8a07b092161e894388056f6d731b67c9.ppt
- Количество слайдов: 24
 Influence of ionic liquid content on properties of dense polymer membranes M. Kohoutová a , A. Sikora b , Š. Hovorka c , A. Randová c, M. Tišmad, P. Izák a Department of Separation Process, Institute of Chemical Process Fundamentals, Rozvojová 135, 165 02 Prague 6, Czech Republic b Institute of Macromolecular Chemistry, Heyrovského nám. 2, 162 06 Prague 6, Czech Republic c Institute of Chemical Technology, Technická 5, 160 00 Prague 6, Czech Republic d. Faculty of Food Technology Osijek, University of Osijek, F. Kuhača 18, 31 000 Osijek, Croatia a http: //www. icpf. cas. cz/
Influence of ionic liquid content on properties of dense polymer membranes M. Kohoutová a , A. Sikora b , Š. Hovorka c , A. Randová c, M. Tišmad, P. Izák a Department of Separation Process, Institute of Chemical Process Fundamentals, Rozvojová 135, 165 02 Prague 6, Czech Republic b Institute of Macromolecular Chemistry, Heyrovského nám. 2, 162 06 Prague 6, Czech Republic c Institute of Chemical Technology, Technická 5, 160 00 Prague 6, Czech Republic d. Faculty of Food Technology Osijek, University of Osijek, F. Kuhača 18, 31 000 Osijek, Croatia a http: //www. icpf. cas. cz/
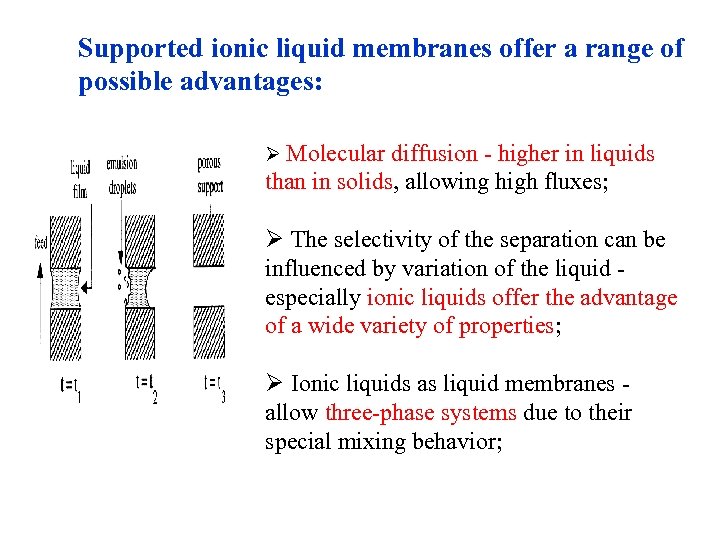 Supported ionic liquid membranes offer a range of possible advantages: Ø Molecular diffusion - higher in liquids than in solids, allowing high fluxes; Ø The selectivity of the separation can be influenced by variation of the liquid especially ionic liquids offer the advantage of a wide variety of properties; Ø Ionic liquids as liquid membranes allow three-phase systems due to their special mixing behavior;
Supported ionic liquid membranes offer a range of possible advantages: Ø Molecular diffusion - higher in liquids than in solids, allowing high fluxes; Ø The selectivity of the separation can be influenced by variation of the liquid especially ionic liquids offer the advantage of a wide variety of properties; Ø Ionic liquids as liquid membranes allow three-phase systems due to their special mixing behavior;
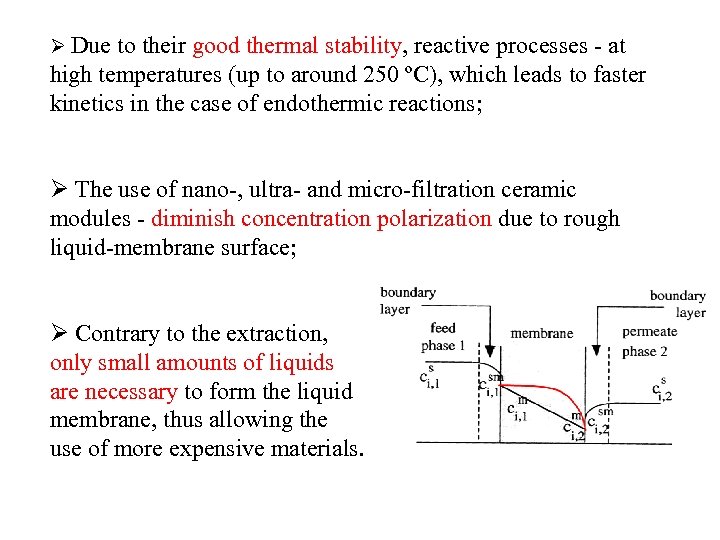 Ø Due to their good thermal stability, reactive processes - at high temperatures (up to around 250 ºC), which leads to faster kinetics in the case of endothermic reactions; Ø The use of nano-, ultra- and micro-filtration ceramic modules - diminish concentration polarization due to rough liquid-membrane surface; Ø Contrary to the extraction, only small amounts of liquids are necessary to form the liquid membrane, thus allowing the use of more expensive materials.
Ø Due to their good thermal stability, reactive processes - at high temperatures (up to around 250 ºC), which leads to faster kinetics in the case of endothermic reactions; Ø The use of nano-, ultra- and micro-filtration ceramic modules - diminish concentration polarization due to rough liquid-membrane surface; Ø Contrary to the extraction, only small amounts of liquids are necessary to form the liquid membrane, thus allowing the use of more expensive materials.
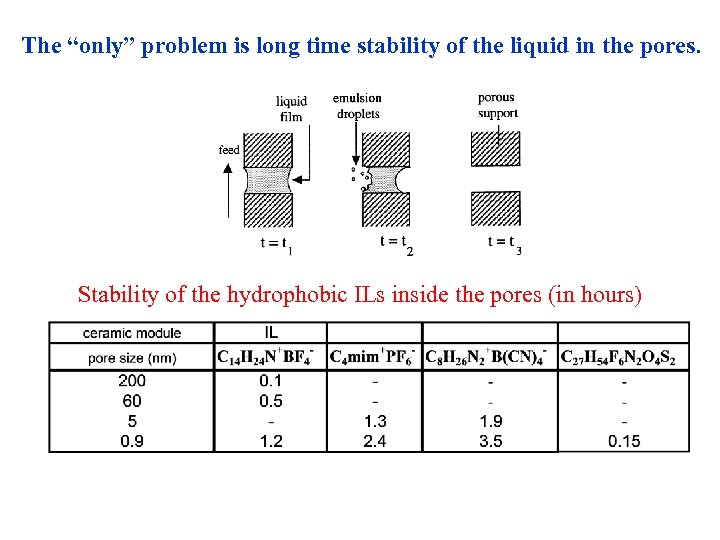 The “only” problem is long time stability of the liquid in the pores. Stability of the hydrophobic ILs inside the pores (in hours)
The “only” problem is long time stability of the liquid in the pores. Stability of the hydrophobic ILs inside the pores (in hours)
 Experimental • Membranes preparation : PDMS Elastosil M 4601 (base & crosslinking catalyst) (Wacker Silicones, Germany) • IL – benzyl-3 -butylimidazolium tetrafluoroborate (Chemada Fine Chemicals, Israel) • 10, 20, 30 wt. % of IL • Average thickness 0. 3 ± 0. 017 mm • Conditions : 37°C, 5 wt. % butan-1 -ol, N 2 flow rate 0. 9 ml/s, 48 hours • Analysis : Finnigan GC Trace Ultra GC – TCD;
Experimental • Membranes preparation : PDMS Elastosil M 4601 (base & crosslinking catalyst) (Wacker Silicones, Germany) • IL – benzyl-3 -butylimidazolium tetrafluoroborate (Chemada Fine Chemicals, Israel) • 10, 20, 30 wt. % of IL • Average thickness 0. 3 ± 0. 017 mm • Conditions : 37°C, 5 wt. % butan-1 -ol, N 2 flow rate 0. 9 ml/s, 48 hours • Analysis : Finnigan GC Trace Ultra GC – TCD;
![Compatibility of PDMS and [BBIM][BF 4] • Tg, IL appeard only in the blend Compatibility of PDMS and [BBIM][BF 4] • Tg, IL appeard only in the blend](https://present5.com/presentation/8a07b092161e894388056f6d731b67c9/image-6.jpg) Compatibility of PDMS and [BBIM][BF 4] • Tg, IL appeard only in the blend with 30 wt. % IL when the sample was cooled and heated slowly Temperature dependences of DSC heat flow of the blend containing 30% of [BBIM][BF 4] • (a) heating mode (10°C/min) preceded by cooling (10°C/min) • (b) heating mode (10°C/min) preceded by cooling (1°C/min) • (c) heating mode (5°C/min) preceded by cooling (1°C/min • PDMS and [BBIM][BF 4] are not compatibile; blend contains amorphous and crystalline phases of PDMS and dispersed phases of [BBIM][BF 4]
Compatibility of PDMS and [BBIM][BF 4] • Tg, IL appeard only in the blend with 30 wt. % IL when the sample was cooled and heated slowly Temperature dependences of DSC heat flow of the blend containing 30% of [BBIM][BF 4] • (a) heating mode (10°C/min) preceded by cooling (10°C/min) • (b) heating mode (10°C/min) preceded by cooling (1°C/min) • (c) heating mode (5°C/min) preceded by cooling (1°C/min • PDMS and [BBIM][BF 4] are not compatibile; blend contains amorphous and crystalline phases of PDMS and dispersed phases of [BBIM][BF 4]
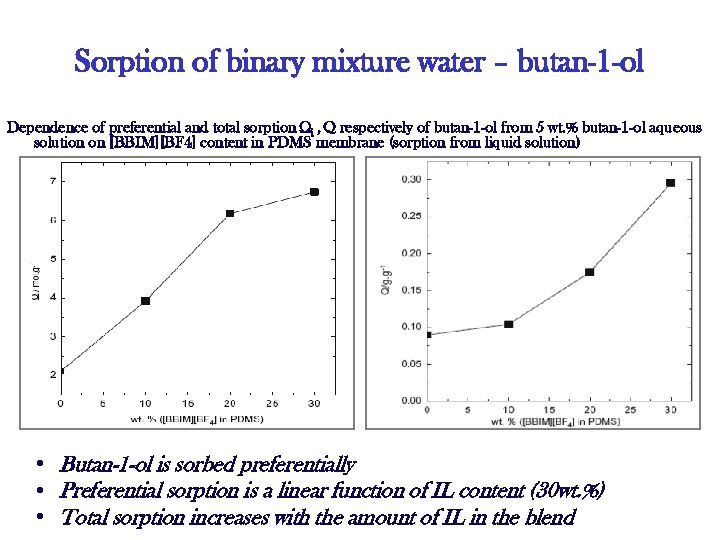 Sorption of binary mixture water – butan-1 -ol Dependence of preferential and total sorption Ωi , Q respectively of butan-1 -ol from 5 wt. % butan-1 -ol aqueous solution on [BBIM][BF 4] content in PDMS membrane (sorption from liquid solution) • Butan-1 -ol is sorbed preferentially • Preferential sorption is a linear function of IL content (30 wt. %) • Total sorption increases with the amount of IL in the blend
Sorption of binary mixture water – butan-1 -ol Dependence of preferential and total sorption Ωi , Q respectively of butan-1 -ol from 5 wt. % butan-1 -ol aqueous solution on [BBIM][BF 4] content in PDMS membrane (sorption from liquid solution) • Butan-1 -ol is sorbed preferentially • Preferential sorption is a linear function of IL content (30 wt. %) • Total sorption increases with the amount of IL in the blend
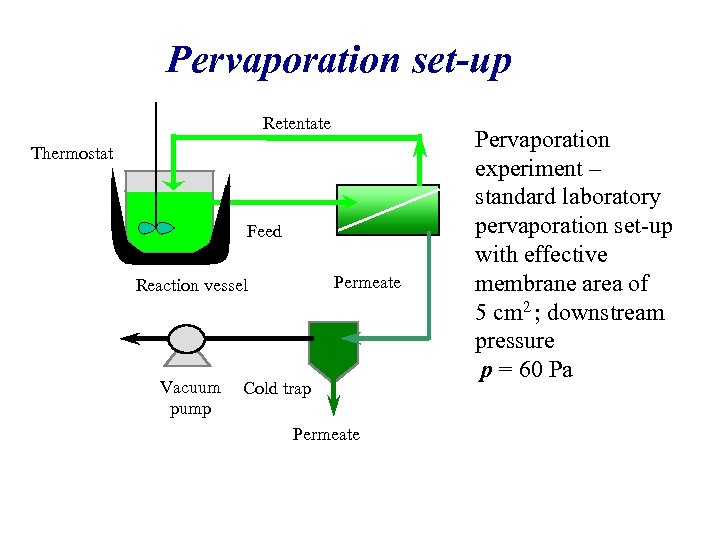 Pervaporation set-up Retentate Thermostat Feed Permeate Reaction vessel Vacuum pump Cold trap Permeate Pervaporation experiment – standard laboratory pervaporation set-up with effective membrane area of 5 cm 2 ; downstream pressure p = 60 Pa
Pervaporation set-up Retentate Thermostat Feed Permeate Reaction vessel Vacuum pump Cold trap Permeate Pervaporation experiment – standard laboratory pervaporation set-up with effective membrane area of 5 cm 2 ; downstream pressure p = 60 Pa
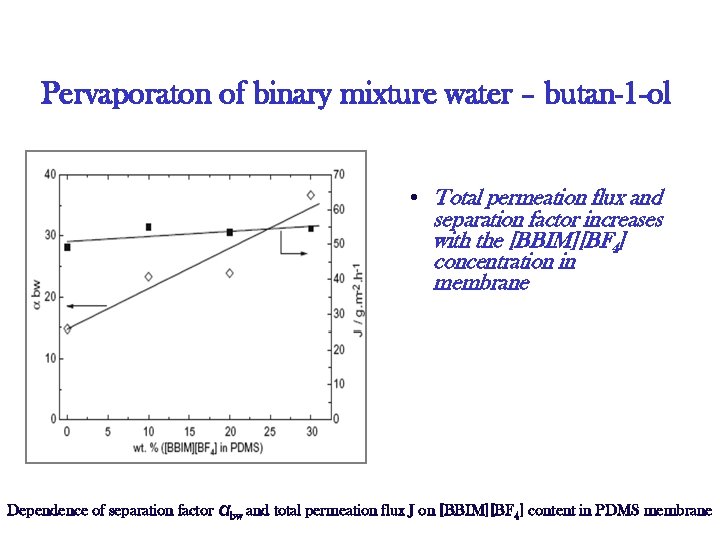 Pervaporaton of binary mixture water – butan-1 -ol • Total permeation flux and separation factor increases with the [BBIM][BF 4] concentration in membrane Dependence of separation factor αbw and total permeation flux J on [BBIM][BF 4] content in PDMS membrane
Pervaporaton of binary mixture water – butan-1 -ol • Total permeation flux and separation factor increases with the [BBIM][BF 4] concentration in membrane Dependence of separation factor αbw and total permeation flux J on [BBIM][BF 4] content in PDMS membrane
 Experimental • As a support matrix for the polymer-IL membrane the ceramic ultrafiltration module made from Ti. O 2 with pore size 60 nm was used. • The PDMS was prepared by mixing a solution of RTV 615 A and RTV 615 B (General Electric) in 10: 1 ratio at 60°C for 0. 5 hour. • 15 wt% of tetrapropylammonium tetracyano-borate ionic liquid and 85 wt% polydimethylsiloxane (IL 1). • 50 wt% of 1 -ethenyl-3 -ethyl-imidazolium hexafluorophosphate ionic liquid was mixed with 50 wt% polydimethylsiloxane (IL 2).
Experimental • As a support matrix for the polymer-IL membrane the ceramic ultrafiltration module made from Ti. O 2 with pore size 60 nm was used. • The PDMS was prepared by mixing a solution of RTV 615 A and RTV 615 B (General Electric) in 10: 1 ratio at 60°C for 0. 5 hour. • 15 wt% of tetrapropylammonium tetracyano-borate ionic liquid and 85 wt% polydimethylsiloxane (IL 1). • 50 wt% of 1 -ethenyl-3 -ethyl-imidazolium hexafluorophosphate ionic liquid was mixed with 50 wt% polydimethylsiloxane (IL 2).
 • The ternary system - practical application in biotransformation processes, where the fermentation broth from Clostridium acetobutylicum is normally used • The compound of interest is biofuel, namely BIObutanol • It is the main product of butan-1 -ol fermentation and it is also the primary inhibitory product affecting the bioconversion
• The ternary system - practical application in biotransformation processes, where the fermentation broth from Clostridium acetobutylicum is normally used • The compound of interest is biofuel, namely BIObutanol • It is the main product of butan-1 -ol fermentation and it is also the primary inhibitory product affecting the bioconversion
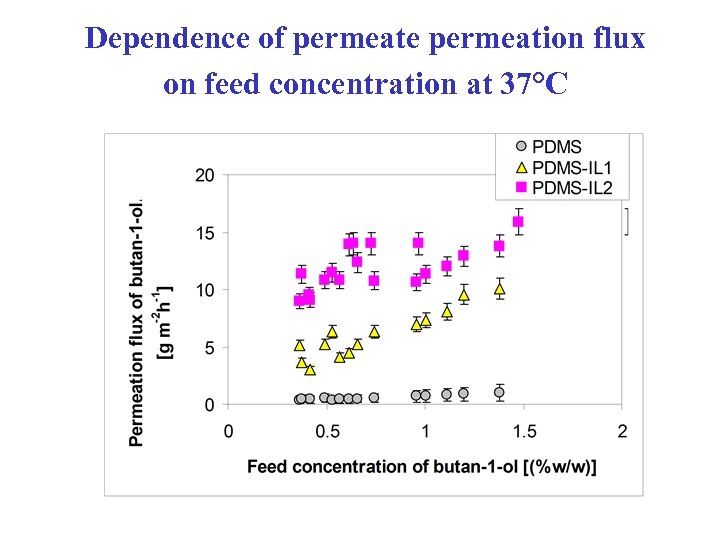 Dependence of permeate permeation flux on feed concentration at 37°C
Dependence of permeate permeation flux on feed concentration at 37°C
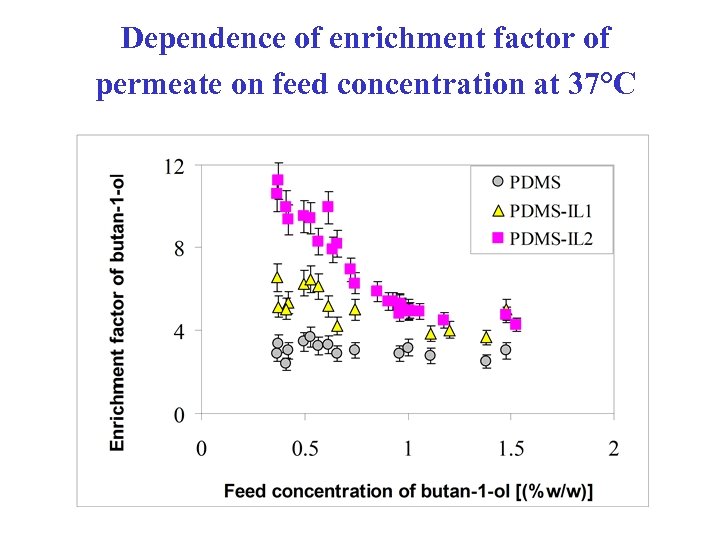 Dependence of enrichment factor of permeate on feed concentration at 37°C
Dependence of enrichment factor of permeate on feed concentration at 37°C
 • The enrichment factor of butan-1 -ol increased from 2. 2 (PDMS) up to 10. 9 (IL 2 -PDMS) (Izák P, Ruth W, Dyson P, Kragl U (2007) Selective Removal of Acetone and Butan-1 ol from Water with Supported Ionic Liquid - Polydimethylsiloxane Membrane by Pervaporation, Chem. Eng. J. , 139/2 (2008) 318 -321) • Fermentation was carried out at 37°C and p. H 4. 5. • Firstly, a continuous fermentation with removal of ABE by pervaporation was measured without any butan-1 -ol addition to test, if the SILM was selective and stable.
• The enrichment factor of butan-1 -ol increased from 2. 2 (PDMS) up to 10. 9 (IL 2 -PDMS) (Izák P, Ruth W, Dyson P, Kragl U (2007) Selective Removal of Acetone and Butan-1 ol from Water with Supported Ionic Liquid - Polydimethylsiloxane Membrane by Pervaporation, Chem. Eng. J. , 139/2 (2008) 318 -321) • Fermentation was carried out at 37°C and p. H 4. 5. • Firstly, a continuous fermentation with removal of ABE by pervaporation was measured without any butan-1 -ol addition to test, if the SILM was selective and stable.
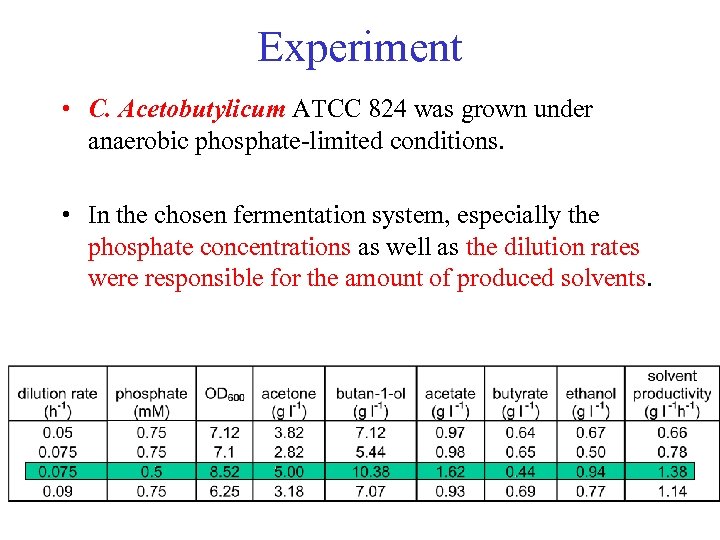 Experiment • C. Acetobutylicum ATCC 824 was grown under anaerobic phosphate-limited conditions. • In the chosen fermentation system, especially the phosphate concentrations as well as the dilution rates were responsible for the amount of produced solvents.
Experiment • C. Acetobutylicum ATCC 824 was grown under anaerobic phosphate-limited conditions. • In the chosen fermentation system, especially the phosphate concentrations as well as the dilution rates were responsible for the amount of produced solvents.
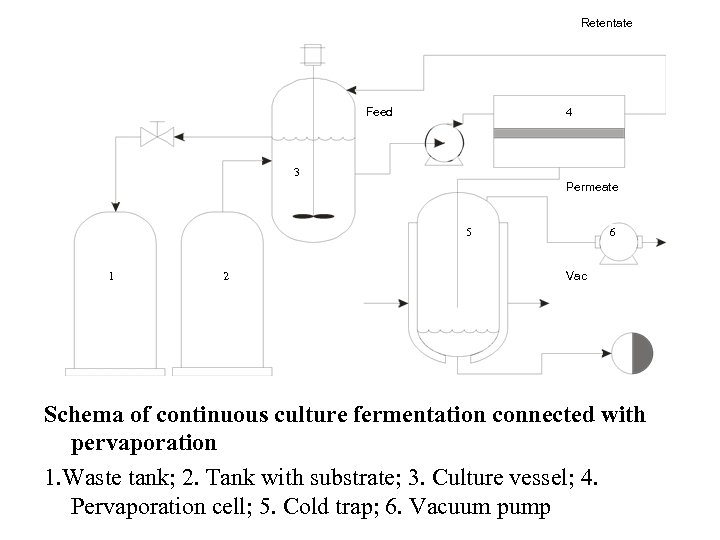 Retentate Feed 4 3 Permeate 5 1 2 6 Vac Schema of continuous culture fermentation connected with pervaporation 1. Waste tank; 2. Tank with substrate; 3. Culture vessel; 4. Pervaporation cell; 5. Cold trap; 6. Vacuum pump
Retentate Feed 4 3 Permeate 5 1 2 6 Vac Schema of continuous culture fermentation connected with pervaporation 1. Waste tank; 2. Tank with substrate; 3. Culture vessel; 4. Pervaporation cell; 5. Cold trap; 6. Vacuum pump
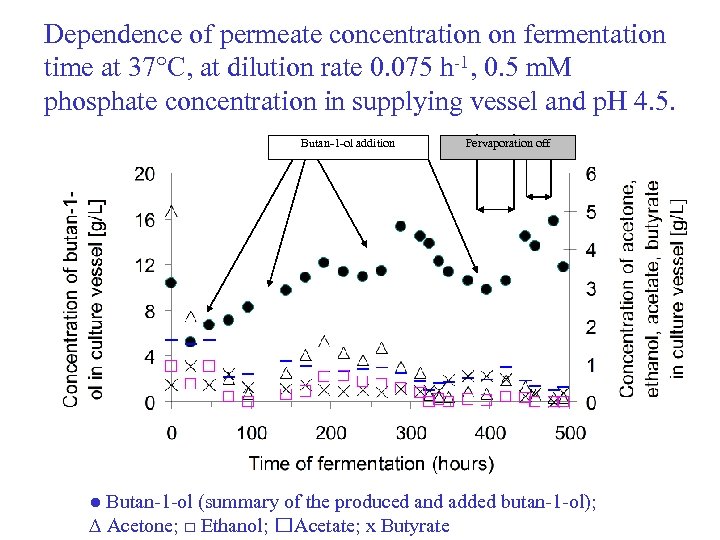 Dependence of permeate concentration on fermentation time at 37°C, at dilution rate 0. 075 h-1, 0. 5 m. M phosphate concentration in supplying vessel and p. H 4. 5. Butan-1 -ol addition Pervaporation off ● Butan-1 -ol (summary of the produced and added butan-1 -ol); ∆ Acetone; □ Ethanol; Acetate; x Butyrate
Dependence of permeate concentration on fermentation time at 37°C, at dilution rate 0. 075 h-1, 0. 5 m. M phosphate concentration in supplying vessel and p. H 4. 5. Butan-1 -ol addition Pervaporation off ● Butan-1 -ol (summary of the produced and added butan-1 -ol); ∆ Acetone; □ Ethanol; Acetate; x Butyrate
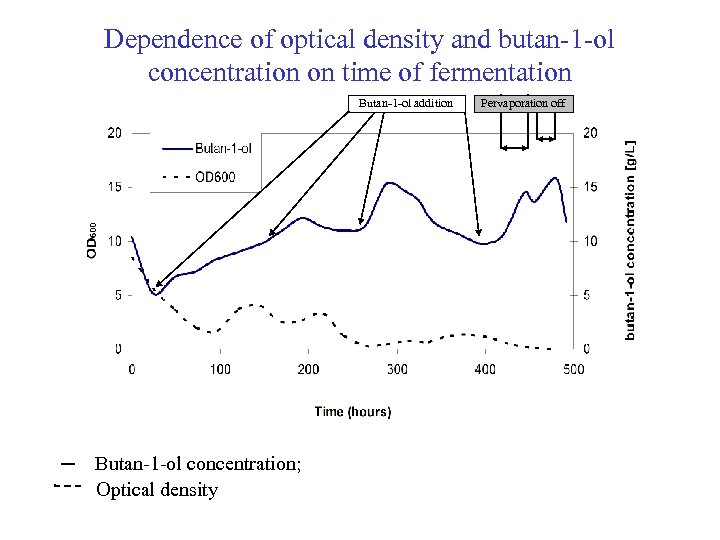 Dependence of optical density and butan-1 -ol concentration on time of fermentation Butan-1 -ol addition ─ Butan-1 -ol concentration; Optical density Pervaporation off
Dependence of optical density and butan-1 -ol concentration on time of fermentation Butan-1 -ol addition ─ Butan-1 -ol concentration; Optical density Pervaporation off
 • After successful tests, the concentration of butan-1 -ol was several times increased to test the SILM under more stringent conditions and to study the effect of pervaporation on the cells. • After 3 months of the experiment we did not observe any change of mass or selectivity of IL in the pores of the ultrafiltration membrane.
• After successful tests, the concentration of butan-1 -ol was several times increased to test the SILM under more stringent conditions and to study the effect of pervaporation on the cells. • After 3 months of the experiment we did not observe any change of mass or selectivity of IL in the pores of the ultrafiltration membrane.
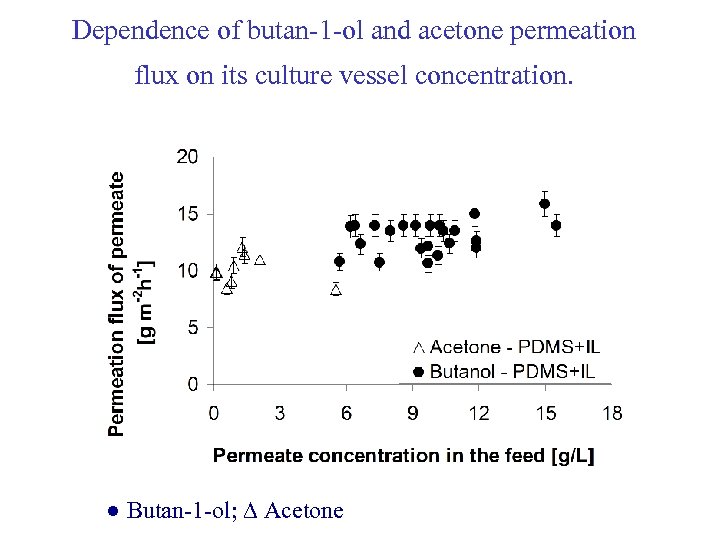 Dependence of butan-1 -ol and acetone permeation flux on its culture vessel concentration. ● Butan-1 -ol; ∆ Acetone
Dependence of butan-1 -ol and acetone permeation flux on its culture vessel concentration. ● Butan-1 -ol; ∆ Acetone
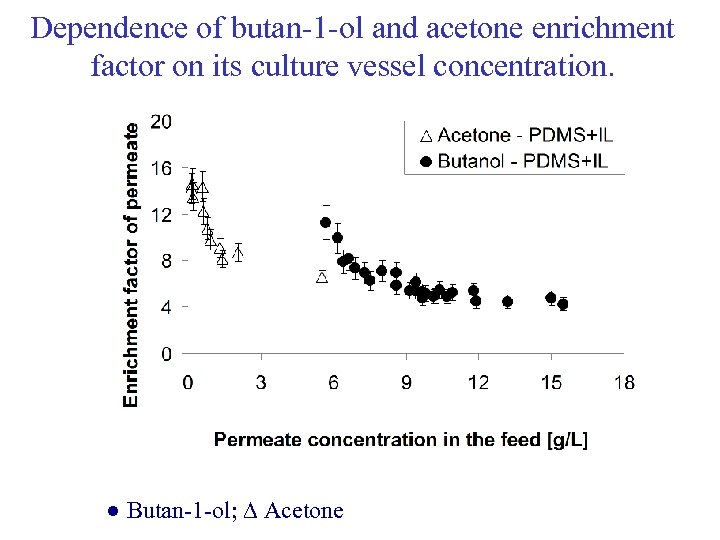 Dependence of butan-1 -ol and acetone enrichment factor on its culture vessel concentration. ● Butan-1 -ol; ∆ Acetone
Dependence of butan-1 -ol and acetone enrichment factor on its culture vessel concentration. ● Butan-1 -ol; ∆ Acetone
 Conclusions • To get more effective ABE removal from fermentor we used pervaporation with IL-PDMS nonporous membrane. • Using this membrane we were able to remove ABE from the culture supernatant more effectively than it was described by others (Qureshi et al. (1992), Soni et al. (1987), Liu et al. (2004)).
Conclusions • To get more effective ABE removal from fermentor we used pervaporation with IL-PDMS nonporous membrane. • Using this membrane we were able to remove ABE from the culture supernatant more effectively than it was described by others (Qureshi et al. (1992), Soni et al. (1987), Liu et al. (2004)).
 Conclusions • The supported ionic liquid membranes were weighted after all experiments and no weight changes were observed – stable SILM. • Higher diffusion coefficient is most probably responsible for higher permeation flux and enrichment factors of butan-1 -ol in IL-PDMS membrane. • If we would run pervaporation with continuous and complete removal of butan-1 -ol from the culture supernatant, it would lead to more stable fermentation process with higher production of BIObutanol.
Conclusions • The supported ionic liquid membranes were weighted after all experiments and no weight changes were observed – stable SILM. • Higher diffusion coefficient is most probably responsible for higher permeation flux and enrichment factors of butan-1 -ol in IL-PDMS membrane. • If we would run pervaporation with continuous and complete removal of butan-1 -ol from the culture supernatant, it would lead to more stable fermentation process with higher production of BIObutanol.
 Acknowledgement Thank you for your attention This research was supported partially by grant No. 104/08/0600 from Czech Science Foundation and Marie Curie Reintegration Fellowships within the 6 th European Community Framework Programme.
Acknowledgement Thank you for your attention This research was supported partially by grant No. 104/08/0600 from Czech Science Foundation and Marie Curie Reintegration Fellowships within the 6 th European Community Framework Programme.


