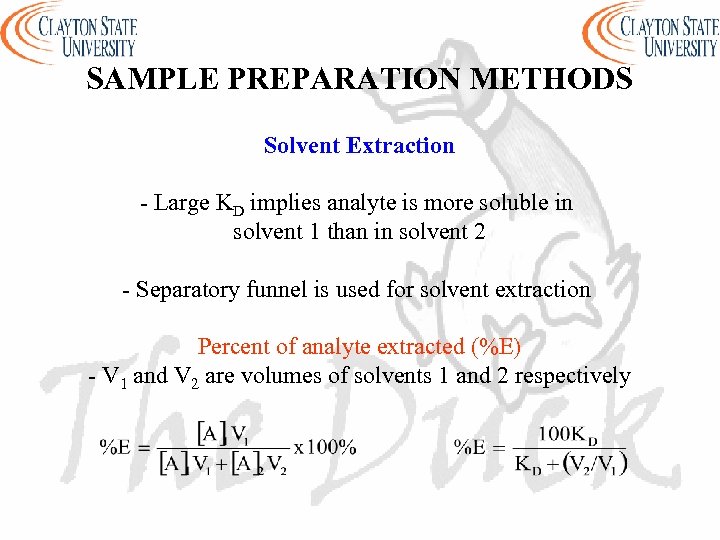 SAMPLE PREPARATION METHODS Solvent Extraction - Large KD implies analyte is more soluble in solvent 1 than in solvent 2 - Separatory funnel is used for solvent extraction Percent of analyte extracted (%E) - V 1 and V 2 are volumes of solvents 1 and 2 respectively
SAMPLE PREPARATION METHODS Solvent Extraction - Large KD implies analyte is more soluble in solvent 1 than in solvent 2 - Separatory funnel is used for solvent extraction Percent of analyte extracted (%E) - V 1 and V 2 are volumes of solvents 1 and 2 respectively
 SAMPLE PREPARATION METHODS Solvent Extraction - Multiple small extractions are more efficient than one large extraction - Extraction instruments are also available Examples Extraction of - pesticides, PCBs, petroluem hydrocarbons from water - fat from milk
SAMPLE PREPARATION METHODS Solvent Extraction - Multiple small extractions are more efficient than one large extraction - Extraction instruments are also available Examples Extraction of - pesticides, PCBs, petroluem hydrocarbons from water - fat from milk
 SAMPLE PREPARATION METHODS Other Extraction Approaches Microwave Assisted Extraction - Heating with microwave energy during extraction Supercritical Fluid Extraction (SFE) - Use of supercritical CO 2 to dissolve organic compounds - Low cost, less toxic, ease of disposal Solid Phase Extraction (SPE) Solid Phase Microextraction (SPME) - The sample is a solid organic material - Extracted by passing sample through a bed of sorbent (extractant)
SAMPLE PREPARATION METHODS Other Extraction Approaches Microwave Assisted Extraction - Heating with microwave energy during extraction Supercritical Fluid Extraction (SFE) - Use of supercritical CO 2 to dissolve organic compounds - Low cost, less toxic, ease of disposal Solid Phase Extraction (SPE) Solid Phase Microextraction (SPME) - The sample is a solid organic material - Extracted by passing sample through a bed of sorbent (extractant)
 STATISTICS - Statistics are needed in designing the correct experiment Analyst must - select the required size of sample - select the number of samples - select the number of replicates - obtain the required accuracy and precision Analyst must also express uncertainty in measured values to - understand any associated limitations - know significant figures
STATISTICS - Statistics are needed in designing the correct experiment Analyst must - select the required size of sample - select the number of samples - select the number of replicates - obtain the required accuracy and precision Analyst must also express uncertainty in measured values to - understand any associated limitations - know significant figures
 STATISTICS Rules For Reporting Results Significant Figures = digits known with certainty + first uncertain digit - The last sig. fig. reflects the precision of the measurement - Report all sig. figs such that only the last figure is uncertain - Round off appropriately (round down, round up, round even)
STATISTICS Rules For Reporting Results Significant Figures = digits known with certainty + first uncertain digit - The last sig. fig. reflects the precision of the measurement - Report all sig. figs such that only the last figure is uncertain - Round off appropriately (round down, round up, round even)
 STATISTICS Rules For Reporting Results - Report least sig. figs for multiplication and division of measurements (greatest number of absolute uncertainty) - Report least decimal places for addition and subtraction of measurements (greatest number of absolute uncertainty) - The characteristic of logarithm has no uncertainty - Does not affect the number of sig. figs. - Discrete objects have no uncertainty - Considered to have infinite number of sig. figs.
STATISTICS Rules For Reporting Results - Report least sig. figs for multiplication and division of measurements (greatest number of absolute uncertainty) - Report least decimal places for addition and subtraction of measurements (greatest number of absolute uncertainty) - The characteristic of logarithm has no uncertainty - Does not affect the number of sig. figs. - Discrete objects have no uncertainty - Considered to have infinite number of sig. figs.
 ACCURACY AND PRECISION - Accuracy is how close a measurement is to the true (accepted) value - True value is evaluated by analyzing known standard samples - Precision is how close replicate measurements on the sample are to each other - Precision is required for accuracy but does not guarantee accuracy - Results should be accurate and precise (reproducible, reliable, truly representative of sample)
ACCURACY AND PRECISION - Accuracy is how close a measurement is to the true (accepted) value - True value is evaluated by analyzing known standard samples - Precision is how close replicate measurements on the sample are to each other - Precision is required for accuracy but does not guarantee accuracy - Results should be accurate and precise (reproducible, reliable, truly representative of sample)
 ERRORS - Two principal types of errors - Determinate (systematic) and indeterminate (random) Determinate (Systematic) Errors - Caused by faults in procedure or instrument - Fault can be found out and corrected - Results in good precision but poor accuracy May be - constant (incorrect calibration of p. H meter or mass balance) - variable (change in volume due to temperature changes) - additive or multiplicative
ERRORS - Two principal types of errors - Determinate (systematic) and indeterminate (random) Determinate (Systematic) Errors - Caused by faults in procedure or instrument - Fault can be found out and corrected - Results in good precision but poor accuracy May be - constant (incorrect calibration of p. H meter or mass balance) - variable (change in volume due to temperature changes) - additive or multiplicative
 ERRORS - Two principal types of errors - Determinate (systematic) and indeterminate (random) Examples of Determinate (Systematic) Errors - Uncalibrated or improperly calibrated mass balances - Improperly calibrated volumetric flasks and pipettes - Analyst error (misreading or inexperience) - Incorrect technique - Malfunctioning instrument (voltage fluctuations, alignment, etc) - Contaminated or impure or decomposed reagents - Interferences
ERRORS - Two principal types of errors - Determinate (systematic) and indeterminate (random) Examples of Determinate (Systematic) Errors - Uncalibrated or improperly calibrated mass balances - Improperly calibrated volumetric flasks and pipettes - Analyst error (misreading or inexperience) - Incorrect technique - Malfunctioning instrument (voltage fluctuations, alignment, etc) - Contaminated or impure or decomposed reagents - Interferences
 ERRORS - Two principal types of errors - Determinate (systematic) and indeterminate (random) To Identify Determinate (Systematic) Errors - Use of standard methods with known accuracy and precision to analyze samples - Run several analysis of a reference analyte whose concentration is known and accepted - Run Standard Operating Procedures (SOPs)
ERRORS - Two principal types of errors - Determinate (systematic) and indeterminate (random) To Identify Determinate (Systematic) Errors - Use of standard methods with known accuracy and precision to analyze samples - Run several analysis of a reference analyte whose concentration is known and accepted - Run Standard Operating Procedures (SOPs)
 ERRORS - Two principal types of errors - Determinate (systematic) and indeterminate (random) Indeterminate (Random) Errors - Sources cannot be identified, avoided, or corrected - Not constant (biased) Examples - Limitations of reading mass balances - Electrical noise in instruments
ERRORS - Two principal types of errors - Determinate (systematic) and indeterminate (random) Indeterminate (Random) Errors - Sources cannot be identified, avoided, or corrected - Not constant (biased) Examples - Limitations of reading mass balances - Electrical noise in instruments
 ERRORS - Random errors are always associated with measurements - No conclusion can be drawn with complete certainty - Scientists use statistics to accept conclusions that have high probability of being correct and to reject conclusions that have low probability of being correct - Random errors follow random distribution and analyzed using laws of probability - Statistics deals with only random errors - Systematic errors should be detected and eliminated
ERRORS - Random errors are always associated with measurements - No conclusion can be drawn with complete certainty - Scientists use statistics to accept conclusions that have high probability of being correct and to reject conclusions that have low probability of being correct - Random errors follow random distribution and analyzed using laws of probability - Statistics deals with only random errors - Systematic errors should be detected and eliminated
 THE GAUSSIAN DISTRIBUTION - Symmetric bell-shaped curve representing the distribution of experimenal data - Results from a number of analysis from a single sample follows the bell-shaped curve - Characterized by mean and standard deviation
THE GAUSSIAN DISTRIBUTION - Symmetric bell-shaped curve representing the distribution of experimenal data - Results from a number of analysis from a single sample follows the bell-shaped curve - Characterized by mean and standard deviation
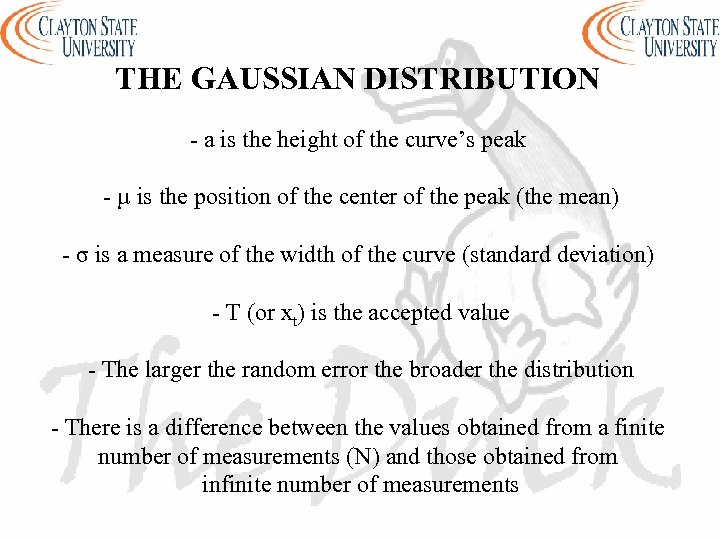 THE GAUSSIAN DISTRIBUTION - a is the height of the curve’s peak - µ is the position of the center of the peak (the mean) - σ is a measure of the width of the curve (standard deviation) - T (or xt) is the accepted value - The larger the random error the broader the distribution - There is a difference between the values obtained from a finite number of measurements (N) and those obtained from infinite number of measurements
THE GAUSSIAN DISTRIBUTION - a is the height of the curve’s peak - µ is the position of the center of the peak (the mean) - σ is a measure of the width of the curve (standard deviation) - T (or xt) is the accepted value - The larger the random error the broader the distribution - There is a difference between the values obtained from a finite number of measurements (N) and those obtained from infinite number of measurements
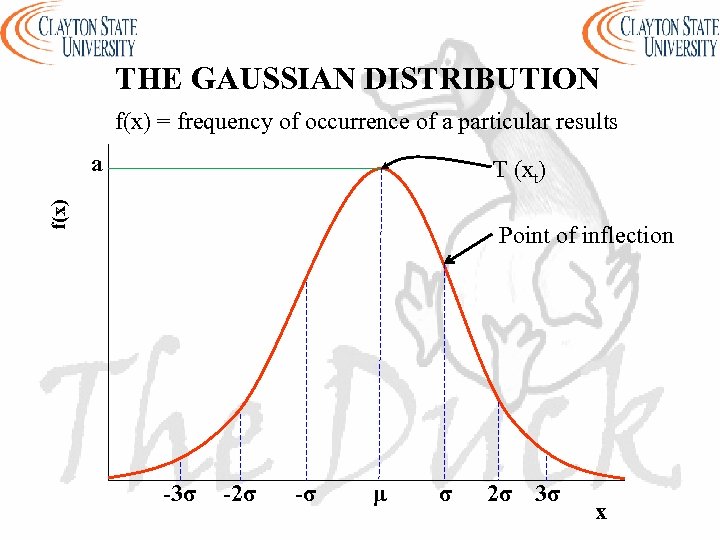 THE GAUSSIAN DISTRIBUTION f(x) = frequency of occurrence of a particular results a f(x) T (xt) Point of inflection -3σ -2σ -σ μ σ 2σ 3σ x
THE GAUSSIAN DISTRIBUTION f(x) = frequency of occurrence of a particular results a f(x) T (xt) Point of inflection -3σ -2σ -σ μ σ 2σ 3σ x
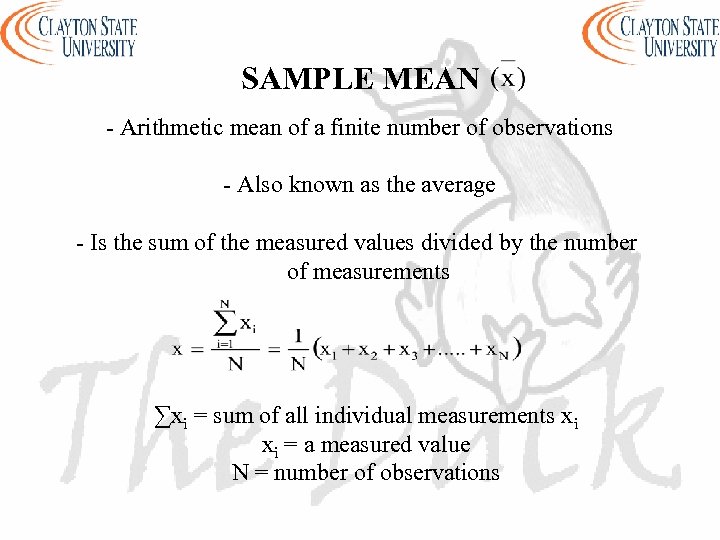 SAMPLE MEAN - Arithmetic mean of a finite number of observations - Also known as the average - Is the sum of the measured values divided by the number of measurements ∑xi = sum of all individual measurements xi xi = a measured value N = number of observations
SAMPLE MEAN - Arithmetic mean of a finite number of observations - Also known as the average - Is the sum of the measured values divided by the number of measurements ∑xi = sum of all individual measurements xi xi = a measured value N = number of observations
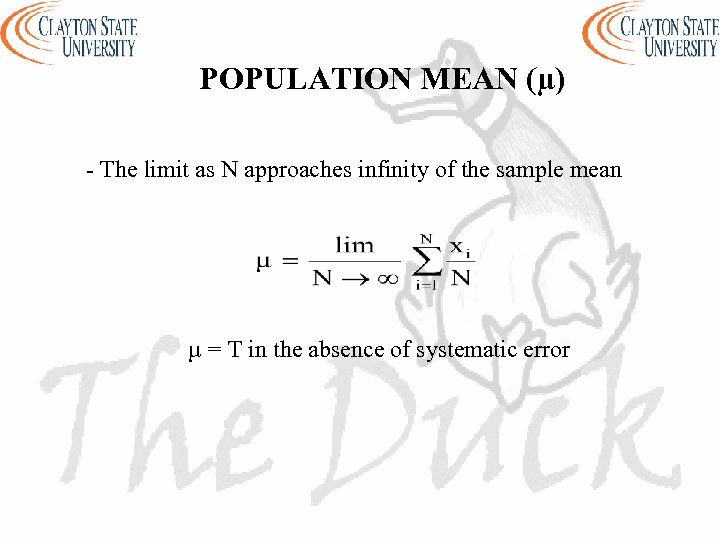 POPULATION MEAN (µ) - The limit as N approaches infinity of the sample mean µ = T in the absence of systematic error
POPULATION MEAN (µ) - The limit as N approaches infinity of the sample mean µ = T in the absence of systematic error
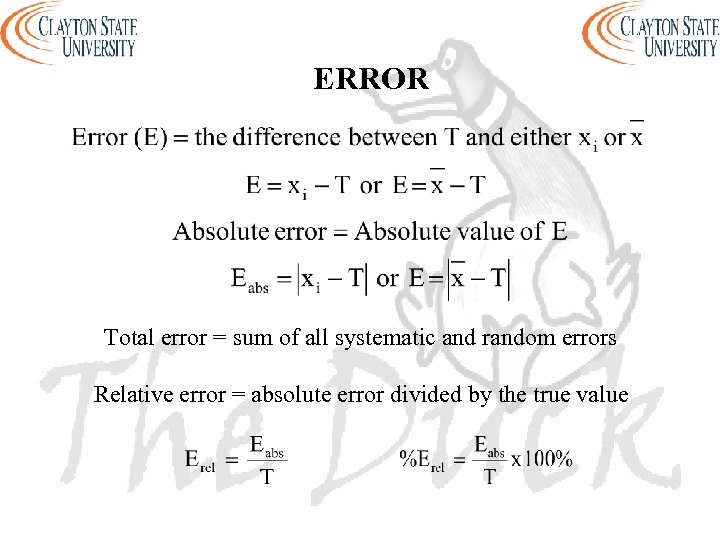 ERROR Total error = sum of all systematic and random errors Relative error = absolute error divided by the true value
ERROR Total error = sum of all systematic and random errors Relative error = absolute error divided by the true value
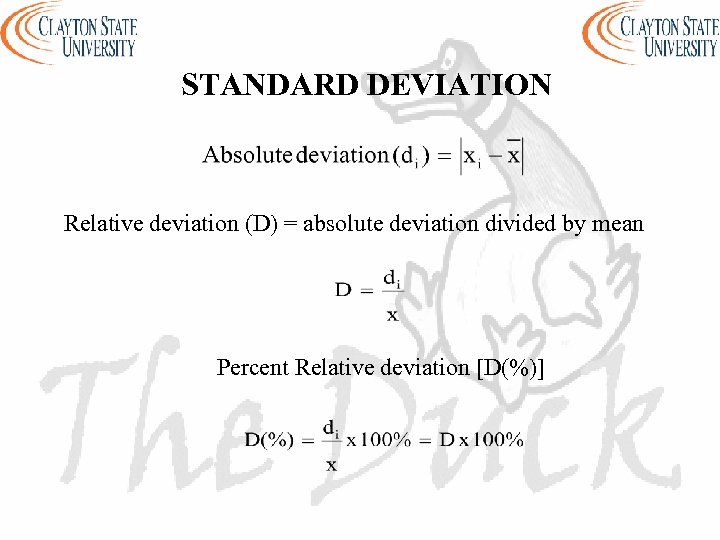 STANDARD DEVIATION Relative deviation (D) = absolute deviation divided by mean Percent Relative deviation [D(%)]
STANDARD DEVIATION Relative deviation (D) = absolute deviation divided by mean Percent Relative deviation [D(%)]
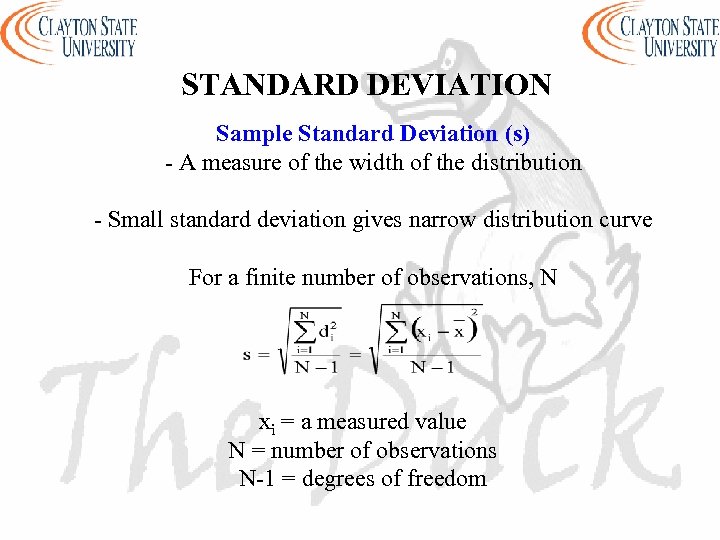 STANDARD DEVIATION Sample Standard Deviation (s) - A measure of the width of the distribution - Small standard deviation gives narrow distribution curve For a finite number of observations, N xi = a measured value N = number of observations N-1 = degrees of freedom
STANDARD DEVIATION Sample Standard Deviation (s) - A measure of the width of the distribution - Small standard deviation gives narrow distribution curve For a finite number of observations, N xi = a measured value N = number of observations N-1 = degrees of freedom
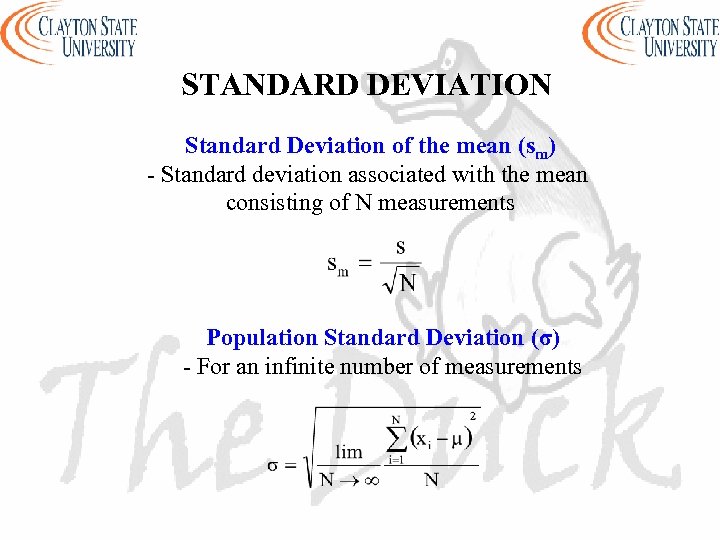 STANDARD DEVIATION Standard Deviation of the mean (sm) - Standard deviation associated with the mean consisting of N measurements Population Standard Deviation (σ) - For an infinite number of measurements
STANDARD DEVIATION Standard Deviation of the mean (sm) - Standard deviation associated with the mean consisting of N measurements Population Standard Deviation (σ) - For an infinite number of measurements
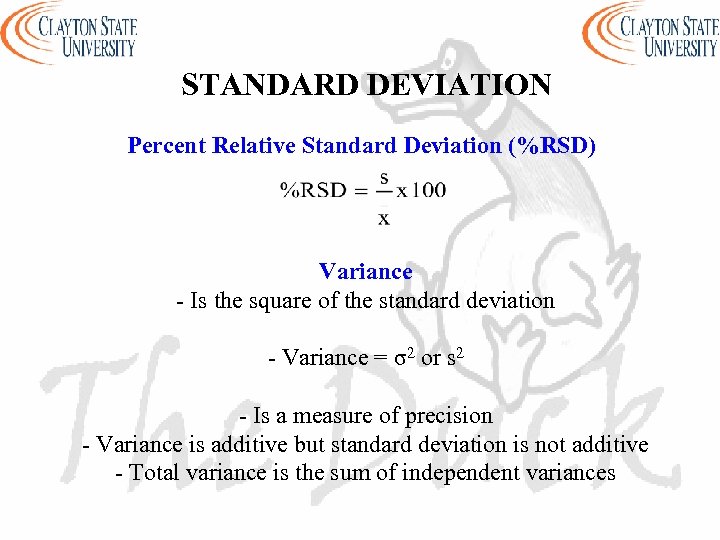 STANDARD DEVIATION Percent Relative Standard Deviation (%RSD) Variance - Is the square of the standard deviation - Variance = σ2 or s 2 - Is a measure of precision - Variance is additive but standard deviation is not additive - Total variance is the sum of independent variances
STANDARD DEVIATION Percent Relative Standard Deviation (%RSD) Variance - Is the square of the standard deviation - Variance = σ2 or s 2 - Is a measure of precision - Variance is additive but standard deviation is not additive - Total variance is the sum of independent variances
 QUANTIFYING RANDOM ERROR Median - The middle number in a series of measurements arranged in increasing order - The average of the two middle numbers if the number of measurements is even Mode - The value that occurs the most frequently Range - The difference between the highest and the lowest values
QUANTIFYING RANDOM ERROR Median - The middle number in a series of measurements arranged in increasing order - The average of the two middle numbers if the number of measurements is even Mode - The value that occurs the most frequently Range - The difference between the highest and the lowest values
 QUANTIFYING RANDOM ERROR - The Gaussian distribution and statistics are used to determine how close the average value of measurements is to the true value - The Gaussian distribution assumes infinite number of measurements for N > 20 - The standard deviation coincides with the point of inflection of the curve (2 inflection points since curve is symmetrical)
QUANTIFYING RANDOM ERROR - The Gaussian distribution and statistics are used to determine how close the average value of measurements is to the true value - The Gaussian distribution assumes infinite number of measurements for N > 20 - The standard deviation coincides with the point of inflection of the curve (2 inflection points since curve is symmetrical)
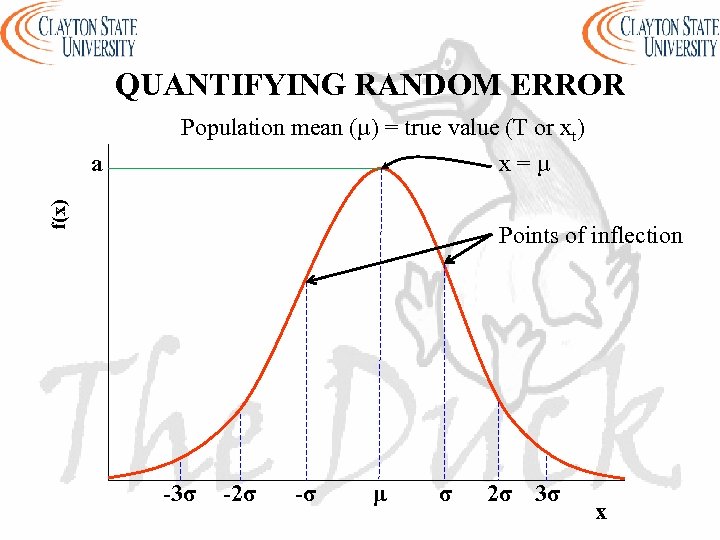 QUANTIFYING RANDOM ERROR Population mean (µ) = true value (T or xt) x=µ f(x) a Points of inflection -3σ -2σ -σ μ σ 2σ 3σ x
QUANTIFYING RANDOM ERROR Population mean (µ) = true value (T or xt) x=µ f(x) a Points of inflection -3σ -2σ -σ μ σ 2σ 3σ x
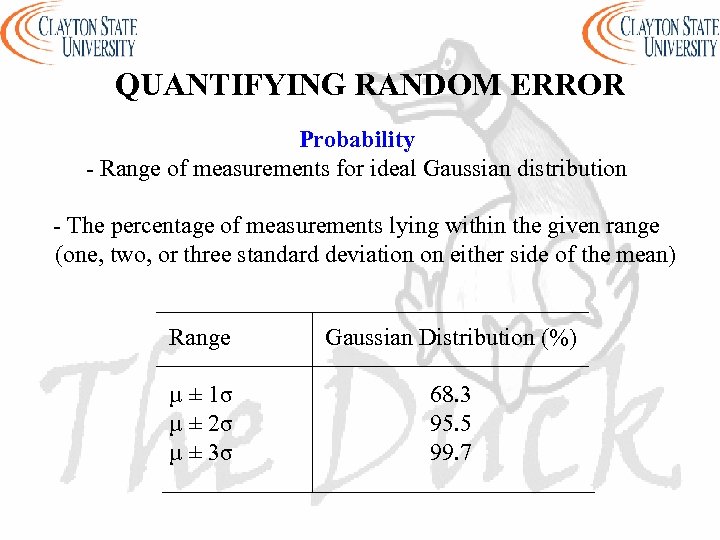 QUANTIFYING RANDOM ERROR Probability - Range of measurements for ideal Gaussian distribution - The percentage of measurements lying within the given range (one, two, or three standard deviation on either side of the mean) Range Gaussian Distribution (%) µ ± 1σ µ ± 2σ µ ± 3σ 68. 3 95. 5 99. 7
QUANTIFYING RANDOM ERROR Probability - Range of measurements for ideal Gaussian distribution - The percentage of measurements lying within the given range (one, two, or three standard deviation on either side of the mean) Range Gaussian Distribution (%) µ ± 1σ µ ± 2σ µ ± 3σ 68. 3 95. 5 99. 7
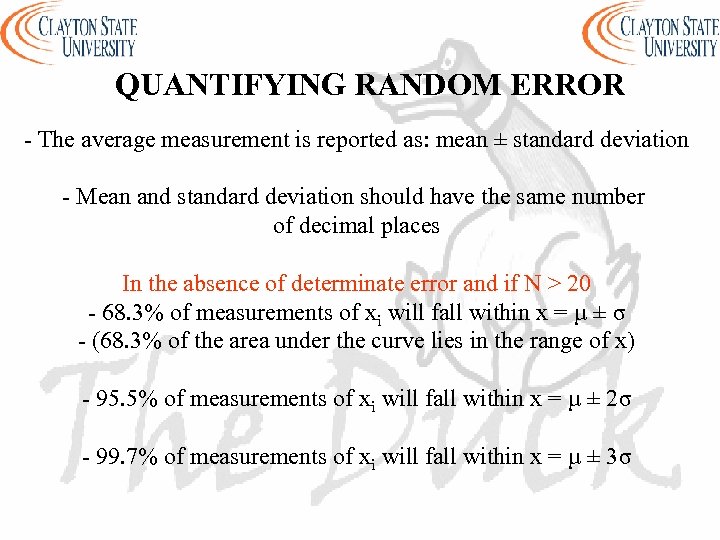 QUANTIFYING RANDOM ERROR - The average measurement is reported as: mean ± standard deviation - Mean and standard deviation should have the same number of decimal places In the absence of determinate error and if N > 20 - 68. 3% of measurements of xi will fall within x = µ ± σ - (68. 3% of the area under the curve lies in the range of x) - 95. 5% of measurements of xi will fall within x = µ ± 2σ - 99. 7% of measurements of xi will fall within x = µ ± 3σ
QUANTIFYING RANDOM ERROR - The average measurement is reported as: mean ± standard deviation - Mean and standard deviation should have the same number of decimal places In the absence of determinate error and if N > 20 - 68. 3% of measurements of xi will fall within x = µ ± σ - (68. 3% of the area under the curve lies in the range of x) - 95. 5% of measurements of xi will fall within x = µ ± 2σ - 99. 7% of measurements of xi will fall within x = µ ± 3σ
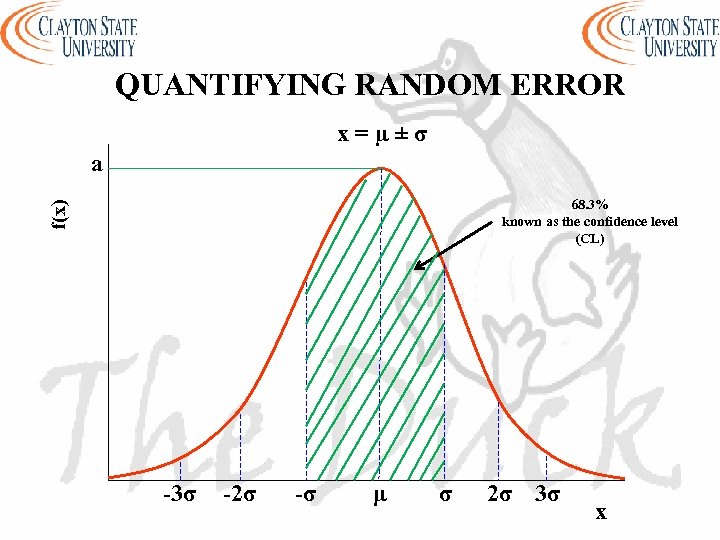 QUANTIFYING RANDOM ERROR x=µ±σ a f(x) 68. 3% known as the confidence level (CL) -3σ -2σ -σ μ σ 2σ 3σ x
QUANTIFYING RANDOM ERROR x=µ±σ a f(x) 68. 3% known as the confidence level (CL) -3σ -2σ -σ μ σ 2σ 3σ x
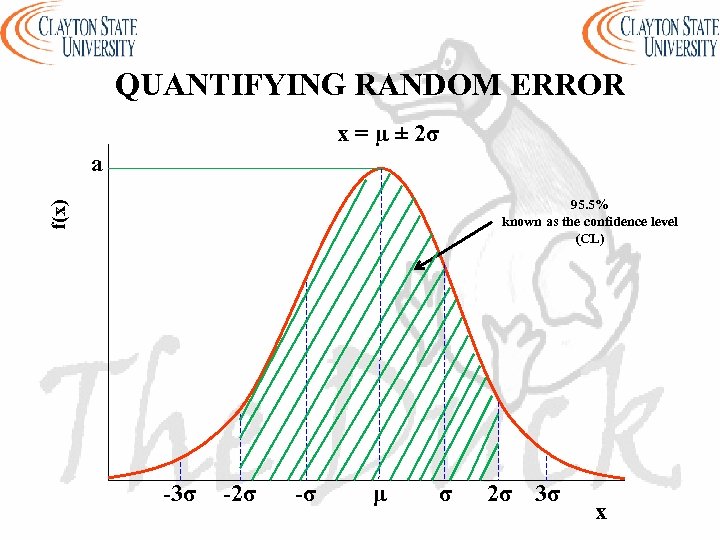 QUANTIFYING RANDOM ERROR x = µ ± 2σ a f(x) 95. 5% known as the confidence level (CL) -3σ -2σ -σ μ σ 2σ 3σ x
QUANTIFYING RANDOM ERROR x = µ ± 2σ a f(x) 95. 5% known as the confidence level (CL) -3σ -2σ -σ μ σ 2σ 3σ x
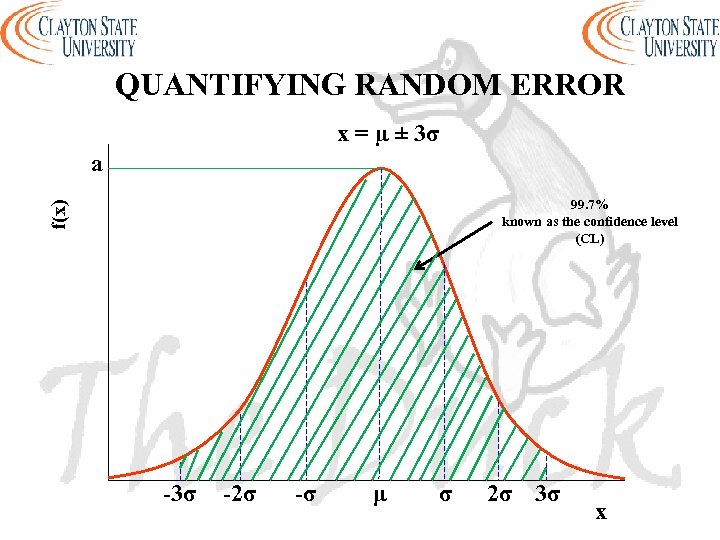 QUANTIFYING RANDOM ERROR x = µ ± 3σ a f(x) 99. 7% known as the confidence level (CL) -3σ -2σ -σ μ σ 2σ 3σ x
QUANTIFYING RANDOM ERROR x = µ ± 3σ a f(x) 99. 7% known as the confidence level (CL) -3σ -2σ -σ μ σ 2σ 3σ x
 QUANTIFYING RANDOM ERROR Short-term Precision - Analysis run at the same time by the same analyst using the same instrument and same chemicals Long-term Precision - Compiled results over several months on a regular basis Repeatability - Short-term precision under same operating conditions
QUANTIFYING RANDOM ERROR Short-term Precision - Analysis run at the same time by the same analyst using the same instrument and same chemicals Long-term Precision - Compiled results over several months on a regular basis Repeatability - Short-term precision under same operating conditions
 QUANTIFYING RANDOM ERROR Reproducibility - Ability of multiple laboratories to obtain same results on a given sample Ruggedness - Degree of reproducibility of results by one laboratory under different conditions (long-term precision) Robustness (Reliability) - Reliable accuracy and precision under small changes in condition
QUANTIFYING RANDOM ERROR Reproducibility - Ability of multiple laboratories to obtain same results on a given sample Ruggedness - Degree of reproducibility of results by one laboratory under different conditions (long-term precision) Robustness (Reliability) - Reliable accuracy and precision under small changes in condition
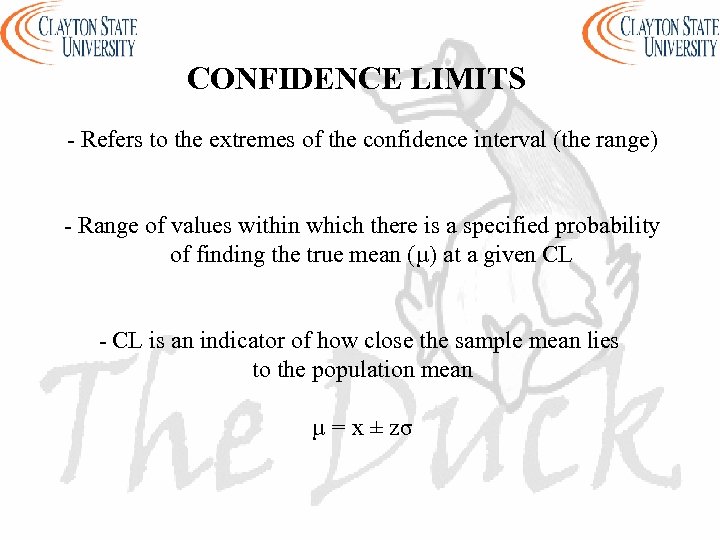 CONFIDENCE LIMITS - Refers to the extremes of the confidence interval (the range) - Range of values within which there is a specified probability of finding the true mean (µ) at a given CL - CL is an indicator of how close the sample mean lies to the population mean µ = x ± zσ
CONFIDENCE LIMITS - Refers to the extremes of the confidence interval (the range) - Range of values within which there is a specified probability of finding the true mean (µ) at a given CL - CL is an indicator of how close the sample mean lies to the population mean µ = x ± zσ
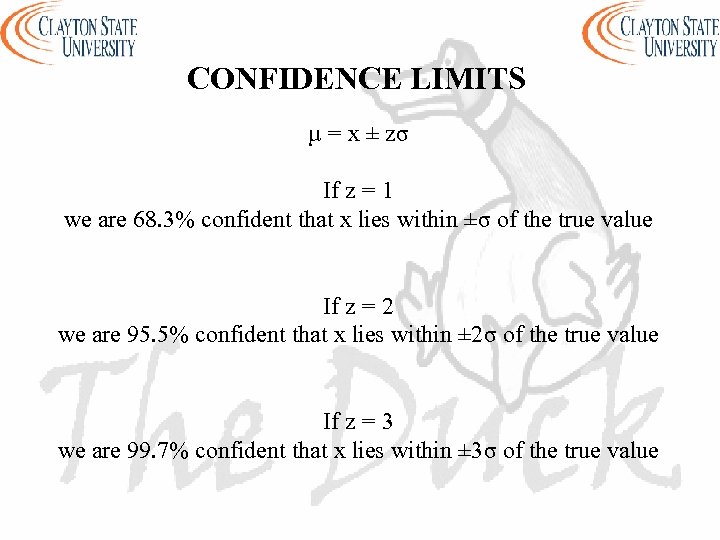 CONFIDENCE LIMITS µ = x ± zσ If z = 1 we are 68. 3% confident that x lies within ±σ of the true value If z = 2 we are 95. 5% confident that x lies within ± 2σ of the true value If z = 3 we are 99. 7% confident that x lies within ± 3σ of the true value
CONFIDENCE LIMITS µ = x ± zσ If z = 1 we are 68. 3% confident that x lies within ±σ of the true value If z = 2 we are 95. 5% confident that x lies within ± 2σ of the true value If z = 3 we are 99. 7% confident that x lies within ± 3σ of the true value
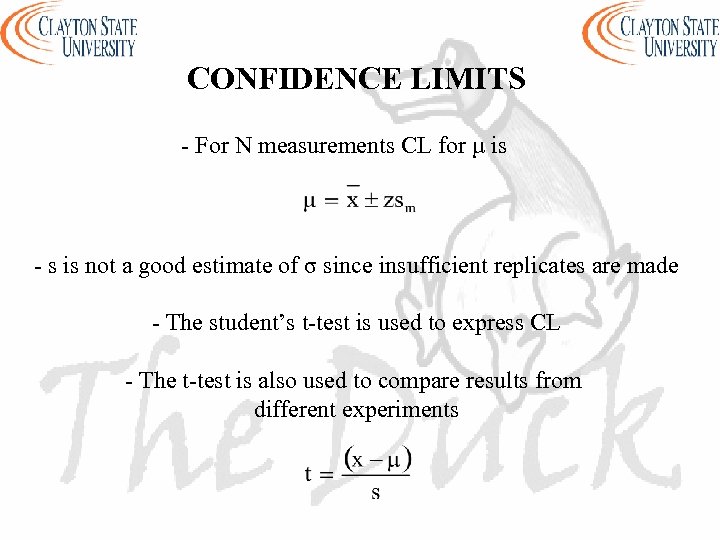 CONFIDENCE LIMITS - For N measurements CL for µ is - s is not a good estimate of σ since insufficient replicates are made - The student’s t-test is used to express CL - The t-test is also used to compare results from different experiments
CONFIDENCE LIMITS - For N measurements CL for µ is - s is not a good estimate of σ since insufficient replicates are made - The student’s t-test is used to express CL - The t-test is also used to compare results from different experiments
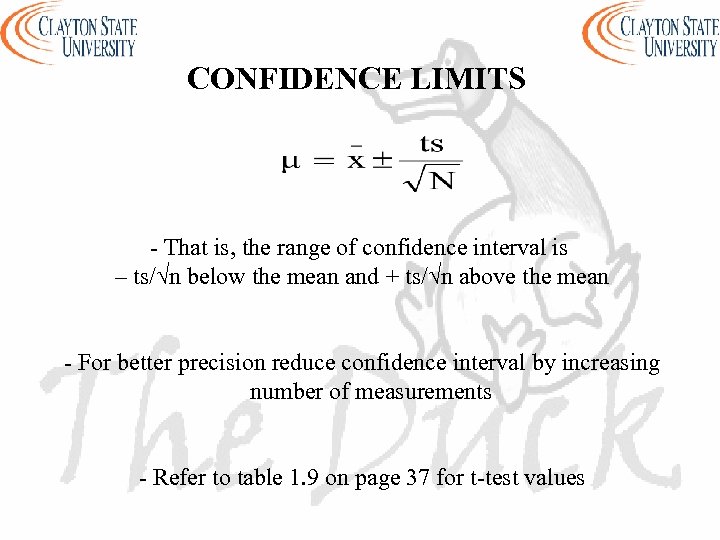 CONFIDENCE LIMITS - That is, the range of confidence interval is – ts/√n below the mean and + ts/√n above the mean - For better precision reduce confidence interval by increasing number of measurements - Refer to table 1. 9 on page 37 for t-test values
CONFIDENCE LIMITS - That is, the range of confidence interval is – ts/√n below the mean and + ts/√n above the mean - For better precision reduce confidence interval by increasing number of measurements - Refer to table 1. 9 on page 37 for t-test values
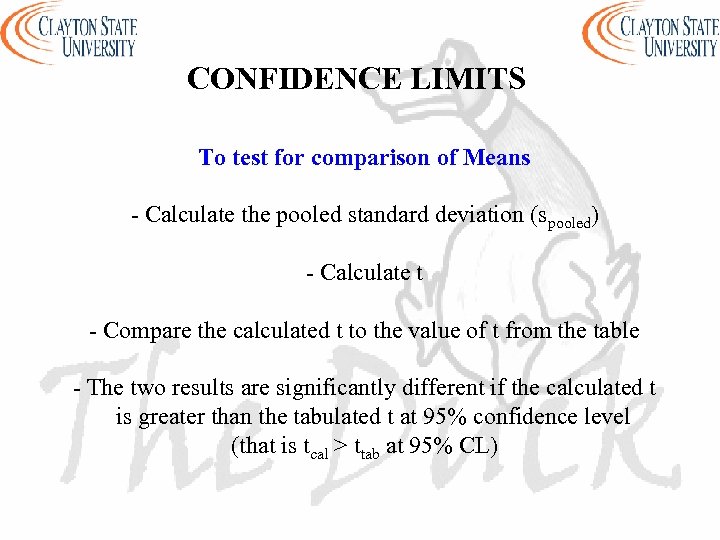 CONFIDENCE LIMITS To test for comparison of Means - Calculate the pooled standard deviation (spooled) - Calculate t - Compare the calculated t to the value of t from the table - The two results are significantly different if the calculated t is greater than the tabulated t at 95% confidence level (that is tcal > ttab at 95% CL)
CONFIDENCE LIMITS To test for comparison of Means - Calculate the pooled standard deviation (spooled) - Calculate t - Compare the calculated t to the value of t from the table - The two results are significantly different if the calculated t is greater than the tabulated t at 95% confidence level (that is tcal > ttab at 95% CL)
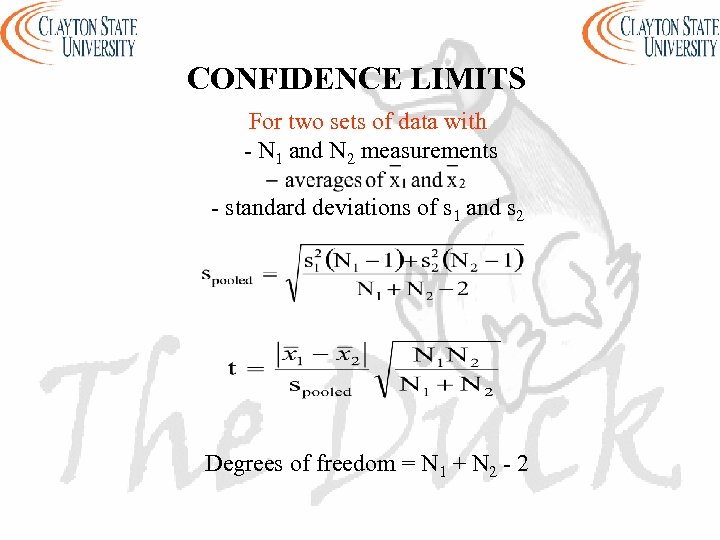 CONFIDENCE LIMITS For two sets of data with - N 1 and N 2 measurements - standard deviations of s 1 and s 2 Degrees of freedom = N 1 + N 2 - 2
CONFIDENCE LIMITS For two sets of data with - N 1 and N 2 measurements - standard deviations of s 1 and s 2 Degrees of freedom = N 1 + N 2 - 2
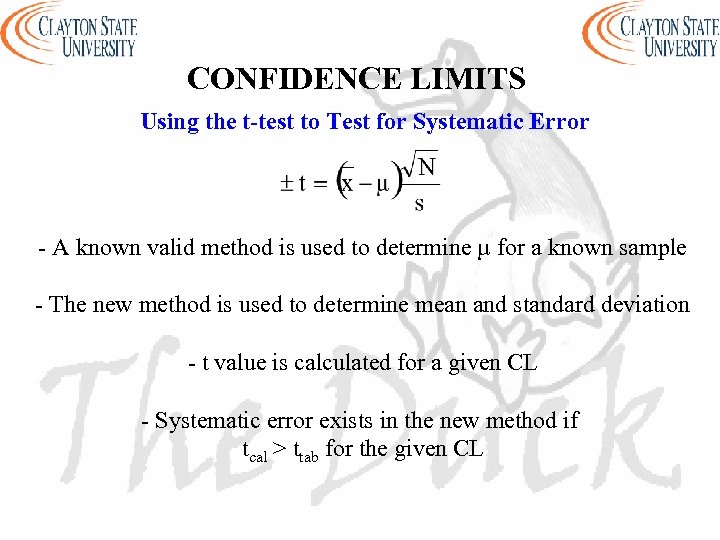 CONFIDENCE LIMITS Using the t-test to Test for Systematic Error - A known valid method is used to determine µ for a known sample - The new method is used to determine mean and standard deviation - t value is calculated for a given CL - Systematic error exists in the new method if tcal > ttab for the given CL
CONFIDENCE LIMITS Using the t-test to Test for Systematic Error - A known valid method is used to determine µ for a known sample - The new method is used to determine mean and standard deviation - t value is calculated for a given CL - Systematic error exists in the new method if tcal > ttab for the given CL
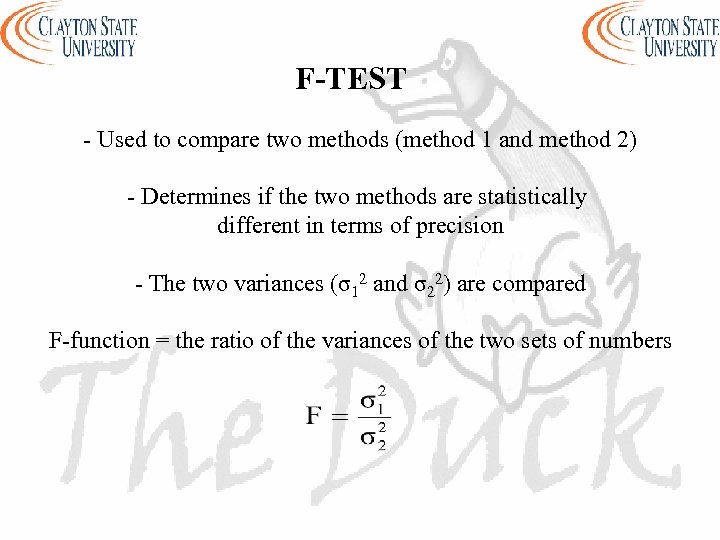 F-TEST - Used to compare two methods (method 1 and method 2) - Determines if the two methods are statistically different in terms of precision - The two variances (σ12 and σ22) are compared F-function = the ratio of the variances of the two sets of numbers
F-TEST - Used to compare two methods (method 1 and method 2) - Determines if the two methods are statistically different in terms of precision - The two variances (σ12 and σ22) are compared F-function = the ratio of the variances of the two sets of numbers
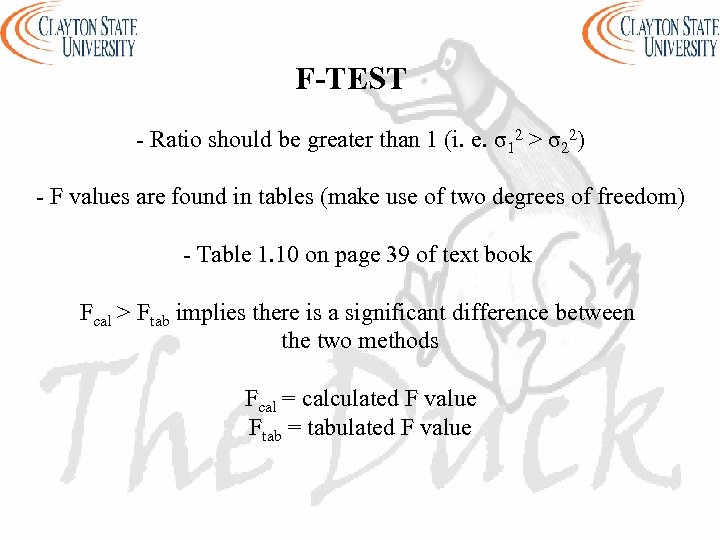 F-TEST - Ratio should be greater than 1 (i. e. σ12 > σ22) - F values are found in tables (make use of two degrees of freedom) - Table 1. 10 on page 39 of text book Fcal > Ftab implies there is a significant difference between the two methods Fcal = calculated F value Ftab = tabulated F value
F-TEST - Ratio should be greater than 1 (i. e. σ12 > σ22) - F values are found in tables (make use of two degrees of freedom) - Table 1. 10 on page 39 of text book Fcal > Ftab implies there is a significant difference between the two methods Fcal = calculated F value Ftab = tabulated F value
 REJECTION OF RESULTS Outlier - A replicate result that is out of the line - A result that is far from other results - Is either the highest value or the lowest value in a set of data - There should be a justification for discarding the outlier - The outlier is rejected if it is > ± 4σ from the mean - The outlier is not included in calculating the mean and standard deviation - A new σ should be calculated that includes outlier if it is < ± 4σ
REJECTION OF RESULTS Outlier - A replicate result that is out of the line - A result that is far from other results - Is either the highest value or the lowest value in a set of data - There should be a justification for discarding the outlier - The outlier is rejected if it is > ± 4σ from the mean - The outlier is not included in calculating the mean and standard deviation - A new σ should be calculated that includes outlier if it is < ± 4σ
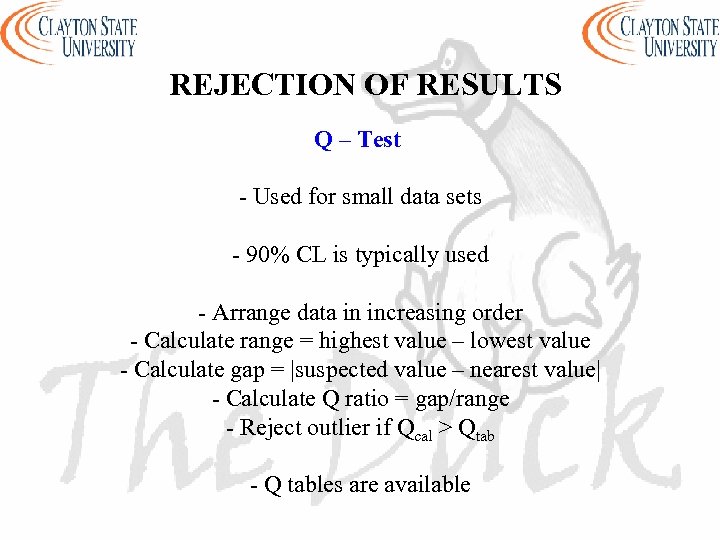 REJECTION OF RESULTS Q – Test - Used for small data sets - 90% CL is typically used - Arrange data in increasing order - Calculate range = highest value – lowest value - Calculate gap = |suspected value – nearest value| - Calculate Q ratio = gap/range - Reject outlier if Qcal > Qtab - Q tables are available
REJECTION OF RESULTS Q – Test - Used for small data sets - 90% CL is typically used - Arrange data in increasing order - Calculate range = highest value – lowest value - Calculate gap = |suspected value – nearest value| - Calculate Q ratio = gap/range - Reject outlier if Qcal > Qtab - Q tables are available
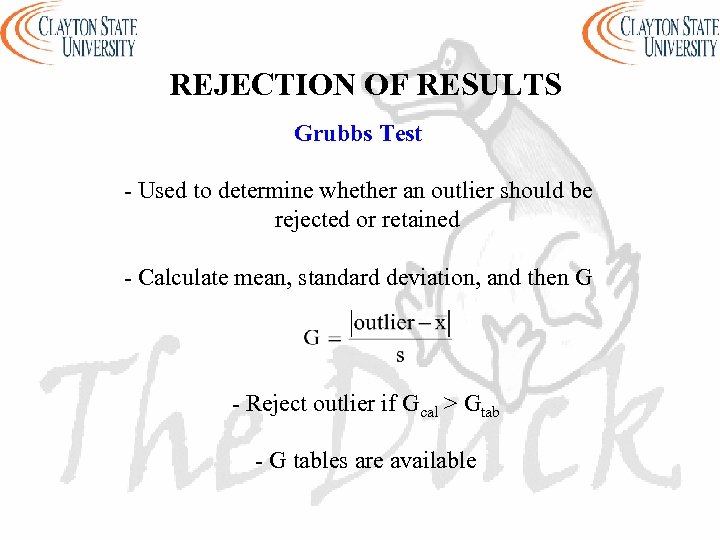 REJECTION OF RESULTS Grubbs Test - Used to determine whether an outlier should be rejected or retained - Calculate mean, standard deviation, and then G - Reject outlier if Gcal > Gtab - G tables are available
REJECTION OF RESULTS Grubbs Test - Used to determine whether an outlier should be rejected or retained - Calculate mean, standard deviation, and then G - Reject outlier if Gcal > Gtab - G tables are available
 PERFORMING THE EXPERIMENT Detector - Records the signal (change in the system that is related to the magnitude of the physical parameter being measured) - Can measure physical, chemical or electrical changes Transducer (Sensor) - Detector that converts nonelectrical signals to electrical signals and vice versa
PERFORMING THE EXPERIMENT Detector - Records the signal (change in the system that is related to the magnitude of the physical parameter being measured) - Can measure physical, chemical or electrical changes Transducer (Sensor) - Detector that converts nonelectrical signals to electrical signals and vice versa
 PERFORMING THE EXPERIMENT Signals and Noise - A detector makes measurements and detector response is converted to an electrical signal - The electrical signal is related to the chemical or physical property being measured, which is related to the amount of analyte - There should be no signal when no analyte is present - Signals should be smooth but are practically not smooth due to noise
PERFORMING THE EXPERIMENT Signals and Noise - A detector makes measurements and detector response is converted to an electrical signal - The electrical signal is related to the chemical or physical property being measured, which is related to the amount of analyte - There should be no signal when no analyte is present - Signals should be smooth but are practically not smooth due to noise
 PERFORMING THE EXPERIMENT Signals and Noise can originate from - Power fluctuations - Radio stations - Electrical motors - Building vibrations - Other instruments nearby
PERFORMING THE EXPERIMENT Signals and Noise can originate from - Power fluctuations - Radio stations - Electrical motors - Building vibrations - Other instruments nearby
 PERFORMING THE EXPERIMENT Signals and Noise - Signal-to-noise ratio (S/N) is a useful tool for comparing methods or instruments - Noise is random and can be treated statistically - Signal can be defined as the average value of measurements - Noise can be defined as the standard deviation
PERFORMING THE EXPERIMENT Signals and Noise - Signal-to-noise ratio (S/N) is a useful tool for comparing methods or instruments - Noise is random and can be treated statistically - Signal can be defined as the average value of measurements - Noise can be defined as the standard deviation
 PERFORMING THE EXPERIMENT Types of Noise 1. White Noise - Two types Thermal Noise - Due to random motions of charge carriers (electrons) which result in voltage fluctuations Shot Noise - When charge carriers cross a junction in an electrical circuit
PERFORMING THE EXPERIMENT Types of Noise 1. White Noise - Two types Thermal Noise - Due to random motions of charge carriers (electrons) which result in voltage fluctuations Shot Noise - When charge carriers cross a junction in an electrical circuit
 PERFORMING THE EXPERIMENT Types of Noise 2. Drift (Flicker) Noise (origin is not well understood) 3. Noise due to surroundings (vibrations) - Signal is enhanced or noise is reduced or both to increase S/N - Hardware and software approaches are available - Another approach is the use of Fourier Transform (FT) or Fast Fourier Transform (FFT) which discriminates signals from noise (FT-IR, FT-NMR, FT-MS)
PERFORMING THE EXPERIMENT Types of Noise 2. Drift (Flicker) Noise (origin is not well understood) 3. Noise due to surroundings (vibrations) - Signal is enhanced or noise is reduced or both to increase S/N - Hardware and software approaches are available - Another approach is the use of Fourier Transform (FT) or Fast Fourier Transform (FFT) which discriminates signals from noise (FT-IR, FT-NMR, FT-MS)
 CALIBRATION CURVES Calibration - The process of establishing the relationship between the measured signals and known concentrations of analyte - Calibration standards: known concentrations of analyte - Calibration standards at different concentrations are prepared and measured - Magnitude of signals are plotted against concentration - Equation relating signal and concentration is obtained and can be used to determine the concentration of unknown analyte after measuring its signal
CALIBRATION CURVES Calibration - The process of establishing the relationship between the measured signals and known concentrations of analyte - Calibration standards: known concentrations of analyte - Calibration standards at different concentrations are prepared and measured - Magnitude of signals are plotted against concentration - Equation relating signal and concentration is obtained and can be used to determine the concentration of unknown analyte after measuring its signal
 CALIBRATION CURVES - Many calibration curves have a linear range with the relation equation in the form y = mx + b - The method of least squares or the spreadsheet may be used - m is the slope and b is the vertical (signal) intercept - The slope is usually the sensitivity of the analytical method - R = correlation coefficient (R 2 is between 0 and 1) - Perfect fit of data (direct relation) if R 2 is closer to 1
CALIBRATION CURVES - Many calibration curves have a linear range with the relation equation in the form y = mx + b - The method of least squares or the spreadsheet may be used - m is the slope and b is the vertical (signal) intercept - The slope is usually the sensitivity of the analytical method - R = correlation coefficient (R 2 is between 0 and 1) - Perfect fit of data (direct relation) if R 2 is closer to 1
 BEST STRAIGHT LINE (METHOD OF LEAST SQUARES) The equation of a straight line y = mx + b m is the slope ( y/ x) b is the y-intercept (where the line crosses the y-axis)
BEST STRAIGHT LINE (METHOD OF LEAST SQUARES) The equation of a straight line y = mx + b m is the slope ( y/ x) b is the y-intercept (where the line crosses the y-axis)
 BEST STRAIGHT LINE (METHOD OF LEAST SQUARES) The method of least squares - finds the best straight line - adjusts the line to minimize the vertical deviations Only vertical deviations are adjusted because - experimental uncertainties in y values > in x values - calculations for minimizing vertical deviations are easier
BEST STRAIGHT LINE (METHOD OF LEAST SQUARES) The method of least squares - finds the best straight line - adjusts the line to minimize the vertical deviations Only vertical deviations are adjusted because - experimental uncertainties in y values > in x values - calculations for minimizing vertical deviations are easier
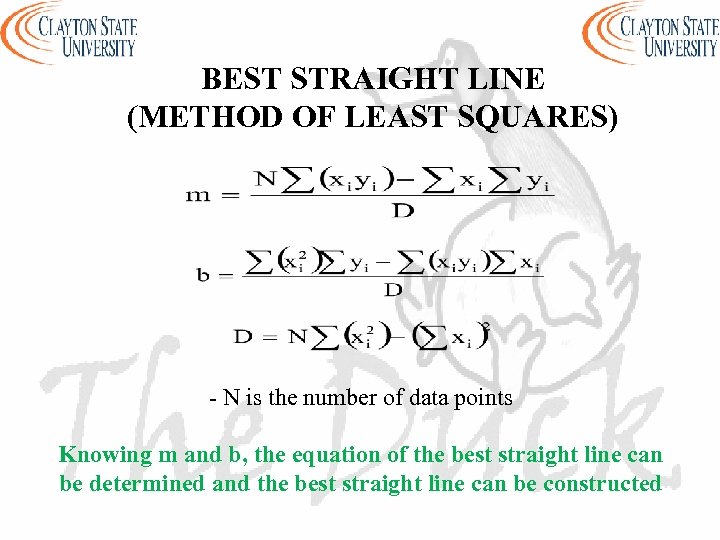 BEST STRAIGHT LINE (METHOD OF LEAST SQUARES) - N is the number of data points Knowing m and b, the equation of the best straight line can be determined and the best straight line can be constructed
BEST STRAIGHT LINE (METHOD OF LEAST SQUARES) - N is the number of data points Knowing m and b, the equation of the best straight line can be determined and the best straight line can be constructed
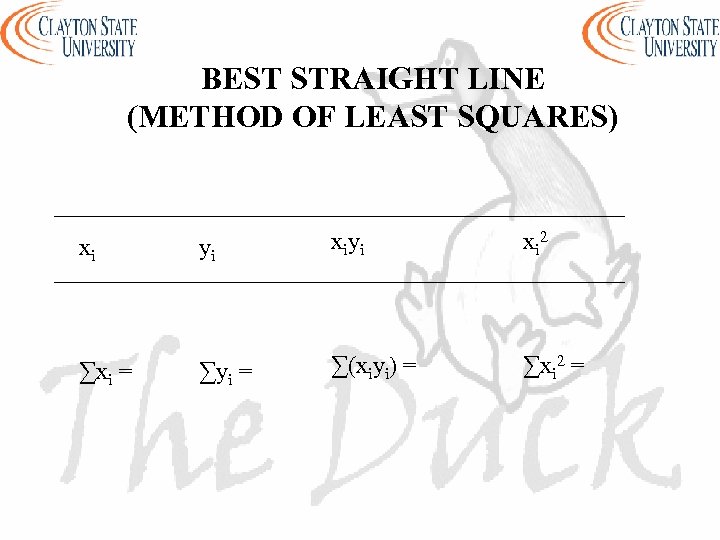 BEST STRAIGHT LINE (METHOD OF LEAST SQUARES) xi yi xi 2 ∑xi = ∑yi = ∑(xiyi) = ∑xi 2 =
BEST STRAIGHT LINE (METHOD OF LEAST SQUARES) xi yi xi 2 ∑xi = ∑yi = ∑(xiyi) = ∑xi 2 =
 ASSESSING THE DATA A good analytical method should be - both accurate and precise - reliable and robust - It is not a good practice to extrapolate above the highest standard or below the lowest standard - These regions may not be in the linear range - Dilute higher concentrations and concentrate lower concentrations of analyte to bring them into the working range
ASSESSING THE DATA A good analytical method should be - both accurate and precise - reliable and robust - It is not a good practice to extrapolate above the highest standard or below the lowest standard - These regions may not be in the linear range - Dilute higher concentrations and concentrate lower concentrations of analyte to bring them into the working range
 ASSESSING THE DATA Limit of Detection (LOD) - The lowest concentration of an analyte that can be detected - Increasing concentration of analyte decreases signal due to noise - Signal can no longer be distinguished from noise at a point - LOD does not necessarily mean concentration can be measured and quantified
ASSESSING THE DATA Limit of Detection (LOD) - The lowest concentration of an analyte that can be detected - Increasing concentration of analyte decreases signal due to noise - Signal can no longer be distinguished from noise at a point - LOD does not necessarily mean concentration can be measured and quantified
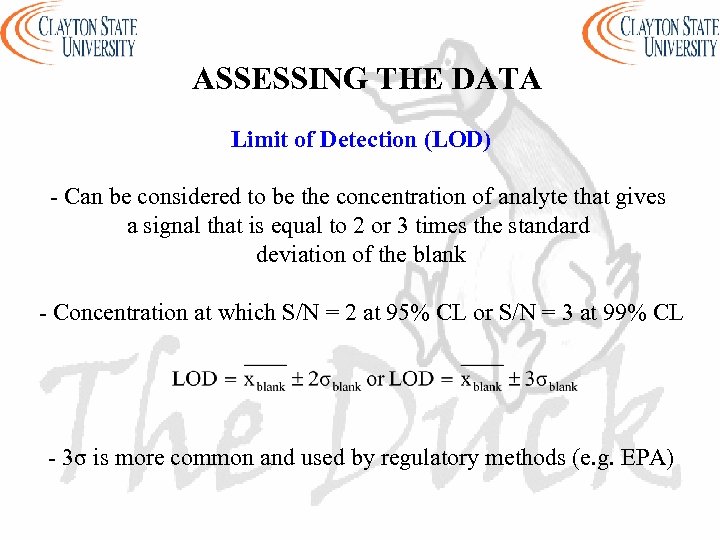 ASSESSING THE DATA Limit of Detection (LOD) - Can be considered to be the concentration of analyte that gives a signal that is equal to 2 or 3 times the standard deviation of the blank - Concentration at which S/N = 2 at 95% CL or S/N = 3 at 99% CL - 3σ is more common and used by regulatory methods (e. g. EPA)
ASSESSING THE DATA Limit of Detection (LOD) - Can be considered to be the concentration of analyte that gives a signal that is equal to 2 or 3 times the standard deviation of the blank - Concentration at which S/N = 2 at 95% CL or S/N = 3 at 99% CL - 3σ is more common and used by regulatory methods (e. g. EPA)
 ASSESSING THE DATA Limit of Quantification (LOQ) - The lowest concentration of an analyte in a sample that can be determined quantitatively with a given accuracy and precision - Precision is poor at or near LOD - LOQ is higher than LOD and has better precision - LOQ is the concentration equivalent to S/N = 10/1 - LOQ is also defined as 10 x σblank
ASSESSING THE DATA Limit of Quantification (LOQ) - The lowest concentration of an analyte in a sample that can be determined quantitatively with a given accuracy and precision - Precision is poor at or near LOD - LOQ is higher than LOD and has better precision - LOQ is the concentration equivalent to S/N = 10/1 - LOQ is also defined as 10 x σblank


