 INFLUENZA 1
INFLUENZA 1
 ‘FLU’ • True influenza – influenza virus A or influenza virus B (or influenza virus C infections - much milder) • Febrile respiratory disease with systemic symptoms caused by a variety of other organisms often called ‘flu’
‘FLU’ • True influenza – influenza virus A or influenza virus B (or influenza virus C infections - much milder) • Febrile respiratory disease with systemic symptoms caused by a variety of other organisms often called ‘flu’
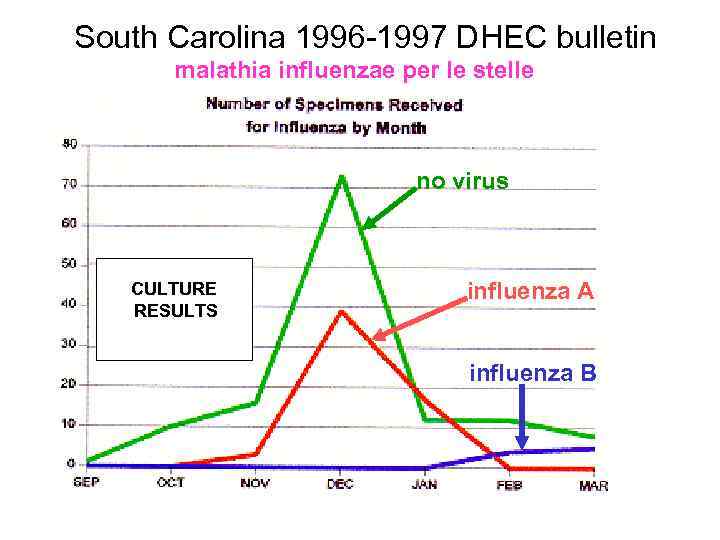 South Carolina 1996 -1997 DHEC bulletin malathia influenzae per le stelle no virus CULTURE RESULTS influenza A influenza B
South Carolina 1996 -1997 DHEC bulletin malathia influenzae per le stelle no virus CULTURE RESULTS influenza A influenza B
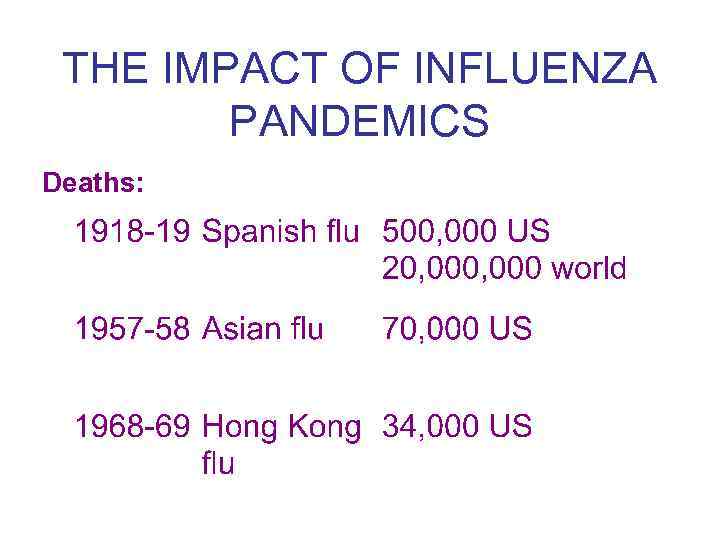 THE IMPACT OF INFLUENZA PANDEMICS Deaths:
THE IMPACT OF INFLUENZA PANDEMICS Deaths:
 THE IMPACT OF INFLUENZA • 1972 -1994 (19 influenza seasons) – >20, 000 US deaths in 11 seasons – >40, 000 US deaths in 6 of these – many more hospitalizations (~110, 000 per year)
THE IMPACT OF INFLUENZA • 1972 -1994 (19 influenza seasons) – >20, 000 US deaths in 11 seasons – >40, 000 US deaths in 6 of these – many more hospitalizations (~110, 000 per year)
 THE IMPACT OF INFLUENZA • recently some increase in morbidity and mortality - possible factors? – more elderly people – CF patients live longer – more high risk neonates – more immunosuppressed patients
THE IMPACT OF INFLUENZA • recently some increase in morbidity and mortality - possible factors? – more elderly people – CF patients live longer – more high risk neonates – more immunosuppressed patients
 ORTHOMYXOVIRUSES • pleomorphic • influenza types A, B, C • febrile, respiratory illness with systemic symptoms
ORTHOMYXOVIRUSES • pleomorphic • influenza types A, B, C • febrile, respiratory illness with systemic symptoms
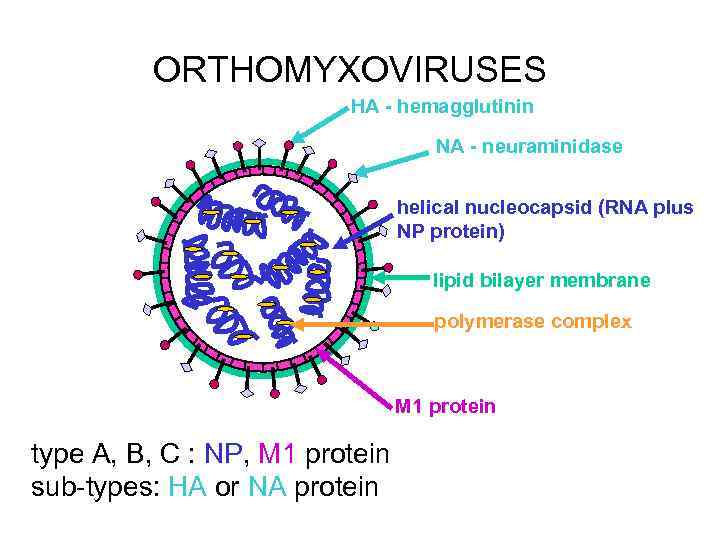 ORTHOMYXOVIRUSES HA - hemagglutinin NA - neuraminidase helical nucleocapsid (RNA plus NP protein) lipid bilayer membrane polymerase complex M 1 protein type A, B, C : NP, M 1 protein sub-types: HA or NA protein
ORTHOMYXOVIRUSES HA - hemagglutinin NA - neuraminidase helical nucleocapsid (RNA plus NP protein) lipid bilayer membrane polymerase complex M 1 protein type A, B, C : NP, M 1 protein sub-types: HA or NA protein
 TRANSMISSION • AEROSOL – 100, 000 TO 1, 000 VIRIONS PER DROPLET • 18 -72 HR INCUBATION • SHEDDING
TRANSMISSION • AEROSOL – 100, 000 TO 1, 000 VIRIONS PER DROPLET • 18 -72 HR INCUBATION • SHEDDING
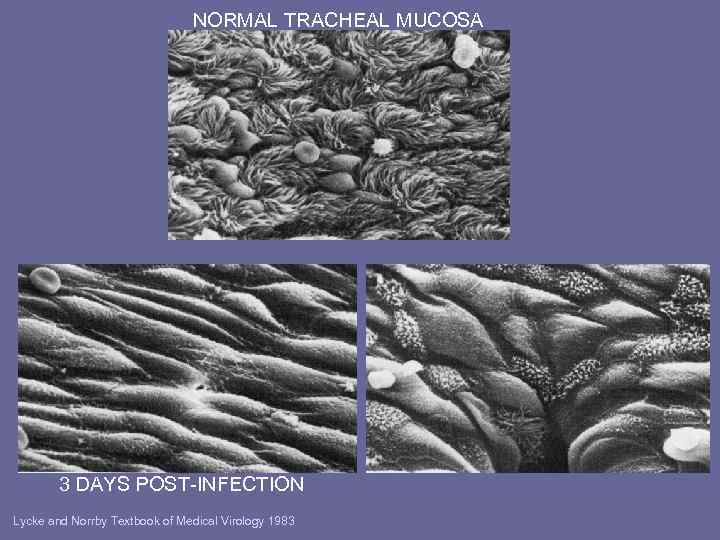 NORMAL TRACHEAL MUCOSA 3 DAYS POST-INFECTION Lycke and Norrby Textbook of Medical Virology 1983
NORMAL TRACHEAL MUCOSA 3 DAYS POST-INFECTION Lycke and Norrby Textbook of Medical Virology 1983
 • DECREASED CLEARANCE • RISK BACTERIAL INFECTION • VIREMIA RARE 11
• DECREASED CLEARANCE • RISK BACTERIAL INFECTION • VIREMIA RARE 11
 RECOVERY • INTERFERON - SIDE EFFECTS INCLUDE: – FEVER, MYALGIA, FATIGUE, MALAISE • CELL-MEDIATED IMMUNE RESPONSE • TISSUE REPAIR – CAN TAKE SOME TIME
RECOVERY • INTERFERON - SIDE EFFECTS INCLUDE: – FEVER, MYALGIA, FATIGUE, MALAISE • CELL-MEDIATED IMMUNE RESPONSE • TISSUE REPAIR – CAN TAKE SOME TIME
 An immunological diversion INTERFERON
An immunological diversion INTERFERON
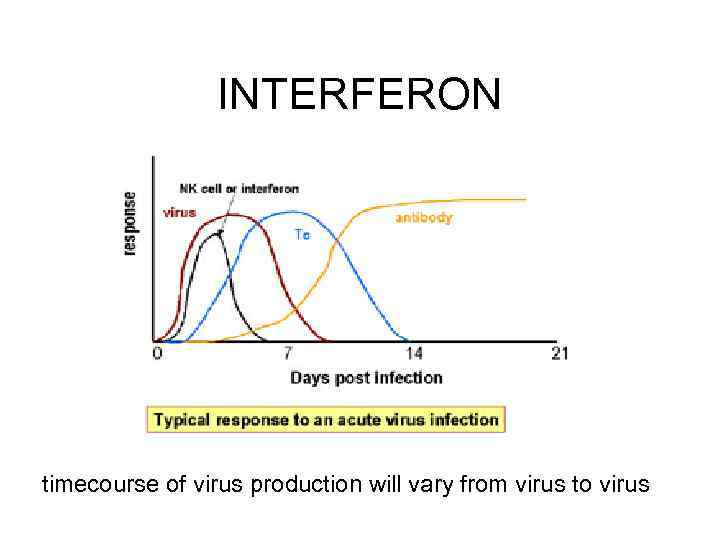 INTERFERON timecourse of virus production will vary from virus to virus
INTERFERON timecourse of virus production will vary from virus to virus
 INTERFERON
INTERFERON
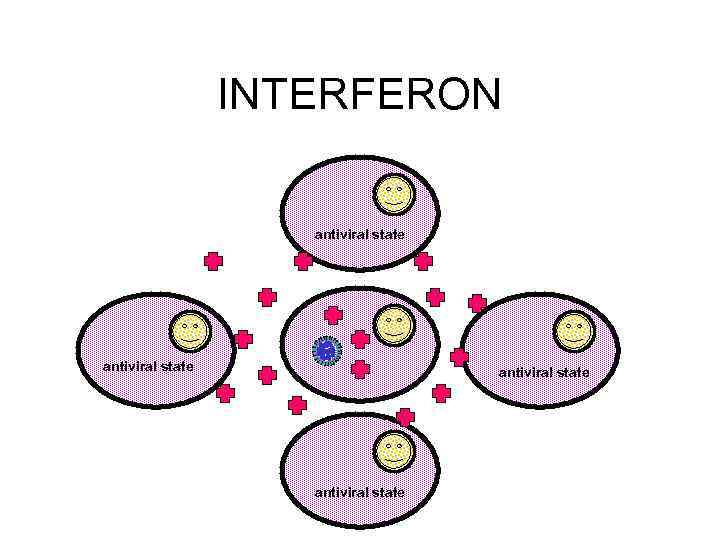 INTERFERON antiviral state
INTERFERON antiviral state
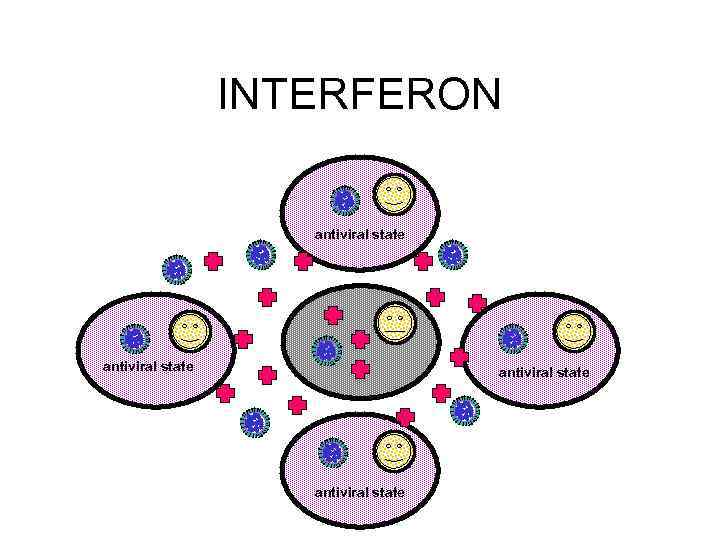 INTERFERON antiviral state
INTERFERON antiviral state
 INTERFERON antiviral state
INTERFERON antiviral state
 INTERFERON THE VIRUSES ARE COMING!
INTERFERON THE VIRUSES ARE COMING!
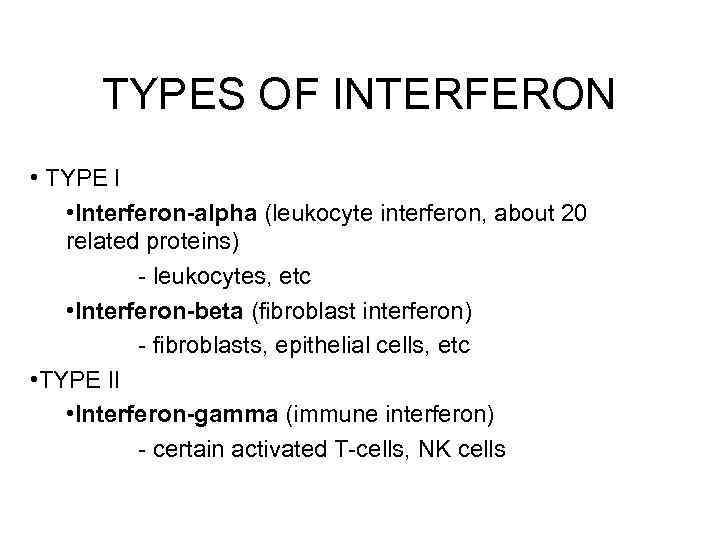 TYPES OF INTERFERON • TYPE I • Interferon-alpha (leukocyte interferon, about 20 related proteins) - leukocytes, etc • Interferon-beta (fibroblast interferon) - fibroblasts, epithelial cells, etc • TYPE II • Interferon-gamma (immune interferon) - certain activated T-cells, NK cells
TYPES OF INTERFERON • TYPE I • Interferon-alpha (leukocyte interferon, about 20 related proteins) - leukocytes, etc • Interferon-beta (fibroblast interferon) - fibroblasts, epithelial cells, etc • TYPE II • Interferon-gamma (immune interferon) - certain activated T-cells, NK cells
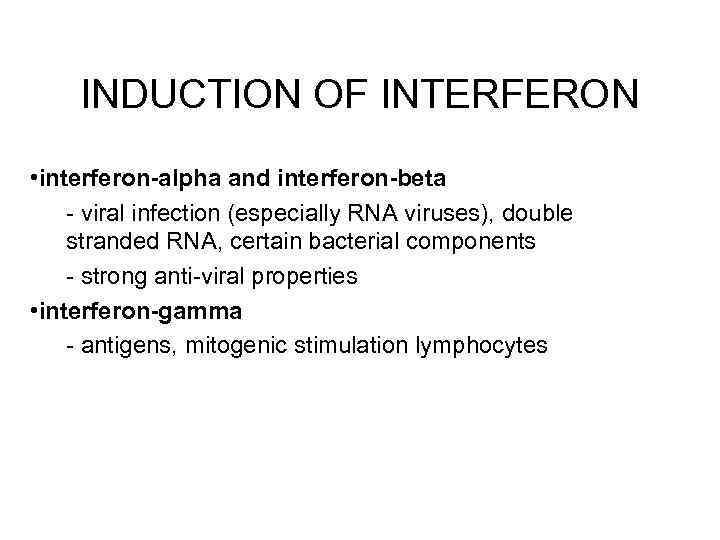 INDUCTION OF INTERFERON • interferon-alpha and interferon-beta - viral infection (especially RNA viruses), double stranded RNA, certain bacterial components - strong anti-viral properties • interferon-gamma - antigens, mitogenic stimulation lymphocytes
INDUCTION OF INTERFERON • interferon-alpha and interferon-beta - viral infection (especially RNA viruses), double stranded RNA, certain bacterial components - strong anti-viral properties • interferon-gamma - antigens, mitogenic stimulation lymphocytes
 INTERFERON • induce various proteins in target cells • many consequences, not all fully understood
INTERFERON • induce various proteins in target cells • many consequences, not all fully understood
 INTERFERON-ALPHA AND INTERFERON-BETA
INTERFERON-ALPHA AND INTERFERON-BETA
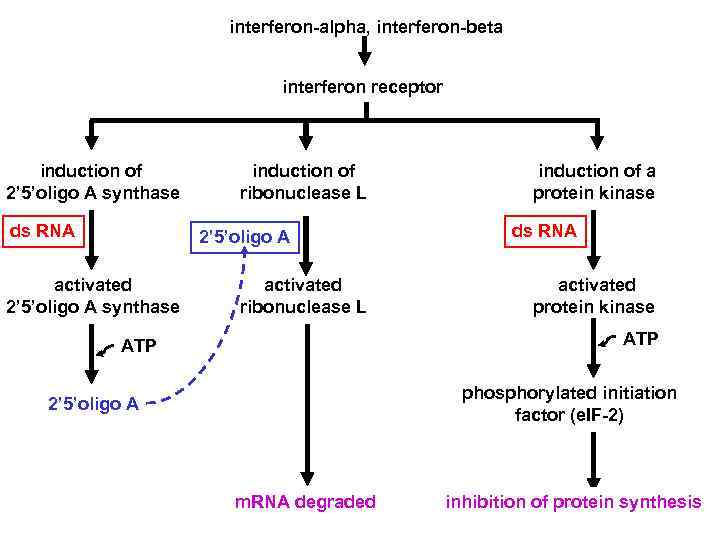 interferon-alpha, interferon-beta interferon receptor induction of 2’ 5’oligo A synthase ds RNA induction of ribonuclease L 2’ 5’oligo A activated 2’ 5’oligo A synthase activated ribonuclease L induction of a protein kinase ds RNA activated protein kinase ATP phosphorylated initiation factor (e. IF-2) 2’ 5’oligo A m. RNA degraded inhibition of protein synthesis 24
interferon-alpha, interferon-beta interferon receptor induction of 2’ 5’oligo A synthase ds RNA induction of ribonuclease L 2’ 5’oligo A activated 2’ 5’oligo A synthase activated ribonuclease L induction of a protein kinase ds RNA activated protein kinase ATP phosphorylated initiation factor (e. IF-2) 2’ 5’oligo A m. RNA degraded inhibition of protein synthesis 24
 interferons • only made when needed
interferons • only made when needed
 OTHER EFFECTS OF INTERFERONS • ALL TYPES – INCREASE MHC I EXPRESSION • CYTOTOXIC T-CELLS – ACTIVATE NK CELLS • CAN KILL VIRALLY INFECTED CELLS
OTHER EFFECTS OF INTERFERONS • ALL TYPES – INCREASE MHC I EXPRESSION • CYTOTOXIC T-CELLS – ACTIVATE NK CELLS • CAN KILL VIRALLY INFECTED CELLS
 OTHER EFFECTS OF INTERFERONS • INTERFERON-GAMMA – INCREASES MHC II EXPRESSION ON APC • HELPER T-CELLS – INCREASES ANTIVIRAL POTENTIAL OF MACROPHAGES • INTRINSIC • EXTRINSIC
OTHER EFFECTS OF INTERFERONS • INTERFERON-GAMMA – INCREASES MHC II EXPRESSION ON APC • HELPER T-CELLS – INCREASES ANTIVIRAL POTENTIAL OF MACROPHAGES • INTRINSIC • EXTRINSIC
 THERAPEUTIC USES OF INTERFERONS • ANTI-VIRAL – e. g. interferon-alpha is currently approved for certain cases of acute and chronic HCV and chronic HBV • MACROPHAGE ACTIVATION – interferon-gamma has been tried for e. g. lepromatous leprosy, leishmaniasis, toxoplasmosis • ANTI-TUMOR – have been used in e. g. melanoma, Kaposi’s sarcoma, CML • MULTIPLE SCLEROSIS – interferon-beta
THERAPEUTIC USES OF INTERFERONS • ANTI-VIRAL – e. g. interferon-alpha is currently approved for certain cases of acute and chronic HCV and chronic HBV • MACROPHAGE ACTIVATION – interferon-gamma has been tried for e. g. lepromatous leprosy, leishmaniasis, toxoplasmosis • ANTI-TUMOR – have been used in e. g. melanoma, Kaposi’s sarcoma, CML • MULTIPLE SCLEROSIS – interferon-beta
 Viral response to host immune system Viruses may : block interferon binding inhibit function of interferon-induced proteins inhibit NK function interfere with MHC I or MHC II expression block complement activation inhibit apoptosis etc!
Viral response to host immune system Viruses may : block interferon binding inhibit function of interferon-induced proteins inhibit NK function interfere with MHC I or MHC II expression block complement activation inhibit apoptosis etc!
 SIDE EFFECTS OF INTERFERONS • • FEVER MALAISE FATIGUE MUSCLE PAINS
SIDE EFFECTS OF INTERFERONS • • FEVER MALAISE FATIGUE MUSCLE PAINS
 BACK TO INFLUENZA
BACK TO INFLUENZA
 PROTECTION AGAINST RE-INFECTION • Ig. G and Ig. A – Ig. G less efficient but lasts longer • antibodies to both HA and NA important – antibody to HA more important (can neutralize)
PROTECTION AGAINST RE-INFECTION • Ig. G and Ig. A – Ig. G less efficient but lasts longer • antibodies to both HA and NA important – antibody to HA more important (can neutralize)
 SYMPTOMS • • • FEVER HEADACHE MYALGIA COUGH RHINITIS OCULAR SYMPTOMS
SYMPTOMS • • • FEVER HEADACHE MYALGIA COUGH RHINITIS OCULAR SYMPTOMS
 CLINICAL FINDINGS • SEVERITY – VERY YOUNG – ELDERLY – IMMUNOCOMPROMISED – HEART OR LUNG DISEASE
CLINICAL FINDINGS • SEVERITY – VERY YOUNG – ELDERLY – IMMUNOCOMPROMISED – HEART OR LUNG DISEASE
 PULMONARY COMPLICATIONS • CROUP (YOUNG CHILDREN) • PRIMARY INFLUENZA VIRUS PNEUMONIA • SECONDARY BACTERIAL INFECTION – Streptococcus pneumoniae – Staphlyococcus aureus – Hemophilus influenzae
PULMONARY COMPLICATIONS • CROUP (YOUNG CHILDREN) • PRIMARY INFLUENZA VIRUS PNEUMONIA • SECONDARY BACTERIAL INFECTION – Streptococcus pneumoniae – Staphlyococcus aureus – Hemophilus influenzae
 NON-PULMONARY COMPLICATIONS • myositis (rare, > in children, > with type B) • cardiac complications • recent studies report encephalopathy – studies of patients <21 yrs in Michigan - 8 cases seen last season • liver and CNS – Reye syndrome • peripheral nervous system – Guillian-Barré syndrome
NON-PULMONARY COMPLICATIONS • myositis (rare, > in children, > with type B) • cardiac complications • recent studies report encephalopathy – studies of patients <21 yrs in Michigan - 8 cases seen last season • liver and CNS – Reye syndrome • peripheral nervous system – Guillian-Barré syndrome
 Reye’s syndrome • • liver - fatty deposits brain - edema vomiting, lethargy, coma risk factors – youth – certain viral infections (influenza, chicken pox) – aspirin
Reye’s syndrome • • liver - fatty deposits brain - edema vomiting, lethargy, coma risk factors – youth – certain viral infections (influenza, chicken pox) – aspirin
 Guillian-Barré syndrome • 1976/77 swine flu vaccine – 35, 000 doses • 354 cases of GBS • 28 GBS-associated deaths • recent vaccines much lower risk
Guillian-Barré syndrome • 1976/77 swine flu vaccine – 35, 000 doses • 354 cases of GBS • 28 GBS-associated deaths • recent vaccines much lower risk
 MORTALITY • MAJOR CAUSES OF INFLUENZA VIRUS- ASSOCIATED DEATH – BACTERIAL PNEUMONIA – CARDIAC FAILURE • 90% OF DEATHS IN THOSE OVER 65 YEARS OF AGE
MORTALITY • MAJOR CAUSES OF INFLUENZA VIRUS- ASSOCIATED DEATH – BACTERIAL PNEUMONIA – CARDIAC FAILURE • 90% OF DEATHS IN THOSE OVER 65 YEARS OF AGE
 DIAGNOSIS • ISOLATION – NOSE, THROAT SWAB – TISSUE CULTURE OR EGGS • SEROLOGY • RAPID TESTS • provisional - clinical picture + outbreak
DIAGNOSIS • ISOLATION – NOSE, THROAT SWAB – TISSUE CULTURE OR EGGS • SEROLOGY • RAPID TESTS • provisional - clinical picture + outbreak
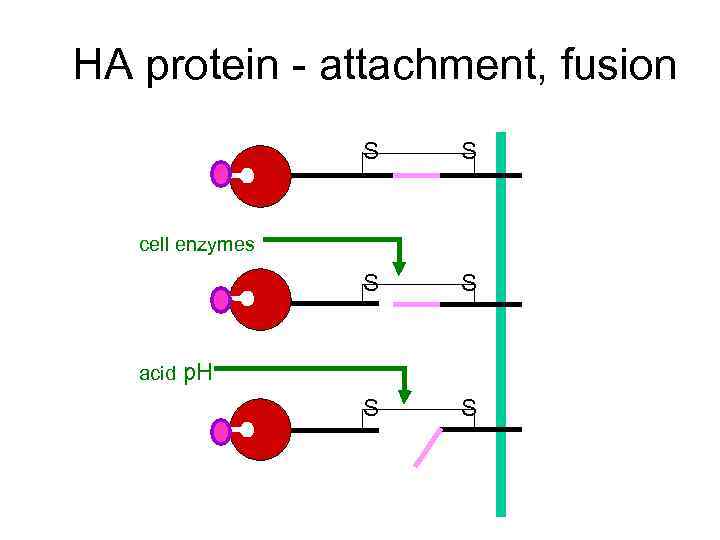 HA protein - attachment, fusion S S S cell enzymes acid p. H
HA protein - attachment, fusion S S S cell enzymes acid p. H
 NA protein - neuraminidase
NA protein - neuraminidase
 ANTIGENIC DRIFT • HA and NA accumulate mutations – RNA virus • immune response no longer protects fully • sporadic outbreaks, limited epidemics
ANTIGENIC DRIFT • HA and NA accumulate mutations – RNA virus • immune response no longer protects fully • sporadic outbreaks, limited epidemics
 ANTIGENIC SHIFT • “new” HA or NA proteins • pre-existing antibodies do not protect • may get pandemics
ANTIGENIC SHIFT • “new” HA or NA proteins • pre-existing antibodies do not protect • may get pandemics
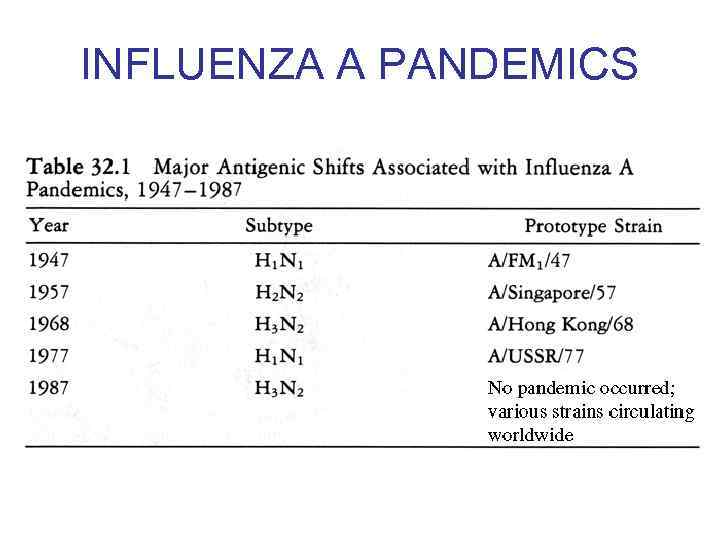 INFLUENZA A PANDEMICS
INFLUENZA A PANDEMICS
 where do “new” HA and NA come from? • 13 types HA • 9 types NA – all circulate in birds • pigs – avian and human
where do “new” HA and NA come from? • 13 types HA • 9 types NA – all circulate in birds • pigs – avian and human
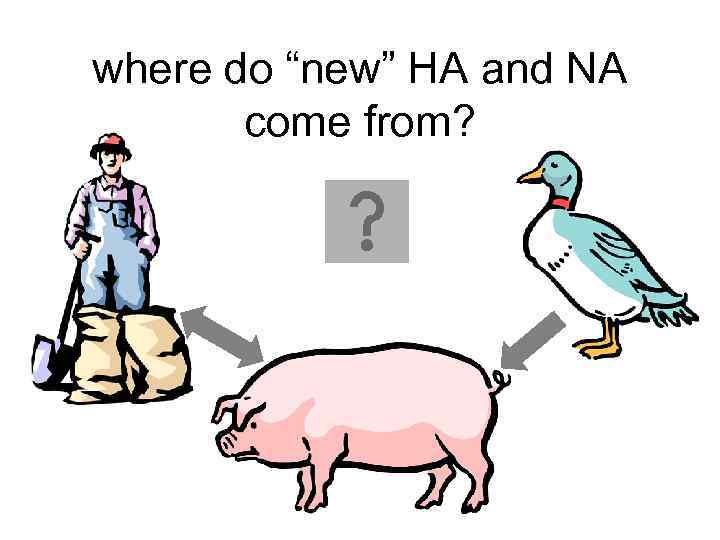 where do “new” HA and NA come from?
where do “new” HA and NA come from?
 why do we not have influenza B pandemics? • so far no shifts have been recorded • no animal reservoir known
why do we not have influenza B pandemics? • so far no shifts have been recorded • no animal reservoir known
 SURVEILLANCE 49 CDC/Katherine Lord
SURVEILLANCE 49 CDC/Katherine Lord
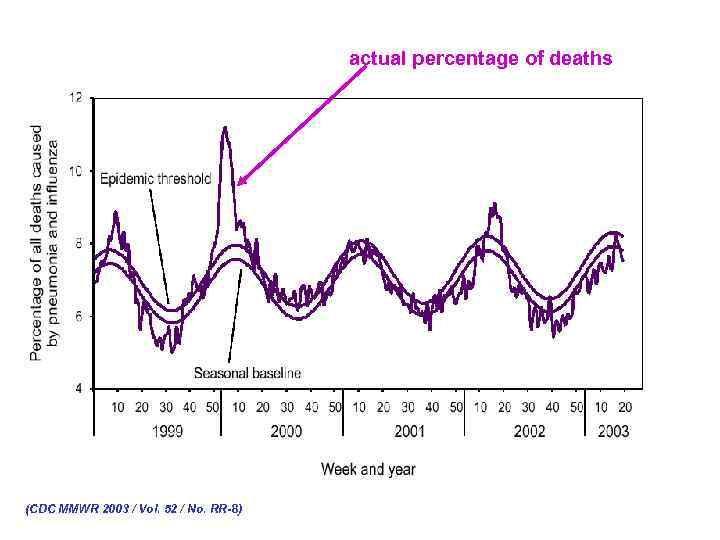 actual percentage of deaths (CDC MMWR 2003 / Vol. 52 / No. RR-8)
actual percentage of deaths (CDC MMWR 2003 / Vol. 52 / No. RR-8)
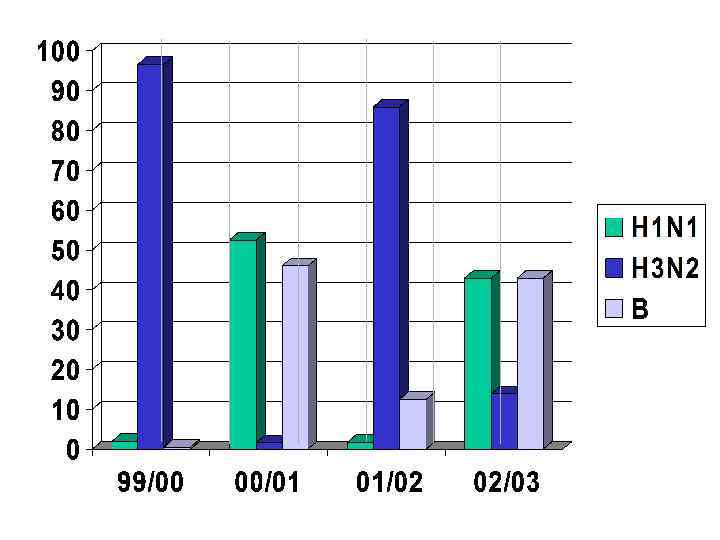
 VACCINE • ‘BEST GUESS’ OF MAIN ANTIGENIC TYPES – CURRENTLY • • type A - H 1 N 1 type A - H 3 N 2 type B each year choose which variant of each subtype is the best to use for optimal protection
VACCINE • ‘BEST GUESS’ OF MAIN ANTIGENIC TYPES – CURRENTLY • • type A - H 1 N 1 type A - H 3 N 2 type B each year choose which variant of each subtype is the best to use for optimal protection
 VACCINE • inactivated • egg grown • sub-unit vaccine for children • reassortant live vaccine approved 2003 – for healthy persons (those not at risk for complications from influenza infection) ages 5 -49 years
VACCINE • inactivated • egg grown • sub-unit vaccine for children • reassortant live vaccine approved 2003 – for healthy persons (those not at risk for complications from influenza infection) ages 5 -49 years
 CDC
CDC
 RECOMMENDATIONS Persons at High Risk for Influenza-Related Complications · $ 65 years · residents of nursing homes and other chronic-care facilities · adults/children who have chronic pulmonary or cardiovascular disorders, including asthma · adults/children who have required regular medical follow-up or hospitalization during the last year because of chronic metabolic diseases (including diabetes mellitus), renal dysfunction, hemoglobinopathies, or immunosuppression (including immunosuppression caused by medications)
RECOMMENDATIONS Persons at High Risk for Influenza-Related Complications · $ 65 years · residents of nursing homes and other chronic-care facilities · adults/children who have chronic pulmonary or cardiovascular disorders, including asthma · adults/children who have required regular medical follow-up or hospitalization during the last year because of chronic metabolic diseases (including diabetes mellitus), renal dysfunction, hemoglobinopathies, or immunosuppression (including immunosuppression caused by medications)
 RECOMMENDATIONS Persons at High Risk for Influenza-Related Complications · children and teenagers (6 mths to 18 yrs) receiving long-term aspirin therapy - might be at risk for developing Reye syndrome after influenza · women who will be in the 2 nd or 3 rd trimester of pregnancy during the influenza season.
RECOMMENDATIONS Persons at High Risk for Influenza-Related Complications · children and teenagers (6 mths to 18 yrs) receiving long-term aspirin therapy - might be at risk for developing Reye syndrome after influenza · women who will be in the 2 nd or 3 rd trimester of pregnancy during the influenza season.
 RECOMMENDATIONS Persons aged 50 -64 years increased prevalence of high-risk conditions from public health point of view, easier to target by age than by high-risk condition (which may not have been discovered)
RECOMMENDATIONS Persons aged 50 -64 years increased prevalence of high-risk conditions from public health point of view, easier to target by age than by high-risk condition (which may not have been discovered)
 RECOMMENDATIONS Persons Who Can Transmit Influenza to Those at High Risk Persons who are clinically or subclinically infected can transmit influenza virus to persons at high risk for complications from influenza.
RECOMMENDATIONS Persons Who Can Transmit Influenza to Those at High Risk Persons who are clinically or subclinically infected can transmit influenza virus to persons at high risk for complications from influenza.
 RECOMMENDATIONS · physicians, nurses, and other personnel in both hospital and outpatient-care settings · employees of nursing homes and chronic-care facilities who have contact with patients or residents · employees of assisted living and other residences for persons in high-risk groups · persons who provide home care to persons in high-risk groups · household members (including children) of persons in high-risk groups.
RECOMMENDATIONS · physicians, nurses, and other personnel in both hospital and outpatient-care settings · employees of nursing homes and chronic-care facilities who have contact with patients or residents · employees of assisted living and other residences for persons in high-risk groups · persons who provide home care to persons in high-risk groups · household members (including children) of persons in high-risk groups.
 RECOMMENDATIONS Children from 0 -23 mths are at increased risk for hospitalization from influenza, vaccination is encouraged for their household contacts and out-of-home caretakers, particularly for contacts of children aged 0– 5 months because influenza vaccines have not been approved for use among children aged <6 months.
RECOMMENDATIONS Children from 0 -23 mths are at increased risk for hospitalization from influenza, vaccination is encouraged for their household contacts and out-of-home caretakers, particularly for contacts of children aged 0– 5 months because influenza vaccines have not been approved for use among children aged <6 months.
 RECOMMENDATIONS • others, including travellers and the general population may wish to be vaccinated
RECOMMENDATIONS • others, including travellers and the general population may wish to be vaccinated
 PREVENTION - DRUGS • RIMANTADINE (M 2) • type A only • AMANTADINE (M 2) • type A only • ZANAMIVIR (NA) • types A and B, not yet approved for prevention • OSELTAMIVIR • types A and B (NA)
PREVENTION - DRUGS • RIMANTADINE (M 2) • type A only • AMANTADINE (M 2) • type A only • ZANAMIVIR (NA) • types A and B, not yet approved for prevention • OSELTAMIVIR • types A and B (NA)
 TREATMENT - DRUGS • RIMANTADINE (M 2) • type A only, needs to be given early • AMANTADINE (M 2) • type A only, needs to be given early • ZANAMIVIR (NA) • types A and B, needs to be given early • OSELTAMIVIR (NA) • types A and B, needs to be given early
TREATMENT - DRUGS • RIMANTADINE (M 2) • type A only, needs to be given early • AMANTADINE (M 2) • type A only, needs to be given early • ZANAMIVIR (NA) • types A and B, needs to be given early • OSELTAMIVIR (NA) • types A and B, needs to be given early
 NA protein - neuraminidase . .
NA protein - neuraminidase . .
 OTHER TREATMENT • REST, LIQUIDS, ANTI-FEBRILE AGENTS (NO ASPIRIN FOR AGES 6 MTHS-18 YRS) • BE AWARE OF COMPLICATIONS AND TREAT APPROPRIATELY
OTHER TREATMENT • REST, LIQUIDS, ANTI-FEBRILE AGENTS (NO ASPIRIN FOR AGES 6 MTHS-18 YRS) • BE AWARE OF COMPLICATIONS AND TREAT APPROPRIATELY
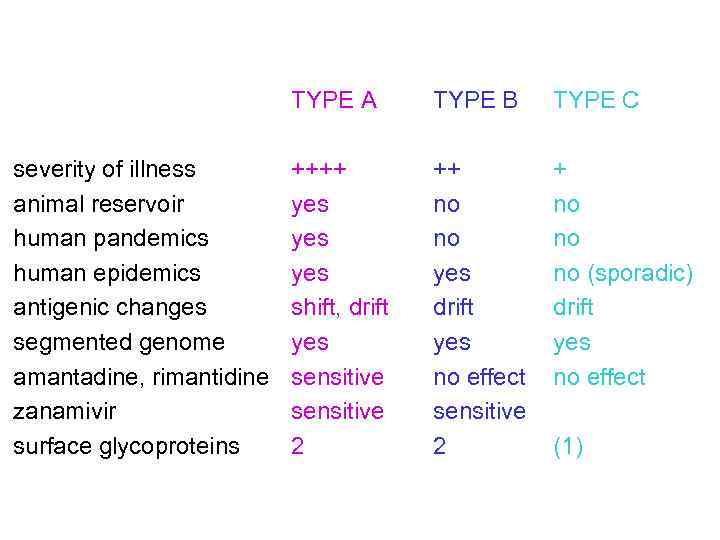 TYPE A severity of illness animal reservoir human pandemics human epidemics antigenic changes segmented genome amantadine, rimantidine zanamivir surface glycoproteins TYPE B TYPE C ++++ yes yes shift, drift yes sensitive 2 ++ no no yes drift yes no effect sensitive 2 + no no no (sporadic) drift yes no effect (1)
TYPE A severity of illness animal reservoir human pandemics human epidemics antigenic changes segmented genome amantadine, rimantidine zanamivir surface glycoproteins TYPE B TYPE C ++++ yes yes shift, drift yes sensitive 2 ++ no no yes drift yes no effect sensitive 2 + no no no (sporadic) drift yes no effect (1)



