c093b3a7e10c11b9d2861637de87c888.ppt
- Количество слайдов: 28
INJECTABLES FOR OSTEOARTHRITIS DR BRENDON AUBREY

AIM OF THIS TALK • What are the injectable options? • How do they work? • What is the current evidence? • Are they legal in sport?

CORTICOSTEROID WHAT IS IT? • Steroid hormone normally produced by the kidneys • Examples of medical injectable corticosteroids: • Betamethasone (Celestone) • Dexamethasone (Decadron) • Triamcinolone acetonide (Kenacort) • Methylprednisolone (Depo-Medrol)
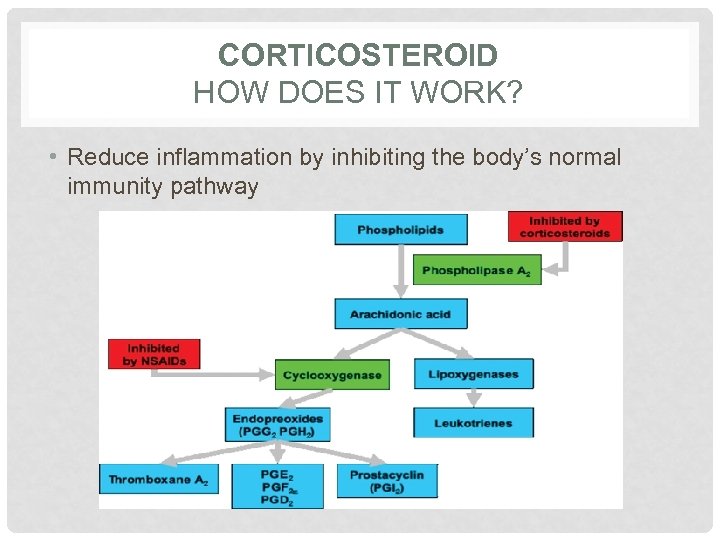
CORTICOSTEROID HOW DOES IT WORK? • Reduce inflammation by inhibiting the body’s normal immunity pathway

CORTICOSTEROID WHAT IS THE EVIDENCE? • Cochrane review 2006 (Bellamy et al) • Pain and function significantly better after 1 week (compared to placebo) • Pain still better up to 3 weeks • Lack of evidence for functional improvement after 1 week • Viscosupplementation was better than corticosteroid between 5 and 13 weeks post injection • Triamcinolone acetonide better than betamethasone (but cost is 4 x greater)
CORTICOSTEROID WHICH PATIENTS MIGHT BENEFIT? • Acute inflammation • Contraindications: • • • Current infection Hypersensitivity Acute haemarthrosis Osteochondral lesions* Diabetes* • Side effects: • Facial flushing • Itchy skin

VISCOSUPPLEMENTATION WHAT IS IT? • Administration of synthetic hyaluronic acid • Examples • • Synvisc/Synvisc One Orthovisc Hyalgan Durolane

VISCOSUPPLEMENTATION HOW DOES IT WORK? • Hyaluronic acid is normally found in synovial joints • In osteoarthritis, the concentration of hyaluronic acid in the joint capsule is reduced • Administration of synthetic hyaluronic acid stimulates the synovial lining to produce better quality synovial fluid • Decreased inflammation • Initial lubricant effect • Increased function

VISCOSUPPLEMENTATION WHAT IS THE EVIDENCE? • Good evidence for symptom relief up to 6 months (Bellamy et al 2009 – Cochrane Review) • Minimal difference between products • Overall appears to be better than corticosteroid, but inferior to PRP and ACS
VISCOSUPPLEMENTATION WHICH PATIENTS MIGHT BENEFIT? • Autologous cellular therapy contraindicated • Current malignancy • Convenience • One injection versus several • Previous success with viscosupplementation • Mild – moderate OA – • Effect is better and more prolonged earlier in the disease • Questionable whether it alters the course of OA progression but does reduce inflammation
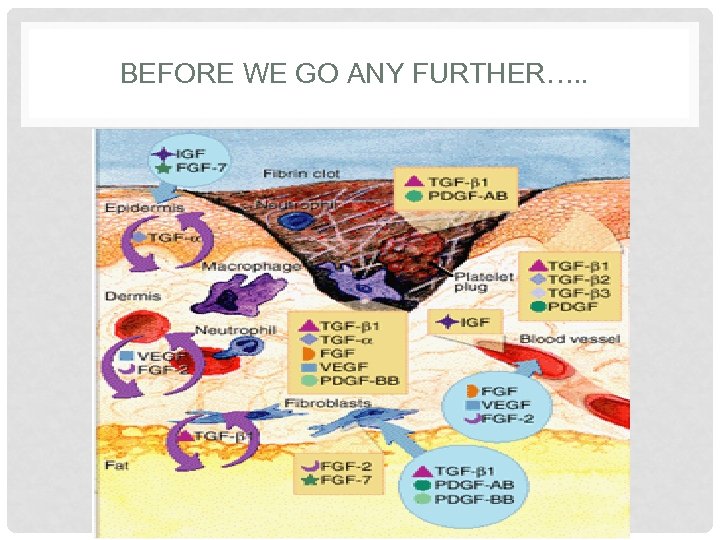
BEFORE WE GO ANY FURTHER…. .

PLATELET RICH PLASMA WHAT IS IT • Autologous injection of platelets • Whole blood is taken and placed in centrifuge, platelets then extracted • Concentrated platelet rich plasma is then injected into the affected joint

PLATELET RICH PLASMA HOW DOES IT WORK? • Platelets play an important role in wound healing • By injecting activated platelets we initiate the wound healing cascade • Platelets release multiple cytokines and growth factors
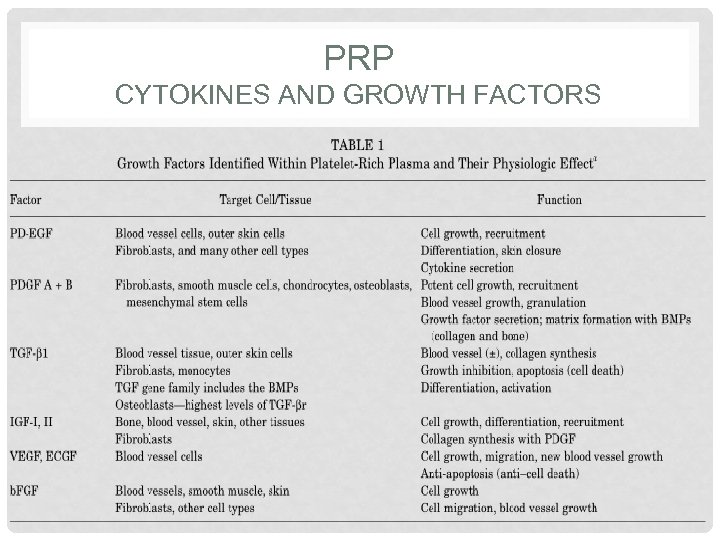
PRP CYTOKINES AND GROWTH FACTORS

PLATELET RICH PLASMA WHAT IS THE EVIDENCE? • Sanchez et al 2008 • PRP improved outcome at 5 weeks • Kon et al 2010 • PRP improved outcomes at 6 and 12 months (although pain and function scores began to decline after 6 months) • Spakova et al 2012 • PRP better than HA at 3 and 6 months • Patel et al 2013 • PRP improved outcomes out to 6 months • Symptoms then began to return but not o baseline

PLATELET RICH PLASMA WHICH PATIENTS MIGHT BENEFIT? • Mild to moderate arthritis • Patients not suitable for surgery • Failure to respond from more conservative measures • Contraindications • Pregnancy • Cancer • Some bleeding disorders
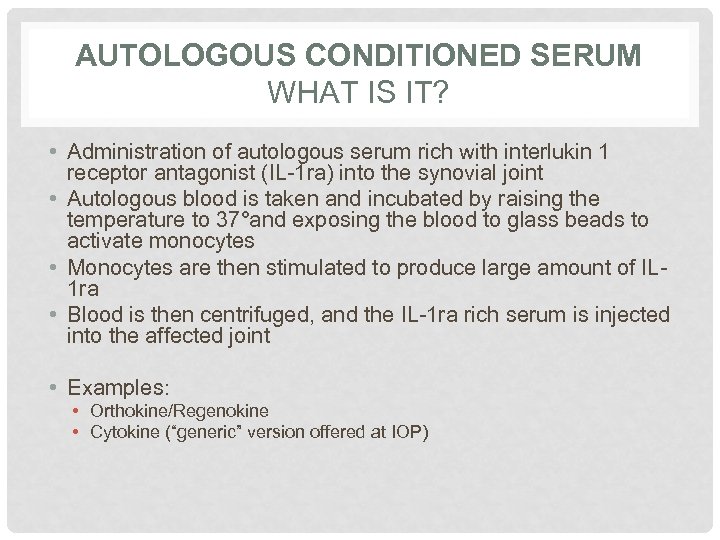
AUTOLOGOUS CONDITIONED SERUM WHAT IS IT? • Administration of autologous serum rich with interlukin 1 receptor antagonist (IL-1 ra) into the synovial joint • Autologous blood is taken and incubated by raising the temperature to 37°and exposing the blood to glass beads to activate monocytes • Monocytes are then stimulated to produce large amount of IL 1 ra • Blood is then centrifuged, and the IL-1 ra rich serum is injected into the affected joint • Examples: • Orthokine/Regenokine • Cytokine (“generic” version offered at IOP)

AUTOLOGOUS CONDITIONED SERUM HOW DOES IT WORK? • Interlukin 1 is a pro-inflammatory cytokine that is thought to be responsible for the progression of arthritis • By injecting large amounts of IL-1 ra it is believed we can slow the progression of cartilage destruction
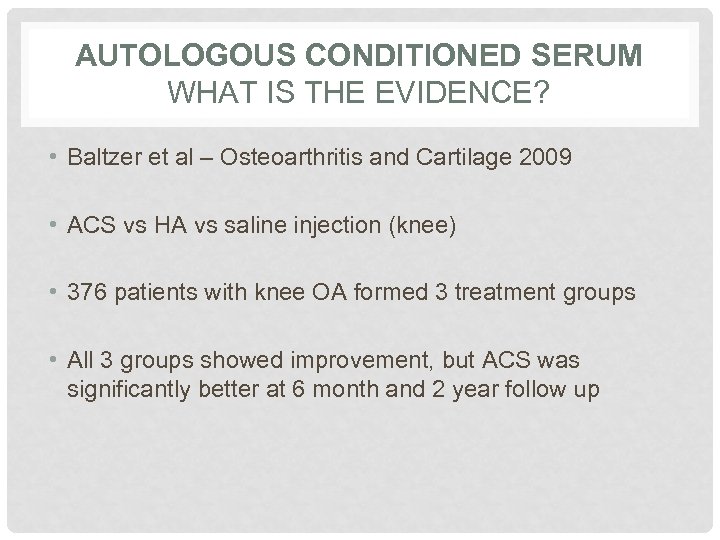
AUTOLOGOUS CONDITIONED SERUM WHAT IS THE EVIDENCE? • Baltzer et al – Osteoarthritis and Cartilage 2009 • ACS vs HA vs saline injection (knee) • 376 patients with knee OA formed 3 treatment groups • All 3 groups showed improvement, but ACS was significantly better at 6 month and 2 year follow up
AUTOLOGOUS CONDITIONED SERUM WHICH PATIENTS MIGHT BENEFIT? • Cost can be a factor • • Custom synringes ~ $600 Cost of appointment/injection Recommended 6 injections ~ $4000 **IOP offer course for $1000
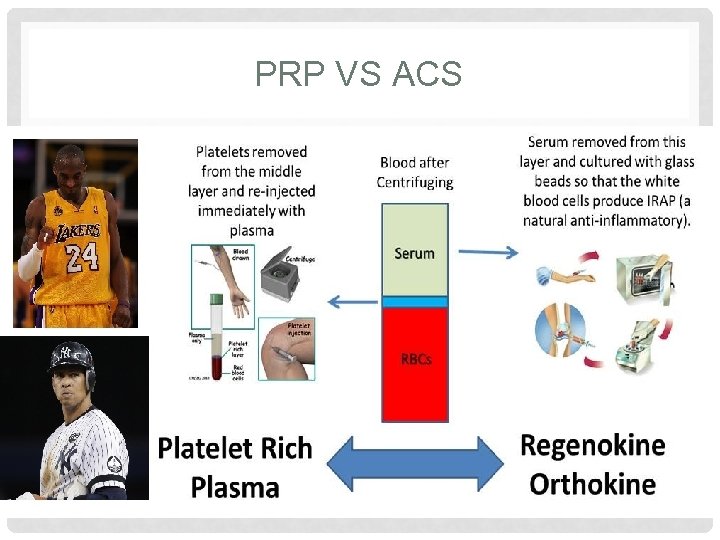
PRP VS ACS

PLATELET-DERIVED GROWTH FACTOR (PDGF) WHAT IS IT? • Synthetic administration of growth factors thought to mediate inflammation and tissue healing • Platelet derived growth factor (PDGF) thought to be the main mediator in tissue healing with this form of treatment

PLATELET-DERIVED GROWTH FACTOR (PDGF) HOW DOES IT WORK? • PDGF engineered in a lab • Injected into the affected joint to try and stimulate local anti-inflammatory and tissue healing response • Potentially leads to halt in arthritic process • Essentially the same mechanism as PRP, just acting at a different level in the tissue healing pathway

PLATELET-DERIVED GROWTH FACTOR (PDGF) WHAT IS THE EVIDENCE? • Very limited • Some good evidence to assist bone grafting on Orthopaedic Surgery (Di. Giovanni et al 2013 JBJS) • Soft tissues and joints mostly studies in animal models • No significant difference between PRP and PDGF • Regranex gel • Topical PDGF for diabetic foot ulcers

IS ALL THIS STUFF LEGAL IN SPORT? ? • Recombinant PDGF • Banned in sport • Thymosin • • Cronulla Sharks alledgedly under investigation for its use Banned under S 2 category Controversial as thymosin is produced naturally by platelets Currently PRP is legal in sport

WADA S 2 CATEGORY PEPTIDE HORMONES, GROWTH FACTORS AND RELATED SUBSTANCES • The following substances, and other substances with similar chemical structure or similar biological effect(s), are prohibited: • Erythropoiesis-Stimulating Agents [e. g. erythropoietin (EPO), darbepoetin (d. EPO), hypoxia-inducible factor (HIF) stabilizers, methoxy polyethylene glycol-epoetin beta (CERA), peginesatide (Hematide)] • Chorionic Gonadotrophin (CG) and Luteinizing Hormone (LH) and their releasing factors, in males • Corticotrophins and their releasing factors • Growth Hormone (GH) and its releasing factors and Insulin -like Growth Factor-1 (IGF-1)
WADA S 2 CATEGORY PEPTIDE HORMONES, GROWTH FACTORS AND RELATED SUBSTANCES • In addition, the following growth factors are prohibited: • Fibroblast Growth Factors (FGFs), Hepatocyte Growth Factor (HGF), Mechano Growth Factors (MGFs), Platelet -Derived Growth Factor (PDGF), Vascular-Endothelial Growth Factor (VEGF) as well as any other growth factor affecting muscle, tendon or ligament protein synthesis/degradation, vascularisation, energy utilization, regenerative capacity or fibre type switching; • and other substances with similar chemical structure or similar biological effect(s).

SUMMARY • Corticosteroid is still a good option for acute exacerbations, but will not alter long term symptoms • Newer injectable options (PRP, ACS, GF’s) have all shown promise • Unable to rank in terms of efficacy, all seem to work • Cost is a major factor • More research required • All injectables can be used in advanced disease to buy time • STEM CELLS ON THE HORIZON……. .
c093b3a7e10c11b9d2861637de87c888.ppt