070bab774ea036433f9ac8bbb4034e94.ppt
- Количество слайдов: 10
 Influenza Vaccine Manufacturing Industry Perspective Tony Colegate Novartis Vaccines and Diagnostics Prepared by Ph. RMA Vaccine Technical Committee for presentation to VRBPAC Meeting 21 February 2008
Influenza Vaccine Manufacturing Industry Perspective Tony Colegate Novartis Vaccines and Diagnostics Prepared by Ph. RMA Vaccine Technical Committee for presentation to VRBPAC Meeting 21 February 2008
 Influenza Vaccine Manufacturing – Critical Factors • Growth potential of seed virus – The quantity of trivalent influenza vaccine that can be produced is limited by the least productive monovalent strain • Timing of strain selection – Available production time is limited due to necessity of distributing and administering vaccine prior to influenza season – Working seeds require at least 4 weeks (from receipt of seed candidate) for development prior to use in large-scale manufacturing • Potency test reagents – Required to determine the potency of monovalent components prior to formulation of trivalent vaccine (SRID) – Must be produced/standardized for new strains • Timing of Annual License Supplement Approval – Product release
Influenza Vaccine Manufacturing – Critical Factors • Growth potential of seed virus – The quantity of trivalent influenza vaccine that can be produced is limited by the least productive monovalent strain • Timing of strain selection – Available production time is limited due to necessity of distributing and administering vaccine prior to influenza season – Working seeds require at least 4 weeks (from receipt of seed candidate) for development prior to use in large-scale manufacturing • Potency test reagents – Required to determine the potency of monovalent components prior to formulation of trivalent vaccine (SRID) – Must be produced/standardized for new strains • Timing of Annual License Supplement Approval – Product release
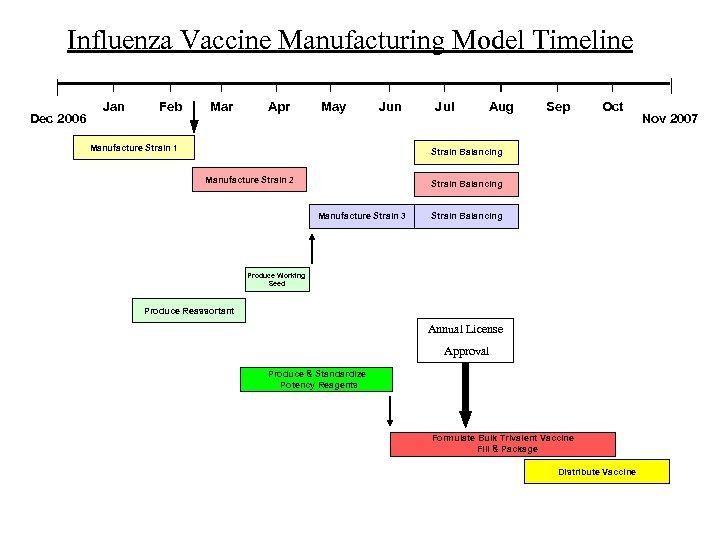 Influenza Vaccine Manufacturing Model Timeline Dec 2006 Jan Feb Mar Apr May Jun Manufacture Strain 1 Jul Aug Sep Oct Strain Balancing Manufacture Strain 2 Strain Balancing Manufacture Strain 3 Strain Balancing Produce Working Seed Produce Reassortant Annual License Approval Produce & Standardize Potency Reagents Formulate Bulk Trivalent Vaccine Fill & Package Distribute Vaccine Nov 2007
Influenza Vaccine Manufacturing Model Timeline Dec 2006 Jan Feb Mar Apr May Jun Manufacture Strain 1 Jul Aug Sep Oct Strain Balancing Manufacture Strain 2 Strain Balancing Manufacture Strain 3 Strain Balancing Produce Working Seed Produce Reassortant Annual License Approval Produce & Standardize Potency Reagents Formulate Bulk Trivalent Vaccine Fill & Package Distribute Vaccine Nov 2007
 Current Manufacturing Status • Production of monovalent strain(s) is underway – Production initiated “at risk of the strain(s) not being selected” for 2008 – 2009 Northern Hemisphere Influenza Vaccine to ensure sufficient vaccine supply. – Based on the publicly available surveillance information, manufacturers have chosen to produce the A/H 1 N 1 A/Solomon Islands/3/2006 and/or B/Florida/4/2006 -like strain(s) at-risk. – However WHO have not recommended A/Solomon Islands/3/2006 for the 2008 – 2009 season. – Three new strains for 2008 – 2009 Northern Hemisphere Influenza season is unprecedented for Northern Hemisphere in the last 20 years and will make it a very challenging year.
Current Manufacturing Status • Production of monovalent strain(s) is underway – Production initiated “at risk of the strain(s) not being selected” for 2008 – 2009 Northern Hemisphere Influenza Vaccine to ensure sufficient vaccine supply. – Based on the publicly available surveillance information, manufacturers have chosen to produce the A/H 1 N 1 A/Solomon Islands/3/2006 and/or B/Florida/4/2006 -like strain(s) at-risk. – However WHO have not recommended A/Solomon Islands/3/2006 for the 2008 – 2009 season. – Three new strains for 2008 – 2009 Northern Hemisphere Influenza season is unprecedented for Northern Hemisphere in the last 20 years and will make it a very challenging year.
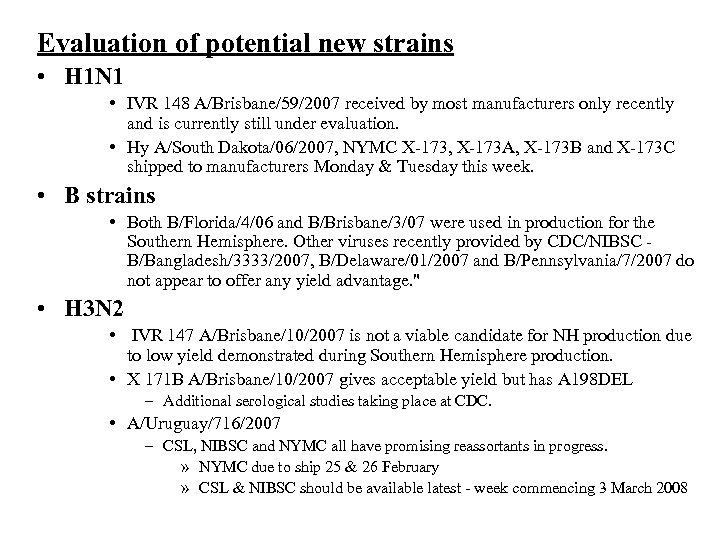 Evaluation of potential new strains • H 1 N 1 • IVR 148 A/Brisbane/59/2007 received by most manufacturers only recently and is currently still under evaluation. • Hy A/South Dakota/06/2007, NYMC X-173, X-173 A, X-173 B and X-173 C shipped to manufacturers Monday & Tuesday this week. • B strains • Both B/Florida/4/06 and B/Brisbane/3/07 were used in production for the Southern Hemisphere. Other viruses recently provided by CDC/NIBSC B/Bangladesh/3333/2007, B/Delaware/01/2007 and B/Pennsylvania/7/2007 do not appear to offer any yield advantage. " • H 3 N 2 • IVR 147 A/Brisbane/10/2007 is not a viable candidate for NH production due to low yield demonstrated during Southern Hemisphere production. • X 171 B A/Brisbane/10/2007 gives acceptable yield but has A 198 DEL – Additional serological studies taking place at CDC. • A/Uruguay/716/2007 – CSL, NIBSC and NYMC all have promising reassortants in progress. » NYMC due to ship 25 & 26 February » CSL & NIBSC should be available latest - week commencing 3 March 2008
Evaluation of potential new strains • H 1 N 1 • IVR 148 A/Brisbane/59/2007 received by most manufacturers only recently and is currently still under evaluation. • Hy A/South Dakota/06/2007, NYMC X-173, X-173 A, X-173 B and X-173 C shipped to manufacturers Monday & Tuesday this week. • B strains • Both B/Florida/4/06 and B/Brisbane/3/07 were used in production for the Southern Hemisphere. Other viruses recently provided by CDC/NIBSC B/Bangladesh/3333/2007, B/Delaware/01/2007 and B/Pennsylvania/7/2007 do not appear to offer any yield advantage. " • H 3 N 2 • IVR 147 A/Brisbane/10/2007 is not a viable candidate for NH production due to low yield demonstrated during Southern Hemisphere production. • X 171 B A/Brisbane/10/2007 gives acceptable yield but has A 198 DEL – Additional serological studies taking place at CDC. • A/Uruguay/716/2007 – CSL, NIBSC and NYMC all have promising reassortants in progress. » NYMC due to ship 25 & 26 February » CSL & NIBSC should be available latest - week commencing 3 March 2008
 Public/Private Cooperation is necessary for successful Influenza Vaccine production & supply • Timely Committee selection of the appropriate antigens – Consideration of antigenic match as well as growth potential • Availability of seed viruses – Especially High Growth Reassortants – Opportunity for manufacturers to evaluate growth characteristics of potential strain candidates • Availability of potency test reagents for all three strains by early June. • Timely approval of Annual License Supplement
Public/Private Cooperation is necessary for successful Influenza Vaccine production & supply • Timely Committee selection of the appropriate antigens – Consideration of antigenic match as well as growth potential • Availability of seed viruses – Especially High Growth Reassortants – Opportunity for manufacturers to evaluate growth characteristics of potential strain candidates • Availability of potency test reagents for all three strains by early June. • Timely approval of Annual License Supplement
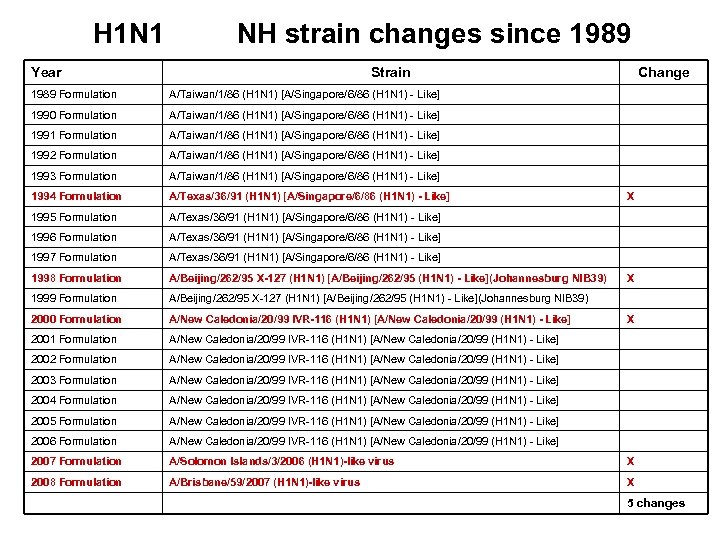 H 1 N 1 NH strain changes since 1989 Year Strain Change 1989 Formulation A/Taiwan/1/86 (H 1 N 1) [A/Singapore/6/86 (H 1 N 1) - Like] 1990 Formulation A/Taiwan/1/86 (H 1 N 1) [A/Singapore/6/86 (H 1 N 1) - Like] 1991 Formulation A/Taiwan/1/86 (H 1 N 1) [A/Singapore/6/86 (H 1 N 1) - Like] 1992 Formulation A/Taiwan/1/86 (H 1 N 1) [A/Singapore/6/86 (H 1 N 1) - Like] 1993 Formulation A/Taiwan/1/86 (H 1 N 1) [A/Singapore/6/86 (H 1 N 1) - Like] 1994 Formulation A/Texas/36/91 (H 1 N 1) [A/Singapore/6/86 (H 1 N 1) - Like] 1995 Formulation A/Texas/36/91 (H 1 N 1) [A/Singapore/6/86 (H 1 N 1) - Like] 1996 Formulation A/Texas/36/91 (H 1 N 1) [A/Singapore/6/86 (H 1 N 1) - Like] 1997 Formulation A/Texas/36/91 (H 1 N 1) [A/Singapore/6/86 (H 1 N 1) - Like] 1998 Formulation A/Beijing/262/95 X-127 (H 1 N 1) [A/Beijing/262/95 (H 1 N 1) - Like](Johannesburg NIB 39) 1999 Formulation A/Beijing/262/95 X-127 (H 1 N 1) [A/Beijing/262/95 (H 1 N 1) - Like](Johannesburg NIB 39) 2000 Formulation A/New Caledonia/20/99 IVR-116 (H 1 N 1) [A/New Caledonia/20/99 (H 1 N 1) - Like] 2001 Formulation A/New Caledonia/20/99 IVR-116 (H 1 N 1) [A/New Caledonia/20/99 (H 1 N 1) - Like] 2002 Formulation A/New Caledonia/20/99 IVR-116 (H 1 N 1) [A/New Caledonia/20/99 (H 1 N 1) - Like] 2003 Formulation A/New Caledonia/20/99 IVR-116 (H 1 N 1) [A/New Caledonia/20/99 (H 1 N 1) - Like] 2004 Formulation A/New Caledonia/20/99 IVR-116 (H 1 N 1) [A/New Caledonia/20/99 (H 1 N 1) - Like] 2005 Formulation A/New Caledonia/20/99 IVR-116 (H 1 N 1) [A/New Caledonia/20/99 (H 1 N 1) - Like] 2006 Formulation A/New Caledonia/20/99 IVR-116 (H 1 N 1) [A/New Caledonia/20/99 (H 1 N 1) - Like] 2007 Formulation A/Solomon Islands/3/2006 (H 1 N 1)-like virus X 2008 Formulation A/Brisbane/59/2007 (H 1 N 1)-like virus X X 5 changes
H 1 N 1 NH strain changes since 1989 Year Strain Change 1989 Formulation A/Taiwan/1/86 (H 1 N 1) [A/Singapore/6/86 (H 1 N 1) - Like] 1990 Formulation A/Taiwan/1/86 (H 1 N 1) [A/Singapore/6/86 (H 1 N 1) - Like] 1991 Formulation A/Taiwan/1/86 (H 1 N 1) [A/Singapore/6/86 (H 1 N 1) - Like] 1992 Formulation A/Taiwan/1/86 (H 1 N 1) [A/Singapore/6/86 (H 1 N 1) - Like] 1993 Formulation A/Taiwan/1/86 (H 1 N 1) [A/Singapore/6/86 (H 1 N 1) - Like] 1994 Formulation A/Texas/36/91 (H 1 N 1) [A/Singapore/6/86 (H 1 N 1) - Like] 1995 Formulation A/Texas/36/91 (H 1 N 1) [A/Singapore/6/86 (H 1 N 1) - Like] 1996 Formulation A/Texas/36/91 (H 1 N 1) [A/Singapore/6/86 (H 1 N 1) - Like] 1997 Formulation A/Texas/36/91 (H 1 N 1) [A/Singapore/6/86 (H 1 N 1) - Like] 1998 Formulation A/Beijing/262/95 X-127 (H 1 N 1) [A/Beijing/262/95 (H 1 N 1) - Like](Johannesburg NIB 39) 1999 Formulation A/Beijing/262/95 X-127 (H 1 N 1) [A/Beijing/262/95 (H 1 N 1) - Like](Johannesburg NIB 39) 2000 Formulation A/New Caledonia/20/99 IVR-116 (H 1 N 1) [A/New Caledonia/20/99 (H 1 N 1) - Like] 2001 Formulation A/New Caledonia/20/99 IVR-116 (H 1 N 1) [A/New Caledonia/20/99 (H 1 N 1) - Like] 2002 Formulation A/New Caledonia/20/99 IVR-116 (H 1 N 1) [A/New Caledonia/20/99 (H 1 N 1) - Like] 2003 Formulation A/New Caledonia/20/99 IVR-116 (H 1 N 1) [A/New Caledonia/20/99 (H 1 N 1) - Like] 2004 Formulation A/New Caledonia/20/99 IVR-116 (H 1 N 1) [A/New Caledonia/20/99 (H 1 N 1) - Like] 2005 Formulation A/New Caledonia/20/99 IVR-116 (H 1 N 1) [A/New Caledonia/20/99 (H 1 N 1) - Like] 2006 Formulation A/New Caledonia/20/99 IVR-116 (H 1 N 1) [A/New Caledonia/20/99 (H 1 N 1) - Like] 2007 Formulation A/Solomon Islands/3/2006 (H 1 N 1)-like virus X 2008 Formulation A/Brisbane/59/2007 (H 1 N 1)-like virus X X 5 changes
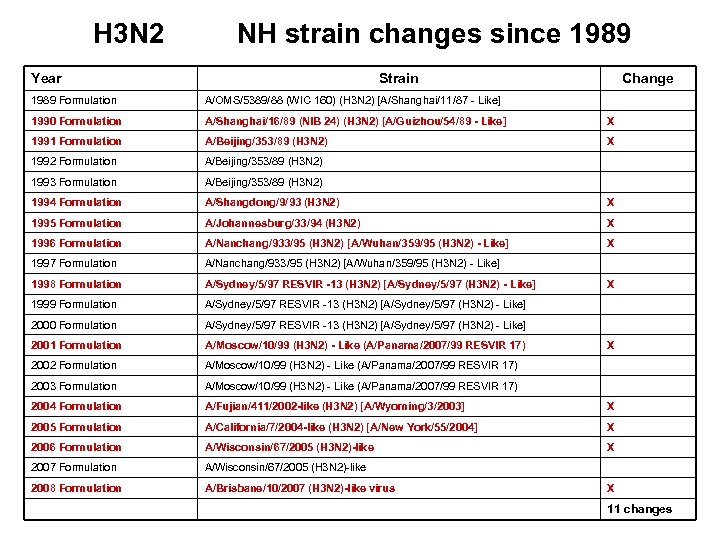 H 3 N 2 NH strain changes since 1989 Year Strain Change 1989 Formulation A/OMS/5389/88 (WIC 160) (H 3 N 2) [A/Shanghai/11/87 - Like] 1990 Formulation A/Shanghai/16/89 (NIB 24) (H 3 N 2) [A/Guizhou/54/89 - Like] X 1991 Formulation A/Beijing/353/89 (H 3 N 2) X 1992 Formulation A/Beijing/353/89 (H 3 N 2) 1993 Formulation A/Beijing/353/89 (H 3 N 2) 1994 Formulation A/Shangdong/9/93 (H 3 N 2) X 1995 Formulation A/Johannesburg/33/94 (H 3 N 2) X 1996 Formulation A/Nanchang/933/95 (H 3 N 2) [A/Wuhan/359/95 (H 3 N 2) - Like] X 1997 Formulation A/Nanchang/933/95 (H 3 N 2) [A/Wuhan/359/95 (H 3 N 2) - Like] 1998 Formulation A/Sydney/5/97 RESVIR -13 (H 3 N 2) [A/Sydney/5/97 (H 3 N 2) - Like] 1999 Formulation A/Sydney/5/97 RESVIR -13 (H 3 N 2) [A/Sydney/5/97 (H 3 N 2) - Like] 2000 Formulation A/Sydney/5/97 RESVIR -13 (H 3 N 2) [A/Sydney/5/97 (H 3 N 2) - Like] 2001 Formulation A/Moscow/10/99 (H 3 N 2) - Like (A/Panama/2007/99 RESVIR 17) 2002 Formulation A/Moscow/10/99 (H 3 N 2) - Like (A/Panama/2007/99 RESVIR 17) 2003 Formulation A/Moscow/10/99 (H 3 N 2) - Like (A/Panama/2007/99 RESVIR 17) 2004 Formulation A/Fujian/411/2002 -like (H 3 N 2) [A/Wyoming/3/2003] X 2005 Formulation A/California/7/2004 -like (H 3 N 2) [A/New York/55/2004] X 2006 Formulation A/Wisconsin/67/2005 (H 3 N 2)-like X 2007 Formulation A/Wisconsin/67/2005 (H 3 N 2)-like 2008 Formulation A/Brisbane/10/2007 (H 3 N 2)-like virus X X X 11 changes
H 3 N 2 NH strain changes since 1989 Year Strain Change 1989 Formulation A/OMS/5389/88 (WIC 160) (H 3 N 2) [A/Shanghai/11/87 - Like] 1990 Formulation A/Shanghai/16/89 (NIB 24) (H 3 N 2) [A/Guizhou/54/89 - Like] X 1991 Formulation A/Beijing/353/89 (H 3 N 2) X 1992 Formulation A/Beijing/353/89 (H 3 N 2) 1993 Formulation A/Beijing/353/89 (H 3 N 2) 1994 Formulation A/Shangdong/9/93 (H 3 N 2) X 1995 Formulation A/Johannesburg/33/94 (H 3 N 2) X 1996 Formulation A/Nanchang/933/95 (H 3 N 2) [A/Wuhan/359/95 (H 3 N 2) - Like] X 1997 Formulation A/Nanchang/933/95 (H 3 N 2) [A/Wuhan/359/95 (H 3 N 2) - Like] 1998 Formulation A/Sydney/5/97 RESVIR -13 (H 3 N 2) [A/Sydney/5/97 (H 3 N 2) - Like] 1999 Formulation A/Sydney/5/97 RESVIR -13 (H 3 N 2) [A/Sydney/5/97 (H 3 N 2) - Like] 2000 Formulation A/Sydney/5/97 RESVIR -13 (H 3 N 2) [A/Sydney/5/97 (H 3 N 2) - Like] 2001 Formulation A/Moscow/10/99 (H 3 N 2) - Like (A/Panama/2007/99 RESVIR 17) 2002 Formulation A/Moscow/10/99 (H 3 N 2) - Like (A/Panama/2007/99 RESVIR 17) 2003 Formulation A/Moscow/10/99 (H 3 N 2) - Like (A/Panama/2007/99 RESVIR 17) 2004 Formulation A/Fujian/411/2002 -like (H 3 N 2) [A/Wyoming/3/2003] X 2005 Formulation A/California/7/2004 -like (H 3 N 2) [A/New York/55/2004] X 2006 Formulation A/Wisconsin/67/2005 (H 3 N 2)-like X 2007 Formulation A/Wisconsin/67/2005 (H 3 N 2)-like 2008 Formulation A/Brisbane/10/2007 (H 3 N 2)-like virus X X X 11 changes
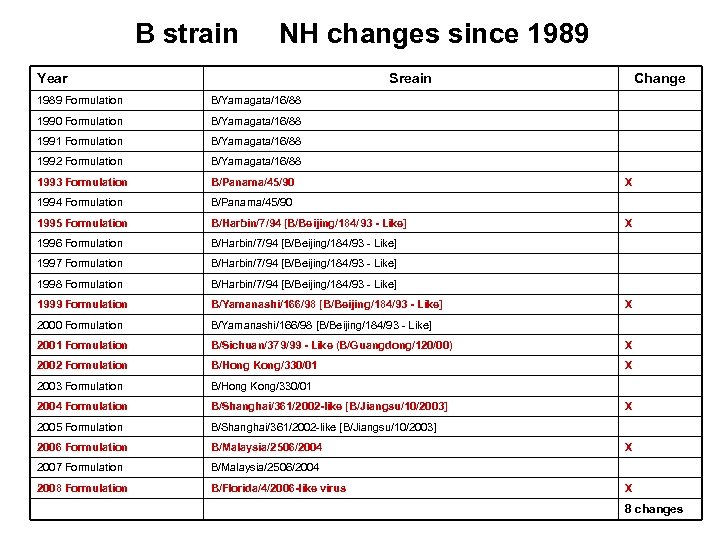 B strain NH changes since 1989 Year Sreain Change 1989 Formulation B/Yamagata/16/88 1990 Formulation B/Yamagata/16/88 1991 Formulation B/Yamagata/16/88 1992 Formulation B/Yamagata/16/88 1993 Formulation B/Panama/45/90 1994 Formulation B/Panama/45/90 1995 Formulation B/Harbin/7/94 [B/Beijing/184/93 - Like] 1996 Formulation B/Harbin/7/94 [B/Beijing/184/93 - Like] 1997 Formulation B/Harbin/7/94 [B/Beijing/184/93 - Like] 1998 Formulation B/Harbin/7/94 [B/Beijing/184/93 - Like] 1999 Formulation B/Yamanashi/166/98 [B/Beijing/184/93 - Like] 2000 Formulation B/Yamanashi/166/98 [B/Beijing/184/93 - Like] 2001 Formulation B/Sichuan/379/99 - Like (B/Guangdong/120/00) X 2002 Formulation B/Hong Kong/330/01 X 2003 Formulation B/Hong Kong/330/01 2004 Formulation B/Shanghai/361/2002 -like [B/Jiangsu/10/2003] 2005 Formulation B/Shanghai/361/2002 -like [B/Jiangsu/10/2003] 2006 Formulation B/Malaysia/2506/2004 2007 Formulation B/Malaysia/2506/2004 2008 Formulation B/Florida/4/2006 -like virus X X X 8 changes
B strain NH changes since 1989 Year Sreain Change 1989 Formulation B/Yamagata/16/88 1990 Formulation B/Yamagata/16/88 1991 Formulation B/Yamagata/16/88 1992 Formulation B/Yamagata/16/88 1993 Formulation B/Panama/45/90 1994 Formulation B/Panama/45/90 1995 Formulation B/Harbin/7/94 [B/Beijing/184/93 - Like] 1996 Formulation B/Harbin/7/94 [B/Beijing/184/93 - Like] 1997 Formulation B/Harbin/7/94 [B/Beijing/184/93 - Like] 1998 Formulation B/Harbin/7/94 [B/Beijing/184/93 - Like] 1999 Formulation B/Yamanashi/166/98 [B/Beijing/184/93 - Like] 2000 Formulation B/Yamanashi/166/98 [B/Beijing/184/93 - Like] 2001 Formulation B/Sichuan/379/99 - Like (B/Guangdong/120/00) X 2002 Formulation B/Hong Kong/330/01 X 2003 Formulation B/Hong Kong/330/01 2004 Formulation B/Shanghai/361/2002 -like [B/Jiangsu/10/2003] 2005 Formulation B/Shanghai/361/2002 -like [B/Jiangsu/10/2003] 2006 Formulation B/Malaysia/2506/2004 2007 Formulation B/Malaysia/2506/2004 2008 Formulation B/Florida/4/2006 -like virus X X X 8 changes
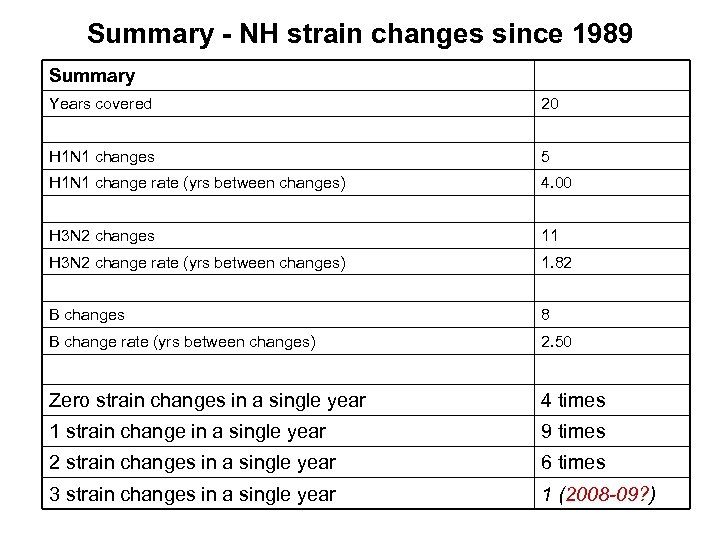 Summary - NH strain changes since 1989 Summary Years covered 20 H 1 N 1 changes 5 H 1 N 1 change rate (yrs between changes) 4. 00 H 3 N 2 changes 11 H 3 N 2 change rate (yrs between changes) 1. 82 B changes 8 B change rate (yrs between changes) 2. 50 Zero strain changes in a single year 4 times 1 strain change in a single year 9 times 2 strain changes in a single year 6 times 3 strain changes in a single year 1 (2008 -09? )
Summary - NH strain changes since 1989 Summary Years covered 20 H 1 N 1 changes 5 H 1 N 1 change rate (yrs between changes) 4. 00 H 3 N 2 changes 11 H 3 N 2 change rate (yrs between changes) 1. 82 B changes 8 B change rate (yrs between changes) 2. 50 Zero strain changes in a single year 4 times 1 strain change in a single year 9 times 2 strain changes in a single year 6 times 3 strain changes in a single year 1 (2008 -09? )


