fbfbd234cbebc8515a578eccdff941b9.ppt
- Количество слайдов: 73
 Infections Following Renal Transplantation and An Update on CMV K. Vinay Ranga MD, MRCP (UK) Associate Professor Internal Medicine / Nephrology UTHSC
Infections Following Renal Transplantation and An Update on CMV K. Vinay Ranga MD, MRCP (UK) Associate Professor Internal Medicine / Nephrology UTHSC
 INFECTION IN IMMUNOSUPPRESSED PATIENTS: BASIC PRINCIPLES • Inflammatory response attenuated by immunosuppression. • may abolish typical signs/symptoms • decreased sensitivity of serological, radiological tests • Effects of established infection may be devastating. • Treatment may have more toxicities ! • Rifampin - decrease CNI • Erythromycin, azoles increase CNI • Synergistic nephrotoxicity - aminoglycosides, Amphotericin B, high dose Co-trimoxazole
INFECTION IN IMMUNOSUPPRESSED PATIENTS: BASIC PRINCIPLES • Inflammatory response attenuated by immunosuppression. • may abolish typical signs/symptoms • decreased sensitivity of serological, radiological tests • Effects of established infection may be devastating. • Treatment may have more toxicities ! • Rifampin - decrease CNI • Erythromycin, azoles increase CNI • Synergistic nephrotoxicity - aminoglycosides, Amphotericin B, high dose Co-trimoxazole
 Infections in Transplant Recipients • Net state of immunosuppression • Epidemiological exposure
Infections in Transplant Recipients • Net state of immunosuppression • Epidemiological exposure
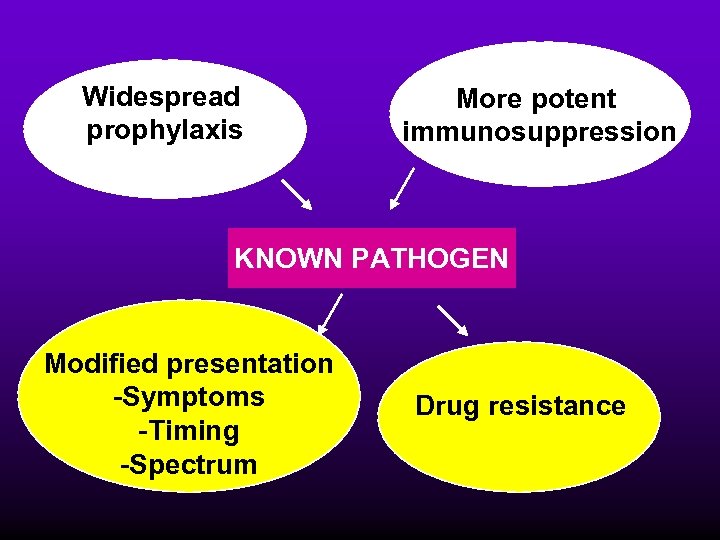 Widespread prophylaxis More potent immunosuppression KNOWN PATHOGEN Modified presentation -Symptoms -Timing -Spectrum Drug resistance
Widespread prophylaxis More potent immunosuppression KNOWN PATHOGEN Modified presentation -Symptoms -Timing -Spectrum Drug resistance
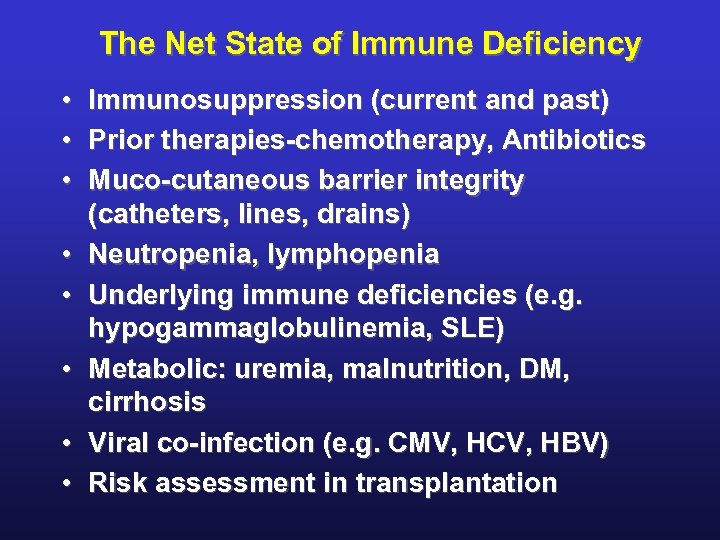 The Net State of Immune Deficiency • Immunosuppression (current and past) • Prior therapies-chemotherapy, Antibiotics • Muco-cutaneous barrier integrity (catheters, lines, drains) • Neutropenia, lymphopenia • Underlying immune deficiencies (e. g. hypogammaglobulinemia, SLE) • Metabolic: uremia, malnutrition, DM, cirrhosis • Viral co-infection (e. g. CMV, HCV, HBV) • Risk assessment in transplantation
The Net State of Immune Deficiency • Immunosuppression (current and past) • Prior therapies-chemotherapy, Antibiotics • Muco-cutaneous barrier integrity (catheters, lines, drains) • Neutropenia, lymphopenia • Underlying immune deficiencies (e. g. hypogammaglobulinemia, SLE) • Metabolic: uremia, malnutrition, DM, cirrhosis • Viral co-infection (e. g. CMV, HCV, HBV) • Risk assessment in transplantation
 Greater infectious risk • Induction therapy– lymphocyte depletion • High-dose steroids • Plasmapheresis • High rejection risk • Early graft rejection • Graft dysfunction • Active/latent D/R infection • Technical complications • Anastomotic leak • Bleeding • Wound infection/poor wound healing • Prolonged intubation/ ICU care • Surgical, vascular or urinary catheters
Greater infectious risk • Induction therapy– lymphocyte depletion • High-dose steroids • Plasmapheresis • High rejection risk • Early graft rejection • Graft dysfunction • Active/latent D/R infection • Technical complications • Anastomotic leak • Bleeding • Wound infection/poor wound healing • Prolonged intubation/ ICU care • Surgical, vascular or urinary catheters
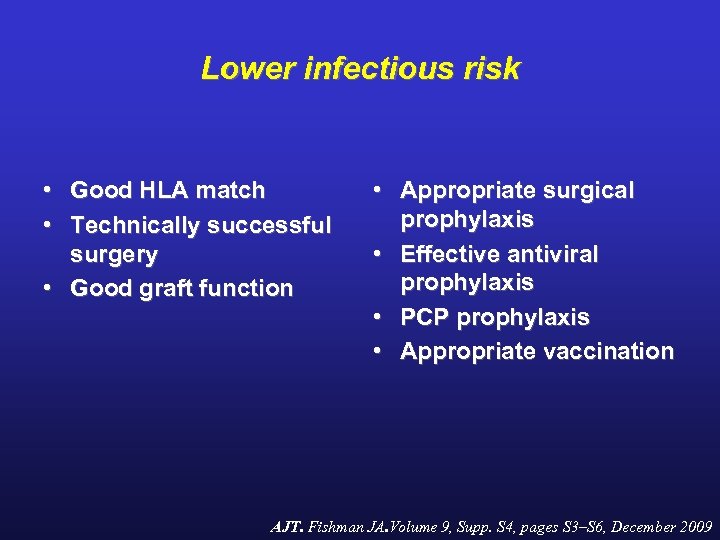 Lower infectious risk • Good HLA match • Technically successful surgery • Good graft function • Appropriate surgical prophylaxis • Effective antiviral prophylaxis • PCP prophylaxis • Appropriate vaccination AJT. Fishman JA. Volume 9, Supp. S 4, pages S 3–S 6, December 2009
Lower infectious risk • Good HLA match • Technically successful surgery • Good graft function • Appropriate surgical prophylaxis • Effective antiviral prophylaxis • PCP prophylaxis • Appropriate vaccination AJT. Fishman JA. Volume 9, Supp. S 4, pages S 3–S 6, December 2009
 Epidemiological Exposure • • Donor derived infections Recipient derived infections Community aquired infections Nosocomial infections
Epidemiological Exposure • • Donor derived infections Recipient derived infections Community aquired infections Nosocomial infections
 EPIDEMIOLOGICAL EXPOSURES Overlapping but distinct exposures Community – CAP, CA-MRSA, Endemic mycosis, respiratory viruses, enteric viruses Nosocomial – MRSA, Aspergillus, MDR gram negatives, VRE Donor – latent or active infection Recipient – latent or active infection
EPIDEMIOLOGICAL EXPOSURES Overlapping but distinct exposures Community – CAP, CA-MRSA, Endemic mycosis, respiratory viruses, enteric viruses Nosocomial – MRSA, Aspergillus, MDR gram negatives, VRE Donor – latent or active infection Recipient – latent or active infection
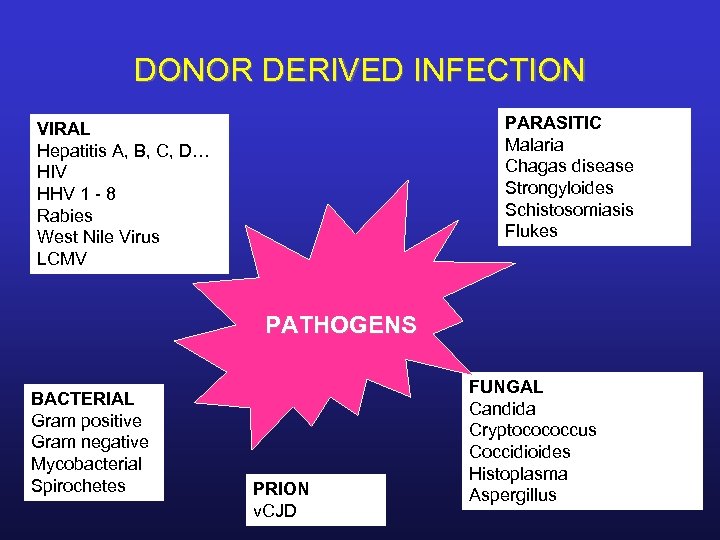 DONOR DERIVED INFECTION PARASITIC Malaria Chagas disease Strongyloides Schistosomiasis Flukes VIRAL Hepatitis A, B, C, D… HIV HHV 1 - 8 Rabies West Nile Virus LCMV PATHOGENS BACTERIAL Gram positive Gram negative Mycobacterial Spirochetes PRION v. CJD FUNGAL Candida Cryptocococcus Coccidioides Histoplasma Aspergillus
DONOR DERIVED INFECTION PARASITIC Malaria Chagas disease Strongyloides Schistosomiasis Flukes VIRAL Hepatitis A, B, C, D… HIV HHV 1 - 8 Rabies West Nile Virus LCMV PATHOGENS BACTERIAL Gram positive Gram negative Mycobacterial Spirochetes PRION v. CJD FUNGAL Candida Cryptocococcus Coccidioides Histoplasma Aspergillus
 HOW DO YOU RECOGNIZE UNUSUAL DONOR TRANSMITTED INFECTION? • These infections often (but not always) occur early post-transplant • They are often clinically very aggressive and may be difficult to diagnose – Rare or unexpected infection, unusual presentation, lack of seroconversion • Infections in multiple recipients of a common donor is often the best (or only) clue
HOW DO YOU RECOGNIZE UNUSUAL DONOR TRANSMITTED INFECTION? • These infections often (but not always) occur early post-transplant • They are often clinically very aggressive and may be difficult to diagnose – Rare or unexpected infection, unusual presentation, lack of seroconversion • Infections in multiple recipients of a common donor is often the best (or only) clue
 WNV and TRANSPLANTATION • Numerous case reports/series of TTWNV • High mortality (~ 30%) • Two instances of organ donor transmission of WNV (2003, 2005) • Most common is community acquired WNV • Immunocompetent : severe disease in 1: 140
WNV and TRANSPLANTATION • Numerous case reports/series of TTWNV • High mortality (~ 30%) • Two instances of organ donor transmission of WNV (2003, 2005) • Most common is community acquired WNV • Immunocompetent : severe disease in 1: 140
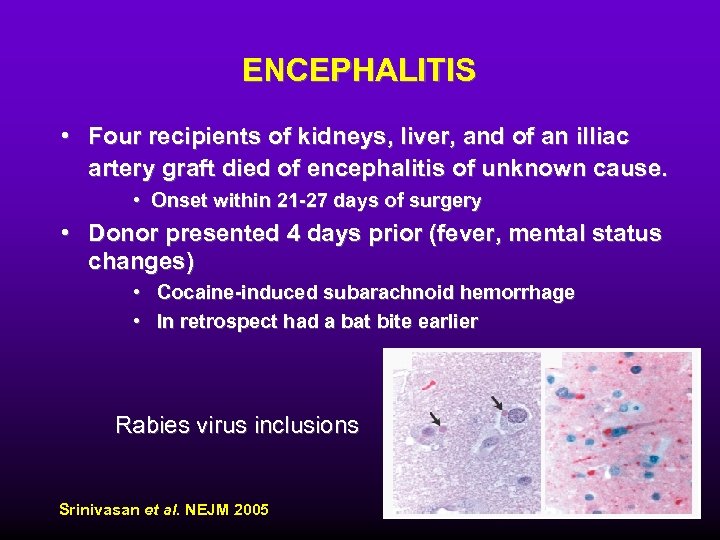 ENCEPHALITIS • Four recipients of kidneys, liver, and of an illiac artery graft died of encephalitis of unknown cause. • Onset within 21 -27 days of surgery • Donor presented 4 days prior (fever, mental status changes) • Cocaine-induced subarachnoid hemorrhage • In retrospect had a bat bite earlier Rabies virus inclusions Srinivasan et al. NEJM 2005
ENCEPHALITIS • Four recipients of kidneys, liver, and of an illiac artery graft died of encephalitis of unknown cause. • Onset within 21 -27 days of surgery • Donor presented 4 days prior (fever, mental status changes) • Cocaine-induced subarachnoid hemorrhage • In retrospect had a bat bite earlier Rabies virus inclusions Srinivasan et al. NEJM 2005
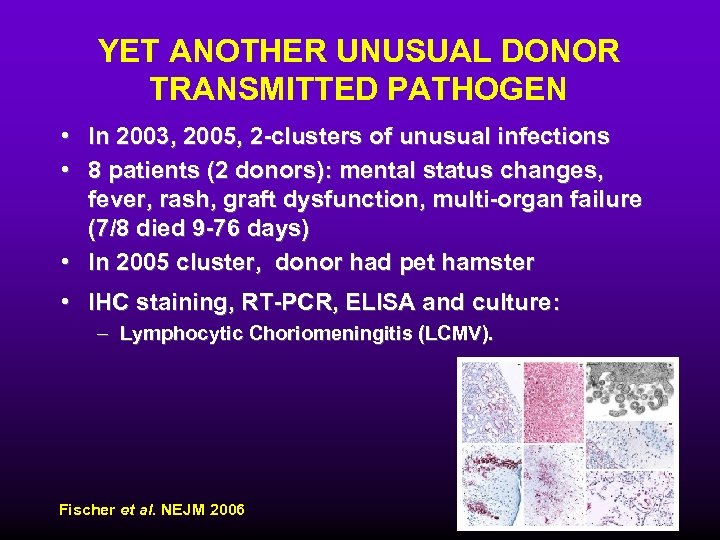 YET ANOTHER UNUSUAL DONOR TRANSMITTED PATHOGEN • In 2003, 2005, 2 -clusters of unusual infections • 8 patients (2 donors): mental status changes, fever, rash, graft dysfunction, multi-organ failure (7/8 died 9 -76 days) • In 2005 cluster, donor had pet hamster • IHC staining, RT-PCR, ELISA and culture: – Lymphocytic Choriomeningitis (LCMV). Fischer et al. NEJM 2006
YET ANOTHER UNUSUAL DONOR TRANSMITTED PATHOGEN • In 2003, 2005, 2 -clusters of unusual infections • 8 patients (2 donors): mental status changes, fever, rash, graft dysfunction, multi-organ failure (7/8 died 9 -76 days) • In 2005 cluster, donor had pet hamster • IHC staining, RT-PCR, ELISA and culture: – Lymphocytic Choriomeningitis (LCMV). Fischer et al. NEJM 2006
 Notable Organ Transplant. Transmitted Infections: Published Literature • • • HIV, 1985 Hepatitis C, 2000 Chagas Disease (T. cruzi), 2001 West Nile Virus (WNV), GA 2002 Lymphocytic Choriomeningitis Virus (LCMV), WI 2003 and MA/RI 2005 Rabies, 2004 WNV, NY/PA 2005 Chagas, 2006 HIV/HCV 2007 Arenavirus 2007
Notable Organ Transplant. Transmitted Infections: Published Literature • • • HIV, 1985 Hepatitis C, 2000 Chagas Disease (T. cruzi), 2001 West Nile Virus (WNV), GA 2002 Lymphocytic Choriomeningitis Virus (LCMV), WI 2003 and MA/RI 2005 Rabies, 2004 WNV, NY/PA 2005 Chagas, 2006 HIV/HCV 2007 Arenavirus 2007
 Aspects of Solid-Organ Transplantation That Should Be Disclosed during the Consent Process • Known risks of transplantation include the rare risks of transmission of HIV, hepatitis B and C virus, and other infectious agents. • Because of the scarcity of organs, organs from expanded-criteria donors, donors after circulatory determination of death, and donors with a slight risk of certain infections are sometimes used. • Patients may decline nonstandard-criteria organs, but doing so may delay their receipt of an organ and possibly even result in death while they are on the waiting list.
Aspects of Solid-Organ Transplantation That Should Be Disclosed during the Consent Process • Known risks of transplantation include the rare risks of transmission of HIV, hepatitis B and C virus, and other infectious agents. • Because of the scarcity of organs, organs from expanded-criteria donors, donors after circulatory determination of death, and donors with a slight risk of certain infections are sometimes used. • Patients may decline nonstandard-criteria organs, but doing so may delay their receipt of an organ and possibly even result in death while they are on the waiting list.
 Aspects of Solid-Organ Transplantation That Should Be Disclosed during the Consent Process • Physicians’ understanding of donor characteristics that confer risk is incomplete and continually evolving. • New risks may arise in the future (e. g. , as new transmittable infections are identified), and all efforts to minimize these risks will be made.
Aspects of Solid-Organ Transplantation That Should Be Disclosed during the Consent Process • Physicians’ understanding of donor characteristics that confer risk is incomplete and continually evolving. • New risks may arise in the future (e. g. , as new transmittable infections are identified), and all efforts to minimize these risks will be made.
 CDC HIGH RISK BEHAVIOR • Men who have had sex with another man in the preceding 5 years. • Persons who report nonmedical intravenous, intramuscular, or subcutaneous injection of drugs in the preceding 5 years. • Persons with hemophilia or related clotting disorders who have received human-derived clotting factor concentrates • Men and women who have engaged in sex in exchange for money or drugs in the preceding 5 years. • Persons who have had sex in the preceding 12 months with any person described above or with a person known or suspected to have HIV infection.
CDC HIGH RISK BEHAVIOR • Men who have had sex with another man in the preceding 5 years. • Persons who report nonmedical intravenous, intramuscular, or subcutaneous injection of drugs in the preceding 5 years. • Persons with hemophilia or related clotting disorders who have received human-derived clotting factor concentrates • Men and women who have engaged in sex in exchange for money or drugs in the preceding 5 years. • Persons who have had sex in the preceding 12 months with any person described above or with a person known or suspected to have HIV infection.
 CDC HIGH RISK BEHAVIOR • Persons who have been exposed in the preceding 12 months to known or suspected HIV-infected blood through percutaneous inoculation or through contact with an open wound, nonintact skin, or mucous membrane. • Inmates of correctional systems. (This exclusion is to address issues such as difficulties with informed consent and increased prevalence of HIV in this population. )
CDC HIGH RISK BEHAVIOR • Persons who have been exposed in the preceding 12 months to known or suspected HIV-infected blood through percutaneous inoculation or through contact with an open wound, nonintact skin, or mucous membrane. • Inmates of correctional systems. (This exclusion is to address issues such as difficulties with informed consent and increased prevalence of HIV in this population. )
 Renal Transplants from CDC High-Risk Donors: What’s the Risk and for Whom Is It Justified? • METHODS: All high-risk donors underwent nucleic acid testing (NAT) for HIV, HCV, and HBV. • Patients were counseled during evaluation and consent obtained rom all recipients highlighting the CDC high-risk status of the organ at the time of transplant. ATC 2010, ABSTRACT # 308
Renal Transplants from CDC High-Risk Donors: What’s the Risk and for Whom Is It Justified? • METHODS: All high-risk donors underwent nucleic acid testing (NAT) for HIV, HCV, and HBV. • Patients were counseled during evaluation and consent obtained rom all recipients highlighting the CDC high-risk status of the organ at the time of transplant. ATC 2010, ABSTRACT # 308
 § Adult recipients were transplanted over the year beginning September, 2008. Given the increased risk, offers were directed towards older and diabetic patients, those with elevated PRA>50, and those already infected with HCV and/or HIV. § All recipients had NAT at transplant and serially at 1, 3, and 6 months
§ Adult recipients were transplanted over the year beginning September, 2008. Given the increased risk, offers were directed towards older and diabetic patients, those with elevated PRA>50, and those already infected with HCV and/or HIV. § All recipients had NAT at transplant and serially at 1, 3, and 6 months
 • CONCLUSIONS: Although NAT reduces the window period between donor viral • infection and detectability, the risk of transmission through transplant with these organs is ill-defined. • Here, with prospective recipient NAT, we show safe use of high-risk kidneys with excellent short-term graft function. • Risk of infection, however low, will clearly not be zero. With continued use of these organs and careful followup, we will be able to further gauge donor risk and match it to recipient need to optimize patient benefit.
• CONCLUSIONS: Although NAT reduces the window period between donor viral • infection and detectability, the risk of transmission through transplant with these organs is ill-defined. • Here, with prospective recipient NAT, we show safe use of high-risk kidneys with excellent short-term graft function. • Risk of infection, however low, will clearly not be zero. With continued use of these organs and careful followup, we will be able to further gauge donor risk and match it to recipient need to optimize patient benefit.
 Donor Screening • Epidemiologic history • Serologic testing for VDRL, HIV, CMV, EBV, HSV, VZV, HBV • (HBs. Ag, anti-HBs. Ag), and HCV • Microbiologic testing of blood and urine • Chest radiography • Known infections (appropriate therapy? ) • Possible infections (e. g. , encephalitis, sepsis) • Special serologic testing, nucleic acid assays, or antigen detection based on epidemiologic factors and recent exposures (e. g. , toxoplasma, West Nile virus, HIV, HCV
Donor Screening • Epidemiologic history • Serologic testing for VDRL, HIV, CMV, EBV, HSV, VZV, HBV • (HBs. Ag, anti-HBs. Ag), and HCV • Microbiologic testing of blood and urine • Chest radiography • Known infections (appropriate therapy? ) • Possible infections (e. g. , encephalitis, sepsis) • Special serologic testing, nucleic acid assays, or antigen detection based on epidemiologic factors and recent exposures (e. g. , toxoplasma, West Nile virus, HIV, HCV
 Additional Donor Testing? • NAT testing – – HIV - reduction in window period from 21 to 7 days HCV - reduction in window period - 70 to 7 days HBV West Nile Virus • Other – TB, Fungal, Chagas/T. cruzi, Strongyloides
Additional Donor Testing? • NAT testing – – HIV - reduction in window period from 21 to 7 days HCV - reduction in window period - 70 to 7 days HBV West Nile Virus • Other – TB, Fungal, Chagas/T. cruzi, Strongyloides
 Recipient Screening • • Epidemiologic history Vaccination history, TB skin test Serologic testing for VDRL, HIV, CMV, EBV, HSV, VZV, HBV (Hbs. Ag, anti-Hbs. Ag), and HCV Microbiologic testing of blood and urine Chest radiography
Recipient Screening • • Epidemiologic history Vaccination history, TB skin test Serologic testing for VDRL, HIV, CMV, EBV, HSV, VZV, HBV (Hbs. Ag, anti-Hbs. Ag), and HCV Microbiologic testing of blood and urine Chest radiography
 Recipient Screening • • Epidemiologic history Vaccination history, TB skin test Serologic testing for VDRL, HIV, CMV, EBV, HSV, VZV, HBV (Hbs. Ag, anti-Hbs. Ag), and HCV Microbiologic testing of blood and urine Chest radiography
Recipient Screening • • Epidemiologic history Vaccination history, TB skin test Serologic testing for VDRL, HIV, CMV, EBV, HSV, VZV, HBV (Hbs. Ag, anti-Hbs. Ag), and HCV Microbiologic testing of blood and urine Chest radiography
 Recipient Screening • Known infections (e. g. , strongyloides, histoplasma, coccidioides, HBV or HCV viral load) Known infections • Past colonization: prophylaxis? • Active infection: appropriate therapy? • Possible infections (e. g. , encephalitis, sepsis) • Special serologic testing, nucleic acid assays, or antigen detection based on epidemiologic factors and recent exposures
Recipient Screening • Known infections (e. g. , strongyloides, histoplasma, coccidioides, HBV or HCV viral load) Known infections • Past colonization: prophylaxis? • Active infection: appropriate therapy? • Possible infections (e. g. , encephalitis, sepsis) • Special serologic testing, nucleic acid assays, or antigen detection based on epidemiologic factors and recent exposures
 Standard Assays • Serologic tests for seroconversion • Microbiologic cultures and susceptibility testing • Quantitative viral-load assay and antigen tests • Histopathological tests and immunostaining
Standard Assays • Serologic tests for seroconversion • Microbiologic cultures and susceptibility testing • Quantitative viral-load assay and antigen tests • Histopathological tests and immunostaining
 Advanced Assays • Multiplex microbiologic assays • Molecular antimicrobial-susceptibility testing • Nonspecific immunoassays for degree of immunosuppression • Intracellular ATP • Biomarkers of rejection (cytokines) • Proteomics
Advanced Assays • Multiplex microbiologic assays • Molecular antimicrobial-susceptibility testing • Nonspecific immunoassays for degree of immunosuppression • Intracellular ATP • Biomarkers of rejection (cytokines) • Proteomics
 Advanced Assays • • Assays of pathogen-specific immunity Cytotoxic lymphocytes Mixed lymphocyte cultures HLA-linked tetramers Intracellular cytokine staining Enzyme-linked immunospot assay Interferon-release assays
Advanced Assays • • Assays of pathogen-specific immunity Cytotoxic lymphocytes Mixed lymphocyte cultures HLA-linked tetramers Intracellular cytokine staining Enzyme-linked immunospot assay Interferon-release assays
 Advanced Assays • Genomics (patterns of gene expression) in: • Immunosuppression • Infection • Rejection • Drug metabolism
Advanced Assays • Genomics (patterns of gene expression) in: • Immunosuppression • Infection • Rejection • Drug metabolism
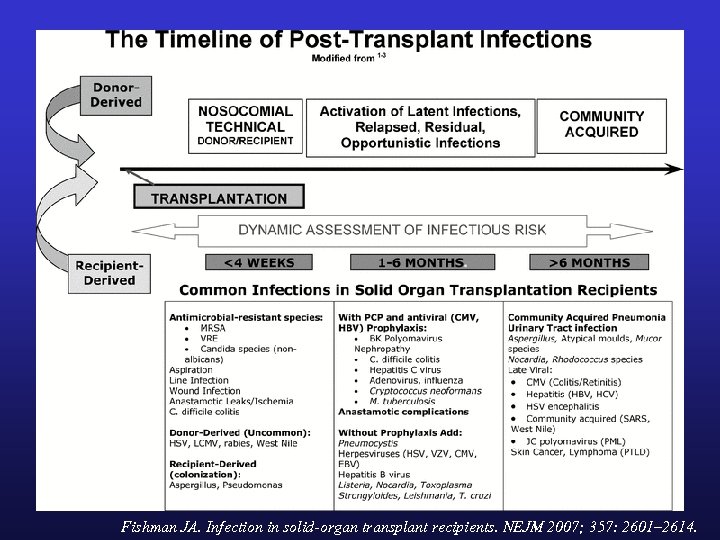 Fishman JA. Infection in solid-organ transplant recipients. NEJM 2007; 357: 2601– 2614.
Fishman JA. Infection in solid-organ transplant recipients. NEJM 2007; 357: 2601– 2614.
 Tx ID TIMELINE: 0 -1 MONTH • Post-op Infections – Technical / anastamotic related infection – Nosocomial pneumonia, wound infection, UTI – MRSA, VRE, Candida, C. difficile • Donor Derived Infection – These are rare but diagnosis can be missed • Recipient derived infection – Ongoing pneumonia – Colonization to infection • Most OI absent: exceptions include certain fungal infections, HSV, occasional others
Tx ID TIMELINE: 0 -1 MONTH • Post-op Infections – Technical / anastamotic related infection – Nosocomial pneumonia, wound infection, UTI – MRSA, VRE, Candida, C. difficile • Donor Derived Infection – These are rare but diagnosis can be missed • Recipient derived infection – Ongoing pneumonia – Colonization to infection • Most OI absent: exceptions include certain fungal infections, HSV, occasional others
 Tx ID TIMELINE 1 -6 MONTH Account for CMV, PCP prophylaxis • BK viruria / viremia, BKVAN • CMV • EBV viremia D+/R • HCV recurrence (OLTx) • C. difficile, fungal, mycobacterial, respiratory virus • VZV post-prophylaxis
Tx ID TIMELINE 1 -6 MONTH Account for CMV, PCP prophylaxis • BK viruria / viremia, BKVAN • CMV • EBV viremia D+/R • HCV recurrence (OLTx) • C. difficile, fungal, mycobacterial, respiratory virus • VZV post-prophylaxis
 Tx ID TIMELINE >6 MONTH • Depends on outcome: good vs. bad graft function. Tapering immunosuppression vs. ongoing high level – problems with rejection • Some patients at ongoing risk of OI despite minimal exposures • Others only get OI if significant exposure
Tx ID TIMELINE >6 MONTH • Depends on outcome: good vs. bad graft function. Tapering immunosuppression vs. ongoing high level – problems with rejection • Some patients at ongoing risk of OI despite minimal exposures • Others only get OI if significant exposure
 Tx ID TIMELINE >6 MONTH • Late viral infections – CMV (colitis, recurrence), BKVAN, PTLD, HCV • Community acquired infection – Respiratory virus, CA-MRSA, atypical mould infection, • Other OI based on exposures
Tx ID TIMELINE >6 MONTH • Late viral infections – CMV (colitis, recurrence), BKVAN, PTLD, HCV • Community acquired infection – Respiratory virus, CA-MRSA, atypical mould infection, • Other OI based on exposures
 FUNGAL INFECTIONS • With improved strategies against CMV, fungal infection have become the most important cause of infectious mortality. • Candida and Aspergillus account for ~ 80% of invasive fungal infections. • Other 20% cryptococcus, other molds, and endemic mycosis.
FUNGAL INFECTIONS • With improved strategies against CMV, fungal infection have become the most important cause of infectious mortality. • Candida and Aspergillus account for ~ 80% of invasive fungal infections. • Other 20% cryptococcus, other molds, and endemic mycosis.
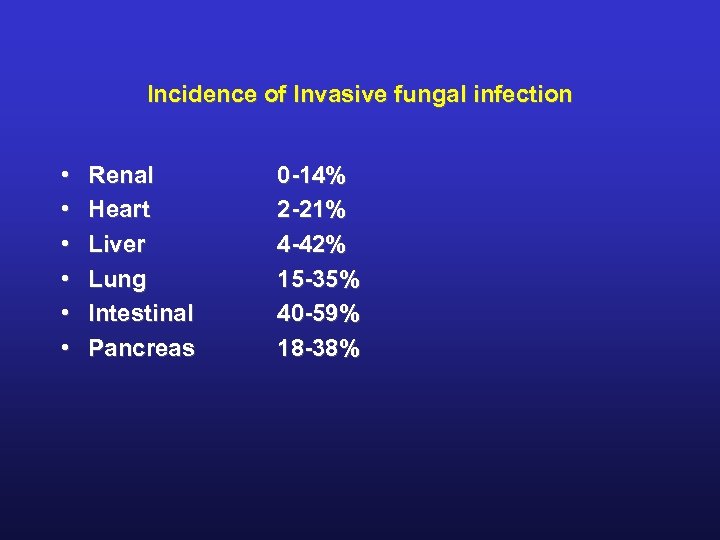 Incidence of Invasive fungal infection • • • Renal Heart Liver Lung Intestinal Pancreas 0 -14% 2 -21% 4 -42% 15 -35% 40 -59% 18 -38%
Incidence of Invasive fungal infection • • • Renal Heart Liver Lung Intestinal Pancreas 0 -14% 2 -21% 4 -42% 15 -35% 40 -59% 18 -38%
 PREVENTION OF INFECTION • • • PCP, UTI, others CMV disease Toxoplasmosis Other herpes viruses Candida Aspergillus Strongyloides Tuberculosis Endemic mycosis — Bactrim — Multiple strategies — Bactrim, Pyrimethamine — Acyclovir — Azoles, echinocandins — Voriconazole, candins, others — Albendazole — INH, ? others — Voriconazole, others
PREVENTION OF INFECTION • • • PCP, UTI, others CMV disease Toxoplasmosis Other herpes viruses Candida Aspergillus Strongyloides Tuberculosis Endemic mycosis — Bactrim — Multiple strategies — Bactrim, Pyrimethamine — Acyclovir — Azoles, echinocandins — Voriconazole, candins, others — Albendazole — INH, ? others — Voriconazole, others
 VACCINATIONS IN TRANSPLANT RECIPIENTS
VACCINATIONS IN TRANSPLANT RECIPIENTS
 PRETRANSPLANT VACCINATION BOOSTERS TO BE GIVEN TO ALL TRANSPLANT RECIPIENTS UNLESS RECENT ADMINISTRATION CAN BE DOCUMENTED 1. Td (Tetanus toxoid, diphtheria) 2. Pneumococcal vaccine 3. Hepatitis B 4. Influenza
PRETRANSPLANT VACCINATION BOOSTERS TO BE GIVEN TO ALL TRANSPLANT RECIPIENTS UNLESS RECENT ADMINISTRATION CAN BE DOCUMENTED 1. Td (Tetanus toxoid, diphtheria) 2. Pneumococcal vaccine 3. Hepatitis B 4. Influenza
 PRETRANSPLANT VACCINATIONS TO BE GIVEN IF SERONEGATIVE OR PAST INFECTION BY HISTORY CANNOT BE DOCUMENTED 1. Measles-mumps-rubella vaccine 2. Polio 3. Varicella 4. Haemophilus influenza type B
PRETRANSPLANT VACCINATIONS TO BE GIVEN IF SERONEGATIVE OR PAST INFECTION BY HISTORY CANNOT BE DOCUMENTED 1. Measles-mumps-rubella vaccine 2. Polio 3. Varicella 4. Haemophilus influenza type B
 INACTIVATED VACCINES THAT ARE CONSIDERED SAFE AND MAY BE GIVEN AS NEEDED POST-TRANSPLANT FOR ANTICIPATED EXPOSURE 1. Anthrax 2. Cholera 3. Rabies vaccine absorbed 4. Human diploid cell rabies vaccine 5. Inactivated typhoid vaccine, capsular polysaccharide parenteral vaccine, or heat phenol-treated parenteral vaccine 6. Japanese encephalitis virus vaccine 7. Meningococcal vaccine 8. Plague vaccine
INACTIVATED VACCINES THAT ARE CONSIDERED SAFE AND MAY BE GIVEN AS NEEDED POST-TRANSPLANT FOR ANTICIPATED EXPOSURE 1. Anthrax 2. Cholera 3. Rabies vaccine absorbed 4. Human diploid cell rabies vaccine 5. Inactivated typhoid vaccine, capsular polysaccharide parenteral vaccine, or heat phenol-treated parenteral vaccine 6. Japanese encephalitis virus vaccine 7. Meningococcal vaccine 8. Plague vaccine
 VACCINES THAT MAY NOT BE GIVEN (LIVE ATTENUATED VACCINES) 1. Bacille Calmette-Guérin (BCG) 2. Measles 3. Mumps 4. Rubella 5. Oral polio 6. Oral typhoid 7. Yellow fever
VACCINES THAT MAY NOT BE GIVEN (LIVE ATTENUATED VACCINES) 1. Bacille Calmette-Guérin (BCG) 2. Measles 3. Mumps 4. Rubella 5. Oral polio 6. Oral typhoid 7. Yellow fever
 WHATS NEW? • • Changing immunosuppression Widespread prophylaxis Emerging infections Evolving infections Donor Derived infection Molecular diagnostics Host-pathogen interactions
WHATS NEW? • • Changing immunosuppression Widespread prophylaxis Emerging infections Evolving infections Donor Derived infection Molecular diagnostics Host-pathogen interactions
 Clinical reactivation Subclinical reactivation
Clinical reactivation Subclinical reactivation
 Cytomegalovirus (CMV) • Largest known virus to infect human beings • greek cyto-, "cell", and -megalo-, "large”. • In humans it is commonly known as HCMV or Human Herpesvirus 5 (HHV-5). • belongs to the Betaherpesvirinae subfamily • Other herpesviruses include Alphaherpesvirinae (HSV 1/2 and VZV) Gammaherpesvirinae (including EBV).
Cytomegalovirus (CMV) • Largest known virus to infect human beings • greek cyto-, "cell", and -megalo-, "large”. • In humans it is commonly known as HCMV or Human Herpesvirus 5 (HHV-5). • belongs to the Betaherpesvirinae subfamily • Other herpesviruses include Alphaherpesvirinae (HSV 1/2 and VZV) Gammaherpesvirinae (including EBV).
 • All herpesviruses share a characteristic ability to remain latent within the body over long periods. Nature Medicine, 6: 2000
• All herpesviruses share a characteristic ability to remain latent within the body over long periods. Nature Medicine, 6: 2000
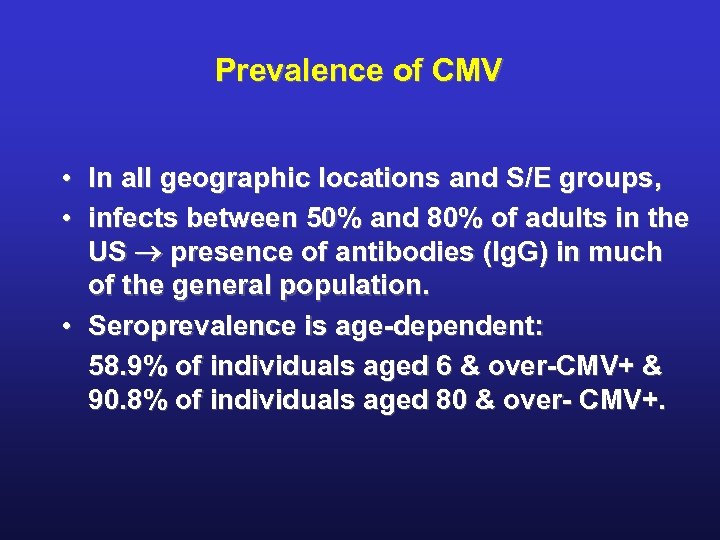 Prevalence of CMV • In all geographic locations and S/E groups, • infects between 50% and 80% of adults in the US presence of antibodies (Ig. G) in much of the general population. • Seroprevalence is age-dependent: 58. 9% of individuals aged 6 & over-CMV+ & 90. 8% of individuals aged 80 & over- CMV+.
Prevalence of CMV • In all geographic locations and S/E groups, • infects between 50% and 80% of adults in the US presence of antibodies (Ig. G) in much of the general population. • Seroprevalence is age-dependent: 58. 9% of individuals aged 6 & over-CMV+ & 90. 8% of individuals aged 80 & over- CMV+.
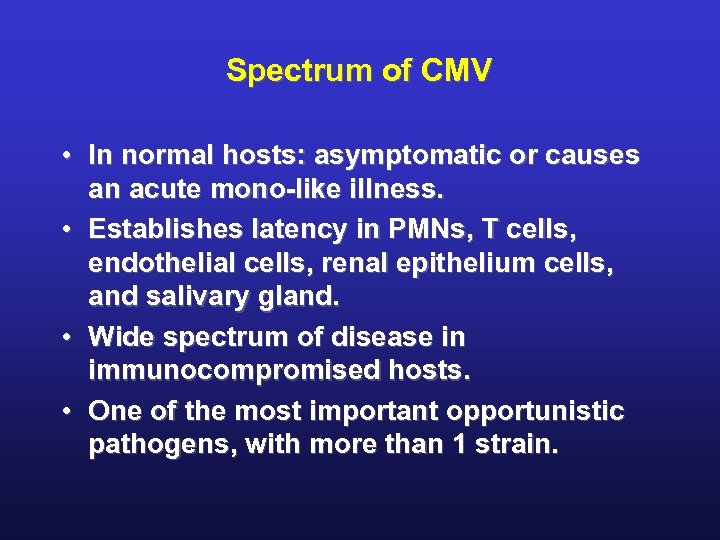 Spectrum of CMV • In normal hosts: asymptomatic or causes an acute mono-like illness. • Establishes latency in PMNs, T cells, endothelial cells, renal epithelium cells, and salivary gland. • Wide spectrum of disease in immunocompromised hosts. • One of the most important opportunistic pathogens, with more than 1 strain.
Spectrum of CMV • In normal hosts: asymptomatic or causes an acute mono-like illness. • Establishes latency in PMNs, T cells, endothelial cells, renal epithelium cells, and salivary gland. • Wide spectrum of disease in immunocompromised hosts. • One of the most important opportunistic pathogens, with more than 1 strain.
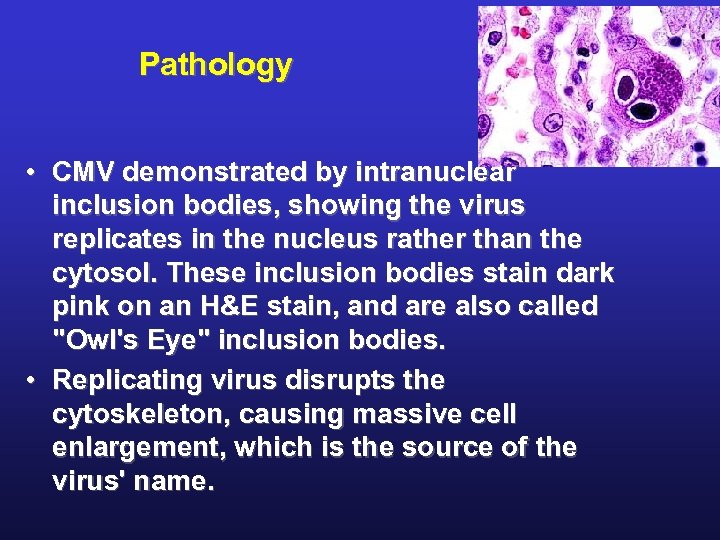 Pathology • CMV demonstrated by intranuclear inclusion bodies, showing the virus replicates in the nucleus rather than the cytosol. These inclusion bodies stain dark pink on an H&E stain, and are also called "Owl's Eye" inclusion bodies. • Replicating virus disrupts the cytoskeleton, causing massive cell enlargement, which is the source of the virus' name.
Pathology • CMV demonstrated by intranuclear inclusion bodies, showing the virus replicates in the nucleus rather than the cytosol. These inclusion bodies stain dark pink on an H&E stain, and are also called "Owl's Eye" inclusion bodies. • Replicating virus disrupts the cytoskeleton, causing massive cell enlargement, which is the source of the virus' name.
 Definitions • CMV infection – Evidence of CMV replication regardless of symptoms • CMV disease – Evidence of CMV infection with attributable symptoms – Viral syndrome with fever and/or malaise, leukopenia, thrombocytopenia, or tissue invasive disease
Definitions • CMV infection – Evidence of CMV replication regardless of symptoms • CMV disease – Evidence of CMV infection with attributable symptoms – Viral syndrome with fever and/or malaise, leukopenia, thrombocytopenia, or tissue invasive disease
 Dynamics of Transplantation Immunosuppression Rejection Infection
Dynamics of Transplantation Immunosuppression Rejection Infection
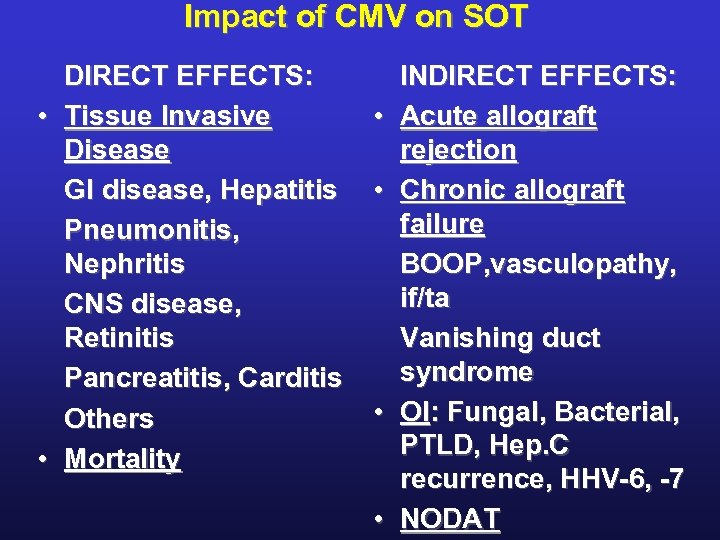 Impact of CMV on SOT DIRECT EFFECTS: • Tissue Invasive Disease GI disease, Hepatitis Pneumonitis, Nephritis CNS disease, Retinitis Pancreatitis, Carditis Others • Mortality • • INDIRECT EFFECTS: Acute allograft rejection Chronic allograft failure BOOP, vasculopathy, if/ta Vanishing duct syndrome OI: Fungal, Bacterial, PTLD, Hep. C recurrence, HHV-6, -7 NODAT
Impact of CMV on SOT DIRECT EFFECTS: • Tissue Invasive Disease GI disease, Hepatitis Pneumonitis, Nephritis CNS disease, Retinitis Pancreatitis, Carditis Others • Mortality • • INDIRECT EFFECTS: Acute allograft rejection Chronic allograft failure BOOP, vasculopathy, if/ta Vanishing duct syndrome OI: Fungal, Bacterial, PTLD, Hep. C recurrence, HHV-6, -7 NODAT
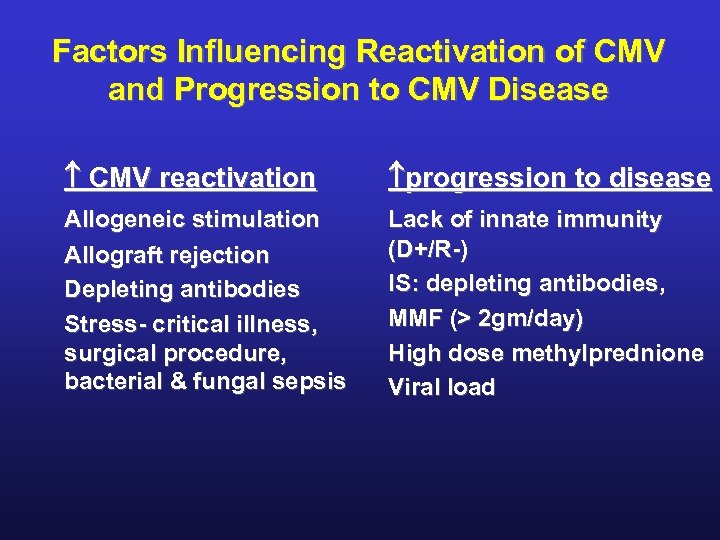 Factors Influencing Reactivation of CMV and Progression to CMV Disease CMV reactivation progression to disease Allogeneic stimulation Allograft rejection Depleting antibodies Stress- critical illness, surgical procedure, bacterial & fungal sepsis Lack of innate immunity (D+/R-) IS: depleting antibodies, MMF (> 2 gm/day) High dose methylprednione Viral load
Factors Influencing Reactivation of CMV and Progression to CMV Disease CMV reactivation progression to disease Allogeneic stimulation Allograft rejection Depleting antibodies Stress- critical illness, surgical procedure, bacterial & fungal sepsis Lack of innate immunity (D+/R-) IS: depleting antibodies, MMF (> 2 gm/day) High dose methylprednione Viral load
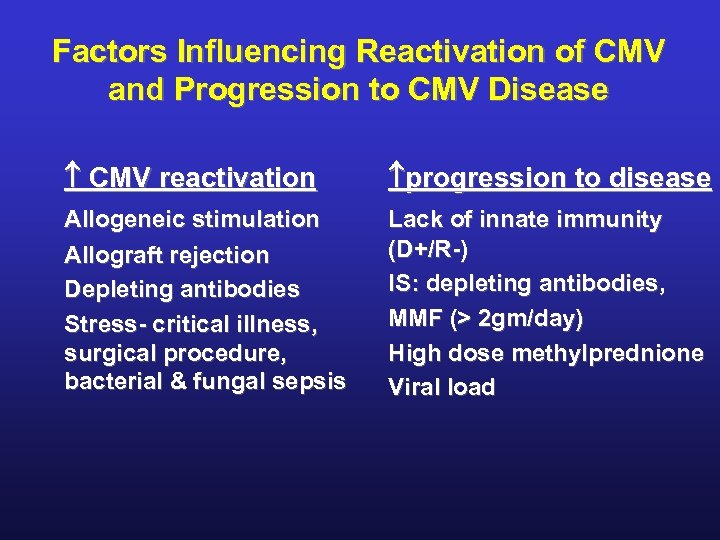 Factors Influencing Reactivation of CMV and Progression to CMV Disease CMV reactivation progression to disease Allogeneic stimulation Allograft rejection Depleting antibodies Stress- critical illness, surgical procedure, bacterial & fungal sepsis Lack of innate immunity (D+/R-) IS: depleting antibodies, MMF (> 2 gm/day) High dose methylprednione Viral load
Factors Influencing Reactivation of CMV and Progression to CMV Disease CMV reactivation progression to disease Allogeneic stimulation Allograft rejection Depleting antibodies Stress- critical illness, surgical procedure, bacterial & fungal sepsis Lack of innate immunity (D+/R-) IS: depleting antibodies, MMF (> 2 gm/day) High dose methylprednione Viral load
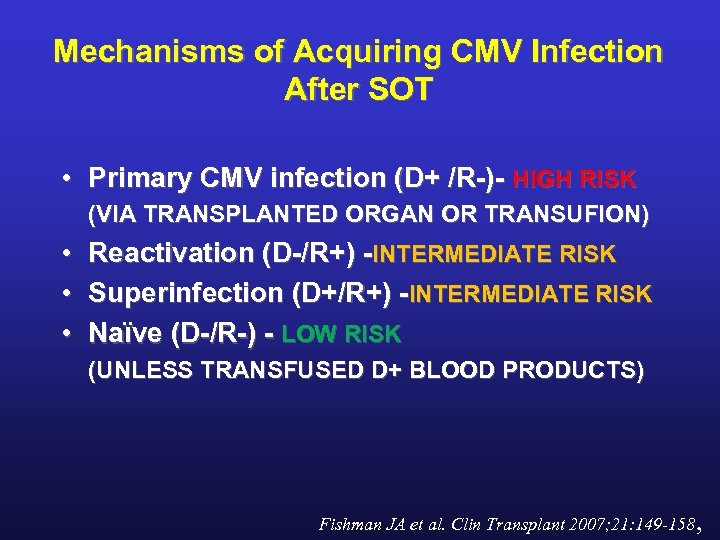 Mechanisms of Acquiring CMV Infection After SOT • Primary CMV infection (D+ /R-)- HIGH RISK (VIA TRANSPLANTED ORGAN OR TRANSUFION) • Reactivation (D-/R+) -INTERMEDIATE RISK • Superinfection (D+/R+) -INTERMEDIATE RISK • Naïve (D-/R-) - LOW RISK (UNLESS TRANSFUSED D+ BLOOD PRODUCTS) Fishman JA et al. Clin Transplant 2007; 21: 149 -158,
Mechanisms of Acquiring CMV Infection After SOT • Primary CMV infection (D+ /R-)- HIGH RISK (VIA TRANSPLANTED ORGAN OR TRANSUFION) • Reactivation (D-/R+) -INTERMEDIATE RISK • Superinfection (D+/R+) -INTERMEDIATE RISK • Naïve (D-/R-) - LOW RISK (UNLESS TRANSFUSED D+ BLOOD PRODUCTS) Fishman JA et al. Clin Transplant 2007; 21: 149 -158,
 Therapeutic Strategies for CMV • UNIVERSAL PROPHYLAXIS: For the whole cohort, typically D+/R- (high risk) • PRE-EMPTIVE THERAPY: For intermediate risk group (R+) vs Universal 2 weekly CMV-Pcr for 6 months, and initiate treatment doses of Valgancyclovir if positive • STANDARD THERAPY: for patients with disease- treat with Vangancyclovir 900 mg bid until symptoms resolved, virologic clearance, or 2 week minimum. Then check Pcr & short course of prophylaxis (1 -3 mo)
Therapeutic Strategies for CMV • UNIVERSAL PROPHYLAXIS: For the whole cohort, typically D+/R- (high risk) • PRE-EMPTIVE THERAPY: For intermediate risk group (R+) vs Universal 2 weekly CMV-Pcr for 6 months, and initiate treatment doses of Valgancyclovir if positive • STANDARD THERAPY: for patients with disease- treat with Vangancyclovir 900 mg bid until symptoms resolved, virologic clearance, or 2 week minimum. Then check Pcr & short course of prophylaxis (1 -3 mo)
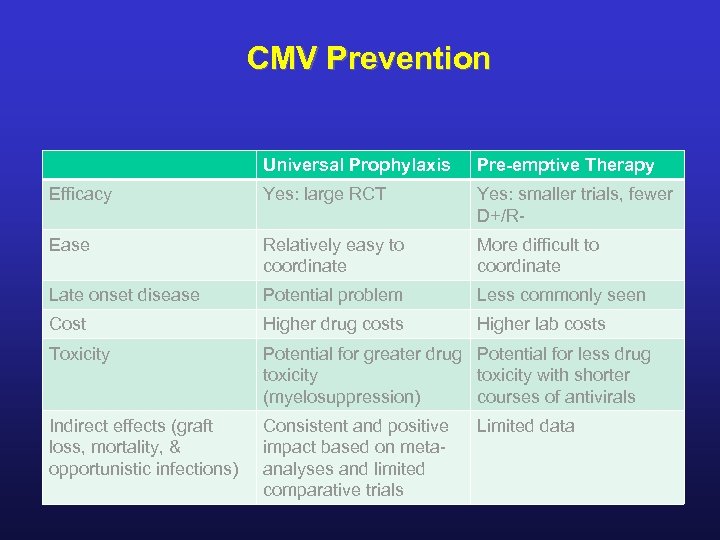 CMV Prevention Universal Prophylaxis Pre-emptive Therapy Efficacy Yes: large RCT Yes: smaller trials, fewer D+/R- Ease Relatively easy to coordinate More difficult to coordinate Late onset disease Potential problem Less commonly seen Cost Higher drug costs Higher lab costs Toxicity Potential for greater drug Potential for less drug toxicity with shorter (myelosuppression) courses of antivirals Indirect effects (graft loss, mortality, & opportunistic infections) Consistent and positive impact based on metaanalyses and limited comparative trials Limited data
CMV Prevention Universal Prophylaxis Pre-emptive Therapy Efficacy Yes: large RCT Yes: smaller trials, fewer D+/R- Ease Relatively easy to coordinate More difficult to coordinate Late onset disease Potential problem Less commonly seen Cost Higher drug costs Higher lab costs Toxicity Potential for greater drug Potential for less drug toxicity with shorter (myelosuppression) courses of antivirals Indirect effects (graft loss, mortality, & opportunistic infections) Consistent and positive impact based on metaanalyses and limited comparative trials Limited data
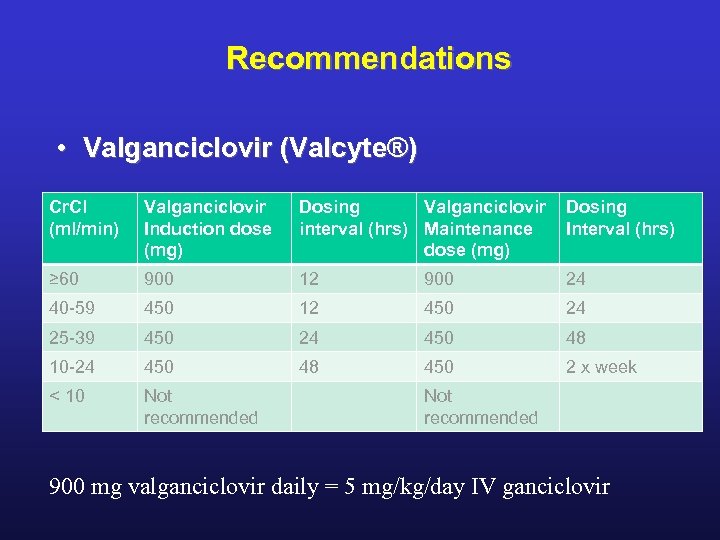 Recommendations • Valganciclovir (Valcyte®) Cr. Cl (ml/min) Valganciclovir Induction dose (mg) Dosing Valganciclovir Dosing interval (hrs) Maintenance Interval (hrs) dose (mg) ≥ 60 900 12 900 24 40 -59 450 12 450 24 25 -39 450 24 450 48 10 -24 450 48 450 2 x week < 10 Not recommended 900 mg valganciclovir daily = 5 mg/kg/day IV ganciclovir
Recommendations • Valganciclovir (Valcyte®) Cr. Cl (ml/min) Valganciclovir Induction dose (mg) Dosing Valganciclovir Dosing interval (hrs) Maintenance Interval (hrs) dose (mg) ≥ 60 900 12 900 24 40 -59 450 12 450 24 25 -39 450 24 450 48 10 -24 450 48 450 2 x week < 10 Not recommended 900 mg valganciclovir daily = 5 mg/kg/day IV ganciclovir
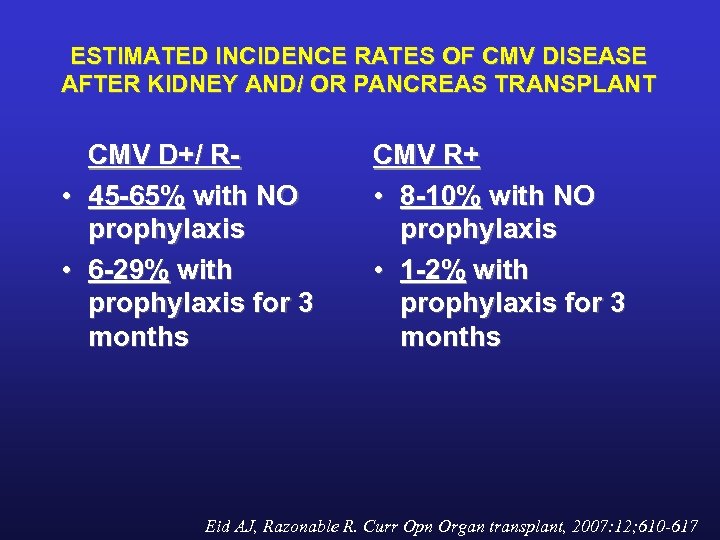 ESTIMATED INCIDENCE RATES OF CMV DISEASE AFTER KIDNEY AND/ OR PANCREAS TRANSPLANT CMV D+/ R • 45 -65% with NO prophylaxis • 6 -29% with prophylaxis for 3 months CMV R+ • 8 -10% with NO prophylaxis • 1 -2% with prophylaxis for 3 months Eid AJ, Razonable R. Curr Opn Organ transplant, 2007: 12; 610 -617
ESTIMATED INCIDENCE RATES OF CMV DISEASE AFTER KIDNEY AND/ OR PANCREAS TRANSPLANT CMV D+/ R • 45 -65% with NO prophylaxis • 6 -29% with prophylaxis for 3 months CMV R+ • 8 -10% with NO prophylaxis • 1 -2% with prophylaxis for 3 months Eid AJ, Razonable R. Curr Opn Organ transplant, 2007: 12; 610 -617
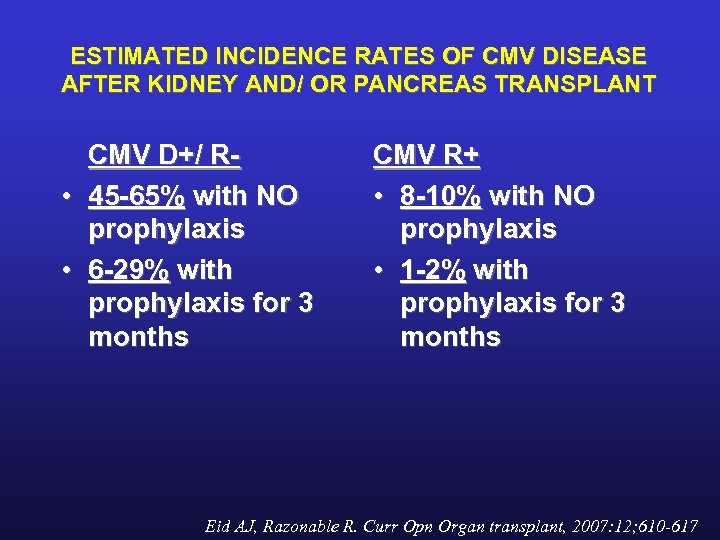 ESTIMATED INCIDENCE RATES OF CMV DISEASE AFTER KIDNEY AND/ OR PANCREAS TRANSPLANT CMV D+/ R • 45 -65% with NO prophylaxis • 6 -29% with prophylaxis for 3 months CMV R+ • 8 -10% with NO prophylaxis • 1 -2% with prophylaxis for 3 months Eid AJ, Razonable R. Curr Opn Organ transplant, 2007: 12; 610 -617
ESTIMATED INCIDENCE RATES OF CMV DISEASE AFTER KIDNEY AND/ OR PANCREAS TRANSPLANT CMV D+/ R • 45 -65% with NO prophylaxis • 6 -29% with prophylaxis for 3 months CMV R+ • 8 -10% with NO prophylaxis • 1 -2% with prophylaxis for 3 months Eid AJ, Razonable R. Curr Opn Organ transplant, 2007: 12; 610 -617
 Oral Valganciclovir Is Noninferior to Intravenous Ganciclovir for the Treatment of Cytomegalovirus Disease in Solid Organ Transplant Recipients: The VICTOR study • Randomized, international trial, txp patients with CMV disease – 900 mg po valganciclovir or 5 mg/kg IV ganciclovir BID for 21 days, followed by 900 mg daily valganciclovir for 28 days • Rate of viremia eradication at Day 21 was 45. 1% for valganciclovir and 48. 4% for ganciclovir (95% CI – 14. 0% to +8. 0%), and at Day 49; 67. 1% and 70. 1% (p=NS) • Treatment success was 77. 4% versus 80. 3% at Day 21 and 85. 4% versus 84. 1% at Day 49 (p = NS) • Baseline viral loads were not different between groups and decreased exponentially with similar half-lives and median time to eradication (21 vs. 19 days, p = 0. 076) Asberg. Am J Transplant 2007; 7: 2106 -13.
Oral Valganciclovir Is Noninferior to Intravenous Ganciclovir for the Treatment of Cytomegalovirus Disease in Solid Organ Transplant Recipients: The VICTOR study • Randomized, international trial, txp patients with CMV disease – 900 mg po valganciclovir or 5 mg/kg IV ganciclovir BID for 21 days, followed by 900 mg daily valganciclovir for 28 days • Rate of viremia eradication at Day 21 was 45. 1% for valganciclovir and 48. 4% for ganciclovir (95% CI – 14. 0% to +8. 0%), and at Day 49; 67. 1% and 70. 1% (p=NS) • Treatment success was 77. 4% versus 80. 3% at Day 21 and 85. 4% versus 84. 1% at Day 49 (p = NS) • Baseline viral loads were not different between groups and decreased exponentially with similar half-lives and median time to eradication (21 vs. 19 days, p = 0. 076) Asberg. Am J Transplant 2007; 7: 2106 -13.
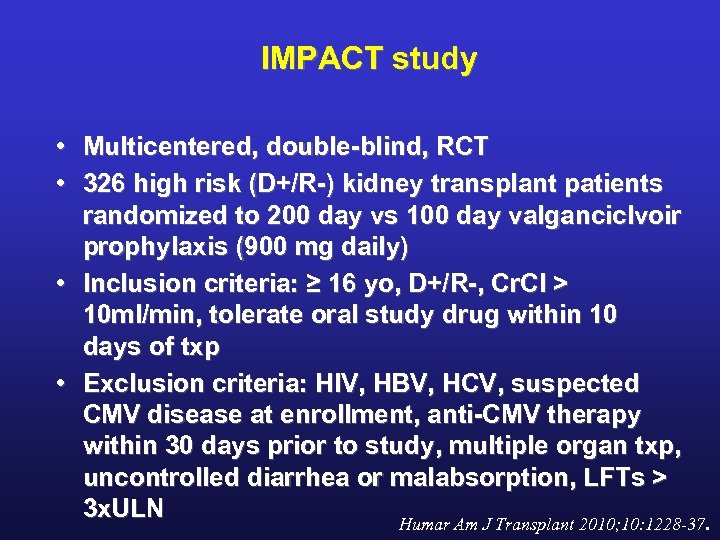 IMPACT study • Multicentered, double-blind, RCT • 326 high risk (D+/R-) kidney transplant patients randomized to 200 day vs 100 day valganciclvoir prophylaxis (900 mg daily) • Inclusion criteria: ≥ 16 yo, D+/R-, Cr. Cl > 10 ml/min, tolerate oral study drug within 10 days of txp • Exclusion criteria: HIV, HBV, HCV, suspected CMV disease at enrollment, anti-CMV therapy within 30 days prior to study, multiple organ txp, uncontrolled diarrhea or malabsorption, LFTs > 3 x. ULN Humar Am J Transplant 2010; 10: 1228 -37.
IMPACT study • Multicentered, double-blind, RCT • 326 high risk (D+/R-) kidney transplant patients randomized to 200 day vs 100 day valganciclvoir prophylaxis (900 mg daily) • Inclusion criteria: ≥ 16 yo, D+/R-, Cr. Cl > 10 ml/min, tolerate oral study drug within 10 days of txp • Exclusion criteria: HIV, HBV, HCV, suspected CMV disease at enrollment, anti-CMV therapy within 30 days prior to study, multiple organ txp, uncontrolled diarrhea or malabsorption, LFTs > 3 x. ULN Humar Am J Transplant 2010; 10: 1228 -37.
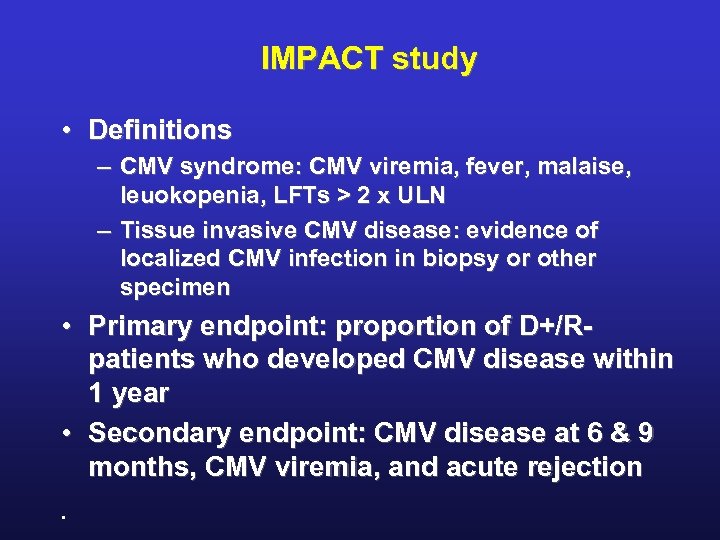 IMPACT study • Definitions – CMV syndrome: CMV viremia, fever, malaise, leuokopenia, LFTs > 2 x ULN – Tissue invasive CMV disease: evidence of localized CMV infection in biopsy or other specimen • Primary endpoint: proportion of D+/R- patients who developed CMV disease within 1 year • Secondary endpoint: CMV disease at 6 & 9 months, CMV viremia, and acute rejection.
IMPACT study • Definitions – CMV syndrome: CMV viremia, fever, malaise, leuokopenia, LFTs > 2 x ULN – Tissue invasive CMV disease: evidence of localized CMV infection in biopsy or other specimen • Primary endpoint: proportion of D+/R- patients who developed CMV disease within 1 year • Secondary endpoint: CMV disease at 6 & 9 months, CMV viremia, and acute rejection.
 IMPACT study • • Results: CMV disease lower in 200 day vs 100 day (16. 1% vs 36. 8%, p <0. 0001) Confirmed CMV viremia lower in 200 day vs 100 day (37. 4% vs 50. 9%, p=0. 015) at month 12 Rejection in 200 day vs 100 day (11% vs 17%, p=0. 114) Incidence of leukopenia 200 vs 100 day was 38% vs. 26% Humar Am J Transplant 2010; 10: 1228 -37.
IMPACT study • • Results: CMV disease lower in 200 day vs 100 day (16. 1% vs 36. 8%, p <0. 0001) Confirmed CMV viremia lower in 200 day vs 100 day (37. 4% vs 50. 9%, p=0. 015) at month 12 Rejection in 200 day vs 100 day (11% vs 17%, p=0. 114) Incidence of leukopenia 200 vs 100 day was 38% vs. 26% Humar Am J Transplant 2010; 10: 1228 -37.
 IMPACT study • Immunosuppressive regimens were not controlled • HLA matching not analyzed • Kidney transplant patients only • Discontinuation of immunosuppression not assessed Humar Am J Transplant 2010; 10: 1228 -37.
IMPACT study • Immunosuppressive regimens were not controlled • HLA matching not analyzed • Kidney transplant patients only • Discontinuation of immunosuppression not assessed Humar Am J Transplant 2010; 10: 1228 -37.
 CMV Resistance • Risk factors – – • • Prolonged antiviral drug exposure Ongoing active viral replication Lack of prior CMV immunity Inadequate antiviral drug delivery Ganciclovir resistance incidence 5 -10% 90% ganciclovir resistant CMV isolates contain UL 97 mutations • Less common UL 54, pol mutation
CMV Resistance • Risk factors – – • • Prolonged antiviral drug exposure Ongoing active viral replication Lack of prior CMV immunity Inadequate antiviral drug delivery Ganciclovir resistance incidence 5 -10% 90% ganciclovir resistant CMV isolates contain UL 97 mutations • Less common UL 54, pol mutation
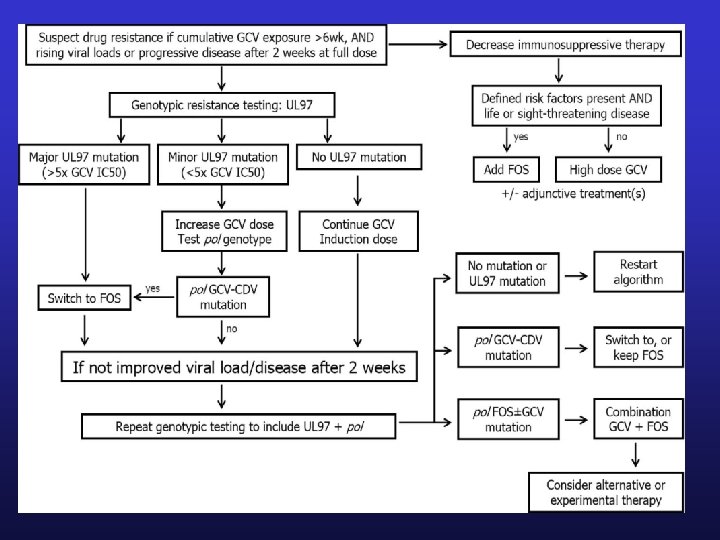
 Foscarnet • Treatment dose – – 90 mg/kg IV every 12 hours x 2 weeks Followed by 120 mg/kg daily for ≥ 2 weeks Infused over 1 hour Pre-treat with 500 mls NSS over 1 hour prior to each dose, and repeat 500 mls NSS over 1 hour after dose • Adjust for renal dysfunction
Foscarnet • Treatment dose – – 90 mg/kg IV every 12 hours x 2 weeks Followed by 120 mg/kg daily for ≥ 2 weeks Infused over 1 hour Pre-treat with 500 mls NSS over 1 hour prior to each dose, and repeat 500 mls NSS over 1 hour after dose • Adjust for renal dysfunction
 Our practice at UTHSC • D+/R- : 900 mg valgancyclovir qd (renal adjusted dose) for 6 months • R+ : 450 mg qd (renal adj) for 90 days OR po Valacyclovir (as below) + q 2 wk CMVPCR for 6 months- if PCR turns +VE, 900 mg bid for 2 -4 wks, then ↓ to 900 mg qd • D-/ R- : Valacyclovir 500 mg qd for 90 days
Our practice at UTHSC • D+/R- : 900 mg valgancyclovir qd (renal adjusted dose) for 6 months • R+ : 450 mg qd (renal adj) for 90 days OR po Valacyclovir (as below) + q 2 wk CMVPCR for 6 months- if PCR turns +VE, 900 mg bid for 2 -4 wks, then ↓ to 900 mg qd • D-/ R- : Valacyclovir 500 mg qd for 90 days
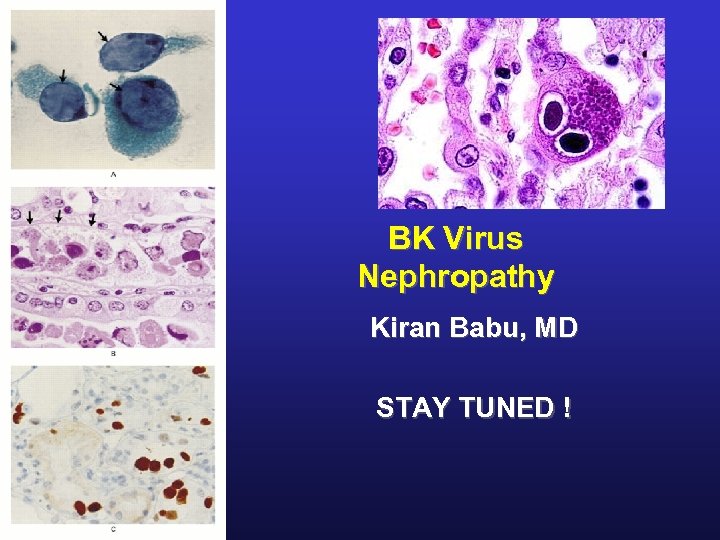 BK Virus Nephropathy Kiran Babu, MD STAY TUNED !
BK Virus Nephropathy Kiran Babu, MD STAY TUNED !


