a06af206a1456e310d2596ced3aaebd5.ppt
- Количество слайдов: 48
 Influenza A Pre-Season Update Dr. Theresa Tam Immunization and Respiratory Infections Division Centre for Infectious Disease Prevention and Control Health Santé Canada al. PHa Teleclass, September 21, 2004
Influenza A Pre-Season Update Dr. Theresa Tam Immunization and Respiratory Infections Division Centre for Infectious Disease Prevention and Control Health Santé Canada al. PHa Teleclass, September 21, 2004
 Outline l l Highlights from the 2003 -2004 season in Canada and worldwide Avian influenza l l H 5 N 1 in Asia H 7 N 3 in British Columbia NACI recommendations for 2004 -2005 Canadian Pandemic Influenza Plan update
Outline l l Highlights from the 2003 -2004 season in Canada and worldwide Avian influenza l l H 5 N 1 in Asia H 7 N 3 in British Columbia NACI recommendations for 2004 -2005 Canadian Pandemic Influenza Plan update
 2003 -2004 Influenza Season in Canada Health Canada Santé Canada
2003 -2004 Influenza Season in Canada Health Canada Santé Canada
 2003 -2004 Season Highlights l l Worldwide Influenza A(H 3 N 2) predominated with cocirculations of A(H 1) and B viruses In Canada l l Early start Relatively severe A(H 3 N 2) predominated Four reports of deaths in children with lab confirmed influenza (7 -14 years) l l IMPACT network reported additional 3 deaths US reported 152 deaths in persons < 18 years (40 states)
2003 -2004 Season Highlights l l Worldwide Influenza A(H 3 N 2) predominated with cocirculations of A(H 1) and B viruses In Canada l l Early start Relatively severe A(H 3 N 2) predominated Four reports of deaths in children with lab confirmed influenza (7 -14 years) l l IMPACT network reported additional 3 deaths US reported 152 deaths in persons < 18 years (40 states)
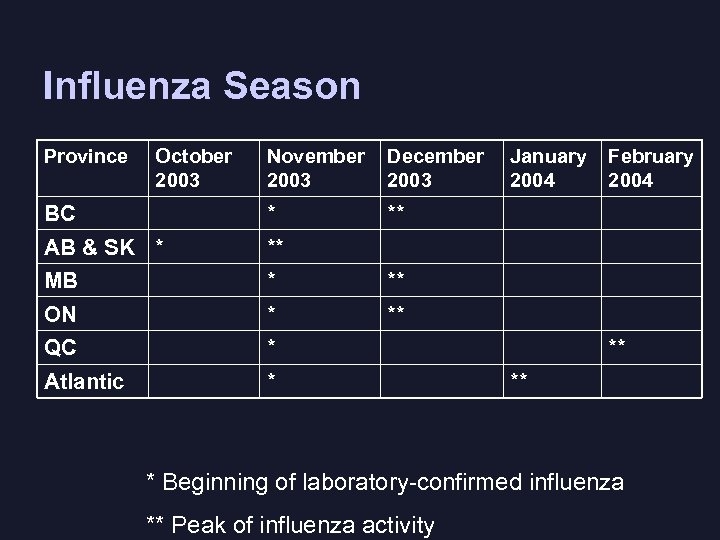 Influenza Season Province October 2003 November 2003 December 2003 BC * ** AB & SK * ** MB * ** ON * ** QC * Atlantic * January 2004 February 2004 ** ** * Beginning of laboratory-confirmed influenza ** Peak of influenza activity
Influenza Season Province October 2003 November 2003 December 2003 BC * ** AB & SK * ** MB * ** ON * ** QC * Atlantic * January 2004 February 2004 ** ** * Beginning of laboratory-confirmed influenza ** Peak of influenza activity
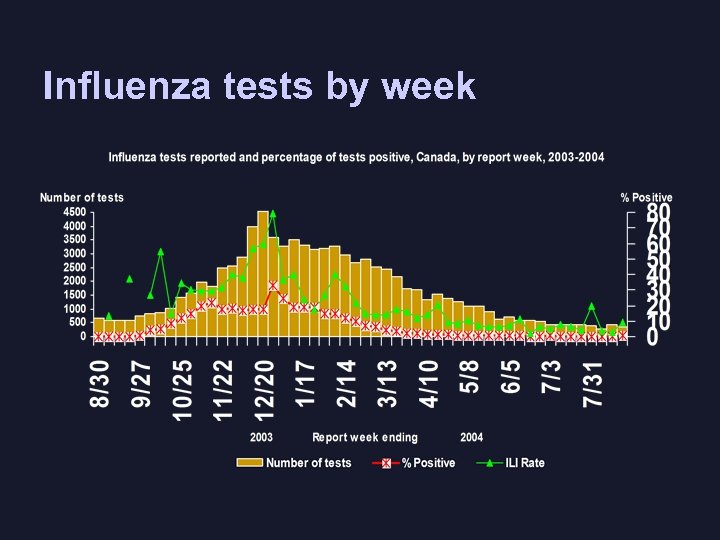 Influenza tests by week
Influenza tests by week
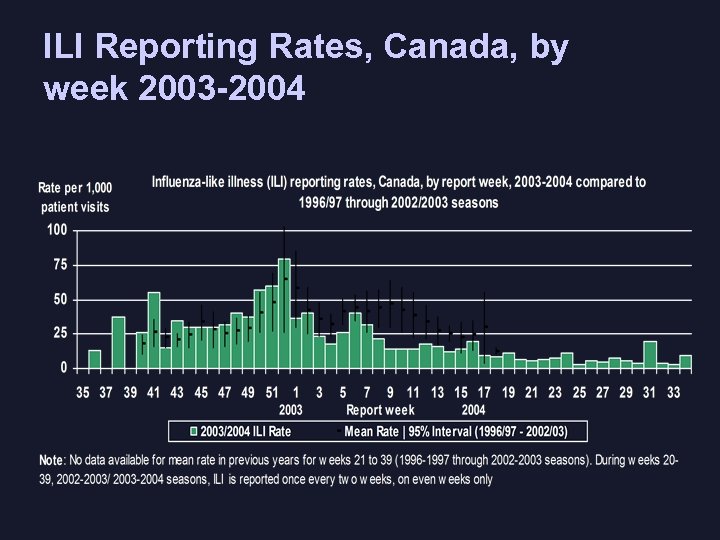 ILI Reporting Rates, Canada, by week 2003 -2004
ILI Reporting Rates, Canada, by week 2003 -2004
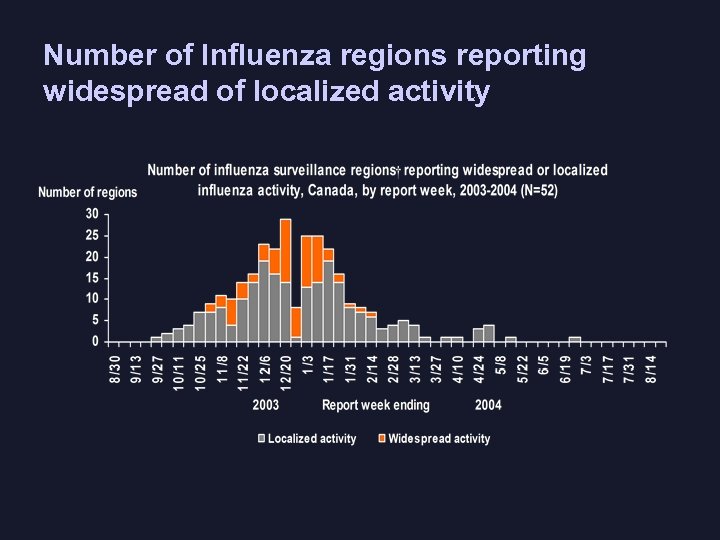 Number of Influenza regions reporting widespread of localized activity
Number of Influenza regions reporting widespread of localized activity
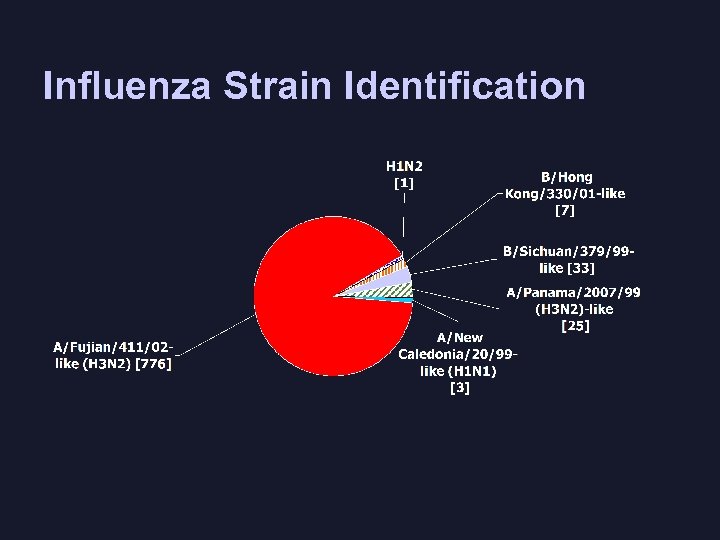 Influenza Strain Identification
Influenza Strain Identification
 Influenza Hospitalizations in Children- Pilot l l l Over 500 children hospitalized with laboratory confirmed influenza in 9 IMPACT centres Weekly admissions ranged over the season, with a peak occurring at week 52 Influenza A was identified in 99% of cases l l 86% under age of 6 years 57% under 2 years one third of cases were in 6 -23 month age-group One third had underlying medical conditions for which annual immunization is recommended
Influenza Hospitalizations in Children- Pilot l l l Over 500 children hospitalized with laboratory confirmed influenza in 9 IMPACT centres Weekly admissions ranged over the season, with a peak occurring at week 52 Influenza A was identified in 99% of cases l l 86% under age of 6 years 57% under 2 years one third of cases were in 6 -23 month age-group One third had underlying medical conditions for which annual immunization is recommended
 Avian Influenza
Avian Influenza
 Human Infections l H 5 N 1 - severe l l H 9 N 2 - mild l l l 1997 Hong Kong: 18 cases; 6 deaths 2003 Hong Kong: 2 cases; 1 death 2004 Vietnam and Thailand: 40 cases; 29 deaths (9 Sep 2004) 1999 Hong Kong: 2 cases (mild) 2003 Hong Kong: 1 case (mild) H 7 N 7 - mild l l 2003 Netherlands: 89 cases; 1 death 2004 Canada: 2 cases
Human Infections l H 5 N 1 - severe l l H 9 N 2 - mild l l l 1997 Hong Kong: 18 cases; 6 deaths 2003 Hong Kong: 2 cases; 1 death 2004 Vietnam and Thailand: 40 cases; 29 deaths (9 Sep 2004) 1999 Hong Kong: 2 cases (mild) 2003 Hong Kong: 1 case (mild) H 7 N 7 - mild l l 2003 Netherlands: 89 cases; 1 death 2004 Canada: 2 cases
 Avian H 5 N 1 in Asia l Continuing presence in Asia since 1996 l l Documented direct avian to human transmission, Hong Kong, 1997 Enzootic and epizootic of unprecedented size and complexity l 9 countries with ongoing outbreaks (most recently in Malaysia) l Ongoing human cases with high case fatality, mostly in healthy children and young adults l Ongoing evolution of the virus’ antigenic, genetic and functional properties l No sustained human to human transmission to date
Avian H 5 N 1 in Asia l Continuing presence in Asia since 1996 l l Documented direct avian to human transmission, Hong Kong, 1997 Enzootic and epizootic of unprecedented size and complexity l 9 countries with ongoing outbreaks (most recently in Malaysia) l Ongoing human cases with high case fatality, mostly in healthy children and young adults l Ongoing evolution of the virus’ antigenic, genetic and functional properties l No sustained human to human transmission to date
 Why are We Concerned? l Increasing countries/areas with avian influenza l l Ongoing human infection with avian H 5 N 1 l l Limited implementation of protective measures Co-Circulating human influenza viruses l l Uncertainties on progress of control Risk of genetic reassortment leading to pandemic strain Majority of human population would have no immunity
Why are We Concerned? l Increasing countries/areas with avian influenza l l Ongoing human infection with avian H 5 N 1 l l Limited implementation of protective measures Co-Circulating human influenza viruses l l Uncertainties on progress of control Risk of genetic reassortment leading to pandemic strain Majority of human population would have no immunity
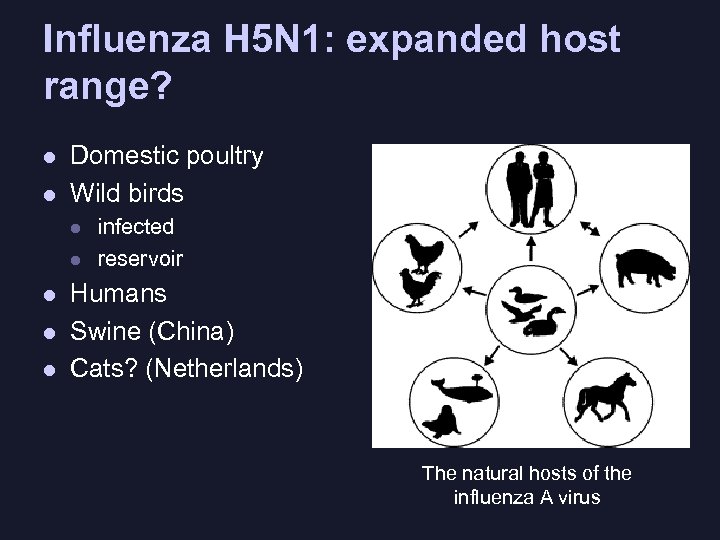 Influenza H 5 N 1: expanded host range? l l Domestic poultry Wild birds l l l infected reservoir Humans Swine (China) Cats? (Netherlands) The natural hosts of the influenza A virus
Influenza H 5 N 1: expanded host range? l l Domestic poultry Wild birds l l l infected reservoir Humans Swine (China) Cats? (Netherlands) The natural hosts of the influenza A virus
 Containing an Initial Outbreak of Novel Influenza – Can this be done? l Hong Kong accomplished this in 1997 l 2004 H 5 N 1 situation much more challenging l Large areas affected in a large number of countries l Slow and incomplete reporting of H 5 N 1 findings l Poor public health infrastructure l Complex political and economic situations l International action required: support for antivirals PPE and compensation may help
Containing an Initial Outbreak of Novel Influenza – Can this be done? l Hong Kong accomplished this in 1997 l 2004 H 5 N 1 situation much more challenging l Large areas affected in a large number of countries l Slow and incomplete reporting of H 5 N 1 findings l Poor public health infrastructure l Complex political and economic situations l International action required: support for antivirals PPE and compensation may help
 Highly Pathogenic Avian Influenza (HPAI) H 7 N 3, BC, 2004 l 42 commercial and 11 backyard premises infected l l l Feb 19 – low path Avian influenza (AI) H 7 first detected in a commercial chicken breeder farm March 8 – HPAI detected on the same farm Mar 11 – HPAI on second farm Approx 19 million birds depopulated Spread likely by movement of people, equipment or birds. Airborne transmission through dust and feathers?
Highly Pathogenic Avian Influenza (HPAI) H 7 N 3, BC, 2004 l 42 commercial and 11 backyard premises infected l l l Feb 19 – low path Avian influenza (AI) H 7 first detected in a commercial chicken breeder farm March 8 – HPAI detected on the same farm Mar 11 – HPAI on second farm Approx 19 million birds depopulated Spread likely by movement of people, equipment or birds. Airborne transmission through dust and feathers?
 Avian H 7 N 3 in BC, 2004 l l l Movement restrictions Susceptible birds within 3 km of infected premises depopulated Active surveillance and testing of flocks; birds tested negative slaughtered through normal commercial channels Depopulation activities suspended on June 4 after 21 days with no new reports. Outbreak declared contained on August 18, 21 days after last infected premise cleaned and disinfected.
Avian H 7 N 3 in BC, 2004 l l l Movement restrictions Susceptible birds within 3 km of infected premises depopulated Active surveillance and testing of flocks; birds tested negative slaughtered through normal commercial channels Depopulation activities suspended on June 4 after 21 days with no new reports. Outbreak declared contained on August 18, 21 days after last infected premise cleaned and disinfected.
 BC Avian H 7 Outbreak Human Health Issues l l 2 cases of lab confirmed human H 7 infections in cullers Surveillance of exposed persons l l l Farm family and workers Persons involved in depopulation of infected poultry Immunization with current “seasonal” flu vaccine Personal protective equipment Antivirals: prophylaxis and treatment Pandemic Influenza Committee guidelines on “Human Health Issues related to Domestic Avian Influenza Outbreaks”
BC Avian H 7 Outbreak Human Health Issues l l 2 cases of lab confirmed human H 7 infections in cullers Surveillance of exposed persons l l l Farm family and workers Persons involved in depopulation of infected poultry Immunization with current “seasonal” flu vaccine Personal protective equipment Antivirals: prophylaxis and treatment Pandemic Influenza Committee guidelines on “Human Health Issues related to Domestic Avian Influenza Outbreaks”
 NACI Recommendations June 15, 2004 Health Canada Santé Canada
NACI Recommendations June 15, 2004 Health Canada Santé Canada
 What’s new in the NACI Statement? l l New vaccine strains Immunization of healthy children 6 -23 months Immunization of cullers involved in depopulation of poultry infected with avian flu Prophylactic use of neuraminidase inhibitors
What’s new in the NACI Statement? l l New vaccine strains Immunization of healthy children 6 -23 months Immunization of cullers involved in depopulation of poultry infected with avian flu Prophylactic use of neuraminidase inhibitors
 Vaccine composition for 20042005 l Trivalent vaccines to be used in Canada will contain the following antigens: l l l A/New Caledonia/20/99 (N 1 H 1)-like A/Wyoming/3/2003 (H 3 N 2) (an A/Fujian 411/2002 (H 3 N 2)-like strain) B/Jiangsu/10/2003 (a B/Shanghai/361/2002 -like strain)
Vaccine composition for 20042005 l Trivalent vaccines to be used in Canada will contain the following antigens: l l l A/New Caledonia/20/99 (N 1 H 1)-like A/Wyoming/3/2003 (H 3 N 2) (an A/Fujian 411/2002 (H 3 N 2)-like strain) B/Jiangsu/10/2003 (a B/Shanghai/361/2002 -like strain)
 Recommendation for children 6 -23 months l l l Increased risk of morbidity – hospitalizations Vaccine efficacy, based on a limited total number of subjects in this age group (<1000), is similar to estimated for the elderly and those with high risk medical conditions. Further study required: l l l Vaccine effectiveness Immunologic response to future encounters with wild virus Adverse events e. g. ORS in first time vaccinees
Recommendation for children 6 -23 months l l l Increased risk of morbidity – hospitalizations Vaccine efficacy, based on a limited total number of subjects in this age group (<1000), is similar to estimated for the elderly and those with high risk medical conditions. Further study required: l l l Vaccine effectiveness Immunologic response to future encounters with wild virus Adverse events e. g. ORS in first time vaccinees
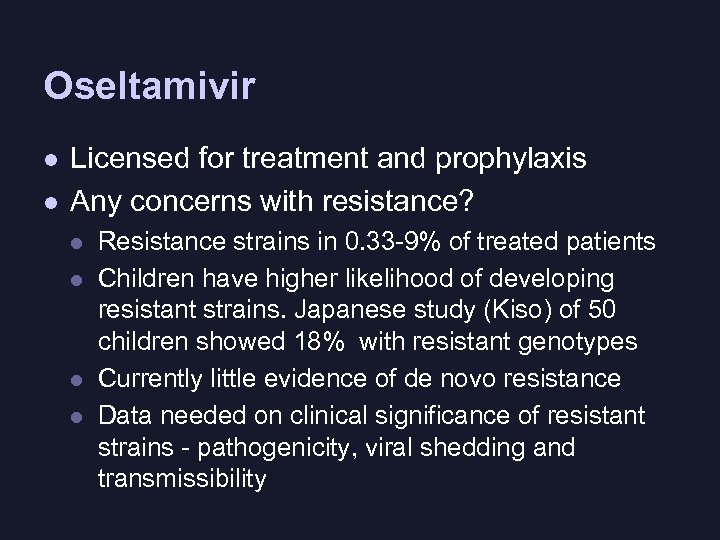 Oseltamivir l l Licensed for treatment and prophylaxis Any concerns with resistance? l l Resistance strains in 0. 33 -9% of treated patients Children have higher likelihood of developing resistant strains. Japanese study (Kiso) of 50 children showed 18% with resistant genotypes Currently little evidence of de novo resistance Data needed on clinical significance of resistant strains - pathogenicity, viral shedding and transmissibility
Oseltamivir l l Licensed for treatment and prophylaxis Any concerns with resistance? l l Resistance strains in 0. 33 -9% of treated patients Children have higher likelihood of developing resistant strains. Japanese study (Kiso) of 50 children showed 18% with resistant genotypes Currently little evidence of de novo resistance Data needed on clinical significance of resistant strains - pathogenicity, viral shedding and transmissibility
 Canadian Pandemic Influenza Plan Update
Canadian Pandemic Influenza Plan Update
 Pandemic Preparedness Milestones l 1988 - 1 st draft plan l 1997 - lessons learnt from Hong Kong “Bird flu” l 1998 to 2002 l l pandemic vaccine contract signed (Sep 2001) l Pandemic Influenza Committee (PIC) established (Mar 2002) l l F/P/T Working Agreement (Mar 2001) : roles and responsibilities Pandemic Plan consultations – 43 organizations (Sep 2002) 2003 l Plan revised in light of SARS experience and approved by Deputy Ministers of Health (Dec 2003) Public Release of the Plan – February 2004
Pandemic Preparedness Milestones l 1988 - 1 st draft plan l 1997 - lessons learnt from Hong Kong “Bird flu” l 1998 to 2002 l l pandemic vaccine contract signed (Sep 2001) l Pandemic Influenza Committee (PIC) established (Mar 2002) l l F/P/T Working Agreement (Mar 2001) : roles and responsibilities Pandemic Plan consultations – 43 organizations (Sep 2002) 2003 l Plan revised in light of SARS experience and approved by Deputy Ministers of Health (Dec 2003) Public Release of the Plan – February 2004
 Canadian Pandemic Influenza Plan (CPIP) l Based on the nationally agreed upon goal l Organized into “components” (framework for national working group activities) l Uses WHO Pandemic Phases l Roles and responsibilities of F/P/T orders of government identified as per Working Agreement l Model for P/T contingency plans l Contains checklists and technical annexes
Canadian Pandemic Influenza Plan (CPIP) l Based on the nationally agreed upon goal l Organized into “components” (framework for national working group activities) l Uses WHO Pandemic Phases l Roles and responsibilities of F/P/T orders of government identified as per Working Agreement l Model for P/T contingency plans l Contains checklists and technical annexes
 Key Strategies and Planning Components l Rapid detection, monitor spread and assess impact l l Reduce spread and impact l l l l Surveillance and lab testing protocols Border measures Public health measures and infection control Vaccines Antivirals Maintaining health services Emergency and social services Maintain public awareness l Risk communication
Key Strategies and Planning Components l Rapid detection, monitor spread and assess impact l l Reduce spread and impact l l l l Surveillance and lab testing protocols Border measures Public health measures and infection control Vaccines Antivirals Maintaining health services Emergency and social services Maintain public awareness l Risk communication
 The Plan: Current activities l l l Using pandemic influenza structures and processes to define Canada’s response to avian influenza (Phase 0, level 2) “Management of Human Health Issues related to Domestic Avian Influenza Outbreaks” Finalize and post new Annexes (2004) l First Nations l Public Health Measures l Surveillance
The Plan: Current activities l l l Using pandemic influenza structures and processes to define Canada’s response to avian influenza (Phase 0, level 2) “Management of Human Health Issues related to Domestic Avian Influenza Outbreaks” Finalize and post new Annexes (2004) l First Nations l Public Health Measures l Surveillance
 The Plan: Current activities - II l Completion of antiviral drug strategy (2004) l Testing domestic vaccine production infrastructure, regulatory processes and clinical trial protocols (2004 -2005, pending funding) l Influenza research agenda (2004) l Further “exercising” of the Plan l Completing the Recovery Section
The Plan: Current activities - II l Completion of antiviral drug strategy (2004) l Testing domestic vaccine production infrastructure, regulatory processes and clinical trial protocols (2004 -2005, pending funding) l Influenza research agenda (2004) l Further “exercising” of the Plan l Completing the Recovery Section
 Public Health and Border Measures l To avert a pandemic or appreciably slow the spread of a novel virus, prior to the development of efficient and sustained human to human transmission
Public Health and Border Measures l To avert a pandemic or appreciably slow the spread of a novel virus, prior to the development of efficient and sustained human to human transmission
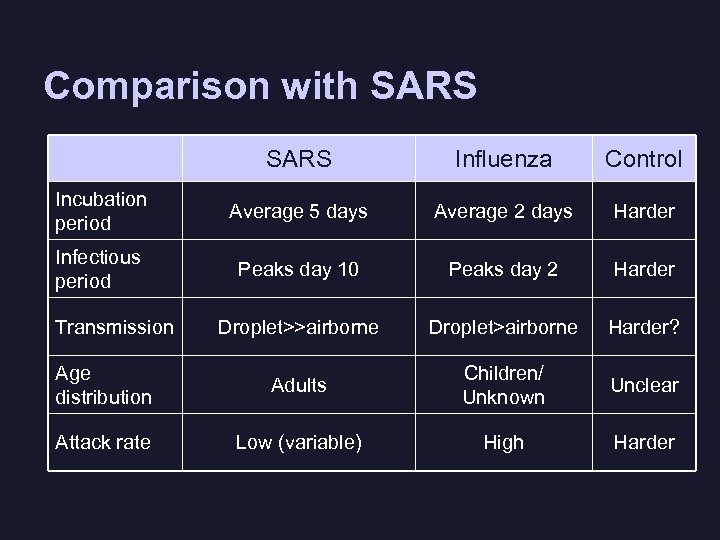 Comparison with SARS Influenza Control Incubation period Average 5 days Average 2 days Harder Infectious period Peaks day 10 Peaks day 2 Harder Droplet>>airborne Droplet>airborne Harder? Age distribution Adults Children/ Unknown Unclear Attack rate Low (variable) High Harder Transmission
Comparison with SARS Influenza Control Incubation period Average 5 days Average 2 days Harder Infectious period Peaks day 10 Peaks day 2 Harder Droplet>>airborne Droplet>airborne Harder? Age distribution Adults Children/ Unknown Unclear Attack rate Low (variable) High Harder Transmission
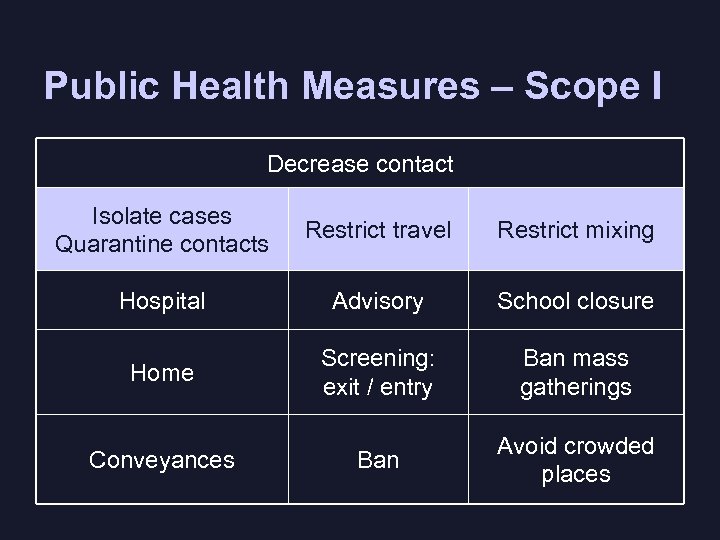 Public Health Measures – Scope I Decrease contact Isolate cases Quarantine contacts Restrict travel Restrict mixing Hospital Advisory School closure Home Screening: exit / entry Ban mass gatherings Conveyances Ban Avoid crowded places
Public Health Measures – Scope I Decrease contact Isolate cases Quarantine contacts Restrict travel Restrict mixing Hospital Advisory School closure Home Screening: exit / entry Ban mass gatherings Conveyances Ban Avoid crowded places
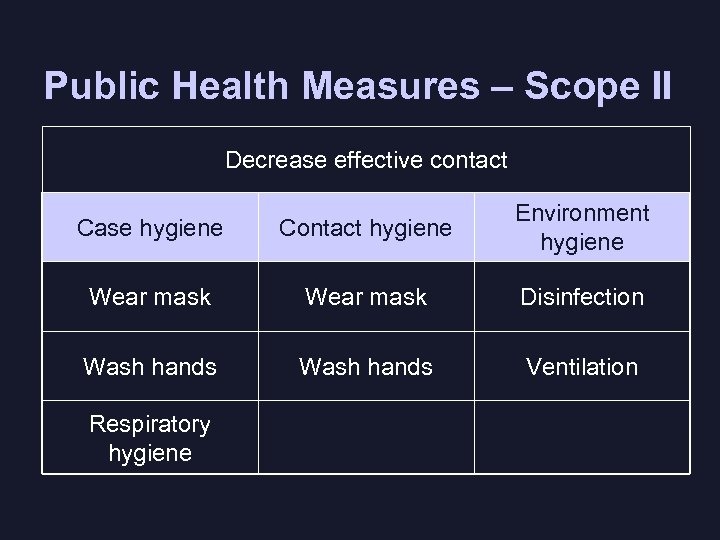 Public Health Measures – Scope II Decrease effective contact Case hygiene Contact hygiene Environment hygiene Wear mask Disinfection Wash hands Ventilation Respiratory hygiene
Public Health Measures – Scope II Decrease effective contact Case hygiene Contact hygiene Environment hygiene Wear mask Disinfection Wash hands Ventilation Respiratory hygiene
 Antivirals l Two main types l l l Neuraminidase inhibitors (e. g. oseltamivir, zanamivir) Amantadine Why use antivirals? l l l Minimise risk of emergence of a novel virus with pandemic potential, through preventing human infection Buying time and limiting spread at the start of a pandemic until vaccine becomes available Minimize health care system disruption and mortality
Antivirals l Two main types l l l Neuraminidase inhibitors (e. g. oseltamivir, zanamivir) Amantadine Why use antivirals? l l l Minimise risk of emergence of a novel virus with pandemic potential, through preventing human infection Buying time and limiting spread at the start of a pandemic until vaccine becomes available Minimize health care system disruption and mortality
 Antivirals – Not A Panacea l l l Global production capacity limited; high cost Ability to use antivirals to limit spread depends on rapid case detection and contact tracing Need to start treatment early Effectiveness on serious illnesses and mortality unknown Prophylaxis may require ongoing use for 6 weeks or longer Antiviral resistance and side effects may limit use
Antivirals – Not A Panacea l l l Global production capacity limited; high cost Ability to use antivirals to limit spread depends on rapid case detection and contact tracing Need to start treatment early Effectiveness on serious illnesses and mortality unknown Prophylaxis may require ongoing use for 6 weeks or longer Antiviral resistance and side effects may limit use
 Antiviral Strategy: Status ü Options for use and stockpiling • • ü Guidelines on use of antivirals in short supply • • ü Neuraminidase inhibitors for treatment and prophylaxis Amantadine for prophylaxis (currently not for stockpiling) Goal oriented For planning purposes Clinical guidelines
Antiviral Strategy: Status ü Options for use and stockpiling • • ü Guidelines on use of antivirals in short supply • • ü Neuraminidase inhibitors for treatment and prophylaxis Amantadine for prophylaxis (currently not for stockpiling) Goal oriented For planning purposes Clinical guidelines
 Current Thinking: Principles l l Antivirals are the only virus-specific intervention prior to vaccine becoming available Priority groups in times of short supply should be determined for planning purposes (but maintain flexibility to change based on epidemiology or local needs) Priority groups should be based on overall goal Use of all anti-influenza drugs available: l l neuraminidase inhibitors for treatment or prophylaxis amantadine for prophylaxis if strain susceptible
Current Thinking: Principles l l Antivirals are the only virus-specific intervention prior to vaccine becoming available Priority groups in times of short supply should be determined for planning purposes (but maintain flexibility to change based on epidemiology or local needs) Priority groups should be based on overall goal Use of all anti-influenza drugs available: l l neuraminidase inhibitors for treatment or prophylaxis amantadine for prophylaxis if strain susceptible
 Current Thinking: Policy Considerations l Security of supply for antiviral drugs should be addressed in the pre-pandemic period. l l Stockpiling of oseltamivir for nationally agreed upon priority groups The F/P/T governments should control the supply and distribution of available antiinfluenza drugs, to the end user, during a pandemic.
Current Thinking: Policy Considerations l Security of supply for antiviral drugs should be addressed in the pre-pandemic period. l l Stockpiling of oseltamivir for nationally agreed upon priority groups The F/P/T governments should control the supply and distribution of available antiinfluenza drugs, to the end user, during a pandemic.
 Overall Goal of Pandemic Preparedness and Response First, to minimize serious illness and overall deaths, and second to minimize societal disruption among Canadians as a result of an influenza pandemic.
Overall Goal of Pandemic Preparedness and Response First, to minimize serious illness and overall deaths, and second to minimize societal disruption among Canadians as a result of an influenza pandemic.
 Current Thinking: National Priorities 1. Tx of persons hospitalized for influenza 2. Tx of ill HCW and ESW 3. Px of “front line” HCW and key health decision makers 4. Tx of high-risk in the community 5. Px of remaining HCW 6. Control outbreaks in high-risk residents of institutions 7. Px of ESW 8. Px of high-risk persons hospitalized for illnesses other than influenza 9. Px of high-risk in the community Need to review definitions and estimates for priority groups
Current Thinking: National Priorities 1. Tx of persons hospitalized for influenza 2. Tx of ill HCW and ESW 3. Px of “front line” HCW and key health decision makers 4. Tx of high-risk in the community 5. Px of remaining HCW 6. Control outbreaks in high-risk residents of institutions 7. Px of ESW 8. Px of high-risk persons hospitalized for illnesses other than influenza 9. Px of high-risk in the community Need to review definitions and estimates for priority groups
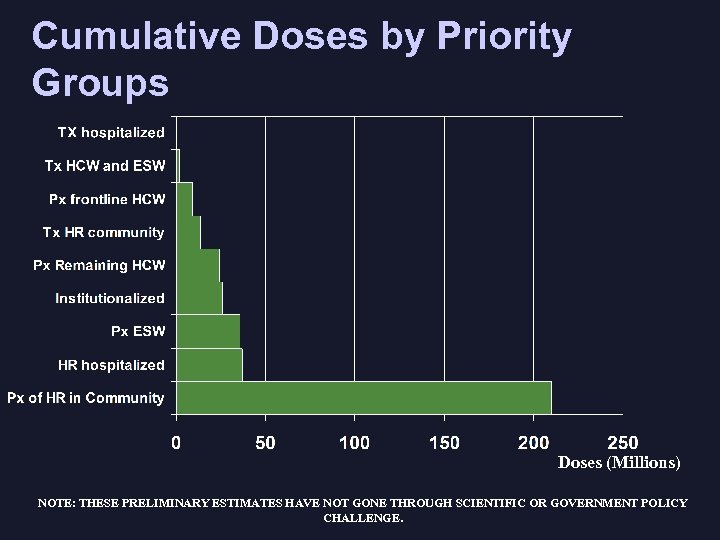 Cumulative Doses by Priority Groups Doses (Millions) NOTE: THESE PRELIMINARY ESTIMATES HAVE NOT GONE THROUGH SCIENTIFIC OR GOVERNMENT POLICY CHALLENGE.
Cumulative Doses by Priority Groups Doses (Millions) NOTE: THESE PRELIMINARY ESTIMATES HAVE NOT GONE THROUGH SCIENTIFIC OR GOVERNMENT POLICY CHALLENGE.
 Current Policy Discussions PIC Priority Groups for pandemic planning compared to those currently being considered in policy discussions: ü Tx Hospitalized ü Tx HCW and ESW ü Px “front line” HCW, key health decision makers ü Tx HR community Approximately equal to q Px Remaining HCW 14 million doses of ü Tx Institutionalized (Px? ) oseltamivir ü Px ESW (post-exposure) q Px HR hospitalized q Px HR community
Current Policy Discussions PIC Priority Groups for pandemic planning compared to those currently being considered in policy discussions: ü Tx Hospitalized ü Tx HCW and ESW ü Px “front line” HCW, key health decision makers ü Tx HR community Approximately equal to q Px Remaining HCW 14 million doses of ü Tx Institutionalized (Px? ) oseltamivir ü Px ESW (post-exposure) q Px HR hospitalized q Px HR community
 Antiviral Use in Phase 0 WHO Discussions l P 0 L 1 – Px of at risk (e. g. cullers), early Tx of symptomatic persons l P 0 L 2 -L 3 – focus on clusters of cases to prevent, reduce or delay spread, early Tx and Px of contacts including HCW, consideration for “intense prophylaxis” around a limited number of small, well defined clusters l Buying time, slowing spread early in a pandemic? ? l International stockpile
Antiviral Use in Phase 0 WHO Discussions l P 0 L 1 – Px of at risk (e. g. cullers), early Tx of symptomatic persons l P 0 L 2 -L 3 – focus on clusters of cases to prevent, reduce or delay spread, early Tx and Px of contacts including HCW, consideration for “intense prophylaxis” around a limited number of small, well defined clusters l Buying time, slowing spread early in a pandemic? ? l International stockpile
 Antiviral Use During Domestic Avian Flu Outbreaks: Prophylaxis l Persons potentially exposed to avian flu: l Involved in outbreak control: culling, disposal, cleaning of infected poultry/materials l Living/working on affected farms with contact to infected materials (those without contact offered early treatment) l Oseltamivir for duration of exposure plus 5 days (6 weeks max, 2 weeks between courses) – off-label use l Post exposure prophylaxis (PEP) for 5 days following significant exposure for those not on continuous prophylaxis l PEP should not be routinely given to close contacts of human cases of avian flu, but may be considered if index case severe or unusual
Antiviral Use During Domestic Avian Flu Outbreaks: Prophylaxis l Persons potentially exposed to avian flu: l Involved in outbreak control: culling, disposal, cleaning of infected poultry/materials l Living/working on affected farms with contact to infected materials (those without contact offered early treatment) l Oseltamivir for duration of exposure plus 5 days (6 weeks max, 2 weeks between courses) – off-label use l Post exposure prophylaxis (PEP) for 5 days following significant exposure for those not on continuous prophylaxis l PEP should not be routinely given to close contacts of human cases of avian flu, but may be considered if index case severe or unusual
 Antiviral Use During Domestic Avian Flu Outbreaks: Treatment l Persons (>= 1 year of age) who develop compatible illness following avian exposure l In light of evidence showing continuing replication of avian influenza virus beyond 48 hours after onset of symptoms, consideration should be given to treating individuals presenting at any point during their illness (i. e. not just during first 48 hours )
Antiviral Use During Domestic Avian Flu Outbreaks: Treatment l Persons (>= 1 year of age) who develop compatible illness following avian exposure l In light of evidence showing continuing replication of avian influenza virus beyond 48 hours after onset of symptoms, consideration should be given to treating individuals presenting at any point during their illness (i. e. not just during first 48 hours )
 Antiviral Strategy: To Do 1. 2. 3. Complete priority group definitions and estimates Funding for stockpile(s) (F/P/T) Implementation issues: 1. 4. Evaluation issues: 1. 5. strategies for delivery, administration, monitoring of distribution, uptake, wastage monitoring for adverse events and resistance Modeling of potential impact Alone and in combination with other potential interventions 2. Containing a localized cluster in P 0 1.
Antiviral Strategy: To Do 1. 2. 3. Complete priority group definitions and estimates Funding for stockpile(s) (F/P/T) Implementation issues: 1. 4. Evaluation issues: 1. 5. strategies for delivery, administration, monitoring of distribution, uptake, wastage monitoring for adverse events and resistance Modeling of potential impact Alone and in combination with other potential interventions 2. Containing a localized cluster in P 0 1.
 Next Steps on Influenza Research l l l Development of national research agenda Collaborative evaluation of the Ontario's Universal Influenza Immunization Program Identifying funding for production and clinical trials with novel influenza vaccine strains (H 5 N 1) Vaccine coverage survey Vaccine effectiveness studies Meeting of the National Vaccine Advisory Committee Washington DC, June 1 -2, 2004
Next Steps on Influenza Research l l l Development of national research agenda Collaborative evaluation of the Ontario's Universal Influenza Immunization Program Identifying funding for production and clinical trials with novel influenza vaccine strains (H 5 N 1) Vaccine coverage survey Vaccine effectiveness studies Meeting of the National Vaccine Advisory Committee Washington DC, June 1 -2, 2004


