6265141bf84aa6221148b71081101827.ppt
- Количество слайдов: 52
 Innovation ● Investigation ● Application A Systematic Analysis of VTE Prophylaxis in the Setting of Cancer Linking Science and Evidence to Clinical Practice— What Do Trials Teach? Program Chairman Craig Kessler, MD MACP Director, Division of Coagulation Lombardi Comprehensive Cancer Center Georgetown University Medical Center Washington, DC
Innovation ● Investigation ● Application A Systematic Analysis of VTE Prophylaxis in the Setting of Cancer Linking Science and Evidence to Clinical Practice— What Do Trials Teach? Program Chairman Craig Kessler, MD MACP Director, Division of Coagulation Lombardi Comprehensive Cancer Center Georgetown University Medical Center Washington, DC
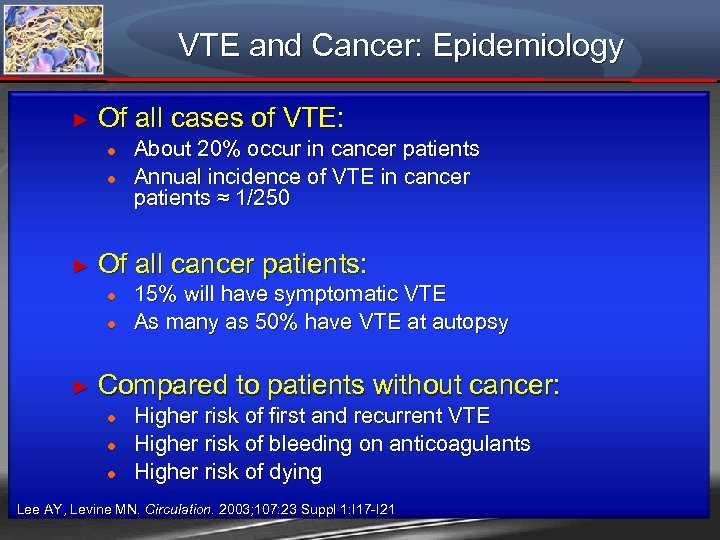 VTE and Cancer: Epidemiology ► Of all cases of VTE: ● ● ► Of all cancer patients: ● ● ► About 20% occur in cancer patients Annual incidence of VTE in cancer patients ≈ 1/250 15% will have symptomatic VTE As many as 50% have VTE at autopsy Compared to patients without cancer: ● ● ● Higher risk of first and recurrent VTE Higher risk of bleeding on anticoagulants Higher risk of dying Lee AY, Levine MN. Circulation. 2003; 107: 23 Suppl 1: I 17 -I 21
VTE and Cancer: Epidemiology ► Of all cases of VTE: ● ● ► Of all cancer patients: ● ● ► About 20% occur in cancer patients Annual incidence of VTE in cancer patients ≈ 1/250 15% will have symptomatic VTE As many as 50% have VTE at autopsy Compared to patients without cancer: ● ● ● Higher risk of first and recurrent VTE Higher risk of bleeding on anticoagulants Higher risk of dying Lee AY, Levine MN. Circulation. 2003; 107: 23 Suppl 1: I 17 -I 21
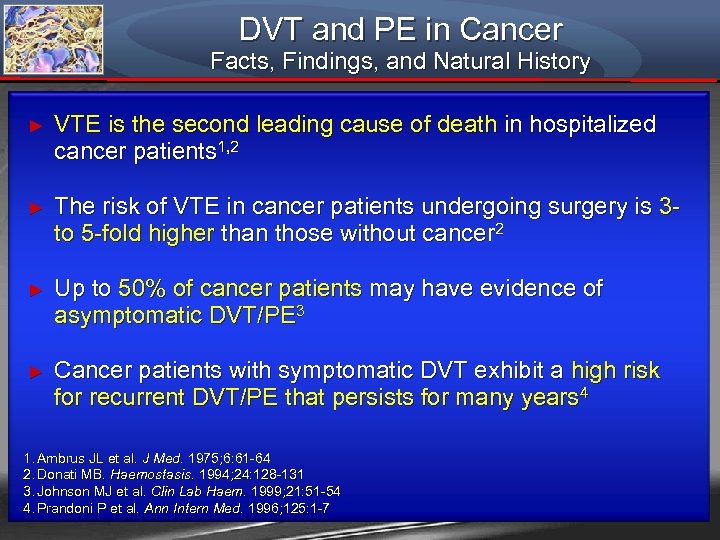 DVT and PE in Cancer Facts, Findings, and Natural History ► VTE is the second leading cause of death in hospitalized cancer patients 1, 2 ► The risk of VTE in cancer patients undergoing surgery is 3 - to 5 -fold higher than those without cancer 2 ► Up to 50% of cancer patients may have evidence of asymptomatic DVT/PE 3 ► Cancer patients with symptomatic DVT exhibit a high risk for recurrent DVT/PE that persists for many years 4 1. Ambrus JL et al. J Med. 1975; 6: 61 -64 2. Donati MB. Haemostasis. 1994; 24: 128 -131 3. Johnson MJ et al. Clin Lab Haem. 1999; 21: 51 -54 4. Prandoni P et al. Ann Intern Med. 1996; 125: 1 -7
DVT and PE in Cancer Facts, Findings, and Natural History ► VTE is the second leading cause of death in hospitalized cancer patients 1, 2 ► The risk of VTE in cancer patients undergoing surgery is 3 - to 5 -fold higher than those without cancer 2 ► Up to 50% of cancer patients may have evidence of asymptomatic DVT/PE 3 ► Cancer patients with symptomatic DVT exhibit a high risk for recurrent DVT/PE that persists for many years 4 1. Ambrus JL et al. J Med. 1975; 6: 61 -64 2. Donati MB. Haemostasis. 1994; 24: 128 -131 3. Johnson MJ et al. Clin Lab Haem. 1999; 21: 51 -54 4. Prandoni P et al. Ann Intern Med. 1996; 125: 1 -7
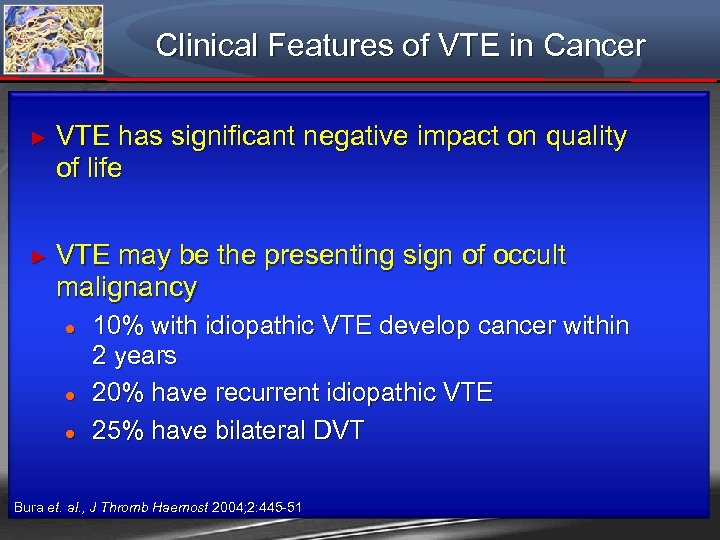 Clinical Features of VTE in Cancer ► VTE has significant negative impact on quality of life ► VTE may be the presenting sign of occult malignancy ● ● ● 10% with idiopathic VTE develop cancer within 2 years 20% have recurrent idiopathic VTE 25% have bilateral DVT Bura et. al. , J Thromb Haemost 2004; 2: 445 -51
Clinical Features of VTE in Cancer ► VTE has significant negative impact on quality of life ► VTE may be the presenting sign of occult malignancy ● ● ● 10% with idiopathic VTE develop cancer within 2 years 20% have recurrent idiopathic VTE 25% have bilateral DVT Bura et. al. , J Thromb Haemost 2004; 2: 445 -51
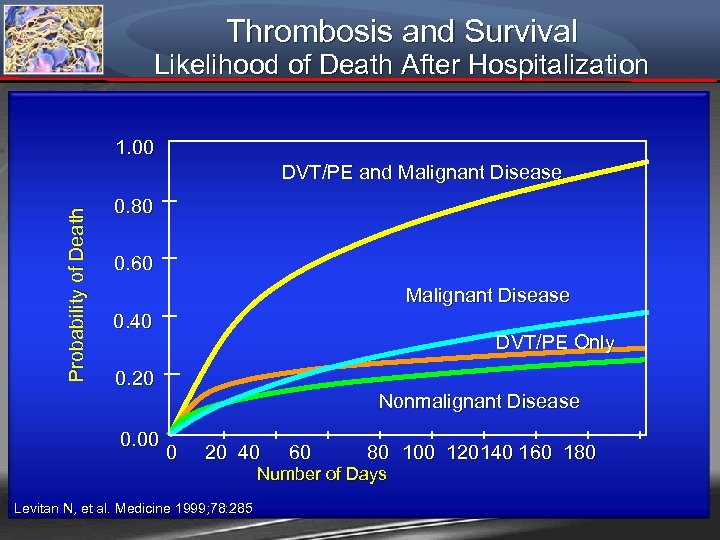 Thrombosis and Survival Likelihood of Death After Hospitalization 1. 00 Probability of Death DVT/PE and Malignant Disease 0. 80 0. 60 Malignant Disease 0. 40 DVT/PE Only 0. 20 0. 00 Nonmalignant Disease 0 20 40 60 80 100 120140 160 180 Number of Days Levitan N, et al. Medicine 1999; 78: 285
Thrombosis and Survival Likelihood of Death After Hospitalization 1. 00 Probability of Death DVT/PE and Malignant Disease 0. 80 0. 60 Malignant Disease 0. 40 DVT/PE Only 0. 20 0. 00 Nonmalignant Disease 0 20 40 60 80 100 120140 160 180 Number of Days Levitan N, et al. Medicine 1999; 78: 285
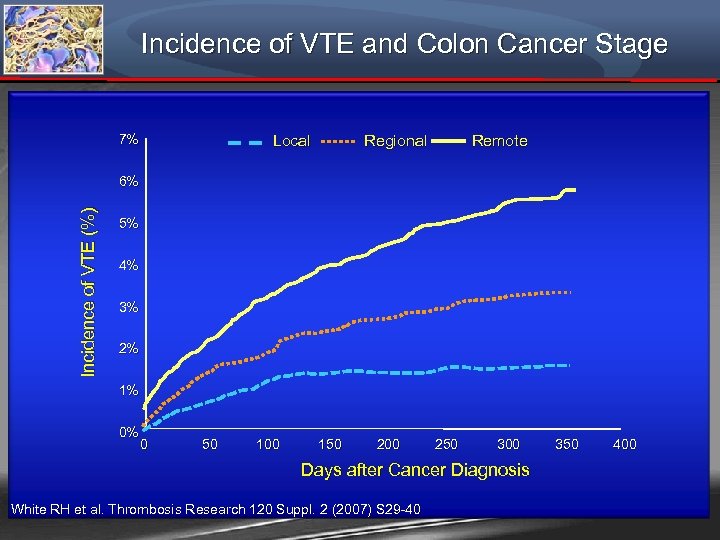 Incidence of VTE and Colon Cancer Stage 7% Local Regional Remote Incidence of VTE (%) 6% 5% 4% 3% 2% 1% 0% 0 50 100 150 200 250 300 350 400 Days after Cancer Diagnosis White RH et al. Thrombosis Research 120 Suppl. 2 (2007) S 29 -40
Incidence of VTE and Colon Cancer Stage 7% Local Regional Remote Incidence of VTE (%) 6% 5% 4% 3% 2% 1% 0% 0 50 100 150 200 250 300 350 400 Days after Cancer Diagnosis White RH et al. Thrombosis Research 120 Suppl. 2 (2007) S 29 -40
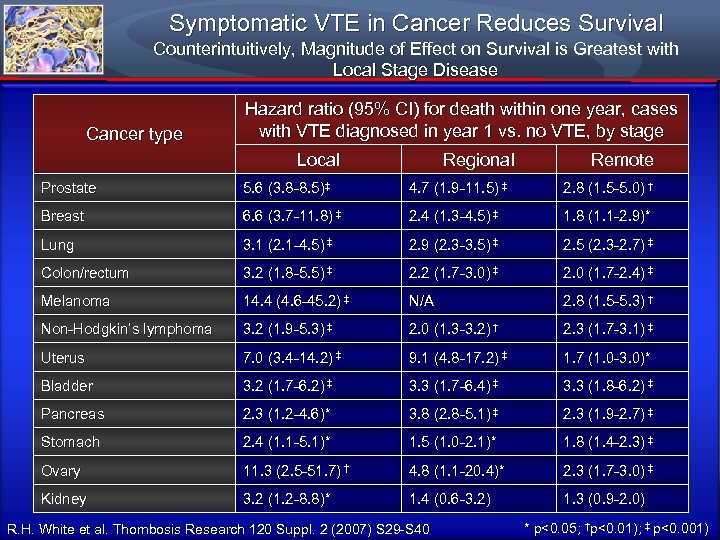 Symptomatic VTE in Cancer Reduces Survival Counterintuitively, Magnitude of Effect on Survival is Greatest with Local Stage Disease Cancer type Hazard ratio (95% CI) for death within one year, cases with VTE diagnosed in year 1 vs. no VTE, by stage Local Regional Remote Prostate 5. 6 (3. 8 -8. 5)‡ 4. 7 (1. 9 -11. 5) ‡ 2. 8 (1. 5 -5. 0) † Breast 6. 6 (3. 7 -11. 8) ‡ 2. 4 (1. 3 -4. 5) ‡ 1. 8 (1. 1 -2. 9)* Lung 3. 1 (2. 1 -4. 5) ‡ 2. 9 (2. 3 -3. 5) ‡ 2. 5 (2. 3 -2. 7) ‡ Colon/rectum 3. 2 (1. 8 -5. 5) ‡ 2. 2 (1. 7 -3. 0) ‡ 2. 0 (1. 7 -2. 4) ‡ Melanoma 14. 4 (4. 6 -45. 2) ‡ N/A 2. 8 (1. 5 -5. 3) † Non-Hodgkin’s lymphoma 3. 2 (1. 9 -5. 3) ‡ 2. 0 (1. 3 -3. 2) † 2. 3 (1. 7 -3. 1) ‡ Uterus 7. 0 (3. 4 -14. 2) ‡ 9. 1 (4. 8 -17. 2) ‡ 1. 7 (1. 0 -3. 0)* Bladder 3. 2 (1. 7 -6. 2) ‡ 3. 3 (1. 7 -6. 4) ‡ 3. 3 (1. 8 -6. 2) ‡ Pancreas 2. 3 (1. 2 -4. 6)* 3. 8 (2. 8 -5. 1) ‡ 2. 3 (1. 9 -2. 7) ‡ Stomach 2. 4 (1. 1 -5. 1)* 1. 5 (1. 0 -2. 1)* 1. 8 (1. 4 -2. 3) ‡ Ovary 11. 3 (2. 5 -51. 7) † 4. 8 (1. 1 -20. 4)* 2. 3 (1. 7 -3. 0) ‡ Kidney 3. 2 (1. 2 -8. 8)* 1. 4 (0. 6 -3. 2) 1. 3 (0. 9 -2. 0) R. H. White et al. Thombosis Research 120 Suppl. 2 (2007) S 29 -S 40 * p<0. 05; †p<0. 01); ‡ p<0. 001)
Symptomatic VTE in Cancer Reduces Survival Counterintuitively, Magnitude of Effect on Survival is Greatest with Local Stage Disease Cancer type Hazard ratio (95% CI) for death within one year, cases with VTE diagnosed in year 1 vs. no VTE, by stage Local Regional Remote Prostate 5. 6 (3. 8 -8. 5)‡ 4. 7 (1. 9 -11. 5) ‡ 2. 8 (1. 5 -5. 0) † Breast 6. 6 (3. 7 -11. 8) ‡ 2. 4 (1. 3 -4. 5) ‡ 1. 8 (1. 1 -2. 9)* Lung 3. 1 (2. 1 -4. 5) ‡ 2. 9 (2. 3 -3. 5) ‡ 2. 5 (2. 3 -2. 7) ‡ Colon/rectum 3. 2 (1. 8 -5. 5) ‡ 2. 2 (1. 7 -3. 0) ‡ 2. 0 (1. 7 -2. 4) ‡ Melanoma 14. 4 (4. 6 -45. 2) ‡ N/A 2. 8 (1. 5 -5. 3) † Non-Hodgkin’s lymphoma 3. 2 (1. 9 -5. 3) ‡ 2. 0 (1. 3 -3. 2) † 2. 3 (1. 7 -3. 1) ‡ Uterus 7. 0 (3. 4 -14. 2) ‡ 9. 1 (4. 8 -17. 2) ‡ 1. 7 (1. 0 -3. 0)* Bladder 3. 2 (1. 7 -6. 2) ‡ 3. 3 (1. 7 -6. 4) ‡ 3. 3 (1. 8 -6. 2) ‡ Pancreas 2. 3 (1. 2 -4. 6)* 3. 8 (2. 8 -5. 1) ‡ 2. 3 (1. 9 -2. 7) ‡ Stomach 2. 4 (1. 1 -5. 1)* 1. 5 (1. 0 -2. 1)* 1. 8 (1. 4 -2. 3) ‡ Ovary 11. 3 (2. 5 -51. 7) † 4. 8 (1. 1 -20. 4)* 2. 3 (1. 7 -3. 0) ‡ Kidney 3. 2 (1. 2 -8. 8)* 1. 4 (0. 6 -3. 2) 1. 3 (0. 9 -2. 0) R. H. White et al. Thombosis Research 120 Suppl. 2 (2007) S 29 -S 40 * p<0. 05; †p<0. 01); ‡ p<0. 001)
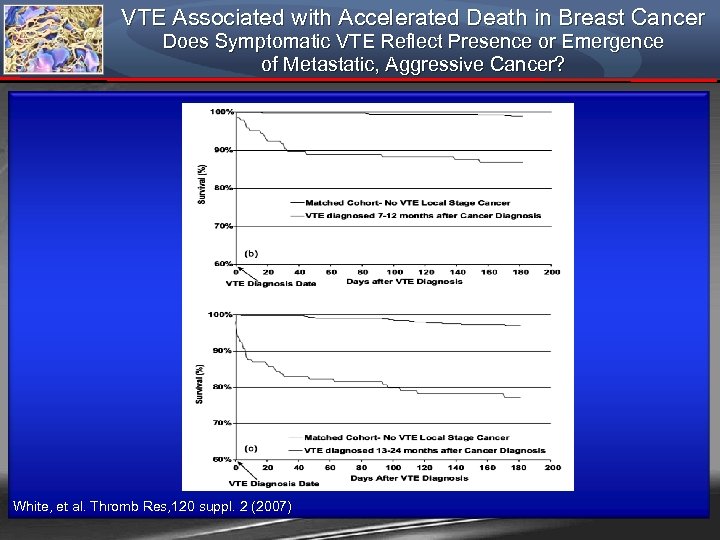 VTE Associated with Accelerated Death in Breast Cancer Does Symptomatic VTE Reflect Presence or Emergence of Metastatic, Aggressive Cancer? White, et al. Thromb Res, 120 suppl. 2 (2007)
VTE Associated with Accelerated Death in Breast Cancer Does Symptomatic VTE Reflect Presence or Emergence of Metastatic, Aggressive Cancer? White, et al. Thromb Res, 120 suppl. 2 (2007)
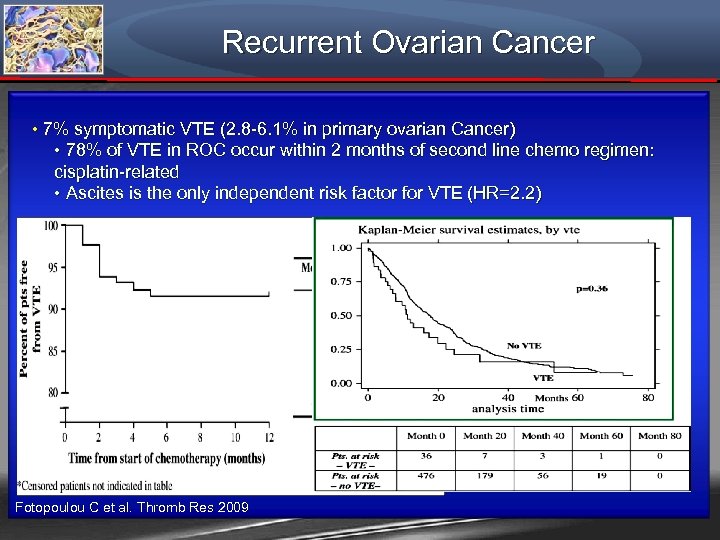 Recurrent Ovarian Cancer • 7% symptomatic VTE (2. 8 -6. 1% in primary ovarian Cancer) • 78% of VTE in ROC occur within 2 months of second line chemo regimen: cisplatin-related • Ascites is the only independent risk factor for VTE (HR=2. 2) Fotopoulou C et al. Thromb Res 2009
Recurrent Ovarian Cancer • 7% symptomatic VTE (2. 8 -6. 1% in primary ovarian Cancer) • 78% of VTE in ROC occur within 2 months of second line chemo regimen: cisplatin-related • Ascites is the only independent risk factor for VTE (HR=2. 2) Fotopoulou C et al. Thromb Res 2009
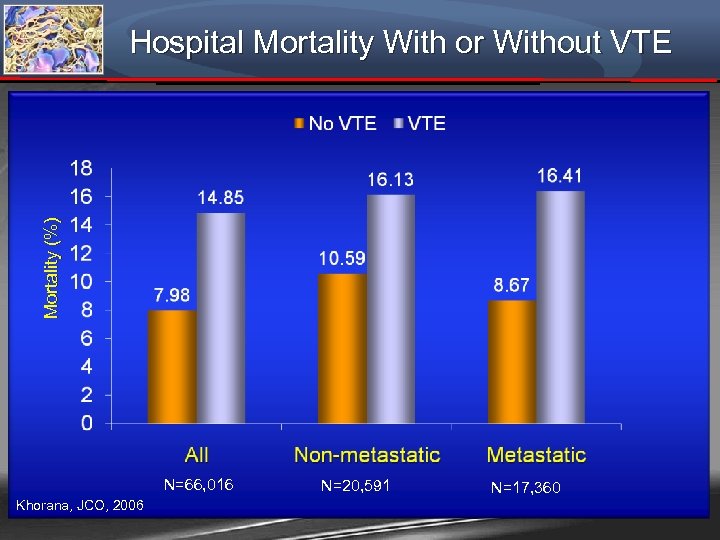 Mortality (%) Hospital Mortality With or Without VTE N=66, 016 Khorana, JCO, 2006 N=20, 591 N=17, 360
Mortality (%) Hospital Mortality With or Without VTE N=66, 016 Khorana, JCO, 2006 N=20, 591 N=17, 360
 Thrombosis Risk In Cancer Primary Prophylaxis ► Medical Inpatients ► Surgery ► Radiotherapy ► Central Venous Catheters
Thrombosis Risk In Cancer Primary Prophylaxis ► Medical Inpatients ► Surgery ► Radiotherapy ► Central Venous Catheters
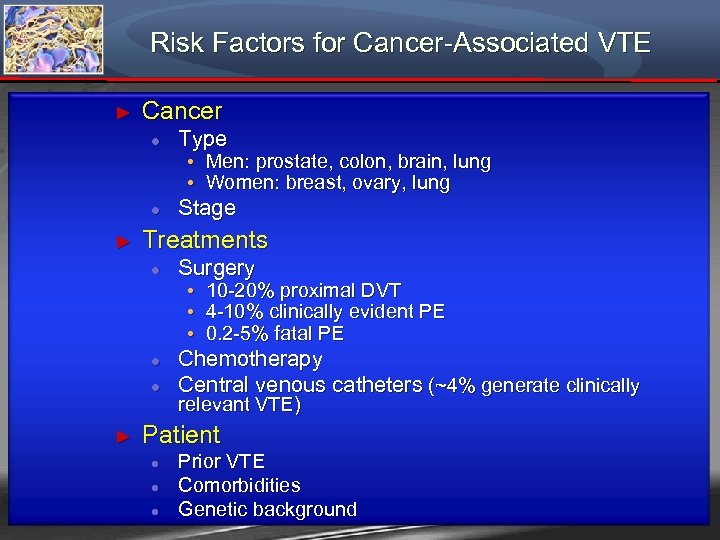 Risk Factors for Cancer-Associated VTE ► Cancer ● ● ► Type Stage • Men: prostate, colon, brain, lung • Women: breast, ovary, lung Treatments ● Surgery ● Chemotherapy Central venous catheters (~4% generate clinically ● ► • 10 -20% proximal DVT • 4 -10% clinically evident PE • 0. 2 -5% fatal PE relevant VTE) Patient ● ● ● Prior VTE Comorbidities Genetic background
Risk Factors for Cancer-Associated VTE ► Cancer ● ● ► Type Stage • Men: prostate, colon, brain, lung • Women: breast, ovary, lung Treatments ● Surgery ● Chemotherapy Central venous catheters (~4% generate clinically ● ► • 10 -20% proximal DVT • 4 -10% clinically evident PE • 0. 2 -5% fatal PE relevant VTE) Patient ● ● ● Prior VTE Comorbidities Genetic background
 Cancer and Thrombosis Medical Inpatients
Cancer and Thrombosis Medical Inpatients
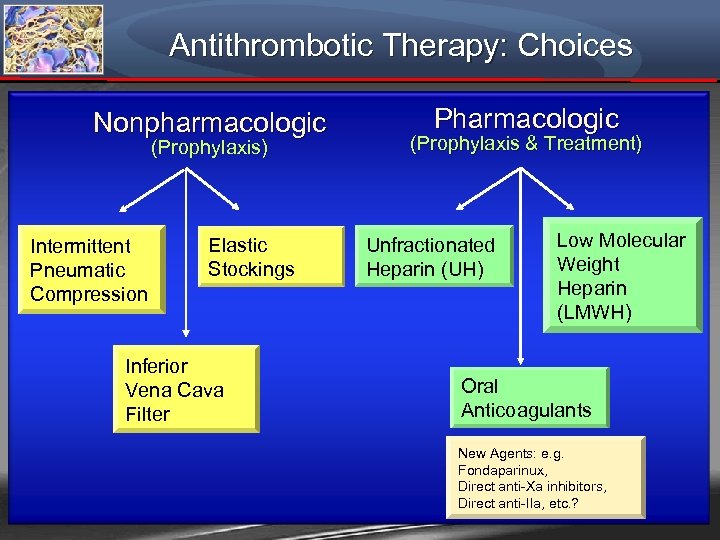 Antithrombotic Therapy: Choices Nonpharmacologic (Prophylaxis) Intermittent Pneumatic Compression Elastic Stockings Inferior Vena Cava Filter Pharmacologic (Prophylaxis & Treatment) Unfractionated Heparin (UH) Low Molecular Weight Heparin (LMWH) Oral Anticoagulants New Agents: e. g. Fondaparinux, Direct anti-Xa inhibitors, Direct anti-IIa, etc. ?
Antithrombotic Therapy: Choices Nonpharmacologic (Prophylaxis) Intermittent Pneumatic Compression Elastic Stockings Inferior Vena Cava Filter Pharmacologic (Prophylaxis & Treatment) Unfractionated Heparin (UH) Low Molecular Weight Heparin (LMWH) Oral Anticoagulants New Agents: e. g. Fondaparinux, Direct anti-Xa inhibitors, Direct anti-IIa, etc. ?
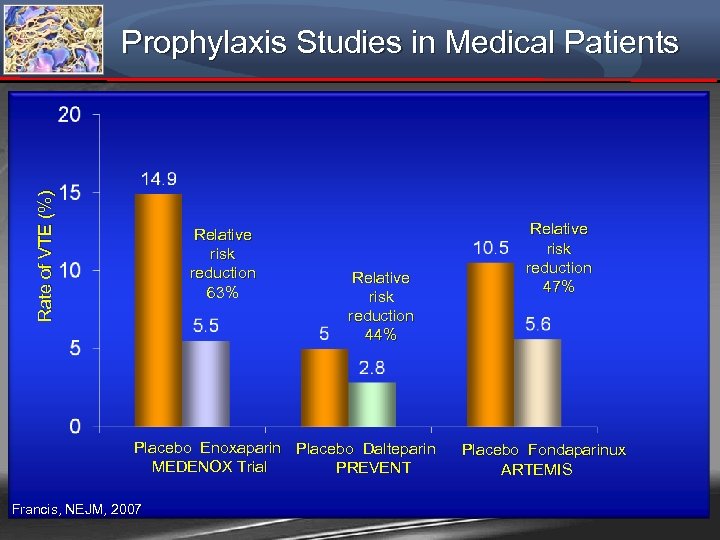 Rate of VTE (%) Prophylaxis Studies in Medical Patients Relative risk reduction 63% Relative risk reduction 44% Placebo Enoxaparin Placebo Dalteparin MEDENOX Trial PREVENT Francis, NEJM, 2007 Relative risk reduction 47% Placebo Fondaparinux ARTEMIS
Rate of VTE (%) Prophylaxis Studies in Medical Patients Relative risk reduction 63% Relative risk reduction 44% Placebo Enoxaparin Placebo Dalteparin MEDENOX Trial PREVENT Francis, NEJM, 2007 Relative risk reduction 47% Placebo Fondaparinux ARTEMIS
 ASCO Guidelines 1. SHOULD HOSPITALIZED PATIENTS WITH CANCER RECEIVE ANTICOAGULATION FOR VTE PROPHYLAXIS? Recommendation. Hospitalized patients with cancer should be considered candidates for VTE prophylaxis with anticoagulants in the absence of bleeding or other contraindications to anticoagulation. Lyman GH et al. J Clin Oncol (25) 2007; 34: 5490 -5505.
ASCO Guidelines 1. SHOULD HOSPITALIZED PATIENTS WITH CANCER RECEIVE ANTICOAGULATION FOR VTE PROPHYLAXIS? Recommendation. Hospitalized patients with cancer should be considered candidates for VTE prophylaxis with anticoagulants in the absence of bleeding or other contraindications to anticoagulation. Lyman GH et al. J Clin Oncol (25) 2007; 34: 5490 -5505.
 Cancer and Thrombosis Surgical Patients
Cancer and Thrombosis Surgical Patients
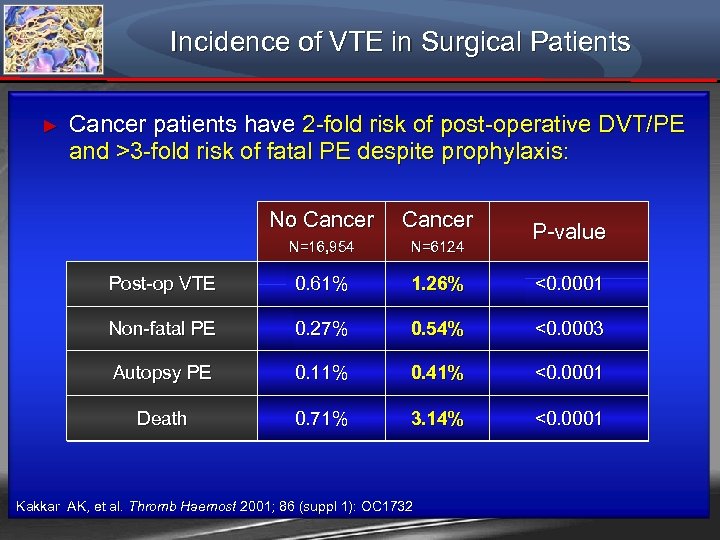 Incidence of VTE in Surgical Patients ► Cancer patients have 2 -fold risk of post-operative DVT/PE and >3 -fold risk of fatal PE despite prophylaxis: No Cancer N=16, 954 N=6124 Post-op VTE 0. 61% 1. 26% <0. 0001 Non-fatal PE 0. 27% 0. 54% <0. 0003 Autopsy PE 0. 11% 0. 41% <0. 0001 Death 0. 71% 3. 14% <0. 0001 Kakkar AK, et al. Thromb Haemost 2001; 86 (suppl 1): OC 1732 P-value
Incidence of VTE in Surgical Patients ► Cancer patients have 2 -fold risk of post-operative DVT/PE and >3 -fold risk of fatal PE despite prophylaxis: No Cancer N=16, 954 N=6124 Post-op VTE 0. 61% 1. 26% <0. 0001 Non-fatal PE 0. 27% 0. 54% <0. 0003 Autopsy PE 0. 11% 0. 41% <0. 0001 Death 0. 71% 3. 14% <0. 0001 Kakkar AK, et al. Thromb Haemost 2001; 86 (suppl 1): OC 1732 P-value
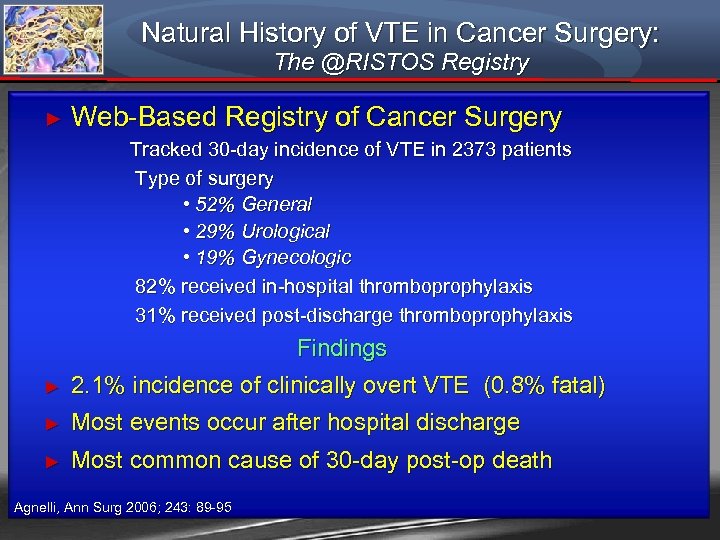 Natural History of VTE in Cancer Surgery: The @RISTOS Registry ► Web-Based Registry of Cancer Surgery Tracked 30 -day incidence of VTE in 2373 patients Type of surgery • 52% General • 29% Urological • 19% Gynecologic 82% received in-hospital thromboprophylaxis 31% received post-discharge thromboprophylaxis Findings ► 2. 1% incidence of clinically overt VTE (0. 8% fatal) ► Most events occur after hospital discharge ► Most common cause of 30 -day post-op death Agnelli, Ann Surg 2006; 243: 89 -95
Natural History of VTE in Cancer Surgery: The @RISTOS Registry ► Web-Based Registry of Cancer Surgery Tracked 30 -day incidence of VTE in 2373 patients Type of surgery • 52% General • 29% Urological • 19% Gynecologic 82% received in-hospital thromboprophylaxis 31% received post-discharge thromboprophylaxis Findings ► 2. 1% incidence of clinically overt VTE (0. 8% fatal) ► Most events occur after hospital discharge ► Most common cause of 30 -day post-op death Agnelli, Ann Surg 2006; 243: 89 -95
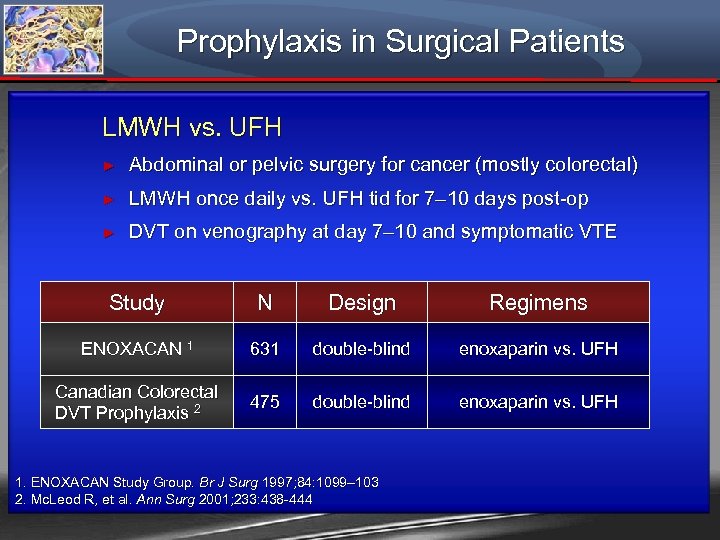 Prophylaxis in Surgical Patients LMWH vs. UFH ► Abdominal or pelvic surgery for cancer (mostly colorectal) ► LMWH once daily vs. UFH tid for 7– 10 days post-op ► DVT on venography at day 7– 10 and symptomatic VTE Study N Design Regimens ENOXACAN 1 631 double-blind enoxaparin vs. UFH Canadian Colorectal DVT Prophylaxis 2 475 double-blind enoxaparin vs. UFH 1. ENOXACAN Study Group. Br J Surg 1997; 84: 1099– 103 2. Mc. Leod R, et al. Ann Surg 2001; 233: 438 -444
Prophylaxis in Surgical Patients LMWH vs. UFH ► Abdominal or pelvic surgery for cancer (mostly colorectal) ► LMWH once daily vs. UFH tid for 7– 10 days post-op ► DVT on venography at day 7– 10 and symptomatic VTE Study N Design Regimens ENOXACAN 1 631 double-blind enoxaparin vs. UFH Canadian Colorectal DVT Prophylaxis 2 475 double-blind enoxaparin vs. UFH 1. ENOXACAN Study Group. Br J Surg 1997; 84: 1099– 103 2. Mc. Leod R, et al. Ann Surg 2001; 233: 438 -444
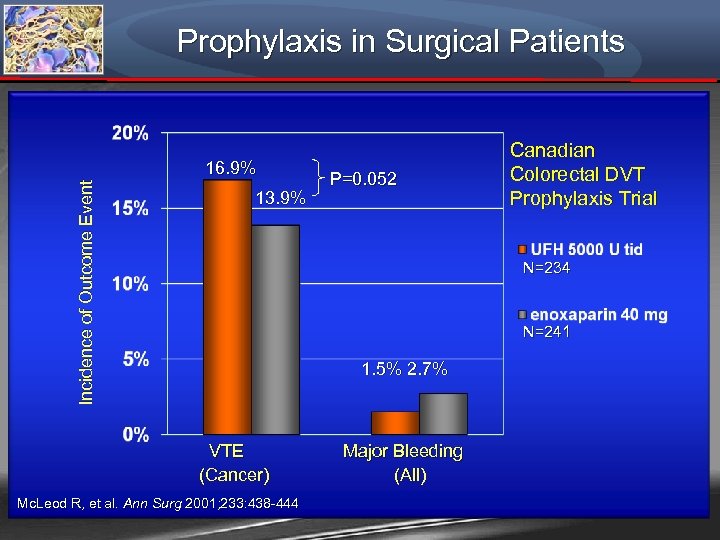 Prophylaxis in Surgical Patients Incidence of Outcome Event 16. 9% 13. 9% P=0. 052 Canadian Colorectal DVT Prophylaxis Trial N=234 N=241 1. 5% 2. 7% VTE Major Bleeding (Cancer) (All) Mc. Leod R, et al. Ann Surg 2001; 233: 438 -444
Prophylaxis in Surgical Patients Incidence of Outcome Event 16. 9% 13. 9% P=0. 052 Canadian Colorectal DVT Prophylaxis Trial N=234 N=241 1. 5% 2. 7% VTE Major Bleeding (Cancer) (All) Mc. Leod R, et al. Ann Surg 2001; 233: 438 -444
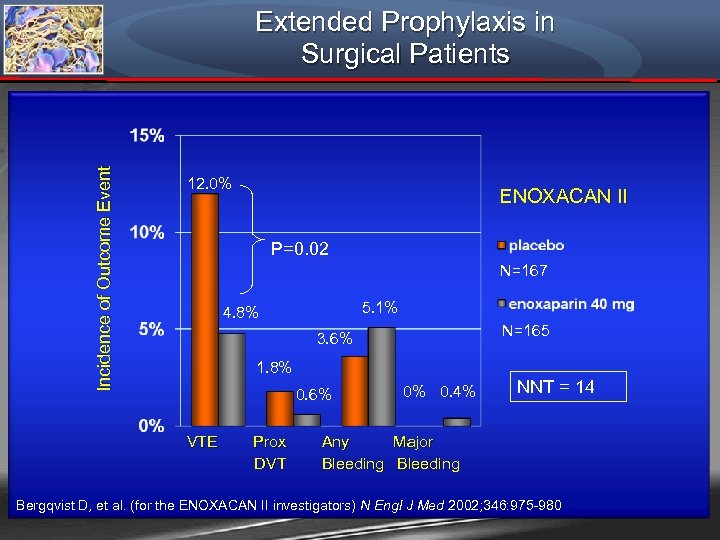 Incidence of Outcome Event Extended Prophylaxis in Surgical Patients 12. 0% ENOXACAN II P=0. 02 N=167 5. 1% 4. 8% N=165 3. 6% 1. 8% 0. 6% 0% 0. 4% NNT = 14 VTE Prox Any Major DVT Bleeding Bergqvist D, et al. (for the ENOXACAN II investigators) N Engl J Med 2002; 346: 975 -980
Incidence of Outcome Event Extended Prophylaxis in Surgical Patients 12. 0% ENOXACAN II P=0. 02 N=167 5. 1% 4. 8% N=165 3. 6% 1. 8% 0. 6% 0% 0. 4% NNT = 14 VTE Prox Any Major DVT Bleeding Bergqvist D, et al. (for the ENOXACAN II investigators) N Engl J Med 2002; 346: 975 -980
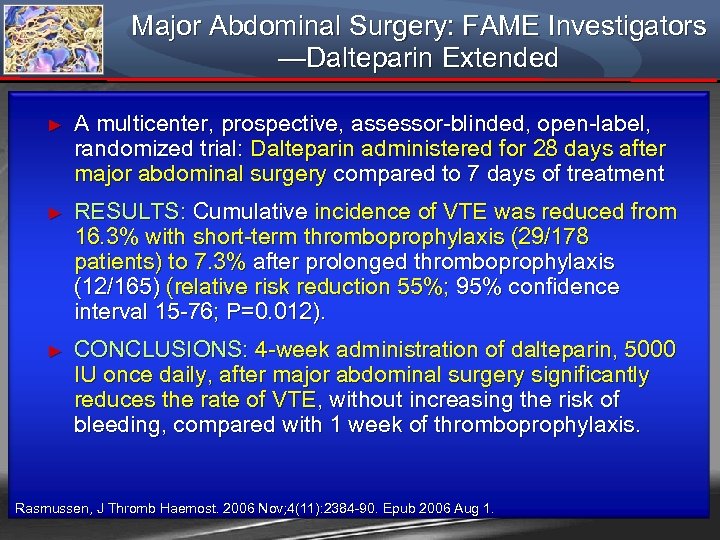 Major Abdominal Surgery: FAME Investigators —Dalteparin Extended ► A multicenter, prospective, assessor-blinded, open-label, randomized trial: Dalteparin administered for 28 days after major abdominal surgery compared to 7 days of treatment ► RESULTS: Cumulative incidence of VTE was reduced from 16. 3% with short-term thromboprophylaxis (29/178 patients) to 7. 3% after prolonged thromboprophylaxis (12/165) (relative risk reduction 55%; 95% confidence interval 15 -76; P=0. 012). ► CONCLUSIONS: 4 -week administration of dalteparin, 5000 IU once daily, after major abdominal surgery significantly reduces the rate of VTE, without increasing the risk of bleeding, compared with 1 week of thromboprophylaxis. Rasmussen, J Thromb Haemost. 2006 Nov; 4(11): 2384 -90. Epub 2006 Aug 1.
Major Abdominal Surgery: FAME Investigators —Dalteparin Extended ► A multicenter, prospective, assessor-blinded, open-label, randomized trial: Dalteparin administered for 28 days after major abdominal surgery compared to 7 days of treatment ► RESULTS: Cumulative incidence of VTE was reduced from 16. 3% with short-term thromboprophylaxis (29/178 patients) to 7. 3% after prolonged thromboprophylaxis (12/165) (relative risk reduction 55%; 95% confidence interval 15 -76; P=0. 012). ► CONCLUSIONS: 4 -week administration of dalteparin, 5000 IU once daily, after major abdominal surgery significantly reduces the rate of VTE, without increasing the risk of bleeding, compared with 1 week of thromboprophylaxis. Rasmussen, J Thromb Haemost. 2006 Nov; 4(11): 2384 -90. Epub 2006 Aug 1.
 ASCO Guidelines: VTE Prophylaxis ► All patients undergoing major surgical intervention for malignant disease should be considered for prophylaxis. ► Patients undergoing laparotomy, laparoscopy, or thoracotomy lasting > 30 min should receive pharmacologic prophylaxis. ► Prophylaxis should be continued at least 7 – 10 days post-op. Prolonged prophylaxis for up to 4 weeks may be considered in patients undergoing major surgery for cancer with high-risk features. Lyman GH et al. J Clin Oncol (25) 2007; 34: 5490 -5505.
ASCO Guidelines: VTE Prophylaxis ► All patients undergoing major surgical intervention for malignant disease should be considered for prophylaxis. ► Patients undergoing laparotomy, laparoscopy, or thoracotomy lasting > 30 min should receive pharmacologic prophylaxis. ► Prophylaxis should be continued at least 7 – 10 days post-op. Prolonged prophylaxis for up to 4 weeks may be considered in patients undergoing major surgery for cancer with high-risk features. Lyman GH et al. J Clin Oncol (25) 2007; 34: 5490 -5505.
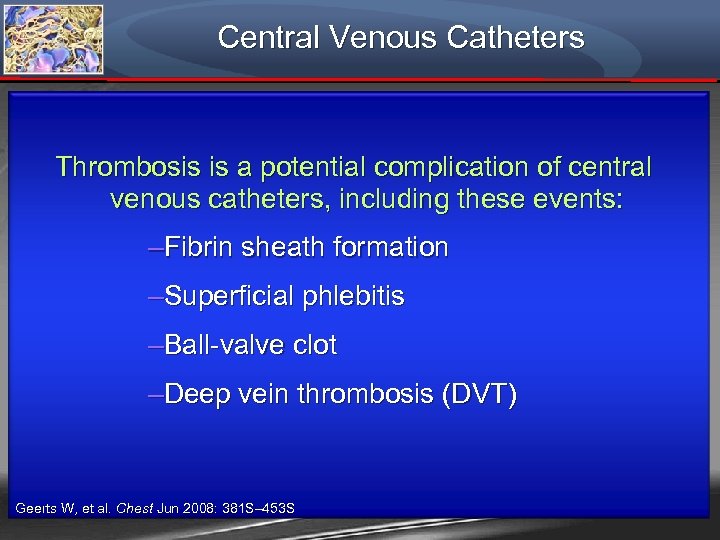 Central Venous Catheters Thrombosis is a potential complication of central venous catheters, including these events: –Fibrin sheath formation –Superficial phlebitis –Ball-valve clot –Deep vein thrombosis (DVT) Geerts W, et al. Chest Jun 2008: 381 S– 453 S
Central Venous Catheters Thrombosis is a potential complication of central venous catheters, including these events: –Fibrin sheath formation –Superficial phlebitis –Ball-valve clot –Deep vein thrombosis (DVT) Geerts W, et al. Chest Jun 2008: 381 S– 453 S
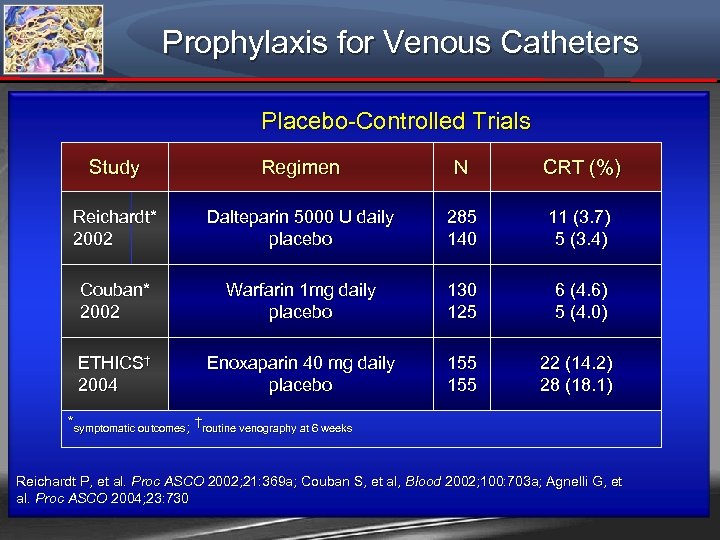 Prophylaxis for Venous Catheters Placebo-Controlled Trials Study Regimen N CRT (%) Reichardt* 2002 Dalteparin 5000 U daily placebo 285 140 11 (3. 7) 5 (3. 4) Couban* 2002 Warfarin 1 mg daily placebo 130 125 6 (4. 6) 5 (4. 0) ETHICS† 2004 Enoxaparin 40 mg daily placebo 155 22 (14. 2) 28 (18. 1) *symptomatic outcomes; †routine venography at 6 weeks Reichardt P, et al. Proc ASCO 2002; 21: 369 a; Couban S, et al, Blood 2002; 100: 703 a; Agnelli G, et al. Proc ASCO 2004; 23: 730
Prophylaxis for Venous Catheters Placebo-Controlled Trials Study Regimen N CRT (%) Reichardt* 2002 Dalteparin 5000 U daily placebo 285 140 11 (3. 7) 5 (3. 4) Couban* 2002 Warfarin 1 mg daily placebo 130 125 6 (4. 6) 5 (4. 0) ETHICS† 2004 Enoxaparin 40 mg daily placebo 155 22 (14. 2) 28 (18. 1) *symptomatic outcomes; †routine venography at 6 weeks Reichardt P, et al. Proc ASCO 2002; 21: 369 a; Couban S, et al, Blood 2002; 100: 703 a; Agnelli G, et al. Proc ASCO 2004; 23: 730
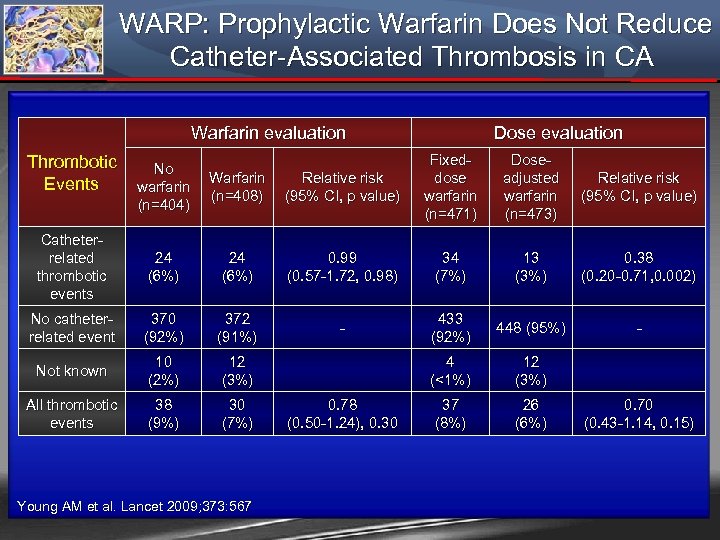 WARP: Prophylactic Warfarin Does Not Reduce Catheter-Associated Thrombosis in CA Warfarin evaluation Thrombotic No Warfarin Relative risk Events warfarin (n=408) (95% CI, p value) (n=404) Dose evaluation Fixeddose warfarin (n=471) Doseadjusted warfarin (n=473) Relative risk (95% CI, p value) Catheterrelated thrombotic events 24 (6%) 0. 99 (0. 57 -1. 72, 0. 98) 34 (7%) 13 (3%) 0. 38 (0. 20 -0. 71, 0. 002) No catheterrelated event 370 (92%) 372 (91%) - 433 (92%) 448 (95%) - Not known 10 (2%) 12 (3%) 4 (<1%) 12 (3%) All thrombotic events 38 (9%) 30 (7%) 37 (8%) 26 (6%) Young AM et al. Lancet 2009; 373: 567 0. 78 (0. 50 -1. 24), 0. 30 0. 70 (0. 43 -1. 14, 0. 15)
WARP: Prophylactic Warfarin Does Not Reduce Catheter-Associated Thrombosis in CA Warfarin evaluation Thrombotic No Warfarin Relative risk Events warfarin (n=408) (95% CI, p value) (n=404) Dose evaluation Fixeddose warfarin (n=471) Doseadjusted warfarin (n=473) Relative risk (95% CI, p value) Catheterrelated thrombotic events 24 (6%) 0. 99 (0. 57 -1. 72, 0. 98) 34 (7%) 13 (3%) 0. 38 (0. 20 -0. 71, 0. 002) No catheterrelated event 370 (92%) 372 (91%) - 433 (92%) 448 (95%) - Not known 10 (2%) 12 (3%) 4 (<1%) 12 (3%) All thrombotic events 38 (9%) 30 (7%) 37 (8%) 26 (6%) Young AM et al. Lancet 2009; 373: 567 0. 78 (0. 50 -1. 24), 0. 30 0. 70 (0. 43 -1. 14, 0. 15)
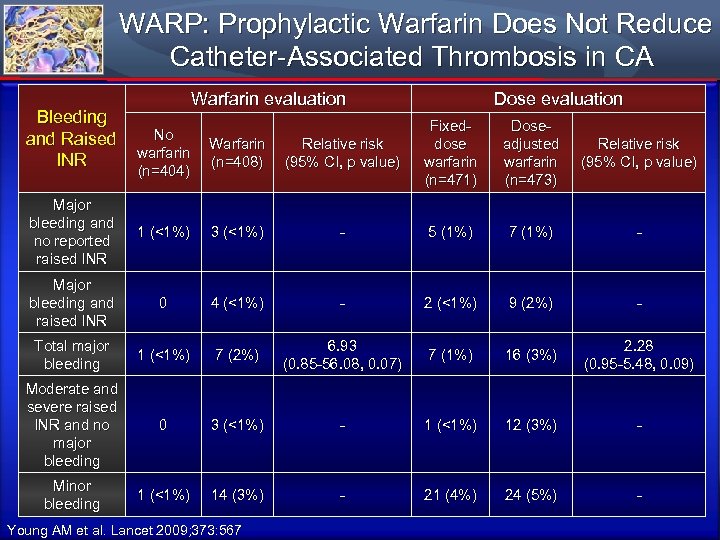 WARP: Prophylactic Warfarin Does Not Reduce Catheter-Associated Thrombosis in CA Warfarin evaluation Bleeding No and Raised Warfarin Relative risk warfarin INR (n=408) (95% CI, p value) (n=404) Dose evaluation Fixeddose warfarin (n=471) Doseadjusted warfarin (n=473) Relative risk (95% CI, p value) Major bleeding and no reported raised INR 1 (<1%) 3 (<1%) - 5 (1%) 7 (1%) - Major bleeding and raised INR 0 4 (<1%) - 2 (<1%) 9 (2%) - Total major bleeding 1 (<1%) 7 (2%) 6. 93 (0. 85 -56. 08, 0. 07) 7 (1%) 16 (3%) 2. 28 (0. 95 -5. 48, 0. 09) Moderate and severe raised INR and no major bleeding 0 3 (<1%) - 1 (<1%) 12 (3%) - Minor bleeding 1 (<1%) 14 (3%) - 21 (4%) 24 (5%) - Young AM et al. Lancet 2009; 373: 567
WARP: Prophylactic Warfarin Does Not Reduce Catheter-Associated Thrombosis in CA Warfarin evaluation Bleeding No and Raised Warfarin Relative risk warfarin INR (n=408) (95% CI, p value) (n=404) Dose evaluation Fixeddose warfarin (n=471) Doseadjusted warfarin (n=473) Relative risk (95% CI, p value) Major bleeding and no reported raised INR 1 (<1%) 3 (<1%) - 5 (1%) 7 (1%) - Major bleeding and raised INR 0 4 (<1%) - 2 (<1%) 9 (2%) - Total major bleeding 1 (<1%) 7 (2%) 6. 93 (0. 85 -56. 08, 0. 07) 7 (1%) 16 (3%) 2. 28 (0. 95 -5. 48, 0. 09) Moderate and severe raised INR and no major bleeding 0 3 (<1%) - 1 (<1%) 12 (3%) - Minor bleeding 1 (<1%) 14 (3%) - 21 (4%) 24 (5%) - Young AM et al. Lancet 2009; 373: 567
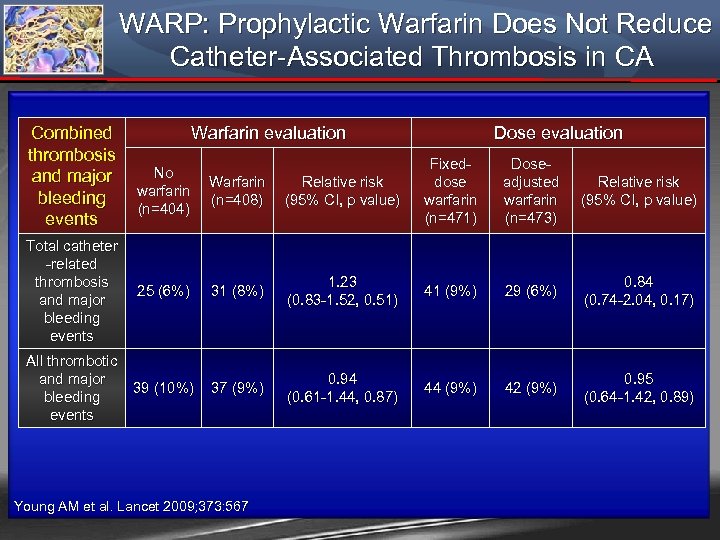 WARP: Prophylactic Warfarin Does Not Reduce Catheter-Associated Thrombosis in CA Combined Warfarin evaluation thrombosis No and major Warfarin Relative risk warfarin bleeding (n=408) (95% CI, p value) (n=404) events Total catheter -related thrombosis and major bleeding events Dose evaluation Fixeddose warfarin (n=471) Doseadjusted warfarin (n=473) Relative risk (95% CI, p value) 25 (6%) 31 (8%) 1. 23 (0. 83 -1. 52, 0. 51) 41 (9%) 29 (6%) 0. 84 (0. 74 -2. 04, 0. 17) All thrombotic and major 39 (10%) bleeding events 37 (9%) 0. 94 (0. 61 -1. 44, 0. 87) 44 (9%) 42 (9%) 0. 95 (0. 64 -1. 42, 0. 89) Young AM et al. Lancet 2009; 373: 567
WARP: Prophylactic Warfarin Does Not Reduce Catheter-Associated Thrombosis in CA Combined Warfarin evaluation thrombosis No and major Warfarin Relative risk warfarin bleeding (n=408) (95% CI, p value) (n=404) events Total catheter -related thrombosis and major bleeding events Dose evaluation Fixeddose warfarin (n=471) Doseadjusted warfarin (n=473) Relative risk (95% CI, p value) 25 (6%) 31 (8%) 1. 23 (0. 83 -1. 52, 0. 51) 41 (9%) 29 (6%) 0. 84 (0. 74 -2. 04, 0. 17) All thrombotic and major 39 (10%) bleeding events 37 (9%) 0. 94 (0. 61 -1. 44, 0. 87) 44 (9%) 42 (9%) 0. 95 (0. 64 -1. 42, 0. 89) Young AM et al. Lancet 2009; 373: 567
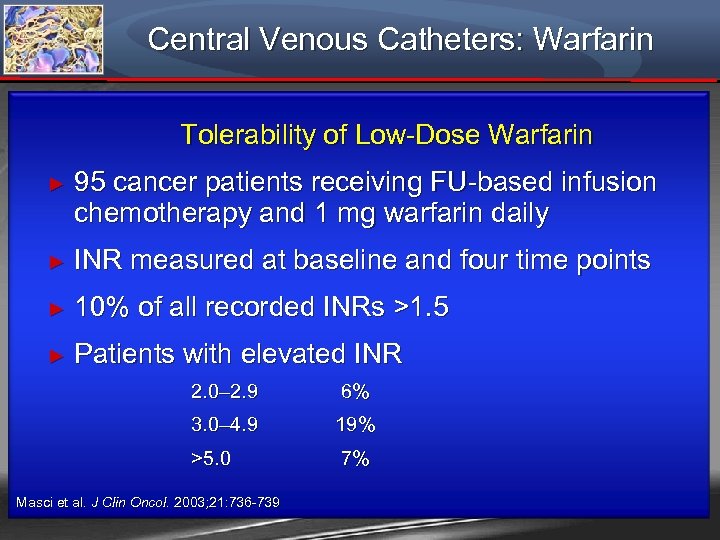 Central Venous Catheters: Warfarin Tolerability of Low-Dose Warfarin ► 95 cancer patients receiving FU-based infusion chemotherapy and 1 mg warfarin daily ► INR measured at baseline and four time points ► 10% of all recorded INRs >1. 5 ► Patients with elevated INR 2. 0– 2. 9 6% 3. 0– 4. 9 19% >5. 0 7% Masci et al. J Clin Oncol. 2003; 21: 736 -739
Central Venous Catheters: Warfarin Tolerability of Low-Dose Warfarin ► 95 cancer patients receiving FU-based infusion chemotherapy and 1 mg warfarin daily ► INR measured at baseline and four time points ► 10% of all recorded INRs >1. 5 ► Patients with elevated INR 2. 0– 2. 9 6% 3. 0– 4. 9 19% >5. 0 7% Masci et al. J Clin Oncol. 2003; 21: 736 -739
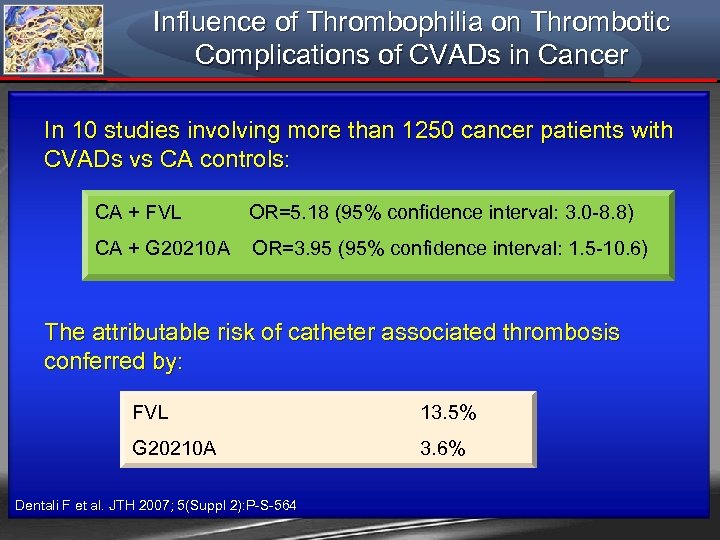 Influence of Thrombophilia on Thrombotic Complications of CVADs in Cancer In 10 studies involving more than 1250 cancer patients with CVADs vs CA controls: CA + FVL OR=5. 18 (95% confidence interval: 3. 0 -8. 8) CA + G 20210 A OR=3. 95 (95% confidence interval: 1. 5 -10. 6) The attributable risk of catheter associated thrombosis conferred by: FVL G 20210 A Dentali F et al. JTH 2007; 5(Suppl 2): P-S-564 13. 5% 3. 6%
Influence of Thrombophilia on Thrombotic Complications of CVADs in Cancer In 10 studies involving more than 1250 cancer patients with CVADs vs CA controls: CA + FVL OR=5. 18 (95% confidence interval: 3. 0 -8. 8) CA + G 20210 A OR=3. 95 (95% confidence interval: 1. 5 -10. 6) The attributable risk of catheter associated thrombosis conferred by: FVL G 20210 A Dentali F et al. JTH 2007; 5(Suppl 2): P-S-564 13. 5% 3. 6%
 8 th ACCP Consensus Guidelines No routine prophylaxis to prevent thrombosis secondary to central venous catheters, including LMWH (2 B) and fixed-dose warfarin (1 B) Revised 2009 NCCN guidelines diverge from this philosophy Chest Jun 2008: 454 S– 545 S
8 th ACCP Consensus Guidelines No routine prophylaxis to prevent thrombosis secondary to central venous catheters, including LMWH (2 B) and fixed-dose warfarin (1 B) Revised 2009 NCCN guidelines diverge from this philosophy Chest Jun 2008: 454 S– 545 S
 Primary Prophylaxis in Cancer Radiotherapy The Ambulatory Patient ► No recommendations from ACCP ► No data from randomized trials (RCTs) ► Weak data from observational studies in high risk tumors (e. g. brain tumors; mucinsecreting adenocarcinomas: Colorectal, pancreatic, lung, renal cell, ovarian) ► Recommendations extrapolated from other groups of patients if additional risk factors present (e. g. , hemiparesis in brain tumors, etc. )
Primary Prophylaxis in Cancer Radiotherapy The Ambulatory Patient ► No recommendations from ACCP ► No data from randomized trials (RCTs) ► Weak data from observational studies in high risk tumors (e. g. brain tumors; mucinsecreting adenocarcinomas: Colorectal, pancreatic, lung, renal cell, ovarian) ► Recommendations extrapolated from other groups of patients if additional risk factors present (e. g. , hemiparesis in brain tumors, etc. )
 Risk Factors for VTE in Medical Oncology Patients ► Tumor type ● Ovary, brain, pancreas, lung, colon ► Stage, grade, and extent of cancer ● ► Type of antineoplastic treatment ● ► Metastatic disease, venous stasis due to bulky disease Multiagent regimens, hormones, anti-VEGF, radiation Miscellaneous VTE risk factors ● Previous VTE, hospitalization, immobility, infection, thrombophilia
Risk Factors for VTE in Medical Oncology Patients ► Tumor type ● Ovary, brain, pancreas, lung, colon ► Stage, grade, and extent of cancer ● ► Type of antineoplastic treatment ● ► Metastatic disease, venous stasis due to bulky disease Multiagent regimens, hormones, anti-VEGF, radiation Miscellaneous VTE risk factors ● Previous VTE, hospitalization, immobility, infection, thrombophilia
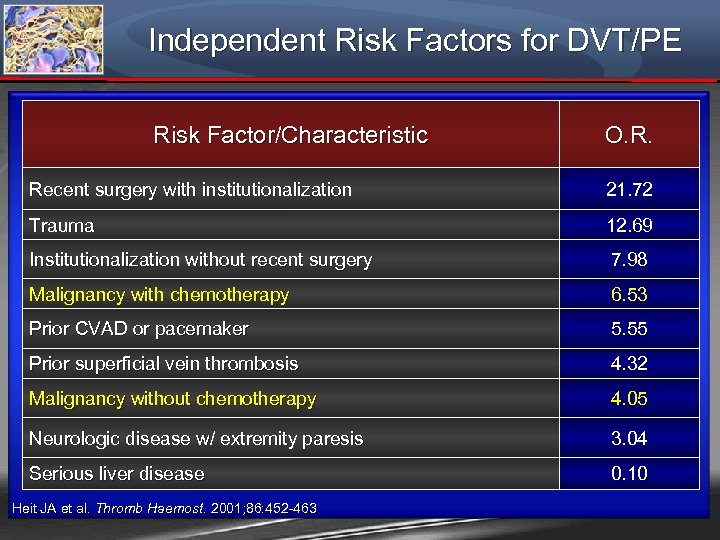 Independent Risk Factors for DVT/PE Risk Factor/Characteristic O. R. Recent surgery with institutionalization 21. 72 Trauma 12. 69 Institutionalization without recent surgery 7. 98 Malignancy with chemotherapy 6. 53 Prior CVAD or pacemaker 5. 55 Prior superficial vein thrombosis 4. 32 Malignancy without chemotherapy 4. 05 Neurologic disease w/ extremity paresis 3. 04 Serious liver disease 0. 10 Heit JA et al. Thromb Haemost. 2001; 86: 452 -463
Independent Risk Factors for DVT/PE Risk Factor/Characteristic O. R. Recent surgery with institutionalization 21. 72 Trauma 12. 69 Institutionalization without recent surgery 7. 98 Malignancy with chemotherapy 6. 53 Prior CVAD or pacemaker 5. 55 Prior superficial vein thrombosis 4. 32 Malignancy without chemotherapy 4. 05 Neurologic disease w/ extremity paresis 3. 04 Serious liver disease 0. 10 Heit JA et al. Thromb Haemost. 2001; 86: 452 -463
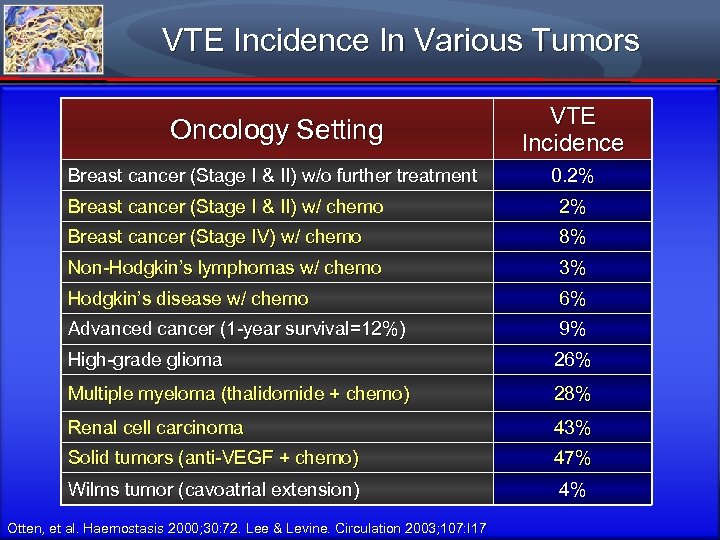 VTE Incidence In Various Tumors Oncology Setting VTE Incidence Breast cancer (Stage I & II) w/o further treatment 0. 2% Breast cancer (Stage I & II) w/ chemo 2% Breast cancer (Stage IV) w/ chemo 8% Non-Hodgkin’s lymphomas w/ chemo 3% Hodgkin’s disease w/ chemo 6% Advanced cancer (1 -year survival=12%) 9% High-grade glioma 26% Multiple myeloma (thalidomide + chemo) 28% Renal cell carcinoma 43% Solid tumors (anti-VEGF + chemo) 47% Wilms tumor (cavoatrial extension) 4% Otten, et al. Haemostasis 2000; 30: 72. Lee & Levine. Circulation 2003; 107: I 17
VTE Incidence In Various Tumors Oncology Setting VTE Incidence Breast cancer (Stage I & II) w/o further treatment 0. 2% Breast cancer (Stage I & II) w/ chemo 2% Breast cancer (Stage IV) w/ chemo 8% Non-Hodgkin’s lymphomas w/ chemo 3% Hodgkin’s disease w/ chemo 6% Advanced cancer (1 -year survival=12%) 9% High-grade glioma 26% Multiple myeloma (thalidomide + chemo) 28% Renal cell carcinoma 43% Solid tumors (anti-VEGF + chemo) 47% Wilms tumor (cavoatrial extension) 4% Otten, et al. Haemostasis 2000; 30: 72. Lee & Levine. Circulation 2003; 107: I 17
 Primary VTE Prophylaxis ► Recommended for hospitalized cancer patients ► Not universally recommended for outpatients, but there are exceptions ● ● New data for certain agents Heterogeneous population Need for risk stratification
Primary VTE Prophylaxis ► Recommended for hospitalized cancer patients ► Not universally recommended for outpatients, but there are exceptions ● ● New data for certain agents Heterogeneous population Need for risk stratification
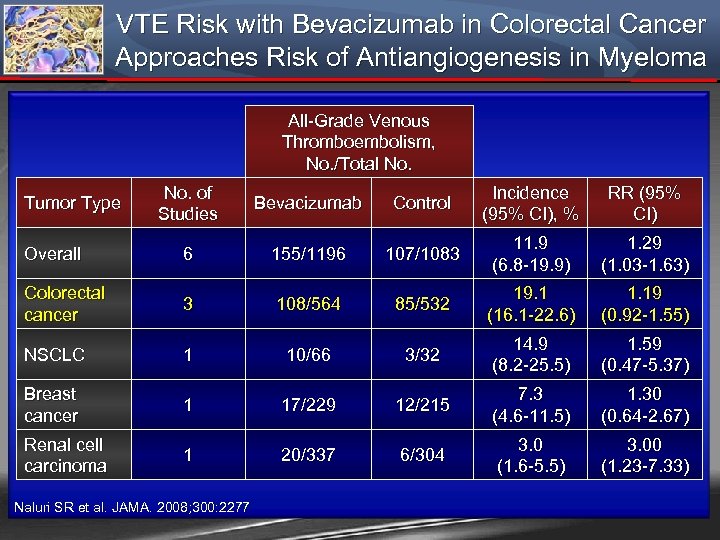 VTE Risk with Bevacizumab in Colorectal Cancer Approaches Risk of Antiangiogenesis in Myeloma All-Grade Venous Thromboembolism, No. /Total No. of Studies Bevacizumab Control Incidence (95% CI), % RR (95% CI) Overall 6 155/1196 107/1083 11. 9 (6. 8 -19. 9) 1. 29 (1. 03 -1. 63) Colorectal cancer 3 108/564 85/532 19. 1 (16. 1 -22. 6) 1. 19 (0. 92 -1. 55) NSCLC 1 10/66 3/32 14. 9 (8. 2 -25. 5) 1. 59 (0. 47 -5. 37) Breast cancer 1 17/229 12/215 7. 3 (4. 6 -11. 5) 1. 30 (0. 64 -2. 67) Renal cell carcinoma 1 20/337 6/304 3. 0 (1. 6 -5. 5) 3. 00 (1. 23 -7. 33) Tumor Type Naluri SR et al. JAMA. 2008; 300: 2277
VTE Risk with Bevacizumab in Colorectal Cancer Approaches Risk of Antiangiogenesis in Myeloma All-Grade Venous Thromboembolism, No. /Total No. of Studies Bevacizumab Control Incidence (95% CI), % RR (95% CI) Overall 6 155/1196 107/1083 11. 9 (6. 8 -19. 9) 1. 29 (1. 03 -1. 63) Colorectal cancer 3 108/564 85/532 19. 1 (16. 1 -22. 6) 1. 19 (0. 92 -1. 55) NSCLC 1 10/66 3/32 14. 9 (8. 2 -25. 5) 1. 59 (0. 47 -5. 37) Breast cancer 1 17/229 12/215 7. 3 (4. 6 -11. 5) 1. 30 (0. 64 -2. 67) Renal cell carcinoma 1 20/337 6/304 3. 0 (1. 6 -5. 5) 3. 00 (1. 23 -7. 33) Tumor Type Naluri SR et al. JAMA. 2008; 300: 2277
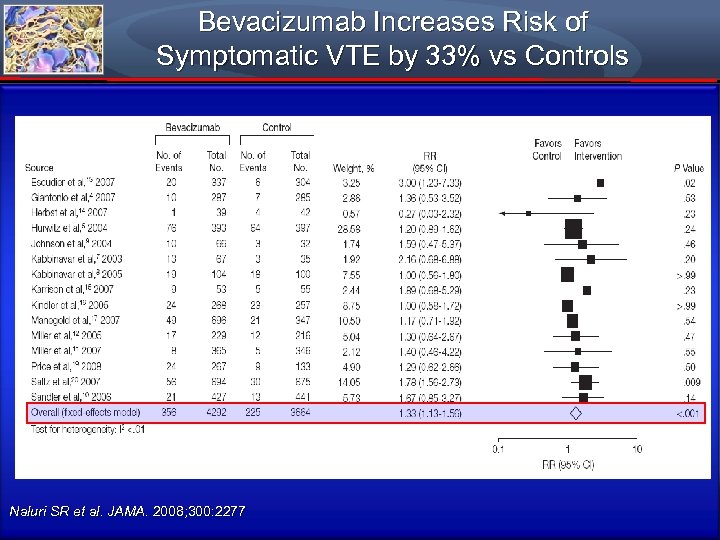 Bevacizumab Increases Risk of Symptomatic VTE by 33% vs Controls Naluri SR et al. JAMA. 2008; 300: 2277
Bevacizumab Increases Risk of Symptomatic VTE by 33% vs Controls Naluri SR et al. JAMA. 2008; 300: 2277
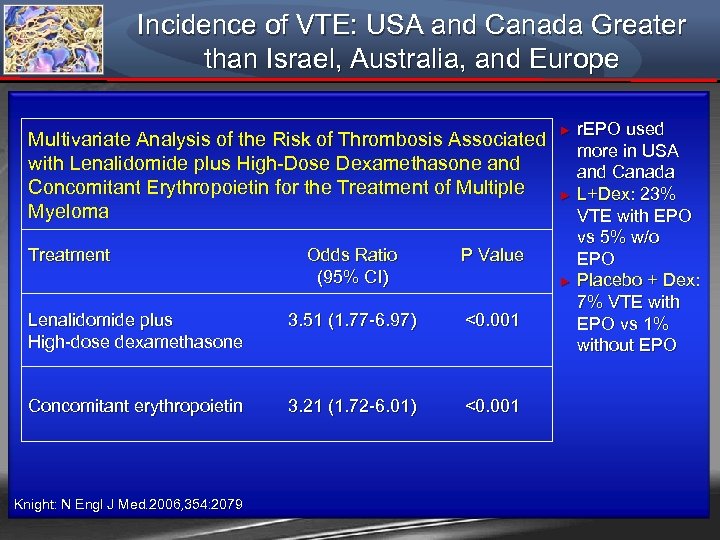 Incidence of VTE: USA and Canada Greater than Israel, Australia, and Europe Multivariate Analysis of the Risk of Thrombosis Associated with Lenalidomide plus High-Dose Dexamethasone and Concomitant Erythropoietin for the Treatment of Multiple Myeloma Treatment Odds Ratio (95% CI) P Value Lenalidomide plus High-dose dexamethasone 3. 51 (1. 77 -6. 97) <0. 001 Concomitant erythropoietin 3. 21 (1. 72 -6. 01) <0. 001 Knight: N Engl J Med. 2006, 354: 2079 r. EPO used more in USA and Canada ► L+Dex: 23% VTE with EPO vs 5% w/o EPO ► Placebo + Dex: 7% VTE with EPO vs 1% without EPO ►
Incidence of VTE: USA and Canada Greater than Israel, Australia, and Europe Multivariate Analysis of the Risk of Thrombosis Associated with Lenalidomide plus High-Dose Dexamethasone and Concomitant Erythropoietin for the Treatment of Multiple Myeloma Treatment Odds Ratio (95% CI) P Value Lenalidomide plus High-dose dexamethasone 3. 51 (1. 77 -6. 97) <0. 001 Concomitant erythropoietin 3. 21 (1. 72 -6. 01) <0. 001 Knight: N Engl J Med. 2006, 354: 2079 r. EPO used more in USA and Canada ► L+Dex: 23% VTE with EPO vs 5% w/o EPO ► Placebo + Dex: 7% VTE with EPO vs 1% without EPO ►
 Oral Anticoagulant Therapy in Cancer Patients: Problematic ► Warfarin therapy is complicated by: ● ● ► Difficulty maintaining tight therapeutic control, due to anorexia, vomiting, drug interactions, etc. Frequent interruptions for thrombocytopenia and procedures Difficulty in venous access for monitoring Increased risk of both recurrence and bleeding Is it reasonable to substitute long-term LMWH for warfarin ? When? How? Why?
Oral Anticoagulant Therapy in Cancer Patients: Problematic ► Warfarin therapy is complicated by: ● ● ► Difficulty maintaining tight therapeutic control, due to anorexia, vomiting, drug interactions, etc. Frequent interruptions for thrombocytopenia and procedures Difficulty in venous access for monitoring Increased risk of both recurrence and bleeding Is it reasonable to substitute long-term LMWH for warfarin ? When? How? Why?
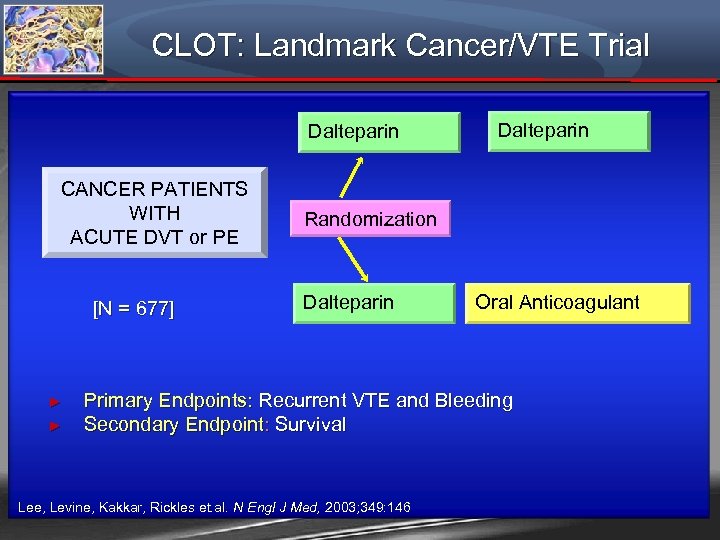 CLOT: Landmark Cancer/VTE Trial Dalteparin CANCER PATIENTS WITH ACUTE DVT or PE [N = 677] ► ► Dalteparin Randomization Dalteparin Oral Anticoagulant Primary Endpoints: Recurrent VTE and Bleeding Secondary Endpoint: Survival Lee, Levine, Kakkar, Rickles et. al. N Engl J Med, 2003; 349: 146
CLOT: Landmark Cancer/VTE Trial Dalteparin CANCER PATIENTS WITH ACUTE DVT or PE [N = 677] ► ► Dalteparin Randomization Dalteparin Oral Anticoagulant Primary Endpoints: Recurrent VTE and Bleeding Secondary Endpoint: Survival Lee, Levine, Kakkar, Rickles et. al. N Engl J Med, 2003; 349: 146
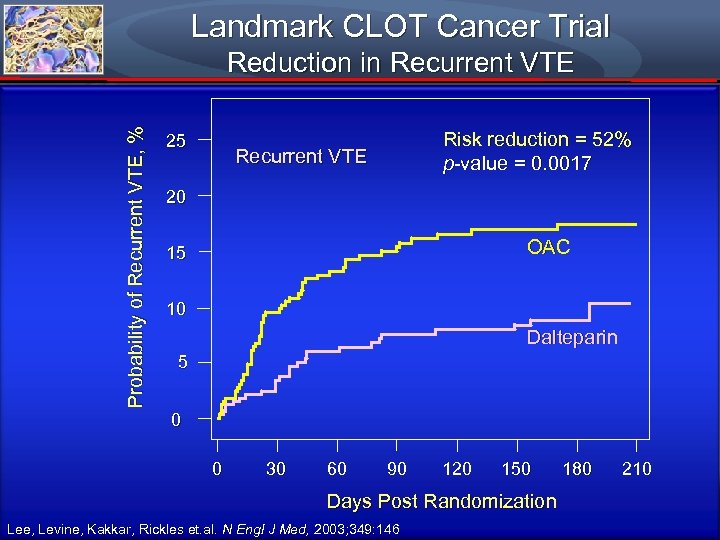 Landmark CLOT Cancer Trial Probability of Recurrent VTE, % Reduction in Recurrent VTE 25 Risk reduction = 52% p-value = 0. 0017 Recurrent VTE 20 OAC 15 10 Dalteparin 5 0 0 30 60 90 120 150 Days Post Randomization Lee, Levine, Kakkar, Rickles et. al. N Engl J Med, 2003; 349: 146 180 210
Landmark CLOT Cancer Trial Probability of Recurrent VTE, % Reduction in Recurrent VTE 25 Risk reduction = 52% p-value = 0. 0017 Recurrent VTE 20 OAC 15 10 Dalteparin 5 0 0 30 60 90 120 150 Days Post Randomization Lee, Levine, Kakkar, Rickles et. al. N Engl J Med, 2003; 349: 146 180 210
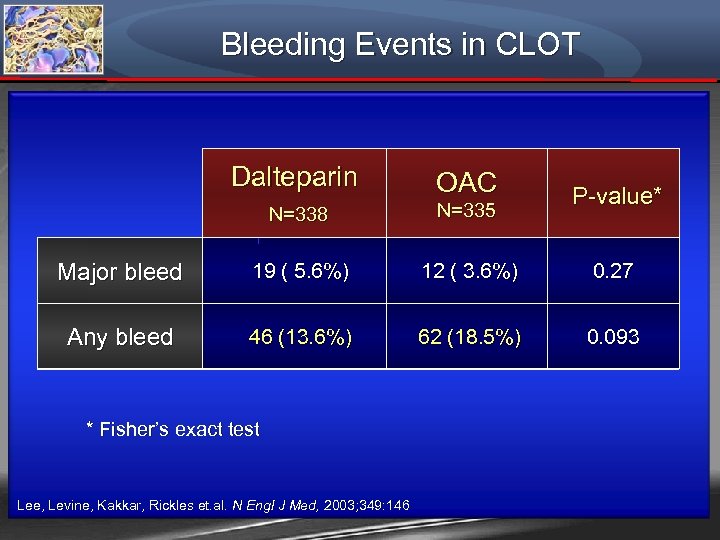 Bleeding Events in CLOT Dalteparin N=338 OAC Major bleed 19 ( 5. 6%) 12 ( 3. 6%) 0. 27 Any bleed 46 (13. 6%) 62 (18. 5%) 0. 093 * Fisher’s exact test Lee, Levine, Kakkar, Rickles et. al. N Engl J Med, 2003; 349: 146 N=335 P-value*
Bleeding Events in CLOT Dalteparin N=338 OAC Major bleed 19 ( 5. 6%) 12 ( 3. 6%) 0. 27 Any bleed 46 (13. 6%) 62 (18. 5%) 0. 093 * Fisher’s exact test Lee, Levine, Kakkar, Rickles et. al. N Engl J Med, 2003; 349: 146 N=335 P-value*
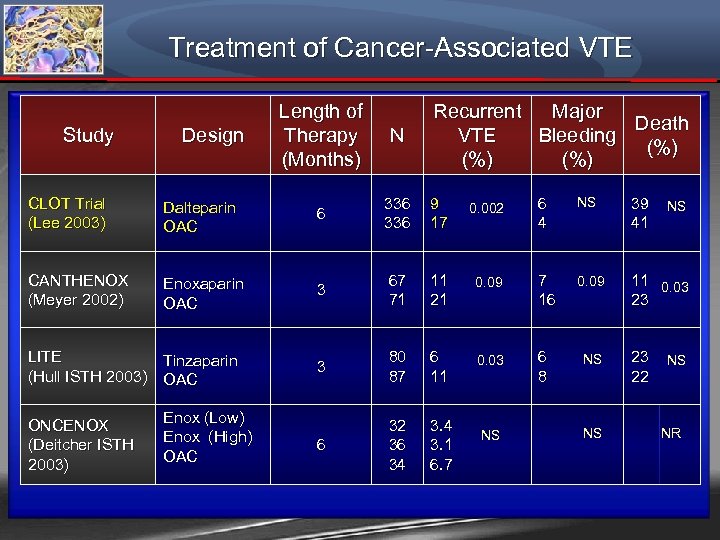 Treatment of Cancer-Associated VTE Study Design Length of Therapy (Months) N Recurrent Major Death VTE Bleeding (%) (%) CLOT Trial (Lee 2003) Dalteparin OAC 6 336 9 17 0. 002 CANTHENOX (Meyer 2002) Enoxaparin OAC 3 67 71 11 21 3 80 87 6 11 6 32 36 34 3. 1 6. 7 LITE Tinzaparin (Hull ISTH 2003) OAC ONCENOX (Deitcher ISTH 2003) Enox (Low) Enox (High) OAC 6 4 NS 39 41 0. 09 7 16 0. 09 11 0. 03 23 0. 03 6 8 NS NS NS 23 22 NS NS NR
Treatment of Cancer-Associated VTE Study Design Length of Therapy (Months) N Recurrent Major Death VTE Bleeding (%) (%) CLOT Trial (Lee 2003) Dalteparin OAC 6 336 9 17 0. 002 CANTHENOX (Meyer 2002) Enoxaparin OAC 3 67 71 11 21 3 80 87 6 11 6 32 36 34 3. 1 6. 7 LITE Tinzaparin (Hull ISTH 2003) OAC ONCENOX (Deitcher ISTH 2003) Enox (Low) Enox (High) OAC 6 4 NS 39 41 0. 09 7 16 0. 09 11 0. 03 23 0. 03 6 8 NS NS NS 23 22 NS NS NR
 Treatment and 2° Prevention of VTE in Cancer – Bottom Line New Development ► New standard of care is LMWH at therapeutic doses for a minimum of 3 -6 months (Grade 1 A recommendation—ACCP) ► NOTE: Dalteparin is only LMWH approved (May, 2007) for both the treatment and secondary prevention of VTE in cancer (NCCN preferred agent) ► Oral anticoagulant therapy to follow for as long as cancer is active (Grade 1 C recommendation—ACCP) Chest Jun 2008: 454 S– 545 S
Treatment and 2° Prevention of VTE in Cancer – Bottom Line New Development ► New standard of care is LMWH at therapeutic doses for a minimum of 3 -6 months (Grade 1 A recommendation—ACCP) ► NOTE: Dalteparin is only LMWH approved (May, 2007) for both the treatment and secondary prevention of VTE in cancer (NCCN preferred agent) ► Oral anticoagulant therapy to follow for as long as cancer is active (Grade 1 C recommendation—ACCP) Chest Jun 2008: 454 S– 545 S
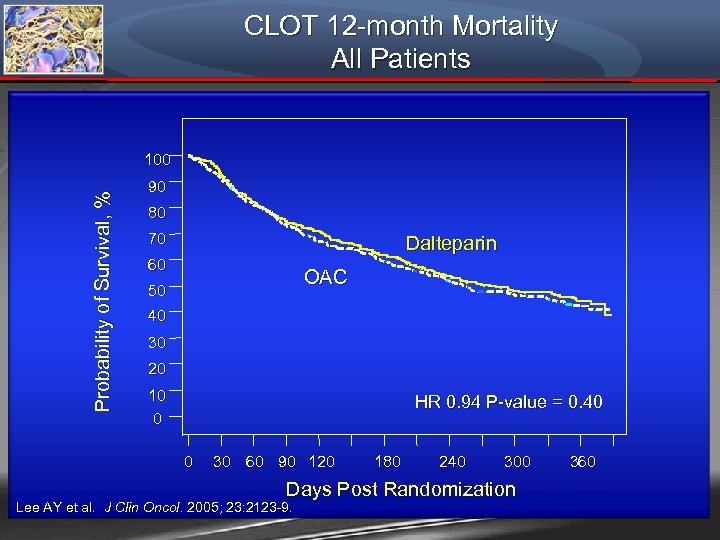 CLOT 12 -month Mortality All Patients Probability of Survival, % 100 90 80 70 Dalteparin 60 OAC 50 40 30 20 10 0 HR 0. 94 P-value = 0. 40 0 30 60 90 120 180 240 300 Days Post Randomization Lee AY et al. J Clin Oncol. 2005; 23: 2123 -9. 360
CLOT 12 -month Mortality All Patients Probability of Survival, % 100 90 80 70 Dalteparin 60 OAC 50 40 30 20 10 0 HR 0. 94 P-value = 0. 40 0 30 60 90 120 180 240 300 Days Post Randomization Lee AY et al. J Clin Oncol. 2005; 23: 2123 -9. 360
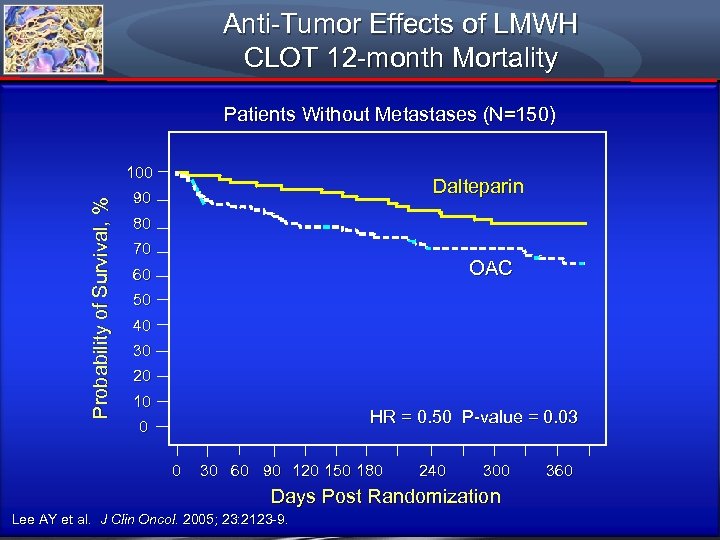 Anti-Tumor Effects of LMWH CLOT 12 -month Mortality Patients Without Metastases (N=150) Probability of Survival, % 100 Dalteparin 90 80 70 OAC 60 50 40 30 20 10 HR = 0. 50 P-value = 0. 03 0 0 30 60 90 120 150 180 240 300 Days Post Randomization Lee AY et al. J Clin Oncol. 2005; 23: 2123 -9. 360
Anti-Tumor Effects of LMWH CLOT 12 -month Mortality Patients Without Metastases (N=150) Probability of Survival, % 100 Dalteparin 90 80 70 OAC 60 50 40 30 20 10 HR = 0. 50 P-value = 0. 03 0 0 30 60 90 120 150 180 240 300 Days Post Randomization Lee AY et al. J Clin Oncol. 2005; 23: 2123 -9. 360
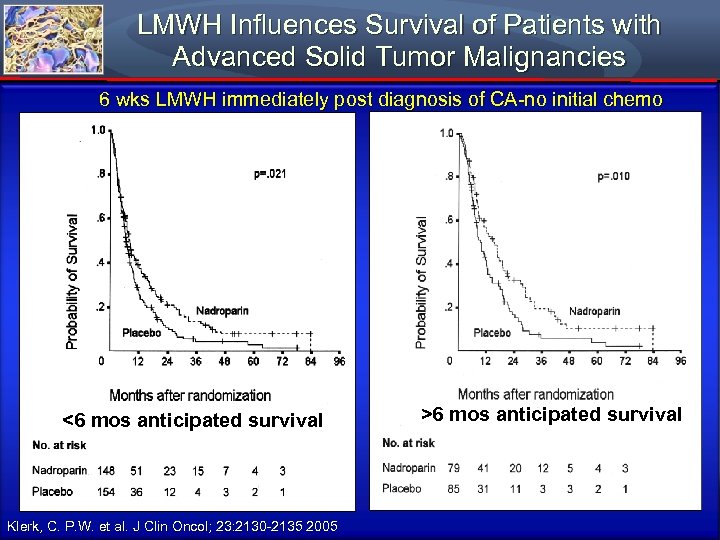 LMWH Influences Survival of Patients with Advanced Solid Tumor Malignancies 6 wks LMWH immediately post diagnosis of CA-no initial chemo <6 mos anticipated survival Klerk, C. P. W. et al. J Clin Oncol; 23: 2130 -2135 2005 >6 mos anticipated survival
LMWH Influences Survival of Patients with Advanced Solid Tumor Malignancies 6 wks LMWH immediately post diagnosis of CA-no initial chemo <6 mos anticipated survival Klerk, C. P. W. et al. J Clin Oncol; 23: 2130 -2135 2005 >6 mos anticipated survival
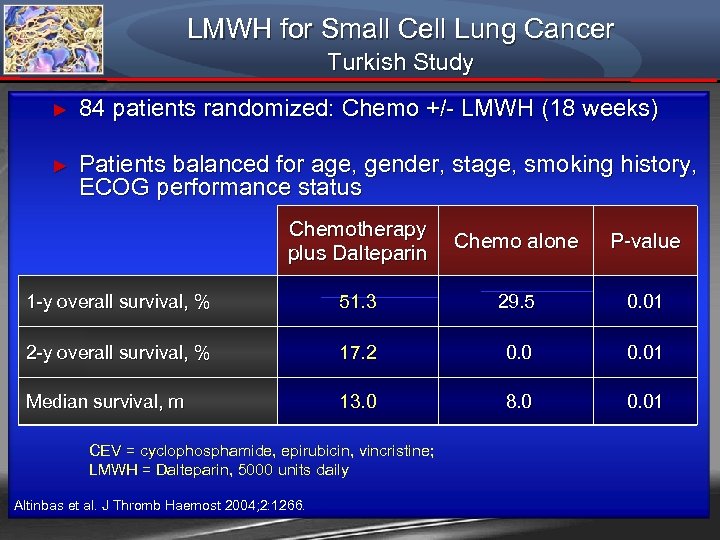 LMWH for Small Cell Lung Cancer Turkish Study ► 84 patients randomized: Chemo +/- LMWH (18 weeks) ► Patients balanced for age, gender, stage, smoking history, ECOG performance status Chemotherapy plus Dalteparin Chemo alone P-value 1 -y overall survival, % 51. 3 29. 5 0. 01 2 -y overall survival, % 17. 2 0. 01 Median survival, m 13. 0 8. 0 0. 01 CEV = cyclophosphamide, epirubicin, vincristine; LMWH = Dalteparin, 5000 units daily Altinbas et al. J Thromb Haemost 2004; 2: 1266.
LMWH for Small Cell Lung Cancer Turkish Study ► 84 patients randomized: Chemo +/- LMWH (18 weeks) ► Patients balanced for age, gender, stage, smoking history, ECOG performance status Chemotherapy plus Dalteparin Chemo alone P-value 1 -y overall survival, % 51. 3 29. 5 0. 01 2 -y overall survival, % 17. 2 0. 01 Median survival, m 13. 0 8. 0 0. 01 CEV = cyclophosphamide, epirubicin, vincristine; LMWH = Dalteparin, 5000 units daily Altinbas et al. J Thromb Haemost 2004; 2: 1266.
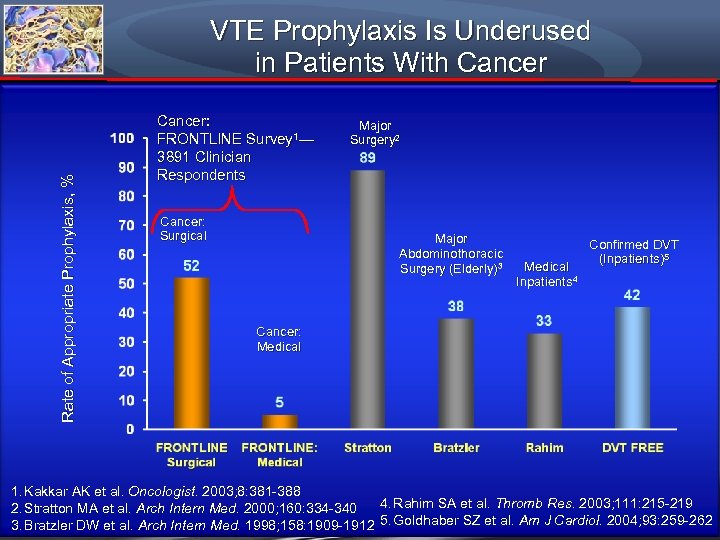 Rate of Appropriate Prophylaxis, % VTE Prophylaxis Is Underused in Patients With Cancer: FRONTLINE Survey 1— 3891 Clinician Respondents Cancer: Surgical Major Surgery 2 Major Abdominothoracic Surgery (Elderly)3 Medical Inpatients 4 Confirmed DVT (Inpatients)5 Cancer: Medical 1. Kakkar AK et al. Oncologist. 2003; 8: 381 -388 4. Rahim SA et al. Thromb Res. 2003; 111: 215 -219 2. Stratton MA et al. Arch Intern Med. 2000; 160: 334 -340 3. Bratzler DW et al. Arch Intern Med. 1998; 158: 1909 -1912 5. Goldhaber SZ et al. Am J Cardiol. 2004; 93: 259 -262
Rate of Appropriate Prophylaxis, % VTE Prophylaxis Is Underused in Patients With Cancer: FRONTLINE Survey 1— 3891 Clinician Respondents Cancer: Surgical Major Surgery 2 Major Abdominothoracic Surgery (Elderly)3 Medical Inpatients 4 Confirmed DVT (Inpatients)5 Cancer: Medical 1. Kakkar AK et al. Oncologist. 2003; 8: 381 -388 4. Rahim SA et al. Thromb Res. 2003; 111: 215 -219 2. Stratton MA et al. Arch Intern Med. 2000; 160: 334 -340 3. Bratzler DW et al. Arch Intern Med. 1998; 158: 1909 -1912 5. Goldhaber SZ et al. Am J Cardiol. 2004; 93: 259 -262
 Conclusions and Summary ► Risk factors for VTE in the setting of cancer have been well characterized: solid tumors, chemotherapy, surgery, thrombocytopenia ► Long-term secondary prevention with LMWH has been shown to produce better outcomes than warfarin ► Guidelines and landmark trials support administration of LMWH in at risk patients ► Cancer patients are under-prophylaxed for VTE ► Health system pharmacists can play a pivotal role in improving clinical outcomes in this patient population
Conclusions and Summary ► Risk factors for VTE in the setting of cancer have been well characterized: solid tumors, chemotherapy, surgery, thrombocytopenia ► Long-term secondary prevention with LMWH has been shown to produce better outcomes than warfarin ► Guidelines and landmark trials support administration of LMWH in at risk patients ► Cancer patients are under-prophylaxed for VTE ► Health system pharmacists can play a pivotal role in improving clinical outcomes in this patient population


