e13005d110ad2ec62935ce0cbbeaed30.ppt
- Количество слайдов: 22
 INSIGHTS Heroin Assisted Treatment (HAT) Professor John STRANG and Dr Teodora GROSHKOVA 16 th November 2010, Lisbon
INSIGHTS Heroin Assisted Treatment (HAT) Professor John STRANG and Dr Teodora GROSHKOVA 16 th November 2010, Lisbon
 Aims of the presentation 1. 2. 3. 4. 5. Why do we need the Insights on HAT? What did we do and where are we at? The evidence base for HAT Current clinical practice Next steps
Aims of the presentation 1. 2. 3. 4. 5. Why do we need the Insights on HAT? What did we do and where are we at? The evidence base for HAT Current clinical practice Next steps
 Acknowledgements International HAT Trials PIs/co-ordinators n Professor dr Ambros Uchtenhagen Contributors, Pharmaceutical companies n Simon Bryson Auralis Ltd, UK University of Zurich, Switzerland n Professor dr. Wim van den Brink Amsterdam Institute for Addiction Research, the Netherlands n Professor Dr Christian Haasen Department of Psychiatry, Hamburg, Germany n Albert Hills Teva UK n Gordon Urquhart Wockhards, UK Professor Marty Schechter University of British Columbia, Canada n Dr Eugenia Oviedo-Joekes University of British Columbia, Canada n Dr Nicola Metrebian National Addiction Centre, UK Other collaborators n Dr Helle Petersen Sundhedsstyrelsen, National Board of Health, Copenhagen, Denmark
Acknowledgements International HAT Trials PIs/co-ordinators n Professor dr Ambros Uchtenhagen Contributors, Pharmaceutical companies n Simon Bryson Auralis Ltd, UK University of Zurich, Switzerland n Professor dr. Wim van den Brink Amsterdam Institute for Addiction Research, the Netherlands n Professor Dr Christian Haasen Department of Psychiatry, Hamburg, Germany n Albert Hills Teva UK n Gordon Urquhart Wockhards, UK Professor Marty Schechter University of British Columbia, Canada n Dr Eugenia Oviedo-Joekes University of British Columbia, Canada n Dr Nicola Metrebian National Addiction Centre, UK Other collaborators n Dr Helle Petersen Sundhedsstyrelsen, National Board of Health, Copenhagen, Denmark
 1. Why Insights on HAT?
1. Why Insights on HAT?
 Background to the HAT Insights n Renewed interest in prescribing diamorphine for heroin addiction n Growing evidence base for HAT n Policy and practice challenges
Background to the HAT Insights n Renewed interest in prescribing diamorphine for heroin addiction n Growing evidence base for HAT n Policy and practice challenges
 HAT trials to date Main paper Hartnoll et al. , 1980 Country England Perneger et al. , 1998 Switzerland Van den Brink et al. , 2003 (a and b) Netherlands March et al. , 2006 Haasen et al. , 2007 (a and b) Spain Germany Oviedo-Joekes et al. , 2009 (+) Canada Strang et al. , 2010 (+) England
HAT trials to date Main paper Hartnoll et al. , 1980 Country England Perneger et al. , 1998 Switzerland Van den Brink et al. , 2003 (a and b) Netherlands March et al. , 2006 Haasen et al. , 2007 (a and b) Spain Germany Oviedo-Joekes et al. , 2009 (+) Canada Strang et al. , 2010 (+) England
 2. What did we do? and where are we now?
2. What did we do? and where are we now?
 Insights Structure (1) Review of the scientific literature on HAT trials n n n n n Retention “Street” heroin and other drug use Health, health-related quality of life, social functioning Criminal offence Safety Cost-effectiveness Long-term trajectories Patients’ perspective Impact of HAT clinics and service provision on local communities (2) Commercial pharmaceutical diamorphine products
Insights Structure (1) Review of the scientific literature on HAT trials n n n n n Retention “Street” heroin and other drug use Health, health-related quality of life, social functioning Criminal offence Safety Cost-effectiveness Long-term trajectories Patients’ perspective Impact of HAT clinics and service provision on local communities (2) Commercial pharmaceutical diamorphine products
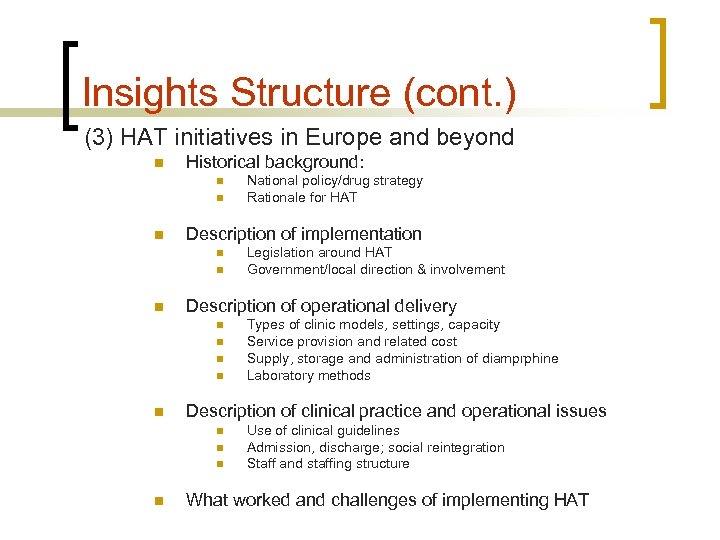 Insights Structure (cont. ) (3) HAT initiatives in Europe and beyond n Historical background: n n n Description of implementation n n n Types of clinic models, settings, capacity Service provision and related cost Supply, storage and administration of diamprphine Laboratory methods Description of clinical practice and operational issues n n Legislation around HAT Government/local direction & involvement Description of operational delivery n n National policy/drug strategy Rationale for HAT Use of clinical guidelines Admission, discharge; social reintegration Staff and staffing structure What worked and challenges of implementing HAT
Insights Structure (cont. ) (3) HAT initiatives in Europe and beyond n Historical background: n n n Description of implementation n n n Types of clinic models, settings, capacity Service provision and related cost Supply, storage and administration of diamprphine Laboratory methods Description of clinical practice and operational issues n n Legislation around HAT Government/local direction & involvement Description of operational delivery n n National policy/drug strategy Rationale for HAT Use of clinical guidelines Admission, discharge; social reintegration Staff and staffing structure What worked and challenges of implementing HAT
 3. The evidence base for HAT Six randomised trials in 6 countries involving >1000 patients over 12 years: Synthesis of findings.
3. The evidence base for HAT Six randomised trials in 6 countries involving >1000 patients over 12 years: Synthesis of findings.
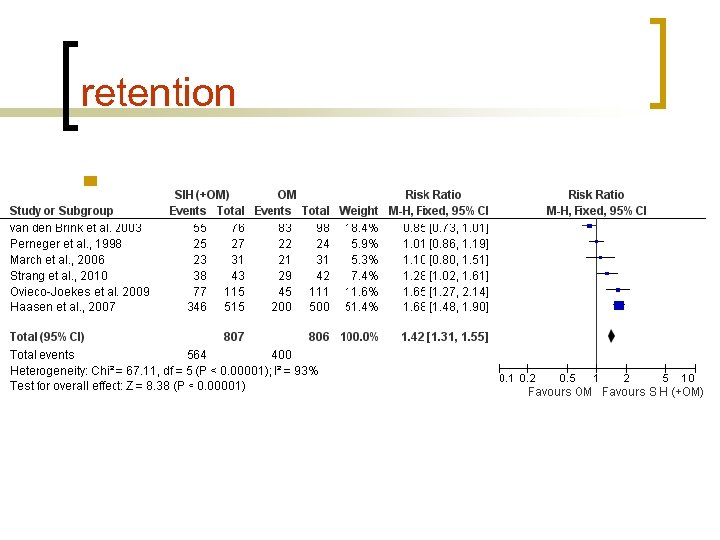
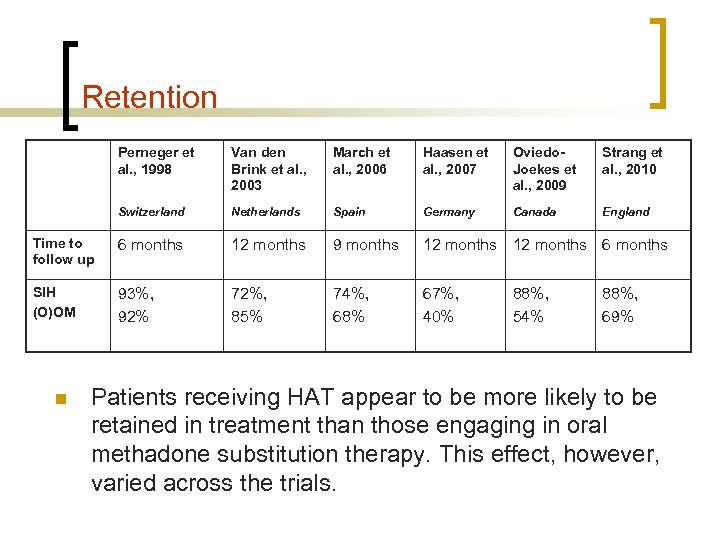 Retention Perneger et al. , 1998 Van den Brink et al. , 2003 March et al. , 2006 Haasen et al. , 2007 Oviedo. Joekes et al. , 2009 Strang et al. , 2010 Switzerland Netherlands Spain Germany Canada England Time to follow up 6 months 12 months 9 months 12 months 6 months SIH (O)OM 93%, 92% 72%, 85% 74%, 68% 67%, 40% n 88%, 54% 88%, 69% Patients receiving HAT appear to be more likely to be retained in treatment than those engaging in oral methadone substitution therapy. This effect, however, varied across the trials.
Retention Perneger et al. , 1998 Van den Brink et al. , 2003 March et al. , 2006 Haasen et al. , 2007 Oviedo. Joekes et al. , 2009 Strang et al. , 2010 Switzerland Netherlands Spain Germany Canada England Time to follow up 6 months 12 months 9 months 12 months 6 months SIH (O)OM 93%, 92% 72%, 85% 74%, 68% 67%, 40% n 88%, 54% 88%, 69% Patients receiving HAT appear to be more likely to be retained in treatment than those engaging in oral methadone substitution therapy. This effect, however, varied across the trials.
 retention n
retention n
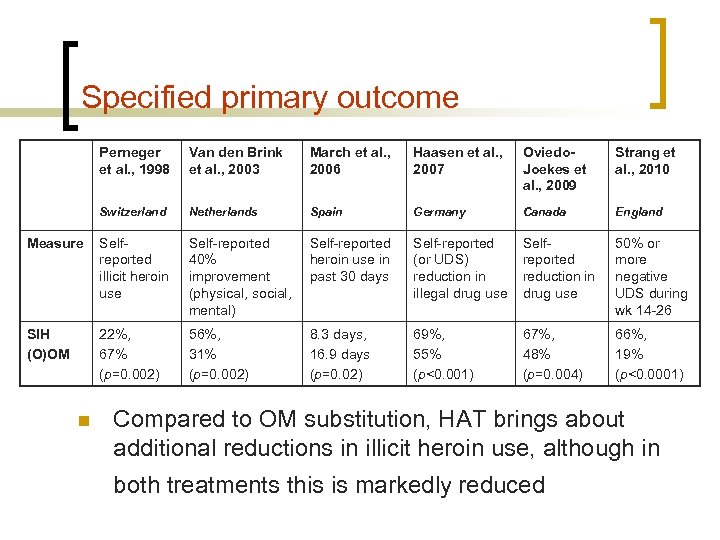 Specified primary outcome Perneger et al. , 1998 Van den Brink et al. , 2003 March et al. , 2006 Haasen et al. , 2007 Oviedo. Joekes et al. , 2009 Strang et al. , 2010 Switzerland Netherlands Spain Germany Canada England Measure Selfreported illicit heroin use Self-reported 40% improvement (physical, social, mental) Self-reported heroin use in past 30 days Self-reported (or UDS) reduction in illegal drug use Selfreported reduction in drug use 50% or more negative UDS during wk 14 -26 SIH (O)OM 22%, 67% (p=0. 002) 56%, 31% (p=0. 002) 8. 3 days, 16. 9 days (p=0. 02) 69%, 55% (p<0. 001) 67%, 48% (p=0. 004) 66%, 19% (p<0. 0001) n Compared to OM substitution, HAT brings about additional reductions in illicit heroin use, although in both treatments this is markedly reduced
Specified primary outcome Perneger et al. , 1998 Van den Brink et al. , 2003 March et al. , 2006 Haasen et al. , 2007 Oviedo. Joekes et al. , 2009 Strang et al. , 2010 Switzerland Netherlands Spain Germany Canada England Measure Selfreported illicit heroin use Self-reported 40% improvement (physical, social, mental) Self-reported heroin use in past 30 days Self-reported (or UDS) reduction in illegal drug use Selfreported reduction in drug use 50% or more negative UDS during wk 14 -26 SIH (O)OM 22%, 67% (p=0. 002) 56%, 31% (p=0. 002) 8. 3 days, 16. 9 days (p=0. 02) 69%, 55% (p<0. 001) 67%, 48% (p=0. 004) 66%, 19% (p<0. 0001) n Compared to OM substitution, HAT brings about additional reductions in illicit heroin use, although in both treatments this is markedly reduced
 primary outcome
primary outcome
 4. Current HAT clinical practice across Europe and beyond
4. Current HAT clinical practice across Europe and beyond
 Key features of the new HAT approach n Consistently (almost entirely, but not completely) now considered as second-line treatment n Direct medical or nursing supervision of all injectable doses
Key features of the new HAT approach n Consistently (almost entirely, but not completely) now considered as second-line treatment n Direct medical or nursing supervision of all injectable doses
 The supervised injecting heroinprescribing clinics n 7 days per week (Spain week-days only); typically 2 -3 blocks of opening hours n Higher daily doses; no take-home injections n Oral take-home supplements n Flexible prescribing - oral take-home conversion on request n Dedicated facility - specific function
The supervised injecting heroinprescribing clinics n 7 days per week (Spain week-days only); typically 2 -3 blocks of opening hours n Higher daily doses; no take-home injections n Oral take-home supplements n Flexible prescribing - oral take-home conversion on request n Dedicated facility - specific function
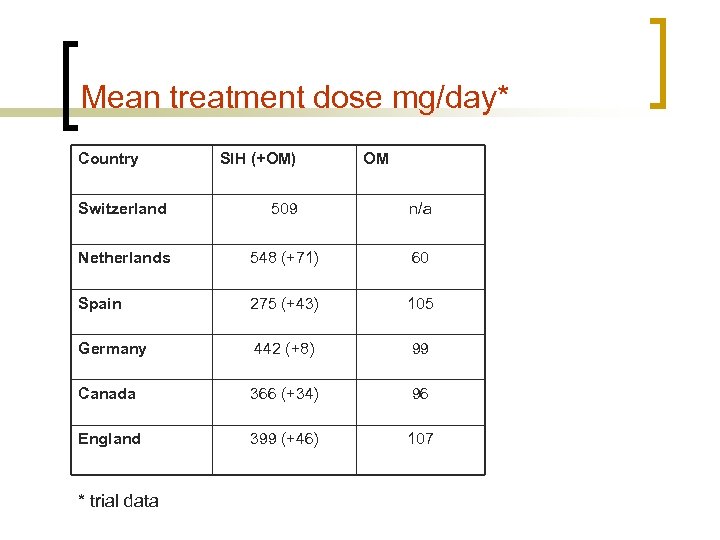 Mean treatment dose mg/day* Country SIH (+OM) OM Switzerland 509 n/a Netherlands 548 (+71) 60 Spain 275 (+43) 105 Germany 442 (+8) 99 Canada 366 (+34) 96 England 399 (+46) 107 * trial data
Mean treatment dose mg/day* Country SIH (+OM) OM Switzerland 509 n/a Netherlands 548 (+71) 60 Spain 275 (+43) 105 Germany 442 (+8) 99 Canada 366 (+34) 96 England 399 (+46) 107 * trial data
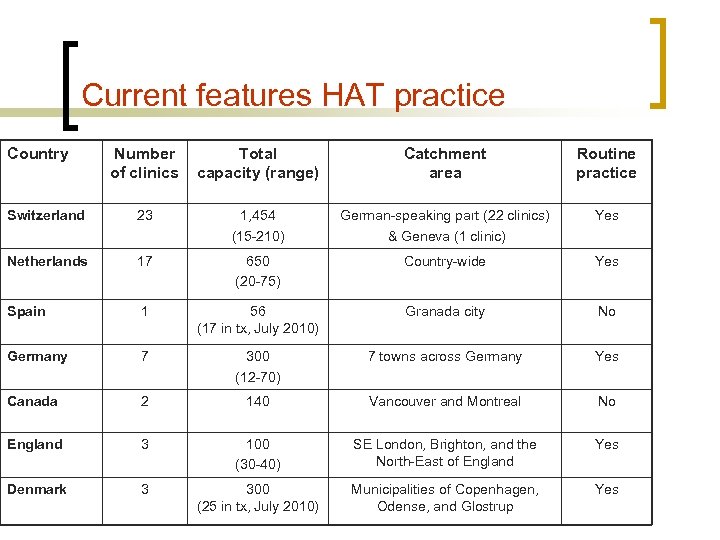 Current features HAT practice Country Number of clinics Total capacity (range) Catchment area Routine practice Switzerland 23 1, 454 (15 -210) German-speaking part (22 clinics) & Geneva (1 clinic) Yes Netherlands 17 650 (20 -75) Country-wide Yes Spain 1 56 (17 in tx, July 2010) Granada city No Germany 7 300 (12 -70) 7 towns across Germany Yes Canada 2 140 Vancouver and Montreal No England 3 100 (30 -40) SE London, Brighton, and the North-East of England Yes Denmark 3 300 (25 in tx, July 2010) Municipalities of Copenhagen, Odense, and Glostrup Yes
Current features HAT practice Country Number of clinics Total capacity (range) Catchment area Routine practice Switzerland 23 1, 454 (15 -210) German-speaking part (22 clinics) & Geneva (1 clinic) Yes Netherlands 17 650 (20 -75) Country-wide Yes Spain 1 56 (17 in tx, July 2010) Granada city No Germany 7 300 (12 -70) 7 towns across Germany Yes Canada 2 140 Vancouver and Montreal No England 3 100 (30 -40) SE London, Brighton, and the North-East of England Yes Denmark 3 300 (25 in tx, July 2010) Municipalities of Copenhagen, Odense, and Glostrup Yes
 5. Next steps
5. Next steps
 Key tasks n Finalising the HAT Insights publication n Drafting completed Chapters to be sent off for checks by international HAT PIs Launch July 2011 n Collaborative paper with EMCDDA n Discussions with EMCDDA around developing a set of minimum clinical standards and link to quality assurance process
Key tasks n Finalising the HAT Insights publication n Drafting completed Chapters to be sent off for checks by international HAT PIs Launch July 2011 n Collaborative paper with EMCDDA n Discussions with EMCDDA around developing a set of minimum clinical standards and link to quality assurance process
 n Thank you
n Thank you


