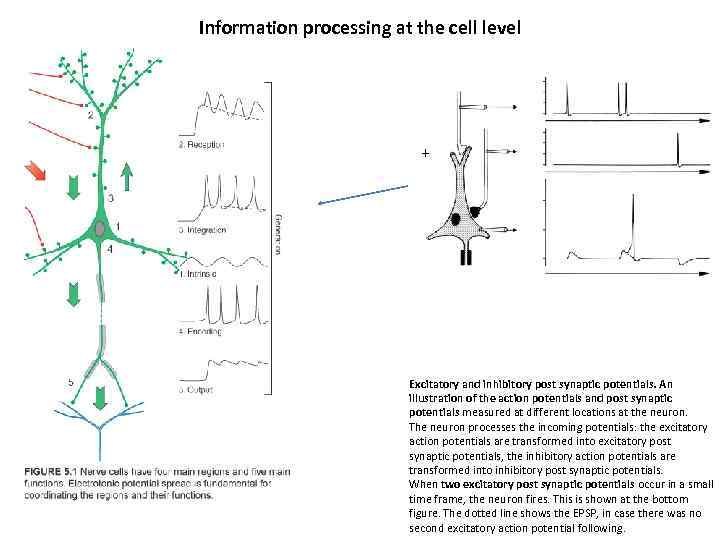
 Information processing at the cell level + - Excitatory and inhibitory post synaptic potentials. An illustration of the action potentials and post synaptic potentials measured at different locations at the neuron. The neuron processes the incoming potentials: the excitatory action potentials are transformed into excitatory post synaptic potentials, the inhibitory action potentials are transformed into inhibitory post synaptic potentials. When two excitatory post synaptic potentials occur in a small time frame, the neuron fires. This is shown at the bottom figure. The dotted line shows the EPSP, in case there was no second excitatory action potential following.
Information processing at the cell level + - Excitatory and inhibitory post synaptic potentials. An illustration of the action potentials and post synaptic potentials measured at different locations at the neuron. The neuron processes the incoming potentials: the excitatory action potentials are transformed into excitatory post synaptic potentials, the inhibitory action potentials are transformed into inhibitory post synaptic potentials. When two excitatory post synaptic potentials occur in a small time frame, the neuron fires. This is shown at the bottom figure. The dotted line shows the EPSP, in case there was no second excitatory action potential following.
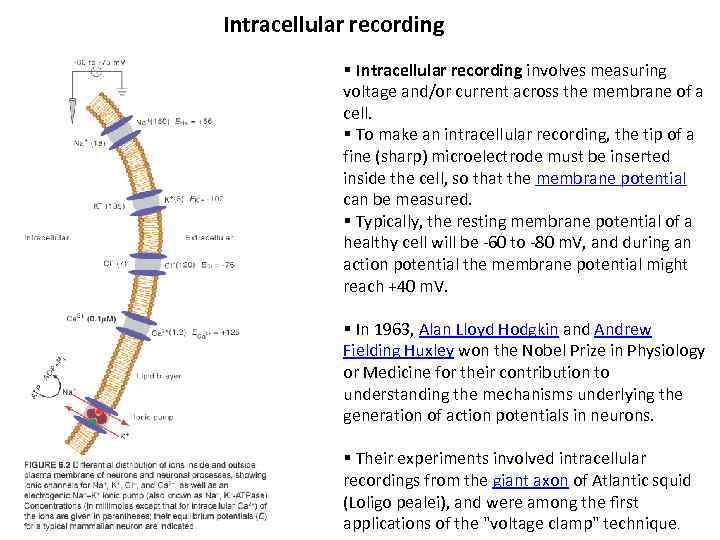 Intracellular recording § Intracellular recording involves measuring voltage and/or current across the membrane of a cell. § To make an intracellular recording, the tip of a fine (sharp) microelectrode must be inserted inside the cell, so that the membrane potential can be measured. § Typically, the resting membrane potential of a healthy cell will be -60 to -80 m. V, and during an action potential the membrane potential might reach +40 m. V. § In 1963, Alan Lloyd Hodgkin and Andrew Fielding Huxley won the Nobel Prize in Physiology or Medicine for their contribution to understanding the mechanisms underlying the generation of action potentials in neurons. § Their experiments involved intracellular recordings from the giant axon of Atlantic squid (Loligo pealei), and were among the first applications of the "voltage clamp" technique.
Intracellular recording § Intracellular recording involves measuring voltage and/or current across the membrane of a cell. § To make an intracellular recording, the tip of a fine (sharp) microelectrode must be inserted inside the cell, so that the membrane potential can be measured. § Typically, the resting membrane potential of a healthy cell will be -60 to -80 m. V, and during an action potential the membrane potential might reach +40 m. V. § In 1963, Alan Lloyd Hodgkin and Andrew Fielding Huxley won the Nobel Prize in Physiology or Medicine for their contribution to understanding the mechanisms underlying the generation of action potentials in neurons. § Their experiments involved intracellular recordings from the giant axon of Atlantic squid (Loligo pealei), and were among the first applications of the "voltage clamp" technique.

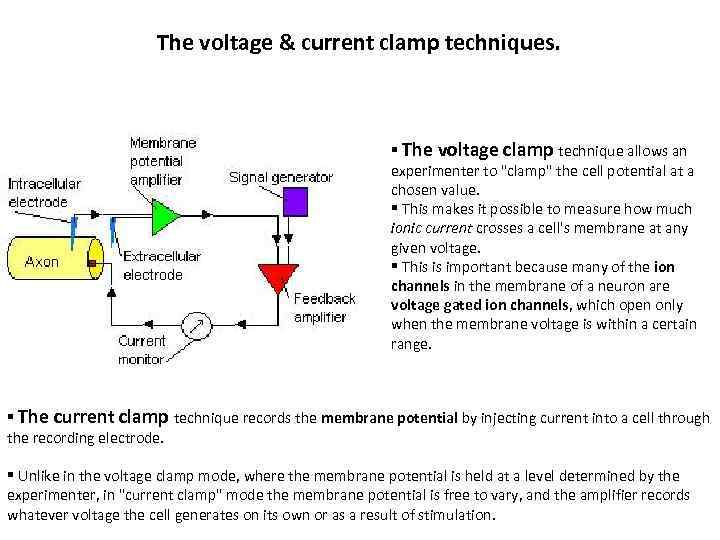 The voltage & current clamp techniques. § The voltage clamp technique allows an experimenter to "clamp" the cell potential at a chosen value. § This makes it possible to measure how much ionic current crosses a cell's membrane at any given voltage. § This is important because many of the ion channels in the membrane of a neuron are voltage gated ion channels, which open only when the membrane voltage is within a certain range. § The current clamp technique records the membrane potential by injecting current into a cell through the recording electrode. § Unlike in the voltage clamp mode, where the membrane potential is held at a level determined by the experimenter, in "current clamp" mode the membrane potential is free to vary, and the amplifier records whatever voltage the cell generates on its own or as a result of stimulation.
The voltage & current clamp techniques. § The voltage clamp technique allows an experimenter to "clamp" the cell potential at a chosen value. § This makes it possible to measure how much ionic current crosses a cell's membrane at any given voltage. § This is important because many of the ion channels in the membrane of a neuron are voltage gated ion channels, which open only when the membrane voltage is within a certain range. § The current clamp technique records the membrane potential by injecting current into a cell through the recording electrode. § Unlike in the voltage clamp mode, where the membrane potential is held at a level determined by the experimenter, in "current clamp" mode the membrane potential is free to vary, and the amplifier records whatever voltage the cell generates on its own or as a result of stimulation.
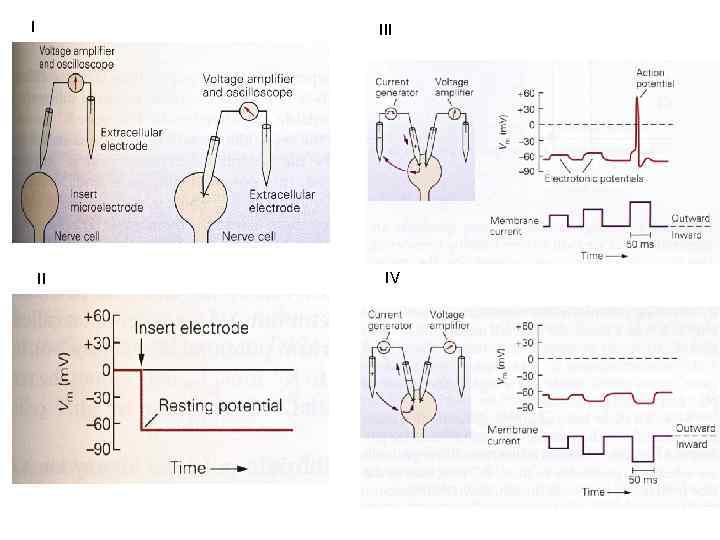 I II IV
I II IV
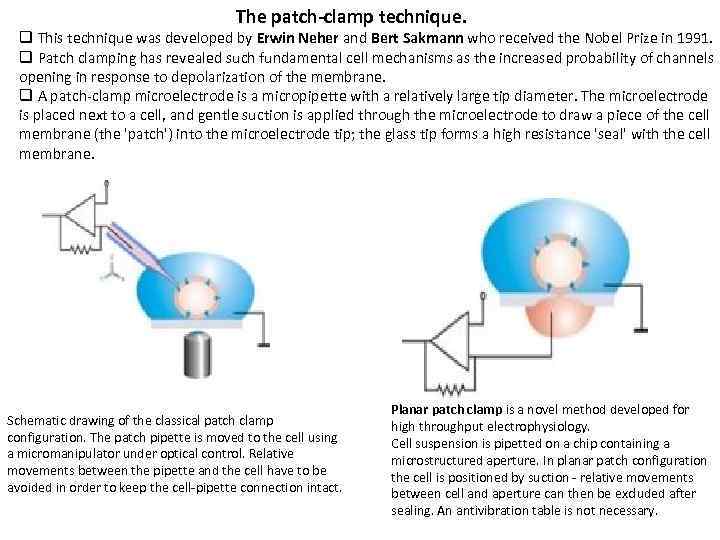 The patch-clamp technique. q This technique was developed by Erwin Neher and Bert Sakmann who received the Nobel Prize in 1991. q Patch clamping has revealed such fundamental cell mechanisms as the increased probability of channels opening in response to depolarization of the membrane. q A patch-clamp microelectrode is a micropipette with a relatively large tip diameter. The microelectrode is placed next to a cell, and gentle suction is applied through the microelectrode to draw a piece of the cell membrane (the 'patch') into the microelectrode tip; the glass tip forms a high resistance 'seal' with the cell membrane. Schematic drawing of the classical patch clamp configuration. The patch pipette is moved to the cell using a micromanipulator under optical control. Relative movements between the pipette and the cell have to be avoided in order to keep the cell-pipette connection intact. Planar patch clamp is a novel method developed for high throughput electrophysiology. Cell suspension is pipetted on a chip containing a microstructured aperture. In planar patch configuration the cell is positioned by suction - relative movements between cell and aperture can then be excluded after sealing. An antivibration table is not necessary.
The patch-clamp technique. q This technique was developed by Erwin Neher and Bert Sakmann who received the Nobel Prize in 1991. q Patch clamping has revealed such fundamental cell mechanisms as the increased probability of channels opening in response to depolarization of the membrane. q A patch-clamp microelectrode is a micropipette with a relatively large tip diameter. The microelectrode is placed next to a cell, and gentle suction is applied through the microelectrode to draw a piece of the cell membrane (the 'patch') into the microelectrode tip; the glass tip forms a high resistance 'seal' with the cell membrane. Schematic drawing of the classical patch clamp configuration. The patch pipette is moved to the cell using a micromanipulator under optical control. Relative movements between the pipette and the cell have to be avoided in order to keep the cell-pipette connection intact. Planar patch clamp is a novel method developed for high throughput electrophysiology. Cell suspension is pipetted on a chip containing a microstructured aperture. In planar patch configuration the cell is positioned by suction - relative movements between cell and aperture can then be excluded after sealing. An antivibration table is not necessary.
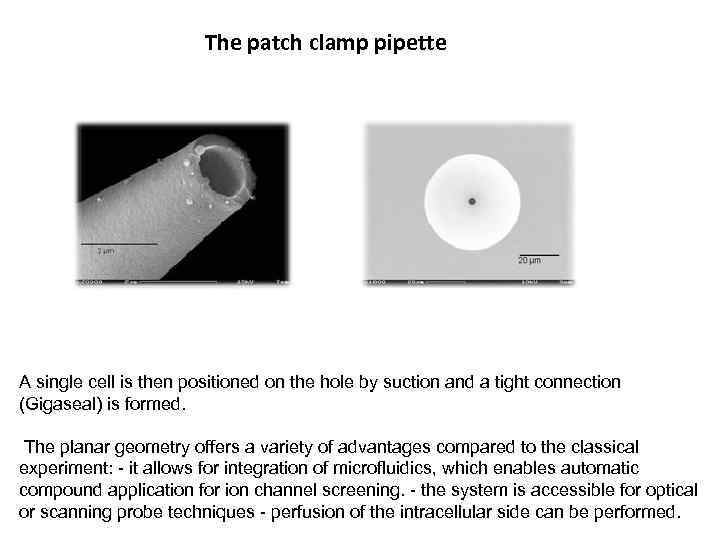 The patch clamp pipette A single cell is then positioned on the hole by suction and a tight connection (Gigaseal) is formed. The planar geometry offers a variety of advantages compared to the classical experiment: - it allows for integration of microfluidics, which enables automatic compound application for ion channel screening. - the system is accessible for optical or scanning probe techniques - perfusion of the intracellular side can be performed.
The patch clamp pipette A single cell is then positioned on the hole by suction and a tight connection (Gigaseal) is formed. The planar geometry offers a variety of advantages compared to the classical experiment: - it allows for integration of microfluidics, which enables automatic compound application for ion channel screening. - the system is accessible for optical or scanning probe techniques - perfusion of the intracellular side can be performed.
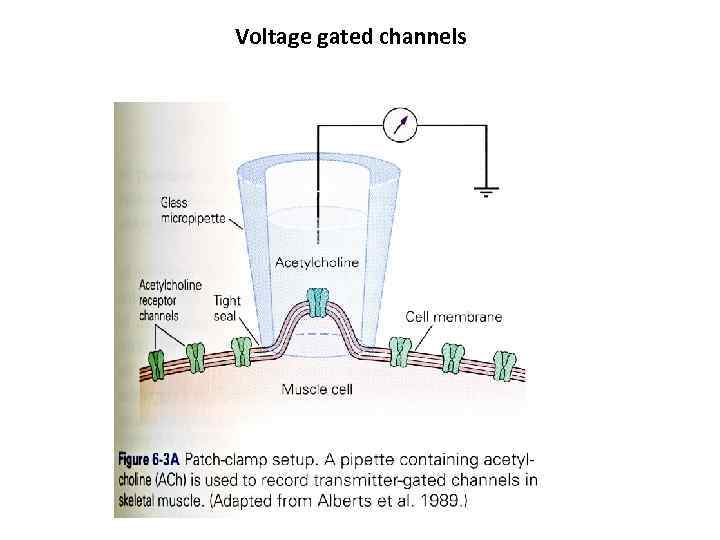 Voltage gated channels
Voltage gated channels
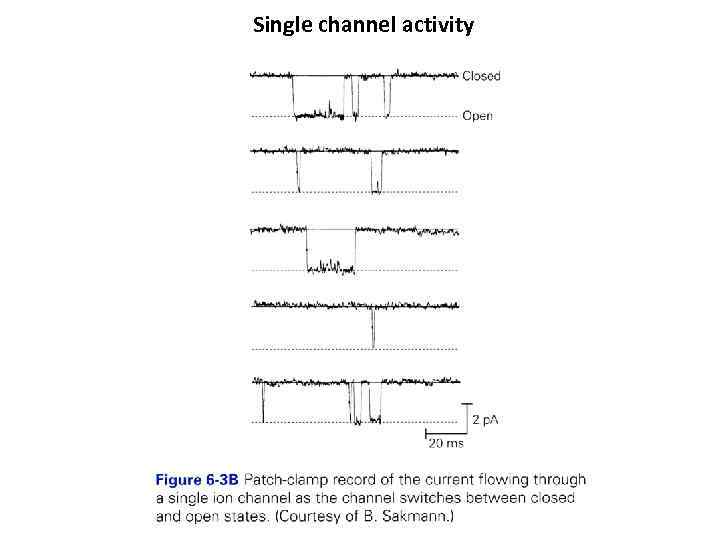 Single channel activity
Single channel activity
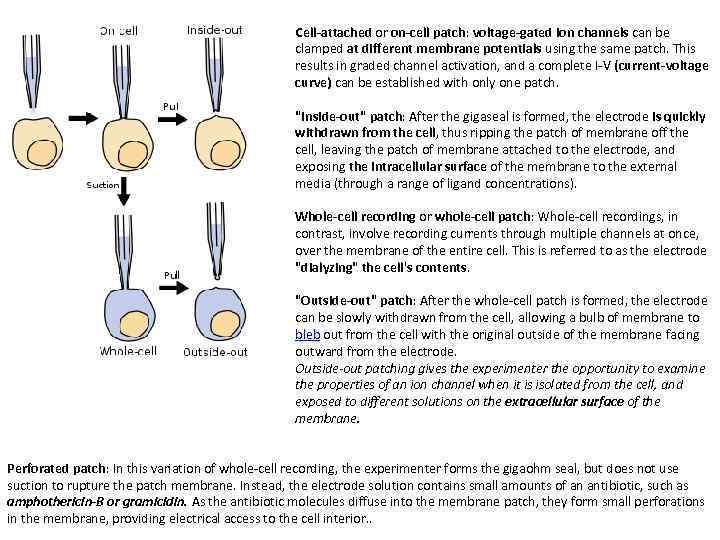 Cell-attached or on-cell patch: voltage-gated ion channels can be clamped at different membrane potentials using the same patch. This results in graded channel activation, and a complete I-V (current-voltage curve) can be established with only one patch. "Inside-out" patch: After the gigaseal is formed, the electrode is quickly withdrawn from the cell, thus ripping the patch of membrane off the cell, leaving the patch of membrane attached to the electrode, and exposing the intracellular surface of the membrane to the external media (through a range of ligand concentrations). Whole-cell recording or whole-cell patch: Whole-cell recordings, in contrast, involve recording currents through multiple channels at once, over the membrane of the entire cell. This is referred to as the electrode "dialyzing" the cell's contents. "Outside-out" patch: After the whole-cell patch is formed, the electrode can be slowly withdrawn from the cell, allowing a bulb of membrane to bleb out from the cell with the original outside of the membrane facing outward from the electrode. Outside-out patching gives the experimenter the opportunity to examine the properties of an ion channel when it is isolated from the cell, and exposed to different solutions on the extracellular surface of the membrane. Perforated patch: In this variation of whole-cell recording, the experimenter forms the gigaohm seal, but does not use suction to rupture the patch membrane. Instead, the electrode solution contains small amounts of an antibiotic, such as amphothericin-B or gramicidin. As the antibiotic molecules diffuse into the membrane patch, they form small perforations in the membrane, providing electrical access to the cell interior. .
Cell-attached or on-cell patch: voltage-gated ion channels can be clamped at different membrane potentials using the same patch. This results in graded channel activation, and a complete I-V (current-voltage curve) can be established with only one patch. "Inside-out" patch: After the gigaseal is formed, the electrode is quickly withdrawn from the cell, thus ripping the patch of membrane off the cell, leaving the patch of membrane attached to the electrode, and exposing the intracellular surface of the membrane to the external media (through a range of ligand concentrations). Whole-cell recording or whole-cell patch: Whole-cell recordings, in contrast, involve recording currents through multiple channels at once, over the membrane of the entire cell. This is referred to as the electrode "dialyzing" the cell's contents. "Outside-out" patch: After the whole-cell patch is formed, the electrode can be slowly withdrawn from the cell, allowing a bulb of membrane to bleb out from the cell with the original outside of the membrane facing outward from the electrode. Outside-out patching gives the experimenter the opportunity to examine the properties of an ion channel when it is isolated from the cell, and exposed to different solutions on the extracellular surface of the membrane. Perforated patch: In this variation of whole-cell recording, the experimenter forms the gigaohm seal, but does not use suction to rupture the patch membrane. Instead, the electrode solution contains small amounts of an antibiotic, such as amphothericin-B or gramicidin. As the antibiotic molecules diffuse into the membrane patch, they form small perforations in the membrane, providing electrical access to the cell interior. .
 Complete Patch Clamp Set-up for Tissue Slices • The Olympus BX 51 WI Fixed Stage Upright Microscope • A range of fluorescence and IR cameras • Fluorescence systems Scientifica Motorised Manipulators Manual Manipulators • Mounting Platforms An Anti-Vibration Table with Faraday Cage. Amplifiers • Perfusion Systems and a range of Temperature Control Items
Complete Patch Clamp Set-up for Tissue Slices • The Olympus BX 51 WI Fixed Stage Upright Microscope • A range of fluorescence and IR cameras • Fluorescence systems Scientifica Motorised Manipulators Manual Manipulators • Mounting Platforms An Anti-Vibration Table with Faraday Cage. Amplifiers • Perfusion Systems and a range of Temperature Control Items
 Extracellular Recordings. q An electrode introduced into the brain of a living animal will detect electrical activity that is generated by the neurons adjacent to the electrode tip. If the electrode is a microelectrode, with a tip size of about 1 micrometer, the electrode will usually detect the activity of at most one neuron. Recording in this way is generally called "single unit" recording. The action potentials recorded are very like the action potentials that are recorded intracellularly, but the signals are very much smaller (typically about 1 m. V). Most recordings of the activity of single neurons in anesthetized animals are made in this way, and all recordings of single neurons in conscious animals. q For example, David Hubel and Torsten Wiesel recorded the activity of single neurons in the primary visual cortex of the anesthetized cat, and were awarded the Nobel Prize in Physiology or Medicine in 1981. q If the electrode tip is slightly larger, then the electrode might record the activity generated by several neurons. This type of recording is often called "multi-unit recording", and is often used in conscious animals to record changes in the activity in a discrete brain area during normal activity. q If the electrode tip is bigger still, generally the activity of individual neurons cannot be distinguished but the electrode will still be able to record a field potential generated by the activity of many cells.
Extracellular Recordings. q An electrode introduced into the brain of a living animal will detect electrical activity that is generated by the neurons adjacent to the electrode tip. If the electrode is a microelectrode, with a tip size of about 1 micrometer, the electrode will usually detect the activity of at most one neuron. Recording in this way is generally called "single unit" recording. The action potentials recorded are very like the action potentials that are recorded intracellularly, but the signals are very much smaller (typically about 1 m. V). Most recordings of the activity of single neurons in anesthetized animals are made in this way, and all recordings of single neurons in conscious animals. q For example, David Hubel and Torsten Wiesel recorded the activity of single neurons in the primary visual cortex of the anesthetized cat, and were awarded the Nobel Prize in Physiology or Medicine in 1981. q If the electrode tip is slightly larger, then the electrode might record the activity generated by several neurons. This type of recording is often called "multi-unit recording", and is often used in conscious animals to record changes in the activity in a discrete brain area during normal activity. q If the electrode tip is bigger still, generally the activity of individual neurons cannot be distinguished but the electrode will still be able to record a field potential generated by the activity of many cells.
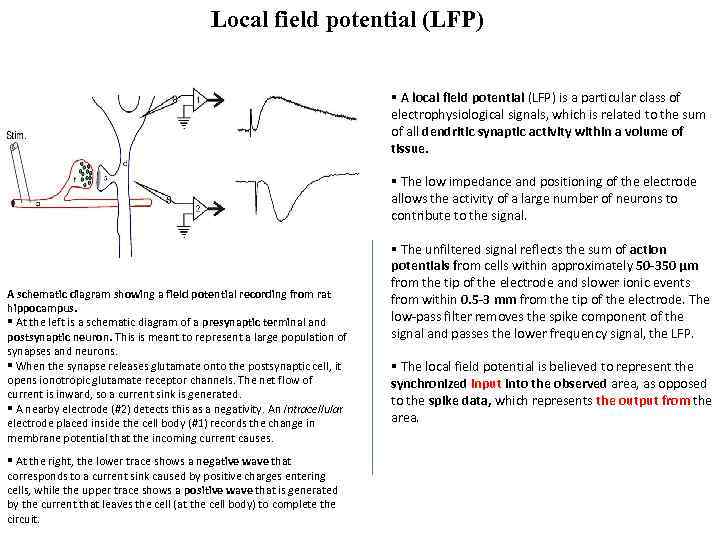 Local field potential (LFP) § A local field potential (LFP) is a particular class of electrophysiological signals, which is related to the sum of all dendritic synaptic activity within a volume of tissue. § The low impedance and positioning of the electrode allows the activity of a large number of neurons to contribute to the signal. A schematic diagram showing a field potential recording from rat hippocampus. § At the left is a schematic diagram of a presynaptic terminal and postsynaptic neuron. This is meant to represent a large population of synapses and neurons. § When the synapse releases glutamate onto the postsynaptic cell, it opens ionotropic glutamate receptor channels. The net flow of current is inward, so a current sink is generated. § A nearby electrode (#2) detects this as a negativity. An intracellular electrode placed inside the cell body (#1) records the change in membrane potential that the incoming current causes. § At the right, the lower trace shows a negative wave that corresponds to a current sink caused by positive charges entering cells, while the upper trace shows a positive wave that is generated by the current that leaves the cell (at the cell body) to complete the circuit. § The unfiltered signal reflects the sum of action potentials from cells within approximately 50 -350 μm from the tip of the electrode and slower ionic events from within 0. 5 -3 mm from the tip of the electrode. The low-pass filter removes the spike component of the signal and passes the lower frequency signal, the LFP. § The local field potential is believed to represent the synchronized input into the observed area, as opposed to the spike data, which represents the output from the area.
Local field potential (LFP) § A local field potential (LFP) is a particular class of electrophysiological signals, which is related to the sum of all dendritic synaptic activity within a volume of tissue. § The low impedance and positioning of the electrode allows the activity of a large number of neurons to contribute to the signal. A schematic diagram showing a field potential recording from rat hippocampus. § At the left is a schematic diagram of a presynaptic terminal and postsynaptic neuron. This is meant to represent a large population of synapses and neurons. § When the synapse releases glutamate onto the postsynaptic cell, it opens ionotropic glutamate receptor channels. The net flow of current is inward, so a current sink is generated. § A nearby electrode (#2) detects this as a negativity. An intracellular electrode placed inside the cell body (#1) records the change in membrane potential that the incoming current causes. § At the right, the lower trace shows a negative wave that corresponds to a current sink caused by positive charges entering cells, while the upper trace shows a positive wave that is generated by the current that leaves the cell (at the cell body) to complete the circuit. § The unfiltered signal reflects the sum of action potentials from cells within approximately 50 -350 μm from the tip of the electrode and slower ionic events from within 0. 5 -3 mm from the tip of the electrode. The low-pass filter removes the spike component of the signal and passes the lower frequency signal, the LFP. § The local field potential is believed to represent the synchronized input into the observed area, as opposed to the spike data, which represents the output from the area.


