c797f5cd3b398778c855a892e0919fd1.ppt
- Количество слайдов: 33

Insert (for PS_1): Bridging Systems Boundaries: Direct vs. Indirect Energy Use • Enlargement of systems boundaries requires: -- Allocation rules for “upstream” activities, e. g. conversion losses/emissions prior to final energy -- Allocation rules for “downstream” activities, e. g. industrial final energy/emissions that end up as consumer products 86025 Energy Systems Analysis Arnulf Grubler
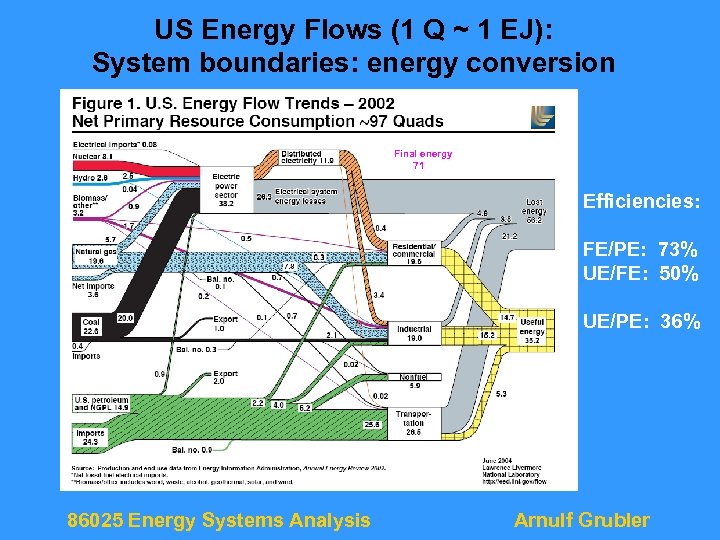
US Energy Flows (1 Q ~ 1 EJ): System boundaries: energy conversion Final energy 71 Efficiencies: FE/PE: 73% UE/FE: 50% UE/PE: 36% 86025 Energy Systems Analysis Arnulf Grubler
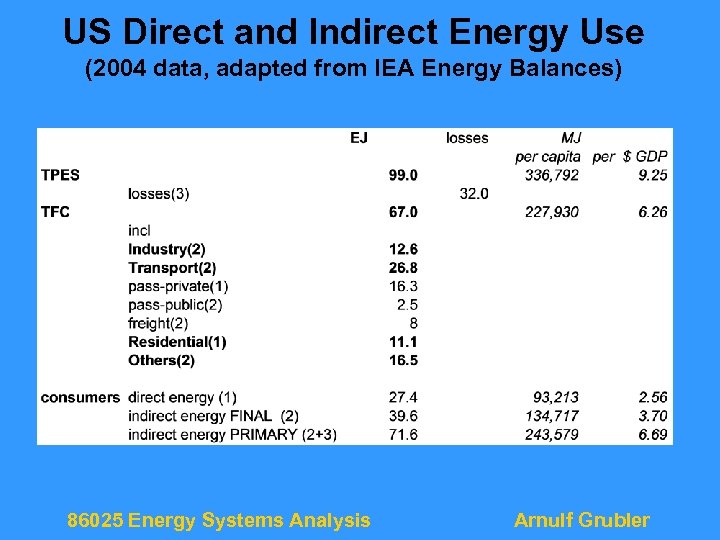
US Direct and Indirect Energy Use (2004 data, adapted from IEA Energy Balances) 86025 Energy Systems Analysis Arnulf Grubler
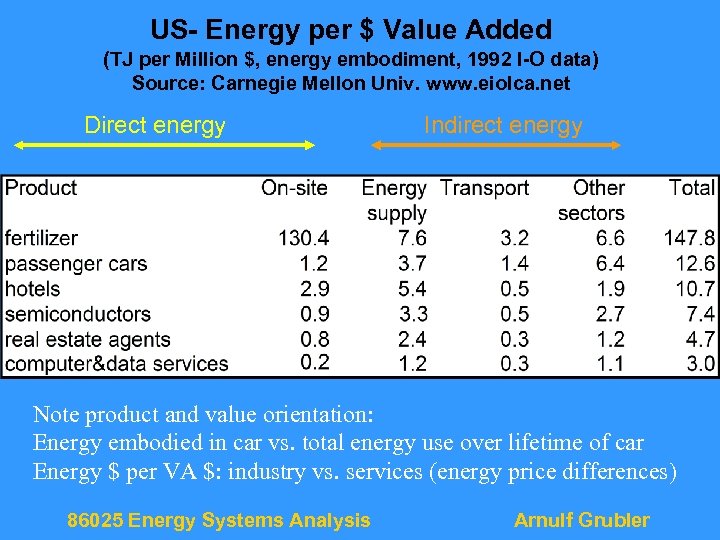
US- Energy per $ Value Added (TJ per Million $, energy embodiment, 1992 I-O data) Source: Carnegie Mellon Univ. www. eiolca. net Direct energy Indirect energy Note product and value orientation: Energy embodied in car vs. total energy use over lifetime of car Energy $ per VA $: industry vs. services (energy price differences) 86025 Energy Systems Analysis Arnulf Grubler
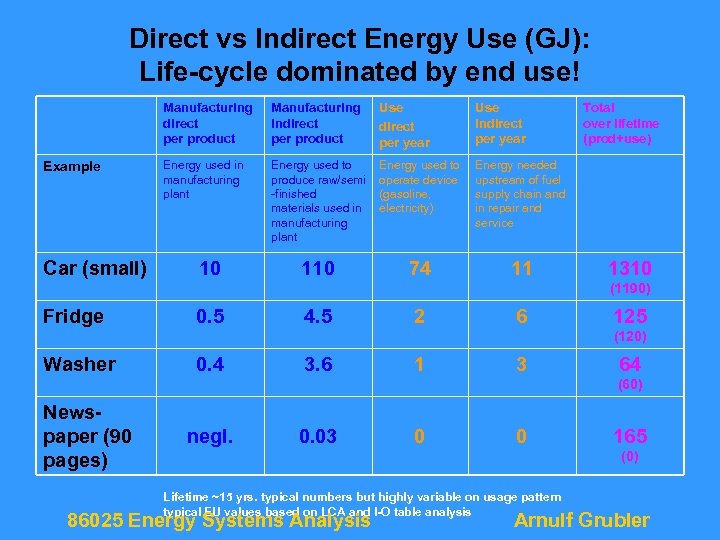
Direct vs Indirect Energy Use (GJ): Life-cycle dominated by end use! Manufacturing direct per product Example Car (small) Manufacturing indirect per product Use direct per year Use indirect per year Energy used in manufacturing plant Energy used to produce raw/semi -finished materials used in manufacturing plant Energy used to operate device (gasoline, electricity) Energy needed upstream of fuel supply chain and in repair and service 110 74 11 10 Total over lifetime (prod+use) 1310 (1190) Fridge 0. 5 4. 5 2 6 125 (120) Washer 0. 4 3. 6 1 3 64 (60) Newspaper (90 pages) negl. 0. 03 0 0 165 (0) Lifetime ~15 yrs. typical numbers but highly variable on usage pattern typical EU values based on LCA and I-O table analysis 86025 Energy Systems Analysis Arnulf Grubler

86025_2 Fundamentals of Energy Systems I 86025 Energy Systems Analysis Arnulf Grubler
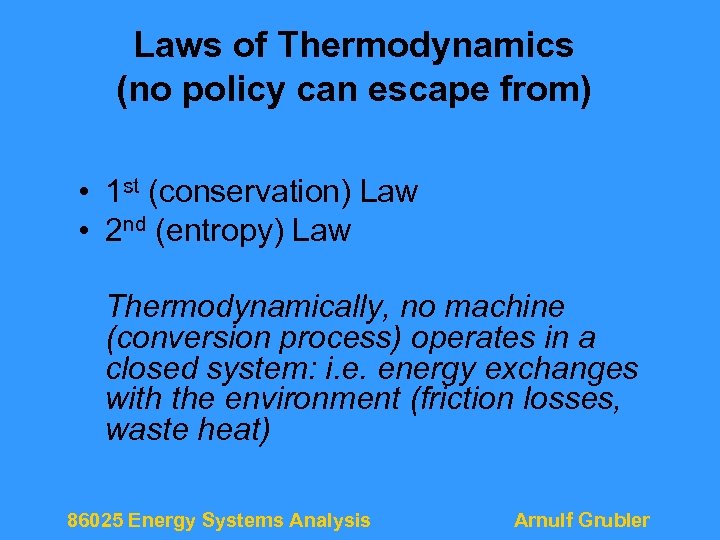
Laws of Thermodynamics (no policy can escape from) • 1 st (conservation) Law • 2 nd (entropy) Law Thermodynamically, no machine (conversion process) operates in a closed system: i. e. energy exchanges with the environment (friction losses, waste heat) 86025 Energy Systems Analysis Arnulf Grubler

Perpetuum Mobile: Impossible in a Thermodynamically Open System 86025 Energy Systems Analysis Arnulf Grubler
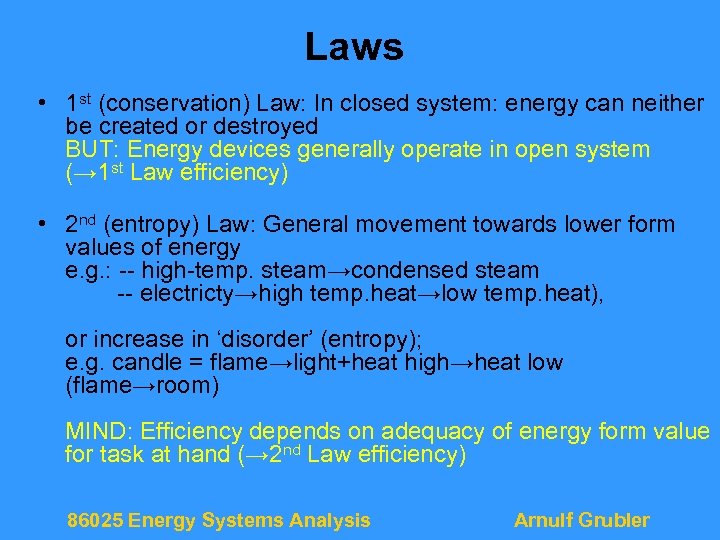
Laws • 1 st (conservation) Law: In closed system: energy can neither be created or destroyed BUT: Energy devices generally operate in open system (→ 1 st Law efficiency) • 2 nd (entropy) Law: General movement towards lower form values of energy e. g. : -- high-temp. steam→condensed steam -- electricty→high temp. heat→low temp. heat), or increase in ‘disorder’ (entropy); e. g. candle = flame→light+heat high→heat low (flame→room) MIND: Efficiency depends on adequacy of energy form value for task at hand (→ 2 nd Law efficiency) 86025 Energy Systems Analysis Arnulf Grubler
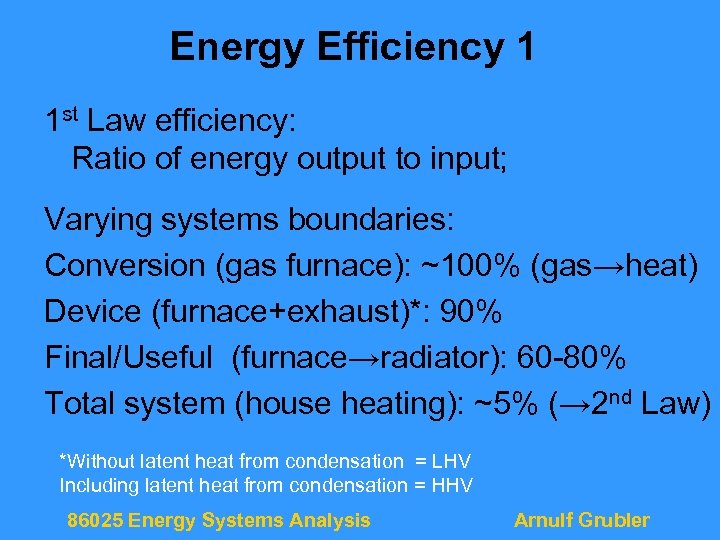
Energy Efficiency 1 1 st Law efficiency: Ratio of energy output to input; Varying systems boundaries: Conversion (gas furnace): ~100% (gas→heat) Device (furnace+exhaust)*: 90% Final/Useful (furnace→radiator): 60 -80% Total system (house heating): ~5% (→ 2 nd Law) *Without latent heat from condensation = LHV Including latent heat from condensation = HHV 86025 Energy Systems Analysis Arnulf Grubler
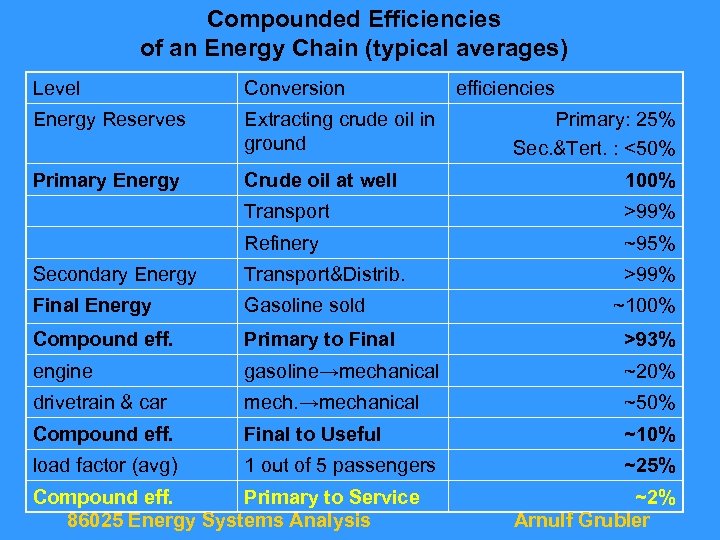
Compounded Efficiencies of an Energy Chain (typical averages) Level Conversion Energy Reserves Extracting crude oil in ground Primary Energy Crude oil at well 100% Transport >99% Refinery ~95% Secondary Energy Transport&Distrib. >99% Final Energy Gasoline sold Compound eff. Primary to Final >93% engine gasoline→mechanical ~20% drivetrain & car mech. →mechanical ~50% Compound eff. Final to Useful ~10% load factor (avg) 1 out of 5 passengers ~25% Compound eff. Primary to Service 86025 Energy Systems Analysis efficiencies Primary: 25% Sec. &Tert. : <50% ~100% ~2% Arnulf Grubler
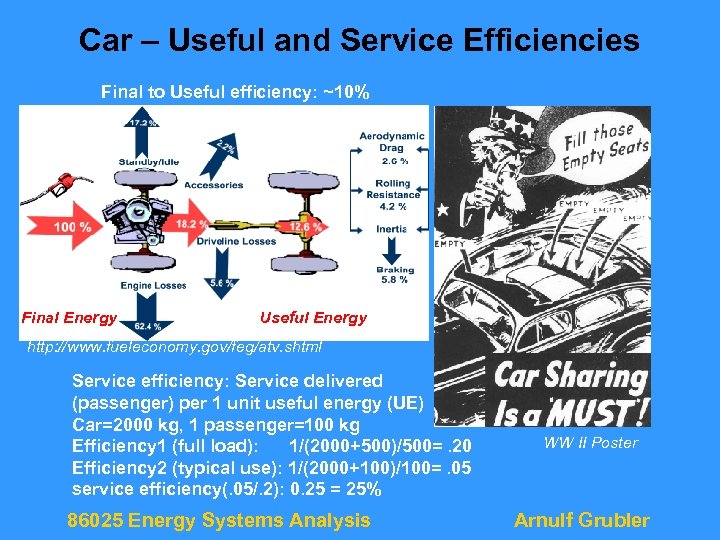
Car – Useful and Service Efficiencies Final to Useful efficiency: ~10% Final Energy Useful Energy http: //www. fueleconomy. gov/feg/atv. shtml Service efficiency: Service delivered (passenger) per 1 unit useful energy (UE) Car=2000 kg, 1 passenger=100 kg Efficiency 1 (full load): 1/(2000+500)/500=. 20 Efficiency 2 (typical use): 1/(2000+100)/100=. 05 service efficiency(. 05/. 2): 0. 25 = 25% 86025 Energy Systems Analysis WW II Poster Arnulf Grubler
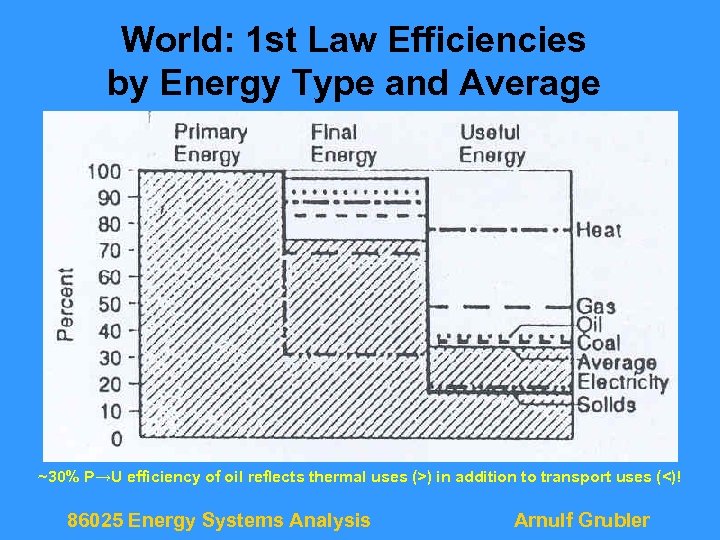
World: 1 st Law Efficiencies by Energy Type and Average ~30% P→U efficiency of oil reflects thermal uses (>) in addition to transport uses (<)! 86025 Energy Systems Analysis Arnulf Grubler
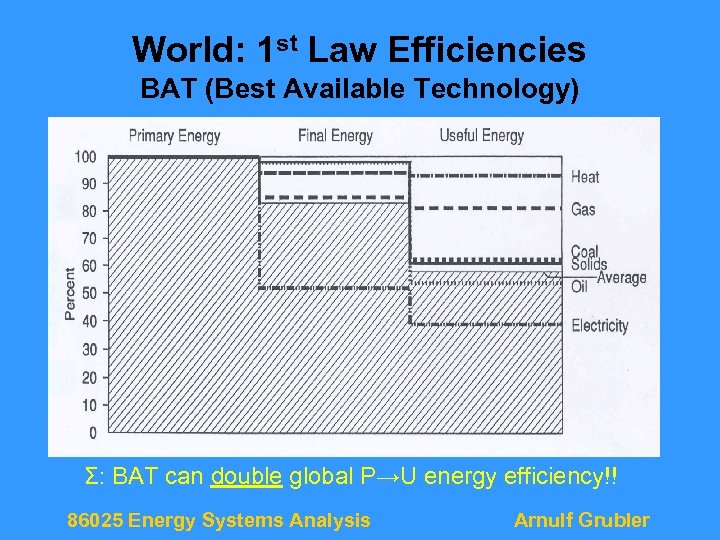
World: 1 st Law Efficiencies BAT (Best Available Technology) Σ: BAT can double global P→U energy efficiency!! 86025 Energy Systems Analysis Arnulf Grubler

Energy Efficiency 2 2 nd Law efficiency: Minimum amount of exergy required for a particular task / actual exergy spent in completing the task Exergy = availability (capacity to do useful work) = inverse of entropy (negentropy) (see candle example from Energy Primer) Hence: Quality and adequacy matters. 86025 Energy Systems Analysis Arnulf Grubler
Conversion of Chemical to Mechanical Energy: The Steam Engine Atmospheric steam engine by Francis Thompson: Motive power by condensing steam http: //www. sciencemuseum. org. uk/exhibitions/energyhall/section 3. asp 86025 Energy Systems Analysis Arnulf Grubler
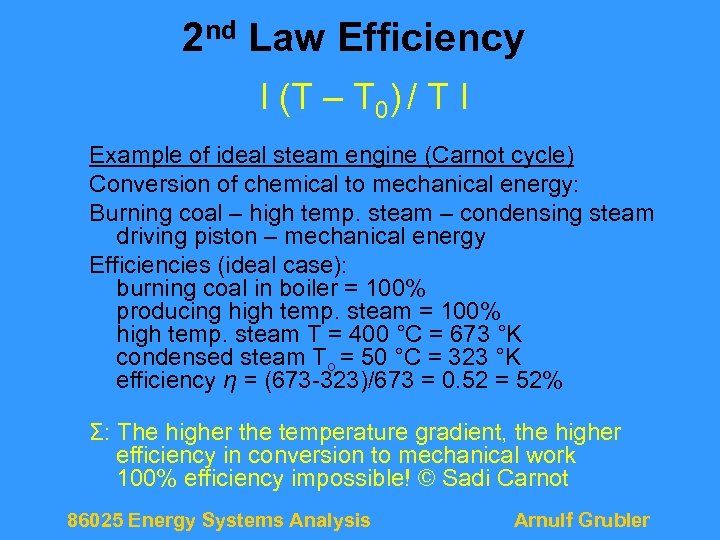
2 nd Law Efficiency I (T – T 0) / T I Example of ideal steam engine (Carnot cycle) Conversion of chemical to mechanical energy: Burning coal – high temp. steam – condensing steam driving piston – mechanical energy Efficiencies (ideal case): burning coal in boiler = 100% producing high temp. steam = 100% high temp. steam T = 400 °C = 673 °K condensed steam To = 50 °C = 323 °K efficiency η = (673 -323)/673 = 0. 52 = 52% Σ: The higher the temperature gradient, the higher efficiency in conversion to mechanical work 100% efficiency impossible! © Sadi Carnot 86025 Energy Systems Analysis Arnulf Grubler
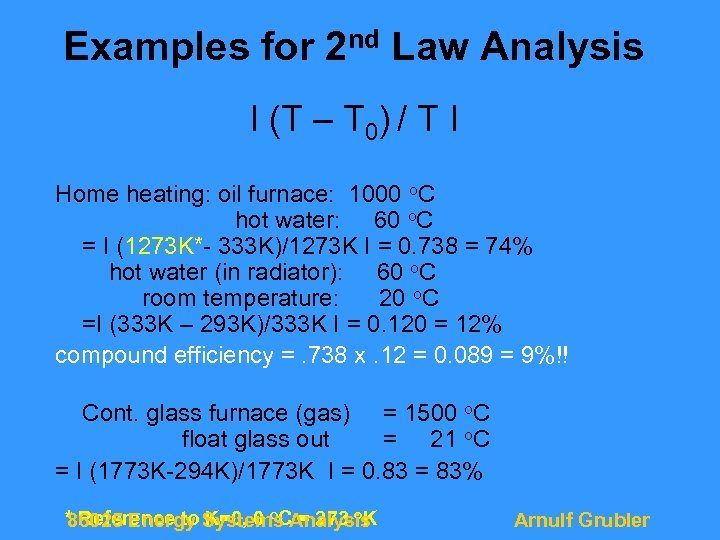
Examples for 2 nd Law Analysis I (T – T 0) / T I Home heating: oil furnace: 1000 o. C hot water: 60 o. C = I (1273 K*- 333 K)/1273 K I = 0. 738 = 74% hot water (in radiator): 60 o. C room temperature: 20 o. C =I (333 K – 293 K)/333 K I = 0. 120 = 12% compound efficiency =. 738 x. 12 = 0. 089 = 9%!! Cont. glass furnace (gas) = 1500 o. C float glass out = 21 o. C = I (1773 K-294 K)/1773 K I = 0. 83 = 83% * Reference to Systems Analysis 86025 Energy K=0, 0 o. C = 273 o. K Arnulf Grubler
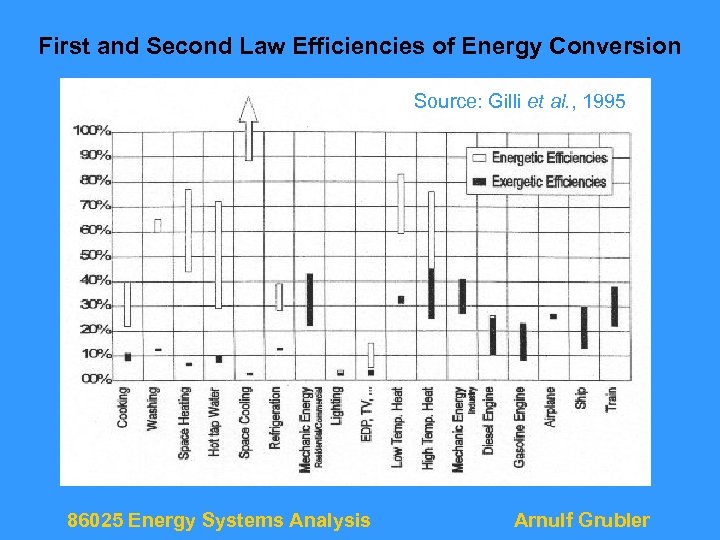
First and Second Law Efficiencies of Energy Conversion Source: Gilli et al. , 1995 86025 Energy Systems Analysis Arnulf Grubler
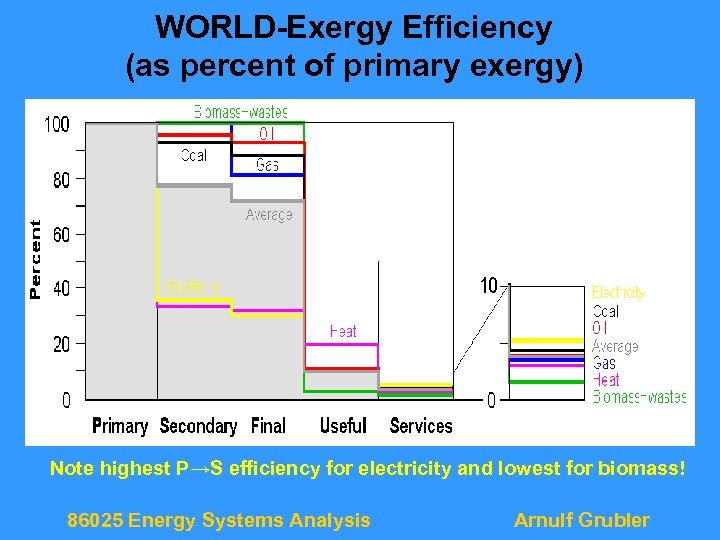
WORLD-Exergy Efficiency (as percent of primary exergy) Note highest P→S efficiency for electricity and lowest for biomass! 86025 Energy Systems Analysis Arnulf Grubler

More on this: • Class server: Boyle/Everett/Ramage: Energy Systems and Sustainability, Oxford University Press 2003 pp. 198 -200 textbook material on S. Carnot) Resources/readings/carnot_summary. pdf Gilli/Nakicenovic/Kurz: First- and Second-Law Efficiencies of the Global and Regional Energy Systems IIASA RR 96 -2 (detailed energy/exergy balances per region/sector; good material also for benchmarking presentations) Resources/readings/naki_gilli_kurz_exergy. pdf • http: //www. taftan. com/thermodynamics (concise summary and equations) • Schaeffer/Wirtshafter, 1992, An Exergy Analysis of the Brazilian Economy… Energy 17(9): 841 -855 (DC perspective) access via Yale library on-line journals Science Direct 86025 Energy Systems Analysis Arnulf Grubler
Implications: Rules of thumb for engineers and policy makers • Largest leverage: Extending system’s boundary for designs and policies • Look at exergy rather than energy alone • Largest possible efficiency gains (x 20): End-use and service efficiency, heat cascading (industrial symbiosis) BUT: • Efficiency not all (→valuation) • Main scope outside energy engineering/policy: Architecture, urban & transport planning, lifestyles, …. 86025 Energy Systems Analysis Arnulf Grubler
Valuation: Multicriteria overall performance • Efficiency (energy, exergy) • Productivity (per service rendered, e. g. value added) = Energy intensity • Costs (money, time, information) • Externalities (social, environmental) • Paramount importance of systems boundaries (“who pays”) 86025 Energy Systems Analysis Arnulf Grubler
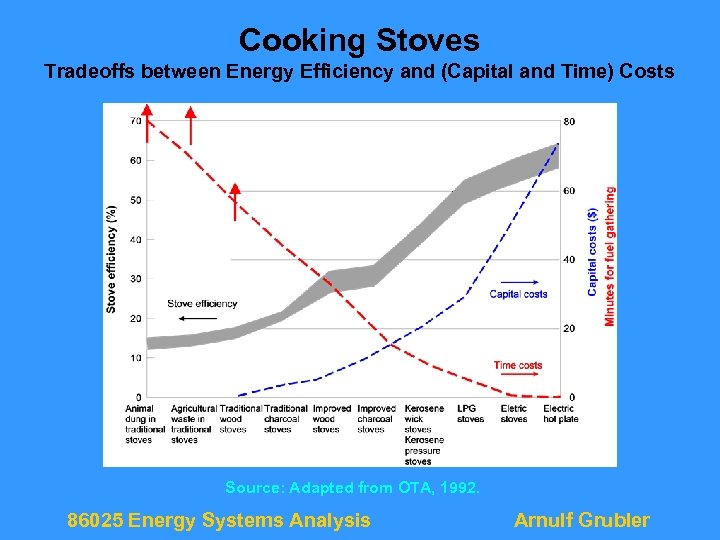
Cooking Stoves Tradeoffs between Energy Efficiency and (Capital and Time) Costs Source: Adapted from OTA, 1992. 86025 Energy Systems Analysis Arnulf Grubler
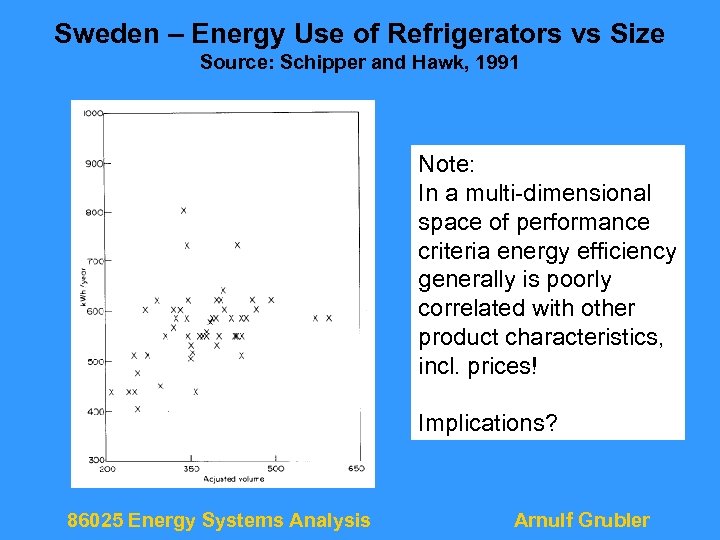
Sweden – Energy Use of Refrigerators vs Size Source: Schipper and Hawk, 1991 Note: In a multi-dimensional space of performance criteria energy efficiency generally is poorly correlated with other product characteristics, incl. prices! Implications? 86025 Energy Systems Analysis Arnulf Grubler
Efficiency: What the Long-run Data Show • Secular trend in efficiency improvement • Secular trend in energy productivity (energy intensity) gains • Complex interplay between radical and incremental technological change as well as structural change • Counter-forces: New demands, new products, slow, or stagnant capital turnover, wrong incentives 86025 Energy Systems Analysis Arnulf Grubler
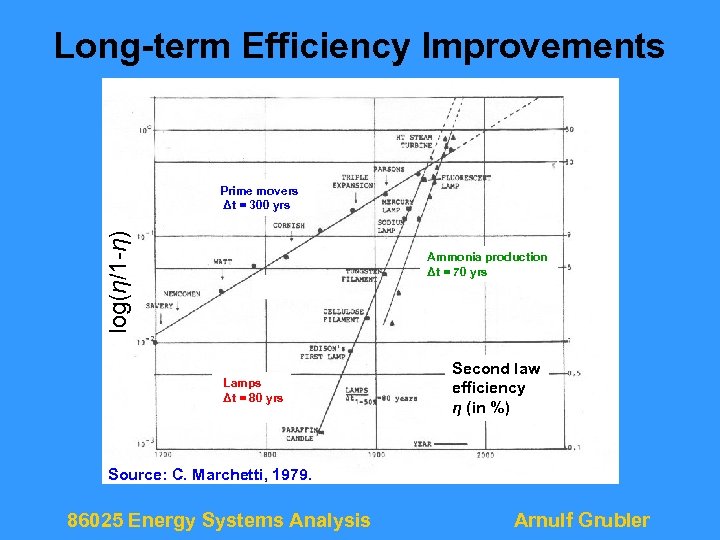
Long-term Efficiency Improvements 1 ε ---- Prime movers Δt = 300 yrs log(η/1 -η) 1 -ε Ammonia production Δt = 70 yrs Lamps Δt = 80 yrs Second law efficiency η efficiency (in %) ε (in %) Source: C. Marchetti, 1979. 86025 Energy Systems Analysis Arnulf Grubler
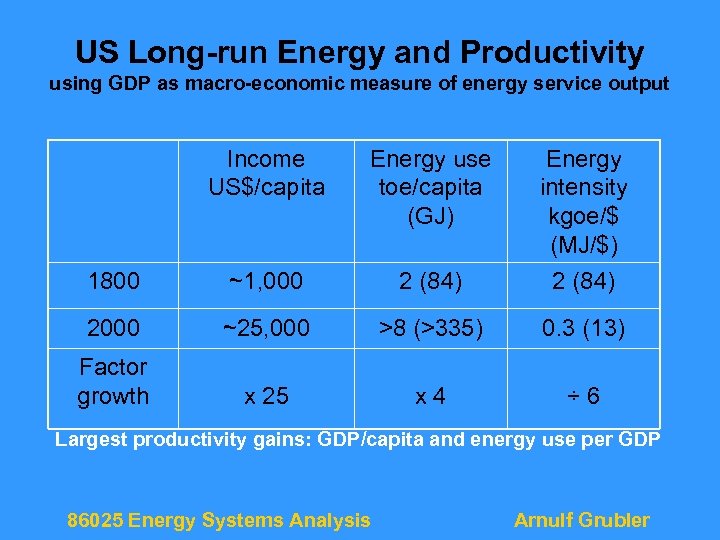
US Long-run Energy and Productivity using GDP as macro-economic measure of energy service output Income US$/capita Energy use toe/capita (GJ) 1800 ~1, 000 2 (84) Energy intensity kgoe/$ (MJ/$) 2 (84) 2000 ~25, 000 >8 (>335) 0. 3 (13) Factor growth x 25 x 4 ÷ 6 Largest productivity gains: GDP/capita and energy use per GDP 86025 Energy Systems Analysis Arnulf Grubler
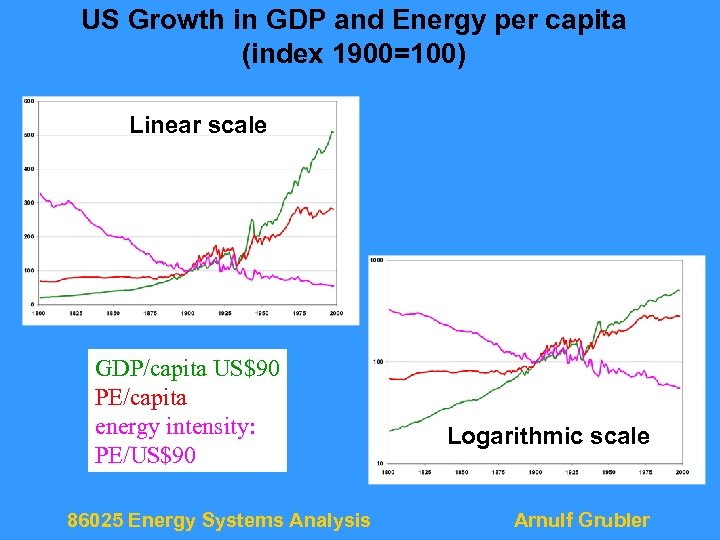
US Growth in GDP and Energy per capita (index 1900=100) Linear scale GDP/capita US$90 PE/capita energy intensity: PE/US$90 86025 Energy Systems Analysis Logarithmic scale Arnulf Grubler
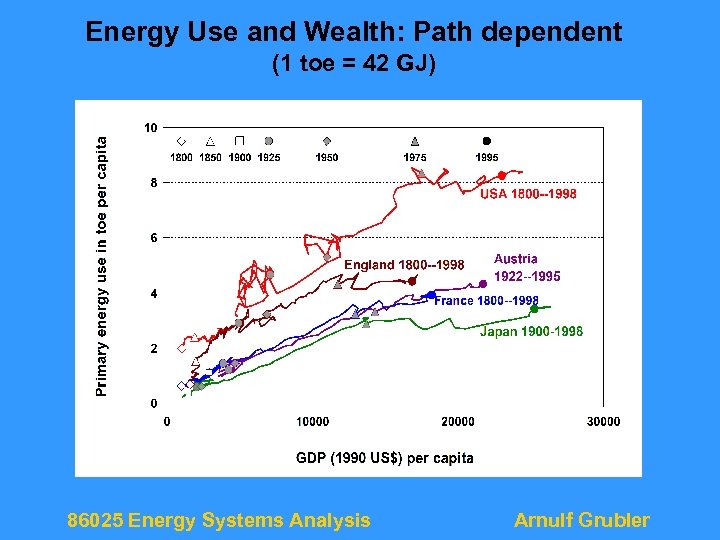
Energy Use and Wealth: Path dependent (1 toe = 42 GJ) 86025 Energy Systems Analysis Arnulf Grubler
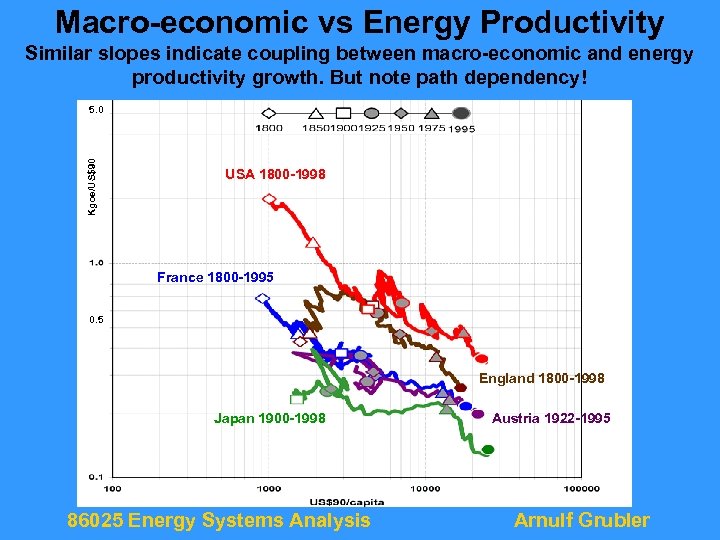
Macro-economic vs Energy Productivity Similar slopes indicate coupling between macro-economic and energy productivity growth. But note path dependency! Kgoe/US$90 5. 0 USA 1800 -1998 France 1800 -1995 0. 5 England 1800 -1998 Japan 1900 -1998 86025 Energy Systems Analysis Austria 1922 -1995 Arnulf Grubler
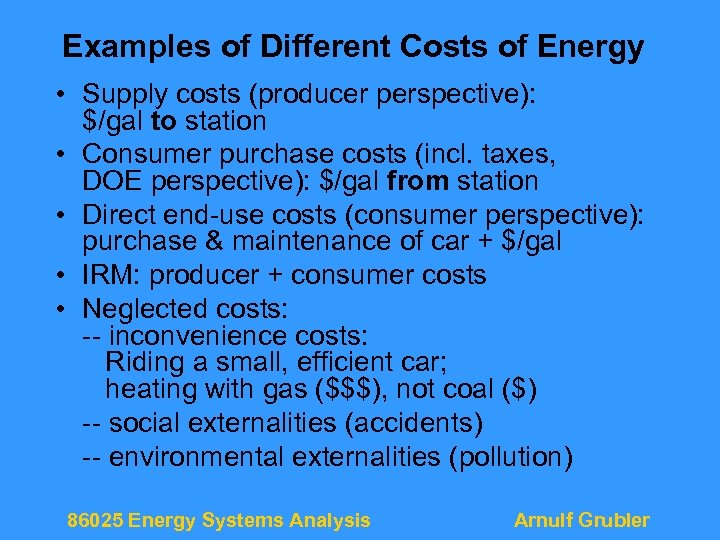
Examples of Different Costs of Energy • Supply costs (producer perspective): $/gal to station • Consumer purchase costs (incl. taxes, DOE perspective): $/gal from station • Direct end-use costs (consumer perspective): purchase & maintenance of car + $/gal • IRM: producer + consumer costs • Neglected costs: -- inconvenience costs: Riding a small, efficient car; heating with gas ($$$), not coal ($) -- social externalities (accidents) -- environmental externalities (pollution) 86025 Energy Systems Analysis Arnulf Grubler
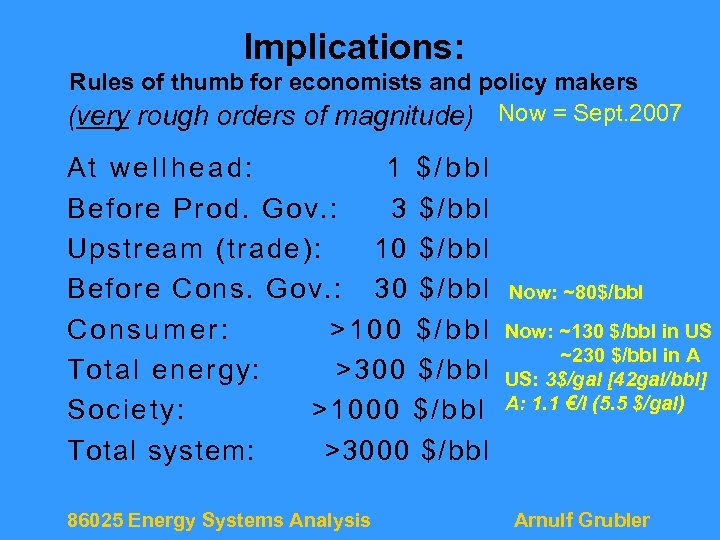
Implications: Rules of thumb for economists and policy makers (very rough orders of magnitude) Now = Sept. 2007 At wellhead: 1 $/bbl Before Prod. Gov. : 3 $/bbl Upstream (trade): 10 $/bbl Before Cons. Gov. : 30 $/bbl Consumer: >100 $/bbl Total energy: >300 $/bbl Society: >1000 $/bbl Total system: >3000 $/bbl 86025 Energy Systems Analysis Now: ~80$/bbl Now: ~130 $/bbl in US ~230 $/bbl in A US: 3$/gal [42 gal/bbl] A: 1. 1 €/l (5. 5 $/gal) Arnulf Grubler
c797f5cd3b398778c855a892e0919fd1.ppt