3a452a95d518d3f52c57ac0ebb181743.ppt
- Количество слайдов: 42
 INFLUENZA A H 1 N 1 PREVENTIVE & CONTROL GUIDELINES 25 Jan 2015
INFLUENZA A H 1 N 1 PREVENTIVE & CONTROL GUIDELINES 25 Jan 2015
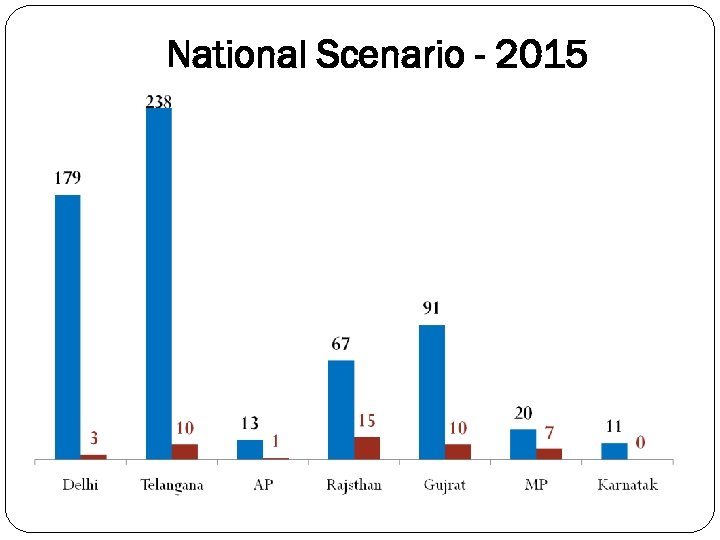 National Scenario - 2015
National Scenario - 2015
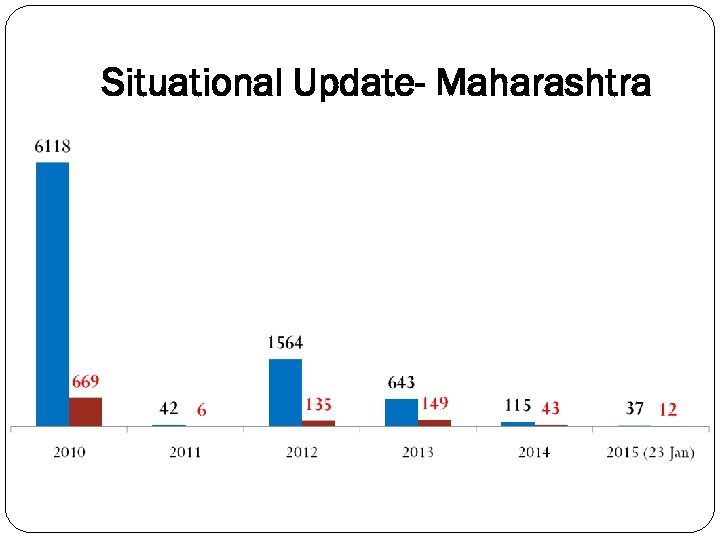 Situational Update- Maharashtra
Situational Update- Maharashtra
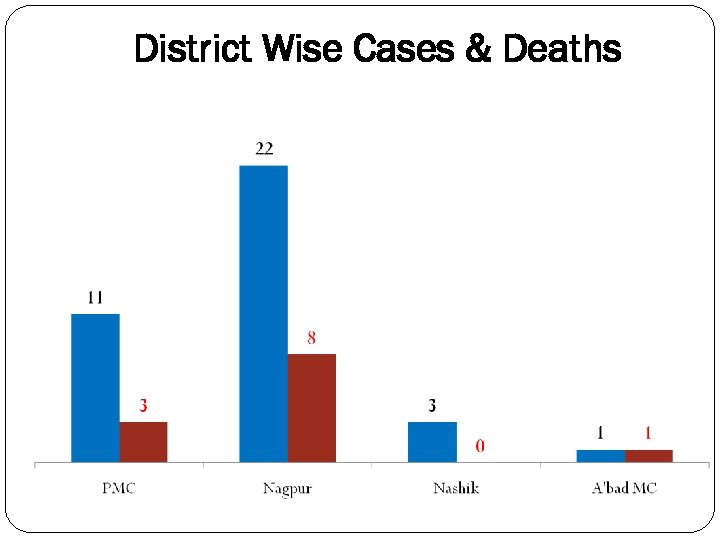 District Wise Cases & Deaths
District Wise Cases & Deaths

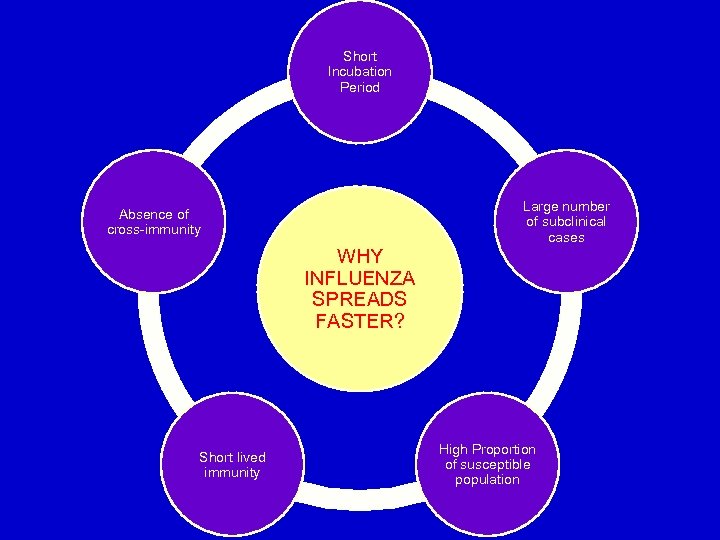 Short Incubation Period Large number of subclinical cases Absence of cross-immunity WHY INFLUENZA SPREADS FASTER? Short lived immunity High Proportion of susceptible population
Short Incubation Period Large number of subclinical cases Absence of cross-immunity WHY INFLUENZA SPREADS FASTER? Short lived immunity High Proportion of susceptible population
 Influenza Surveillance
Influenza Surveillance
 INFLUENZA H 1 N 1 • Incubation Period- 1 to 7 days. • Infectious Period- 1 day prior to the onset of illness to 7 days after onset. • Close Contact -Close Contact is defined within 6 feet of an ill person who is a confirmed, probable or suspected case of influenza A H 1 N 1 virus infection during the infectious period.
INFLUENZA H 1 N 1 • Incubation Period- 1 to 7 days. • Infectious Period- 1 day prior to the onset of illness to 7 days after onset. • Close Contact -Close Contact is defined within 6 feet of an ill person who is a confirmed, probable or suspected case of influenza A H 1 N 1 virus infection during the infectious period.
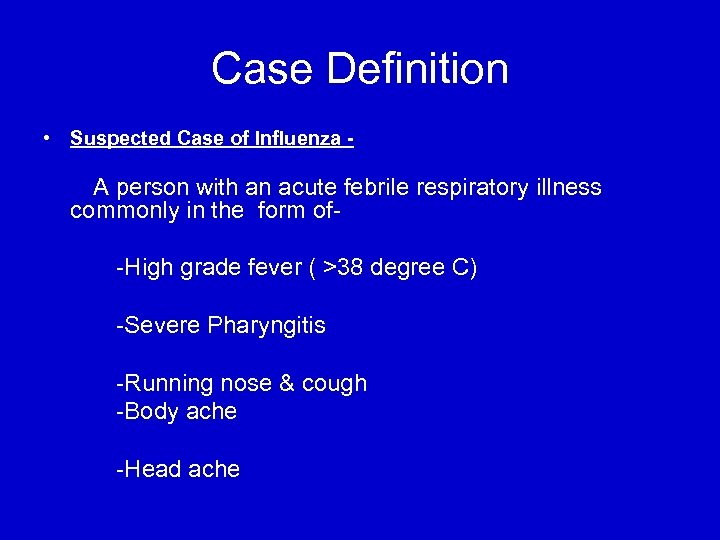 Case Definition • Suspected Case of Influenza - A person with an acute febrile respiratory illness commonly in the form of-High grade fever ( >38 degree C) -Severe Pharyngitis -Running nose & cough -Body ache -Head ache
Case Definition • Suspected Case of Influenza - A person with an acute febrile respiratory illness commonly in the form of-High grade fever ( >38 degree C) -Severe Pharyngitis -Running nose & cough -Body ache -Head ache
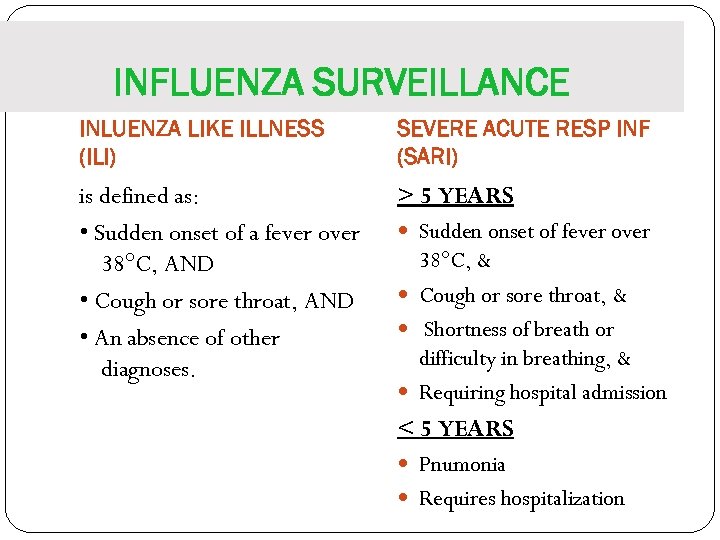 INFLUENZA SURVEILLANCE INLUENZA LIKE ILLNESS (ILI) SEVERE ACUTE RESP INF (SARI) is defined as: • Sudden onset of a fever over 38°C, AND • Cough or sore throat, AND • An absence of other diagnoses. > 5 YEARS Sudden onset of fever over 38°C, & Cough or sore throat, & Shortness of breath or difficulty in breathing, & Requiring hospital admission < 5 YEARS Pnumonia Requires hospitalization
INFLUENZA SURVEILLANCE INLUENZA LIKE ILLNESS (ILI) SEVERE ACUTE RESP INF (SARI) is defined as: • Sudden onset of a fever over 38°C, AND • Cough or sore throat, AND • An absence of other diagnoses. > 5 YEARS Sudden onset of fever over 38°C, & Cough or sore throat, & Shortness of breath or difficulty in breathing, & Requiring hospital admission < 5 YEARS Pnumonia Requires hospitalization
 Surveillance Guidelines • Active surveillance for ILI at all levels. • Focus on – Schools, Hostels, Anganwadis Ashramshalas, Orphanages, madarasas. • Report clusters of ILI to district/divisional & state authority immediately. • ANMs should screen pregnant mothers for ILI during routine check up in ANC clinic. • Tackle clusters effectively to avoid further spread.
Surveillance Guidelines • Active surveillance for ILI at all levels. • Focus on – Schools, Hostels, Anganwadis Ashramshalas, Orphanages, madarasas. • Report clusters of ILI to district/divisional & state authority immediately. • ANMs should screen pregnant mothers for ILI during routine check up in ANC clinic. • Tackle clusters effectively to avoid further spread.
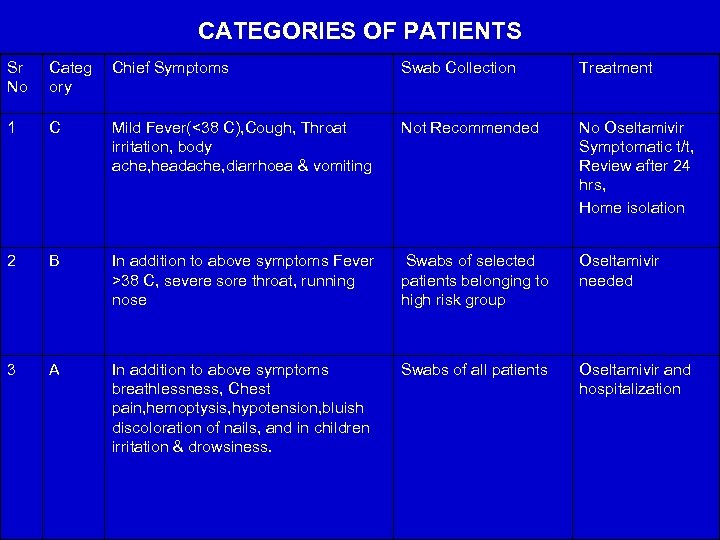 CATEGORIES OF PATIENTS Sr No Categ ory Chief Symptoms Swab Collection Treatment 1 C Mild Fever(<38 C), Cough, Throat irritation, body ache, headache, diarrhoea & vomiting Not Recommended No Oseltamivir Symptomatic t/t, Review after 24 hrs, Home isolation 2 B In addition to above symptoms Fever >38 C, severe sore throat, running nose Swabs of selected patients belonging to high risk group Oseltamivir needed 3 A In addition to above symptoms breathlessness, Chest pain, hemoptysis, hypotension, bluish discoloration of nails, and in children irritation & drowsiness. Swabs of all patients Oseltamivir and hospitalization
CATEGORIES OF PATIENTS Sr No Categ ory Chief Symptoms Swab Collection Treatment 1 C Mild Fever(<38 C), Cough, Throat irritation, body ache, headache, diarrhoea & vomiting Not Recommended No Oseltamivir Symptomatic t/t, Review after 24 hrs, Home isolation 2 B In addition to above symptoms Fever >38 C, severe sore throat, running nose Swabs of selected patients belonging to high risk group Oseltamivir needed 3 A In addition to above symptoms breathlessness, Chest pain, hemoptysis, hypotension, bluish discoloration of nails, and in children irritation & drowsiness. Swabs of all patients Oseltamivir and hospitalization
 High Risk Patients • • Children below 5 years of age. Persons above 65 years of age. Pregnant Women. Persons having lung, heart, liver, kidney diseases. Persons with blood & neurological disorders. • HIV/AIDS patients. • Patients on long term steroid treatment.
High Risk Patients • • Children below 5 years of age. Persons above 65 years of age. Pregnant Women. Persons having lung, heart, liver, kidney diseases. Persons with blood & neurological disorders. • HIV/AIDS patients. • Patients on long term steroid treatment.
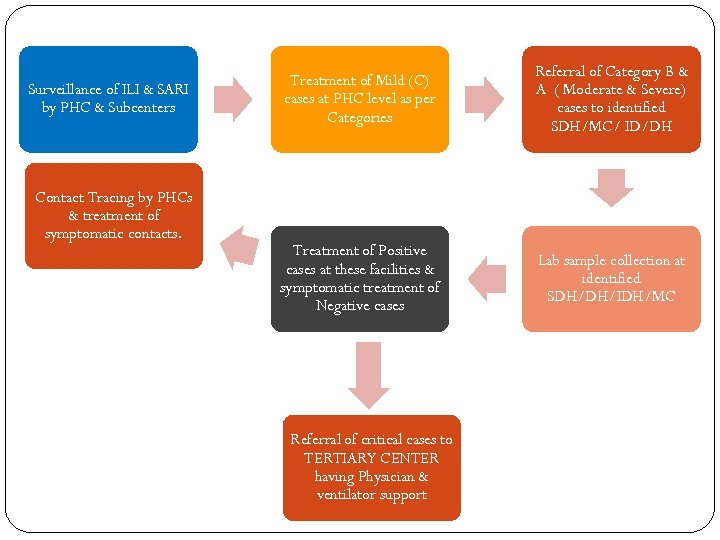 Surveillance of ILI & SARI by PHC & Subcenters Contact Tracing by PHCs & treatment of symptomatic contacts. Treatment of Mild (C) cases at PHC level as per Categories Referral of Category B & A ( Moderate & Severe) cases to identified SDH/MC/ ID/DH Treatment of Positive cases at these facilities & symptomatic treatment of Negative cases Lab sample collection at identified SDH/DH/IDH/MC Referral of critical cases to TERTIARY CENTER having Physician & ventilator support
Surveillance of ILI & SARI by PHC & Subcenters Contact Tracing by PHCs & treatment of symptomatic contacts. Treatment of Mild (C) cases at PHC level as per Categories Referral of Category B & A ( Moderate & Severe) cases to identified SDH/MC/ ID/DH Treatment of Positive cases at these facilities & symptomatic treatment of Negative cases Lab sample collection at identified SDH/DH/IDH/MC Referral of critical cases to TERTIARY CENTER having Physician & ventilator support
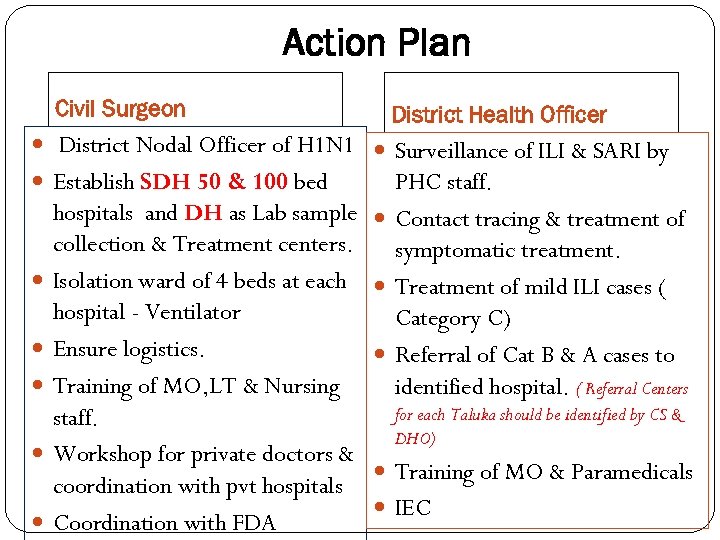 Action Plan Civil Surgeon District Health Officer District Nodal Officer of H 1 N 1 Surveillance of ILI & SARI by Establish SDH 50 & 100 bed PHC staff. hospitals and DH as Lab sample collection & Treatment centers. Isolation ward of 4 beds at each hospital - Ventilator Ensure logistics. Training of MO, LT & Nursing staff. Workshop for private doctors & coordination with pvt hospitals Coordination with FDA Contact tracing & treatment of symptomatic treatment. Treatment of mild ILI cases ( Category C) Referral of Cat B & A cases to identified hospital. ( Referral Centers for each Taluka should be identified by CS & DHO) Training of MO & Paramedicals IEC
Action Plan Civil Surgeon District Health Officer District Nodal Officer of H 1 N 1 Surveillance of ILI & SARI by Establish SDH 50 & 100 bed PHC staff. hospitals and DH as Lab sample collection & Treatment centers. Isolation ward of 4 beds at each hospital - Ventilator Ensure logistics. Training of MO, LT & Nursing staff. Workshop for private doctors & coordination with pvt hospitals Coordination with FDA Contact tracing & treatment of symptomatic treatment. Treatment of mild ILI cases ( Category C) Referral of Cat B & A cases to identified hospital. ( Referral Centers for each Taluka should be identified by CS & DHO) Training of MO & Paramedicals IEC
 ISOLATION WARD GUIDELINES
ISOLATION WARD GUIDELINES

 Guidelines For IIWs-1 • Separate ward for positive & suspected patients. • Distance of 6 feet between two beds. • Isolation ward should have following facilities 1. Oxygen cylinders with accessories. 2. Pulse Oxymeter. 3. Electric & foot suction machine. 4. Emergency tray 5. Ventilators with trained staff.
Guidelines For IIWs-1 • Separate ward for positive & suspected patients. • Distance of 6 feet between two beds. • Isolation ward should have following facilities 1. Oxygen cylinders with accessories. 2. Pulse Oxymeter. 3. Electric & foot suction machine. 4. Emergency tray 5. Ventilators with trained staff.
 Guidelines For IIWs-2 • Well ventilated ward with exhaust fan. • Disinfection measures & Biomedical waste management according to standard guideline. • One separate on road ambulance. • Sufficient stock of Tamiflu, PPE, VTM. • Trained staff & doctors to operate all instruments. • At least one on call physician available for 24 hours.
Guidelines For IIWs-2 • Well ventilated ward with exhaust fan. • Disinfection measures & Biomedical waste management according to standard guideline. • One separate on road ambulance. • Sufficient stock of Tamiflu, PPE, VTM. • Trained staff & doctors to operate all instruments. • At least one on call physician available for 24 hours.
 LAB SAMPLE COLLECTION
LAB SAMPLE COLLECTION
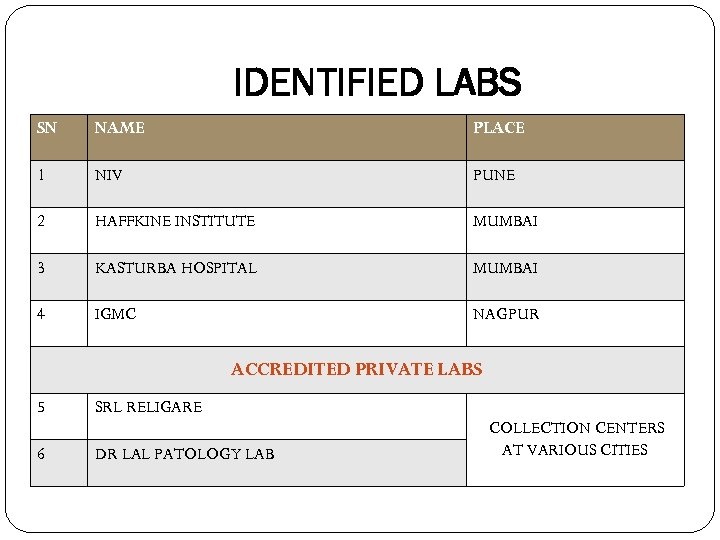 IDENTIFIED LABS SN NAME PLACE 1 NIV PUNE 2 HAFFKINE INSTITUTE MUMBAI 3 KASTURBA HOSPITAL MUMBAI 4 IGMC NAGPUR ACCREDITED PRIVATE LABS 5 6 SRL RELIGARE DR LAL PATOLOGY LAB COLLECTION CENTERS AT VARIOUS CITIES
IDENTIFIED LABS SN NAME PLACE 1 NIV PUNE 2 HAFFKINE INSTITUTE MUMBAI 3 KASTURBA HOSPITAL MUMBAI 4 IGMC NAGPUR ACCREDITED PRIVATE LABS 5 6 SRL RELIGARE DR LAL PATOLOGY LAB COLLECTION CENTERS AT VARIOUS CITIES
 Guidelines About Sample Collection Swab should be accompanied with detailed clinical history of patient. Preferably prior to giving Oseltamivir. No swab collection on OPD basis. Swabs of admitted patients only. In clusters of ILI, send only 5% samples. Do not take samples of asymptomatic contacts. Do not collect swabs after more than 8 days of onset of illness. If required do other tests also – eg Dengue, Malaria, Lepto etc.
Guidelines About Sample Collection Swab should be accompanied with detailed clinical history of patient. Preferably prior to giving Oseltamivir. No swab collection on OPD basis. Swabs of admitted patients only. In clusters of ILI, send only 5% samples. Do not take samples of asymptomatic contacts. Do not collect swabs after more than 8 days of onset of illness. If required do other tests also – eg Dengue, Malaria, Lepto etc.
 Sample Collection & Laboratory Diagnosis-1 • • • What sample to be collected? Nasopharyngeal/oropharyngeal swabs. Brochoalveolar lavage. Tracheal aspirates. Nasopharyngeal/oropharyngeal aspirates as washes. Samples should be collected in VTM.
Sample Collection & Laboratory Diagnosis-1 • • • What sample to be collected? Nasopharyngeal/oropharyngeal swabs. Brochoalveolar lavage. Tracheal aspirates. Nasopharyngeal/oropharyngeal aspirates as washes. Samples should be collected in VTM.
 Sample Collection & Laboratory Diagnosis-2 When to collect Specimens -As soon as possible after symptoms begin -Before administration of antiviral medications. • Full complement of PPE should be worn before initiating sample collection.
Sample Collection & Laboratory Diagnosis-2 When to collect Specimens -As soon as possible after symptoms begin -Before administration of antiviral medications. • Full complement of PPE should be worn before initiating sample collection.
 Transportation of Samples • All samples should be kept at 2 -8 degree Celsius until they can be placed at -70 C. • Samples transported on dry ice in triple packaging. • Clear labels with patient’s complete information. • Samples should be sent to NIV, Pune, IGMC Nagpur, Kasturba Hospital Mumbai or Haffkine Mumbai within 24 hrs.
Transportation of Samples • All samples should be kept at 2 -8 degree Celsius until they can be placed at -70 C. • Samples transported on dry ice in triple packaging. • Clear labels with patient’s complete information. • Samples should be sent to NIV, Pune, IGMC Nagpur, Kasturba Hospital Mumbai or Haffkine Mumbai within 24 hrs.
 TREATMENT PROTOCOL
TREATMENT PROTOCOL

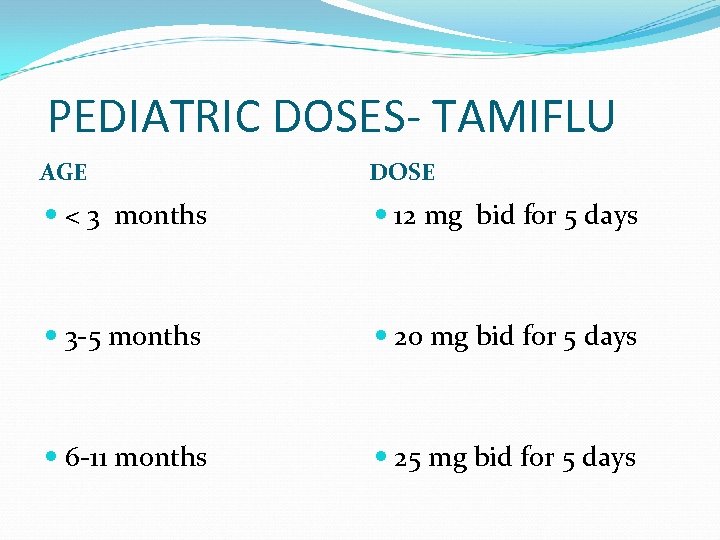 PEDIATRIC DOSES- TAMIFLU AGE DOSE < 3 months 12 mg bid for 5 days 3 -5 months 20 mg bid for 5 days 6 -11 months 25 mg bid for 5 days
PEDIATRIC DOSES- TAMIFLU AGE DOSE < 3 months 12 mg bid for 5 days 3 -5 months 20 mg bid for 5 days 6 -11 months 25 mg bid for 5 days
 No Need of chemoprophylaxis. . Guidelines for Close Contacts • Search meticulously for all close contacts of every positive case of Influenza A H 1 N 1 case. • Start Oseltamivir in therapeutic dose to all close contacts with Influenza like symptoms. • Keep asymptomatic contacts under surveillance for 10 days, if any one of them develop Influenza like symptoms within that period start Oseltamivir in therapeutic dose. • Don’t give Oseltamivir to close contacts who are asymptomatic during the entire period of observation.
No Need of chemoprophylaxis. . Guidelines for Close Contacts • Search meticulously for all close contacts of every positive case of Influenza A H 1 N 1 case. • Start Oseltamivir in therapeutic dose to all close contacts with Influenza like symptoms. • Keep asymptomatic contacts under surveillance for 10 days, if any one of them develop Influenza like symptoms within that period start Oseltamivir in therapeutic dose. • Don’t give Oseltamivir to close contacts who are asymptomatic during the entire period of observation.
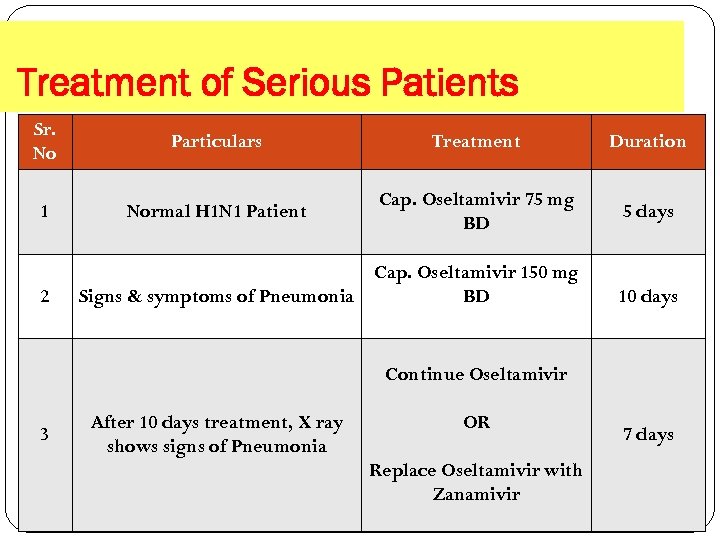 Treatment of Serious Patients Sr. No Particulars Treatment Duration 1 Normal H 1 N 1 Patient Cap. Oseltamivir 75 mg BD 5 days 2 Cap. Oseltamivir 150 mg Signs & symptoms of Pneumonia BD 10 days Continue Oseltamivir 3 After 10 days treatment, X ray shows signs of Pneumonia OR Replace Oseltamivir with Zanamivir 7 days
Treatment of Serious Patients Sr. No Particulars Treatment Duration 1 Normal H 1 N 1 Patient Cap. Oseltamivir 75 mg BD 5 days 2 Cap. Oseltamivir 150 mg Signs & symptoms of Pneumonia BD 10 days Continue Oseltamivir 3 After 10 days treatment, X ray shows signs of Pneumonia OR Replace Oseltamivir with Zanamivir 7 days
 Antiviral drugs in Pregnancy • Category C medication. • No clinical studies to assess safety. • It should be used when potential benefit justifies the potential risk to the embryo/fetus. • So far no adverse effects have been reported. • Some prefer Zanamivir over Oseltamivir as systemic absorption is minimal.
Antiviral drugs in Pregnancy • Category C medication. • No clinical studies to assess safety. • It should be used when potential benefit justifies the potential risk to the embryo/fetus. • So far no adverse effects have been reported. • Some prefer Zanamivir over Oseltamivir as systemic absorption is minimal.
 Warning Signs- Children • • • Fast breathing/trouble breathing. Bluish skin colour. Not drinking enough fluids/eating food. Increased irritability. Flu like symptoms improve but later return with fever and worse cough. • Fever with rash. EMERGENCY WARNING SIGNS NEED URGENT MEDICAL ATTENTION.
Warning Signs- Children • • • Fast breathing/trouble breathing. Bluish skin colour. Not drinking enough fluids/eating food. Increased irritability. Flu like symptoms improve but later return with fever and worse cough. • Fever with rash. EMERGENCY WARNING SIGNS NEED URGENT MEDICAL ATTENTION.
 Warning Signs- Adults • • • Difficulty breathing/shortness of breath. Pain /pressure in the chest or abdomen. Sudden dizziness. Confusion. Severe or persistent vomiting. EMERGENCY WARNING SIGNS NEED URGENT MEDICAL ATTENTION.
Warning Signs- Adults • • • Difficulty breathing/shortness of breath. Pain /pressure in the chest or abdomen. Sudden dizziness. Confusion. Severe or persistent vomiting. EMERGENCY WARNING SIGNS NEED URGENT MEDICAL ATTENTION.

 Pregnant Women • Active Screening of pregnant women during routine ANC check up by MOs & paramedical staff for ILI. • Timely initiation of Oseltamivir in symptomatic pregnant women.
Pregnant Women • Active Screening of pregnant women during routine ANC check up by MOs & paramedical staff for ILI. • Timely initiation of Oseltamivir in symptomatic pregnant women.
 Influenza- Educating the Public • Covering nose & mouth with a tissue/handkerchief when coughing / sneezing Dispose the tissue in the trash after use. • Hand washing with soap & water-especially after coughing/sneezing. • Cleaning hands with alcohol based hand cleaners. • Avoiding close contact with sick people. • Avoiding touching eyes , nose or mouth with unwashed hands. • Avoid hand shaking & spitting. • If sick with Influenza staying home away from work/school & limit contact with others to keep from infecting them. • No need to use mask by common public. • If you found more people suffering from Flu like symptoms from your area, inform concerned health authority.
Influenza- Educating the Public • Covering nose & mouth with a tissue/handkerchief when coughing / sneezing Dispose the tissue in the trash after use. • Hand washing with soap & water-especially after coughing/sneezing. • Cleaning hands with alcohol based hand cleaners. • Avoiding close contact with sick people. • Avoiding touching eyes , nose or mouth with unwashed hands. • Avoid hand shaking & spitting. • If sick with Influenza staying home away from work/school & limit contact with others to keep from infecting them. • No need to use mask by common public. • If you found more people suffering from Flu like symptoms from your area, inform concerned health authority.


 Use of Masks • No need of mask for common people. • Tissue or handkerchief is sufficient to cover nose/mouth while sneezing or coughing. • Improper use of disposable mask spreads infection.
Use of Masks • No need of mask for common people. • Tissue or handkerchief is sufficient to cover nose/mouth while sneezing or coughing. • Improper use of disposable mask spreads infection.
 Guidelines for Educational Institutions • Avoid large gatherings. • Active screening of flu like symptoms by teacher. • Students, teaching/non teaching staff with ILI- ask for medical consultation & home isolation. • No need of medical certificate for such preventive absentees. • Identify students with high risk condition. • Regular cleaning of area with ordinary cleaner. • Hostel- regular check up of students & staff. Closure of school not advised. • Local district administration can take decision after reviewing the situation. • Display Do’s and Don’ts prominently.
Guidelines for Educational Institutions • Avoid large gatherings. • Active screening of flu like symptoms by teacher. • Students, teaching/non teaching staff with ILI- ask for medical consultation & home isolation. • No need of medical certificate for such preventive absentees. • Identify students with high risk condition. • Regular cleaning of area with ordinary cleaner. • Hostel- regular check up of students & staff. Closure of school not advised. • Local district administration can take decision after reviewing the situation. • Display Do’s and Don’ts prominently.
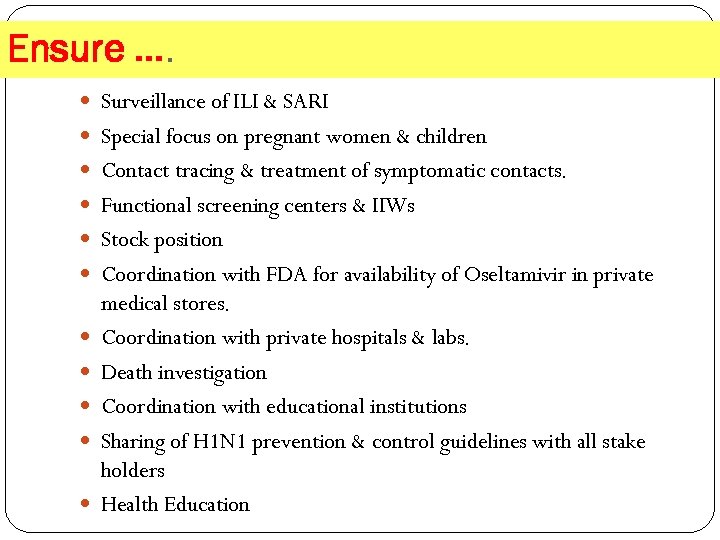 Ensure …. Surveillance of ILI & SARI Special focus on pregnant women & children Contact tracing & treatment of symptomatic contacts. Functional screening centers & IIWs Stock position Coordination with FDA for availability of Oseltamivir in private medical stores. Coordination with private hospitals & labs. Death investigation Coordination with educational institutions Sharing of H 1 N 1 prevention & control guidelines with all stake holders Health Education
Ensure …. Surveillance of ILI & SARI Special focus on pregnant women & children Contact tracing & treatment of symptomatic contacts. Functional screening centers & IIWs Stock position Coordination with FDA for availability of Oseltamivir in private medical stores. Coordination with private hospitals & labs. Death investigation Coordination with educational institutions Sharing of H 1 N 1 prevention & control guidelines with all stake holders Health Education
 Thanks…!
Thanks…!


