f74ff007a25cc40bb807ce414f1058eb.ppt
- Количество слайдов: 25

Industry role in building clinical evidence James Shannon, Head of Global Development Giovanni Della Cioppa, Global Head of Methodology & Innovation – Global Development Management Board AIFA spring conference 30 March 2007

Agenda § Pharma has a leading role in fighting disease…but disease is not conquered yet § The Pharma environment is challenging § Health authorities and industry have a common goal § The future will bring new demands 2 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007
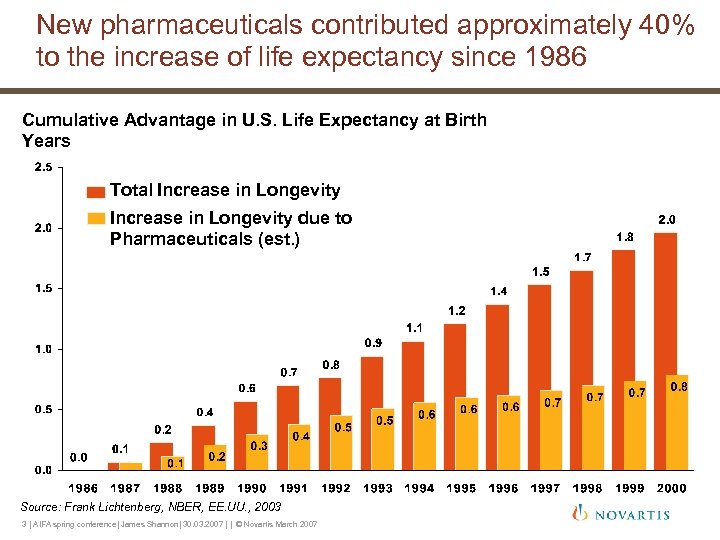
New pharmaceuticals contributed approximately 40% to the increase of life expectancy since 1986 Cumulative Advantage in U. S. Life Expectancy at Birth Years Total Increase in Longevity due to Pharmaceuticals (est. ) Source: Frank Lichtenberg, NBER, EE. UU. , 2003 3 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007
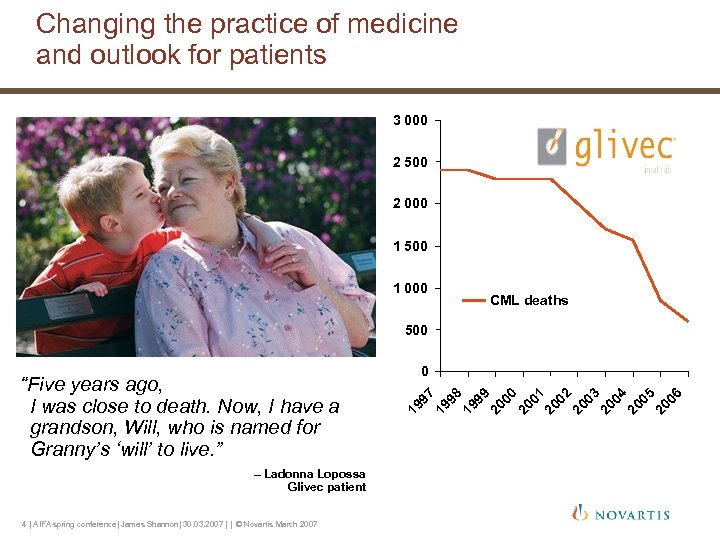
Changing the practice of medicine and outlook for patients 3 000 2 500 2 000 1 500 1 000 CML deaths 500 – Ladonna Lopossa Glivec patient 4 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007 4 20 05 20 06 3 20 0 2 20 0 1 20 0 0 20 0 9 20 0 8 19 9 7 0 19 9 “Five years ago, I was close to death. Now, I have a grandson, Will, who is named for Granny’s ‘will’ to live. ”
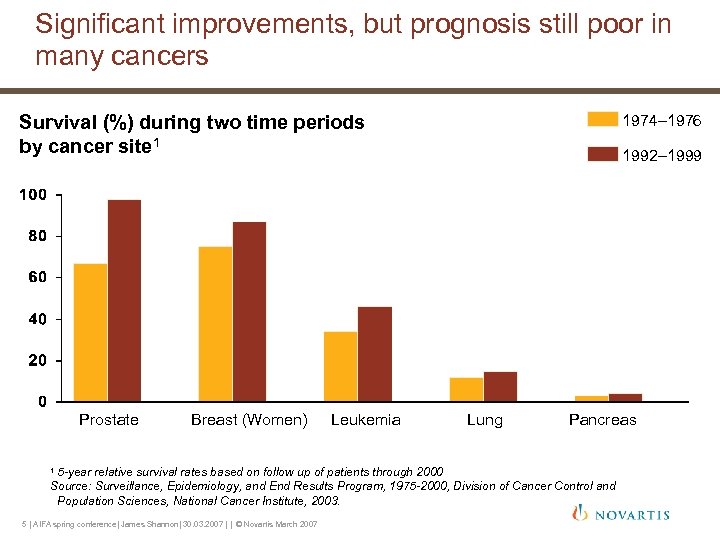
Significant improvements, but prognosis still poor in many cancers Survival (%) during two time periods by cancer site 1 Prostate Breast (Women) Leukemia 1974– 1976 1992– 1999 Lung Pancreas 5 -year relative survival rates based on follow up of patients through 2000 Source: Surveillance, Epidemiology, and End Results Program, 1975 -2000, Division of Cancer Control and Population Sciences, National Cancer Institute, 2003. 1 5 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007
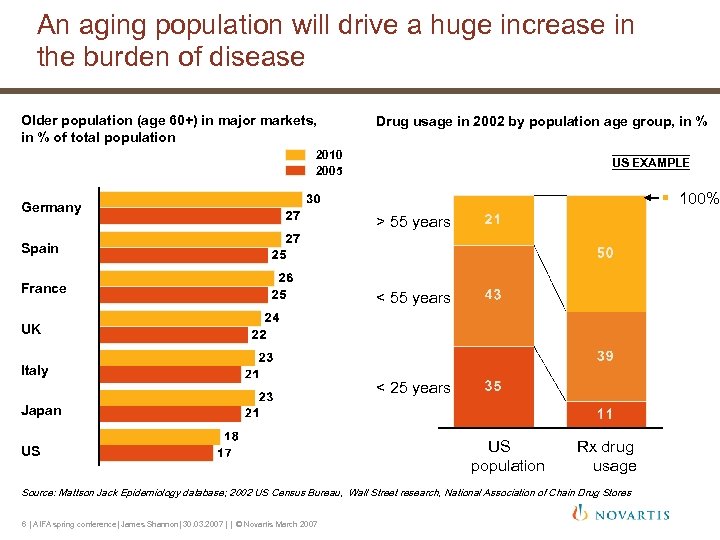
An aging population will drive a huge increase in the burden of disease Older population (age 60+) in major markets, in % of total population Drug usage in 2002 by population age group, in % 2010 2005 Germany US EXAMPLE § 100% > 55 years Spain France < 55 years UK Italy < 25 years Japan US US population Rx drug usage Source: Mattson Jack Epidemiology database; 2002 US Census Bureau, Wall Street research, National Association of Chain Drug Stores 6 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007
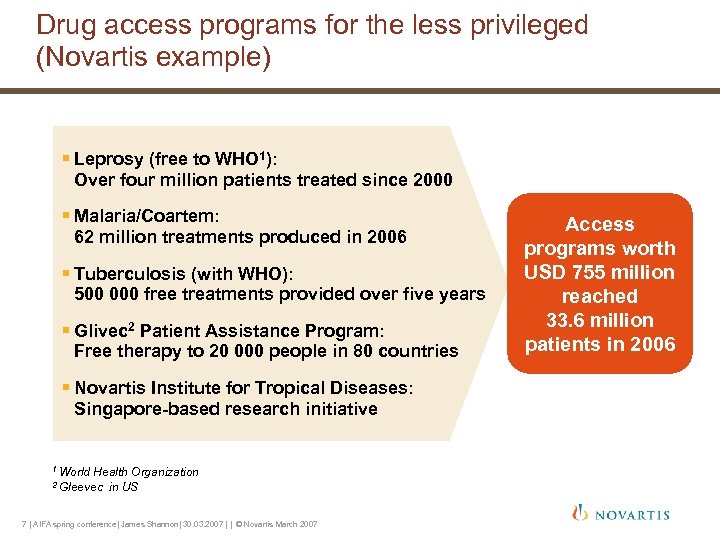
Drug access programs for the less privileged (Novartis example) § Leprosy (free to WHO 1): Over four million patients treated since 2000 § Malaria/Coartem: 62 million treatments produced in 2006 § Tuberculosis (with WHO): 500 000 free treatments provided over five years § Glivec 2 Patient Assistance Program: Free therapy to 20 000 people in 80 countries § Novartis Institute for Tropical Diseases: Singapore-based research initiative 1 World Health Organization in US 2 Gleevec 7 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007 Access programs worth USD 755 million reached 33. 6 million patients in 2006

Agenda § Pharma has a leading role in fighting disease…but disease is not conquered yet § The Pharma environment is challenging § Health authorities and industry have a common goal § The future will bring new demands 8 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007
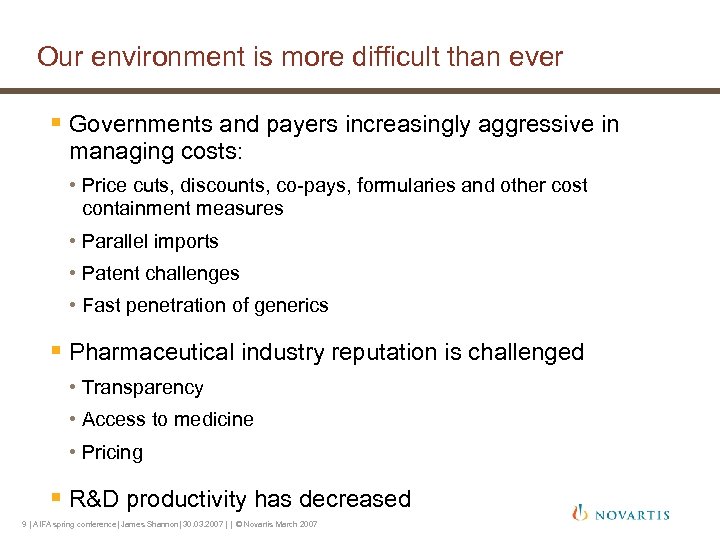
Our environment is more difficult than ever § Governments and payers increasingly aggressive in managing costs: • Price cuts, discounts, co-pays, formularies and other cost containment measures • Parallel imports • Patent challenges • Fast penetration of generics § Pharmaceutical industry reputation is challenged • Transparency • Access to medicine • Pricing § R&D productivity has decreased 9 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007
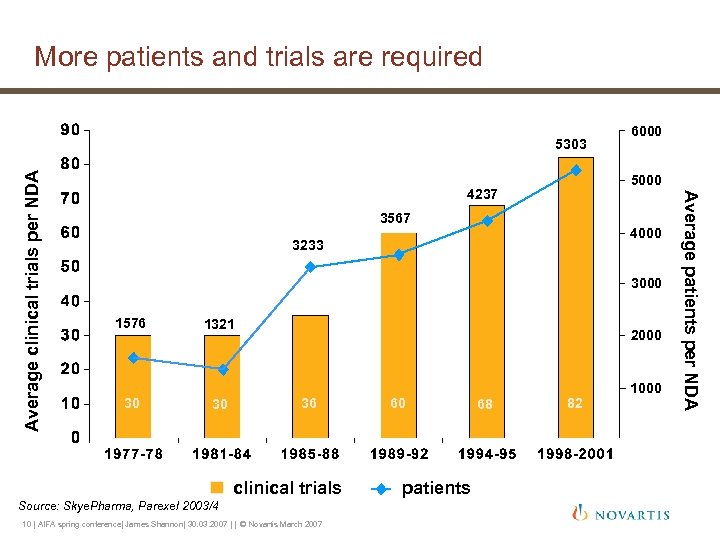
More patients and trials are required 6000 5000 4237 3567 4000 3233 3000 1576 30 1321 30 2000 36 clinical trials Source: Skye. Pharma, Parexel 2003/4 10 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007 60 patients 68 82 1000 Average patients per NDA Average clinical trials per NDA 5303
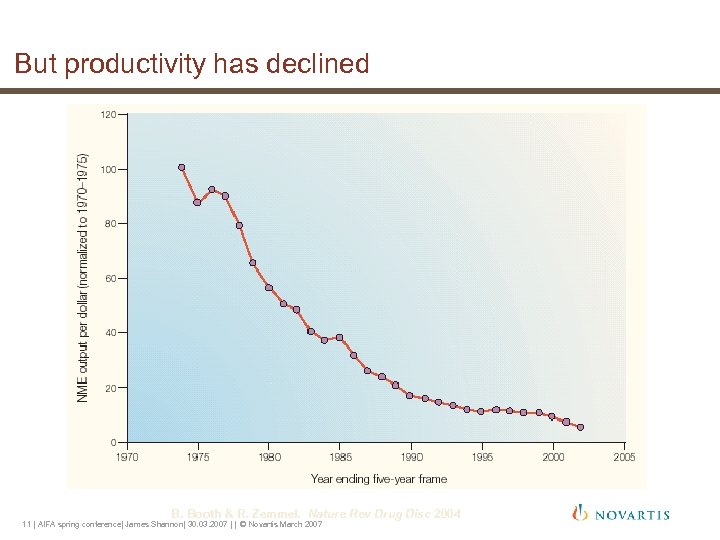
But productivity has declined B. Booth & R. Zemmel. Nature Rev Drug Disc 2004 11 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007

Agenda § Pharma has a leading role in fighting disease…but disease is not conquered yet § The Pharma environment is challenging § Health authorities and industry have a common goal § The future will bring new demands 12 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007
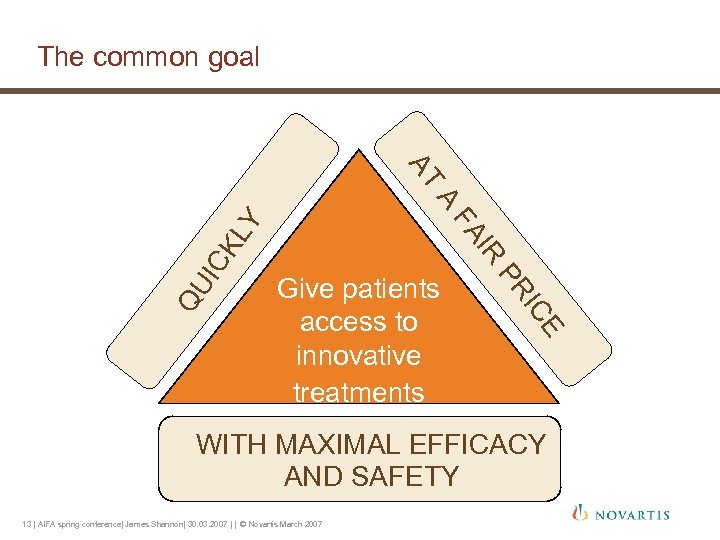
The common goal AT CK PR E IC QU I IR FA LY A Give patients access to innovative treatments WITH MAXIMAL EFFICACY AND SAFETY 13 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007
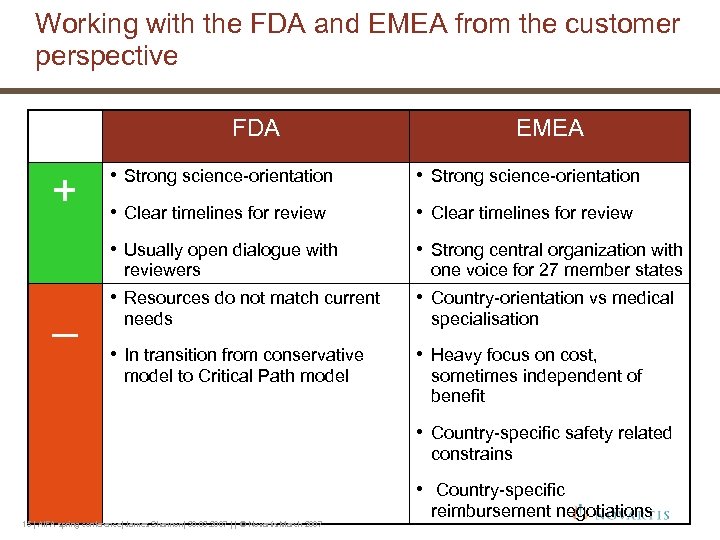
Working with the FDA and EMEA from the customer perspective FDA EMEA • Strong science-orientation • Clear timelines for review • Usually open dialogue with • Strong central organization with • Resources do not match current • Country-orientation vs medical • In transition from conservative + • Strong science-orientation • Heavy focus on cost, reviewers _ needs model to Critical Path model one voice for 27 member states specialisation sometimes independent of benefit • Country-specific safety related constrains • Country-specific 15 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007 reimbursement negotiations

Agenda § Pharma has a leading role in fighting disease…but disease is not conquered yet § The Pharma environment is challenging § Health authorities and industry have a common goal § The future will bring new demands 16 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007
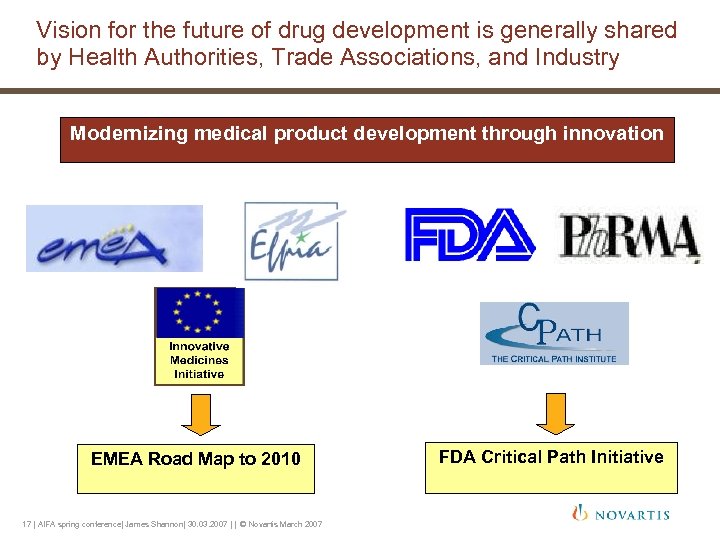
Vision for the future of drug development is generally shared by Health Authorities, Trade Associations, and Industry Modernizing medical product development through innovation EMEA Road Map to 2010 17 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007 FDA Critical Path Initiative
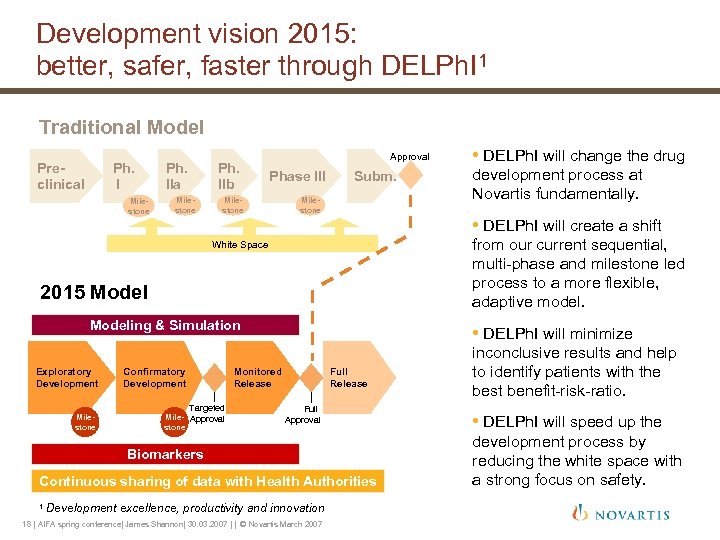
Development vision 2015: better, safer, faster through DELPh. I 1 Traditional Model Preclinical Ph. I Milestone Ph. IIa Ph. IIb Milestone Approval Phase III Milestone Subm. Milestone from our current sequential, multi-phase and milestone led process to a more flexible, adaptive model. 2015 Modeling & Simulation Confirmatory Development • DELPh. I will minimize Monitored Release Targeted Milestone Mile- Approval stone Full Release Full Approval Biomarkers Continuous sharing of data with Health Authorities 1 development process at Novartis fundamentally. • DELPh. I will create a shift White Space Exploratory Development • DELPh. I will change the drug Development excellence, productivity and innovation 18 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007 inconclusive results and help to identify patients with the best benefit-risk-ratio. • DELPh. I will speed up the development process by reducing the white space with a strong focus on safety.
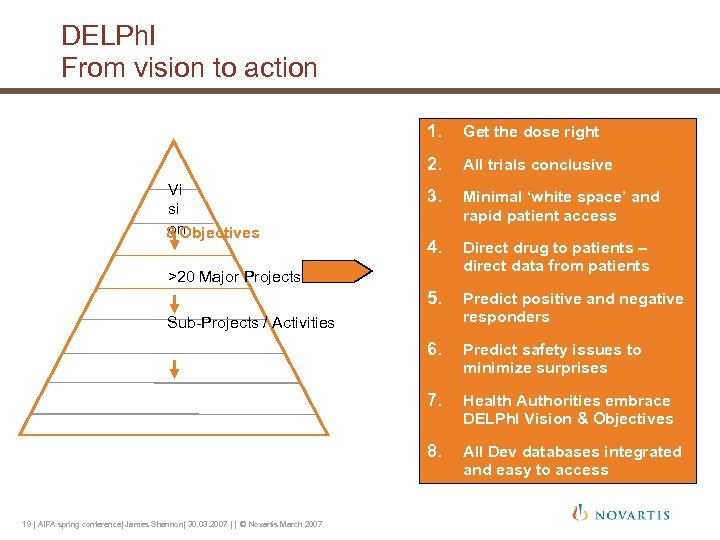
DELPh. I From vision to action 1. 2. All trials conclusive 3. Minimal ‘white space’ and rapid patient access 4. Direct drug to patients – direct data from patients 5. Predict positive and negative responders 6. Predict safety issues to minimize surprises 7. Health Authorities embrace DELPh. I Vision & Objectives 8. Vi si on 8 Objectives Get the dose right All Dev databases integrated and easy to access >20 Major Projects Sub-Projects / Activities 19 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007
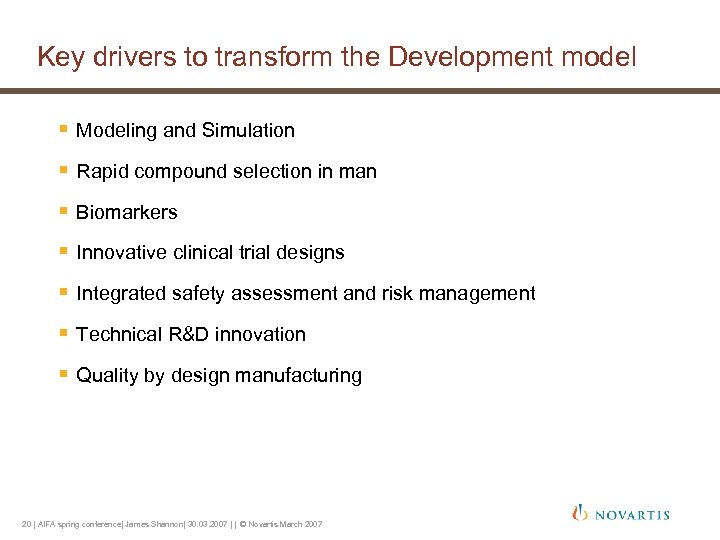
Key drivers to transform the Development model § Modeling and Simulation § Rapid compound selection in man § Biomarkers § Innovative clinical trial designs § Integrated safety assessment and risk management § Technical R&D innovation § Quality by design manufacturing 20 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007
Innovative trial designs are in implementation phase § Adaptive trial design methodology workshops with both the EMEA and the FDA § Novartis has embarked on implementation of adaptive designs in multiple registration programs (5 on-going, up to 10 by the end of 2007) • Adaptive designs: to adapt dose, target population, sample size etc. • Seamless adaptive designs: phase I/II, phase II/III, phase III/IV 21 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007
Redefining end-points Approval of anti-Ig. E in severe asthma Exacerbations FEV 1 22 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007
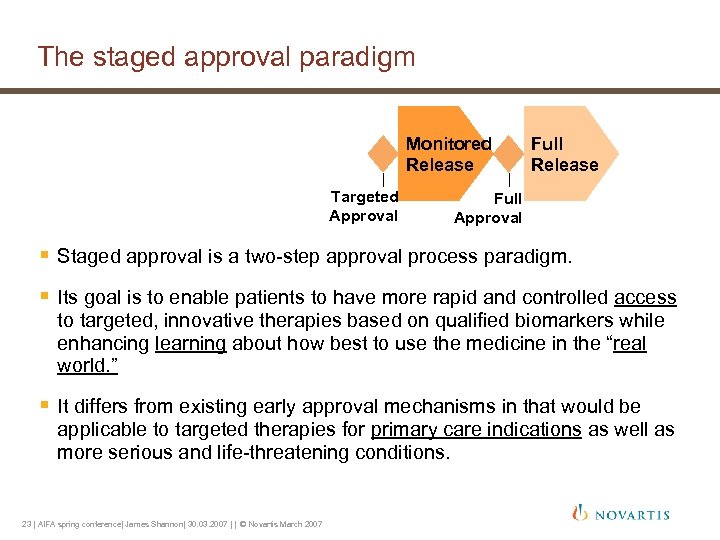
The staged approval paradigm Monitored Release Targeted Approval Full Release Full Approval § Staged approval is a two-step approval process paradigm. § Its goal is to enable patients to have more rapid and controlled access to targeted, innovative therapies based on qualified biomarkers while enhancing learning about how best to use the medicine in the “real world. ” § It differs from existing early approval mechanisms in that would be applicable to targeted therapies for primary care indications as well as more serious and life-threatening conditions. 23 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007
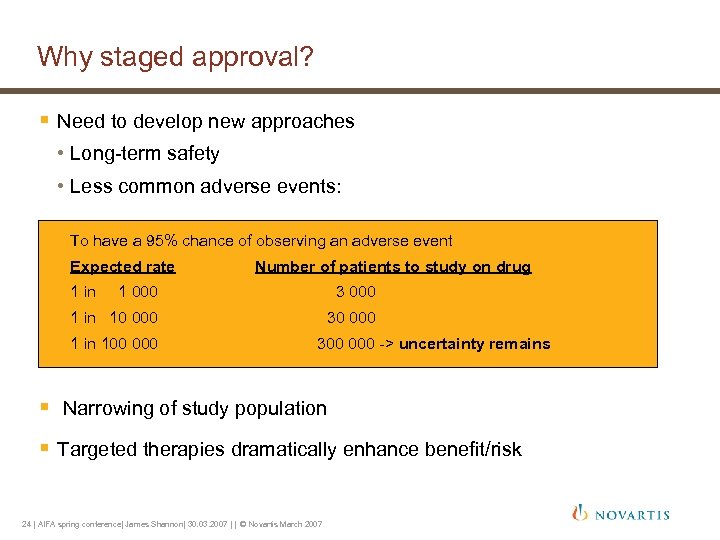
Why staged approval? § Need to develop new approaches • Long-term safety • Less common adverse events: To have a 95% chance of observing an adverse event Expected rate 1 in Number of patients to study on drug 1 000 3 000 1 in 10 000 30 000 1 in 100 000 300 000 -> uncertainty remains § Narrowing of study population § Targeted therapies dramatically enhance benefit/risk 24 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007
Experience to date EU Conditional Approval § Very limited experience; application of approach and definition of “serious” indication not fully understood by industry US Subpart H (Accelerated Approval) § Widely applied to oncology and HIV/AIDs drugs, but some indication of reluctance on the part of FDA to utilize this approach Can these mechanisms be utilized for primary care indications? 25 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007

Critical success factors to achieve staged approval Advances in science must justify change in policy § Identification and qualification of biomarkers as surrogate endpoints § Advancement of predictive safety § Advancement of pharmacovigilance methods Staged approval must translate into a viable business opportunity § Reimbursement, pricing, formulary acceptance must be adequate to support the proposed approach 26 | AIFA spring conference| James Shannon| 30. 03. 2007 | | © Novartis March 2007
f74ff007a25cc40bb807ce414f1058eb.ppt