9bf61d0605ec7f2dd06f4c25bff896af.ppt
- Количество слайдов: 33
 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Risk-Based Development for Quality by Design Ken Morris Purdue University Department of Industrial and Physical Pharmacy FDA SAB Manufacturing Sub-Committee September, 17 th, 2003
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Risk-Based Development for Quality by Design Ken Morris Purdue University Department of Industrial and Physical Pharmacy FDA SAB Manufacturing Sub-Committee September, 17 th, 2003
 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Pharmaceutical c. GMPs for the 21 st Century: A Risk-Based Approach A science and risk-based approach to product quality regulation incorporating an integrated quality systems approach 1. Risk-based orientation 2. Science-based policies and standards 3. Integrated quality systems orientation 4. International cooperation 5. Strong Public Health Protection
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Pharmaceutical c. GMPs for the 21 st Century: A Risk-Based Approach A science and risk-based approach to product quality regulation incorporating an integrated quality systems approach 1. Risk-based orientation 2. Science-based policies and standards 3. Integrated quality systems orientation 4. International cooperation 5. Strong Public Health Protection
 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Pharmaceutical c. GMPs for the 21 st Century: A Risk-Based Approach What’s New? • Good science isn’t new, we all do it now • Some technologies, techniques, and models are • Computers • Sensors • Chemometrics • Phenomenological and Fundemental Models • The mutual FDA-Industry-Academic recognition of the technical “way forward “ in application of the state of the science
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Pharmaceutical c. GMPs for the 21 st Century: A Risk-Based Approach What’s New? • Good science isn’t new, we all do it now • Some technologies, techniques, and models are • Computers • Sensors • Chemometrics • Phenomenological and Fundemental Models • The mutual FDA-Industry-Academic recognition of the technical “way forward “ in application of the state of the science
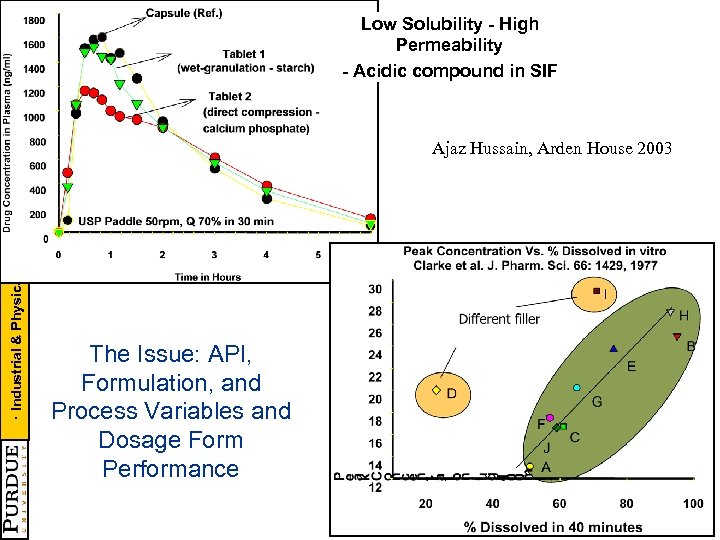 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Low Solubility - High Permeability - Acidic compound in SIF Ajaz Hussain, Arden House 2003 The Issue: API, Formulation, and Process Variables and Dosage Form Performance
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Low Solubility - High Permeability - Acidic compound in SIF Ajaz Hussain, Arden House 2003 The Issue: API, Formulation, and Process Variables and Dosage Form Performance
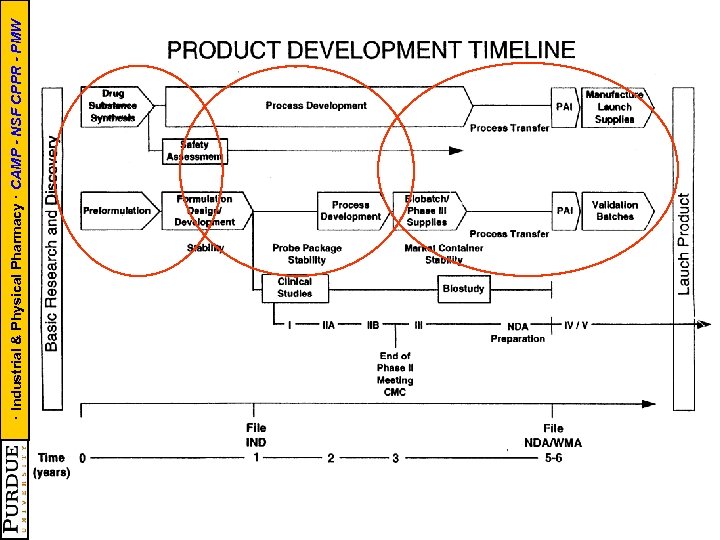 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW
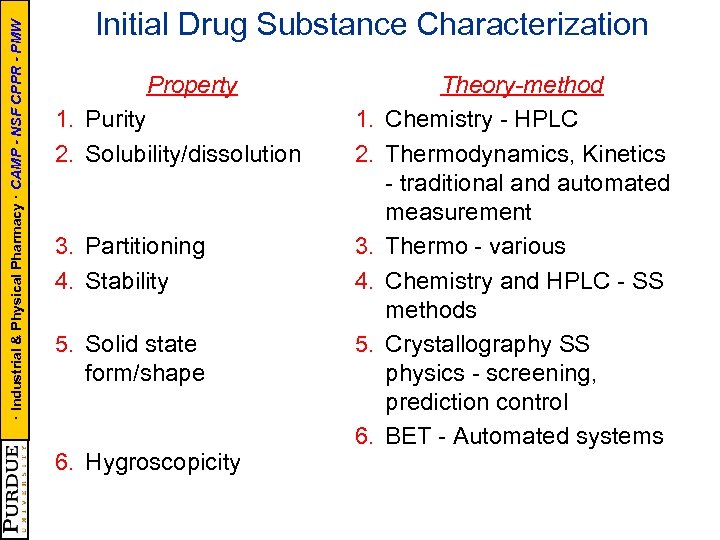 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Initial Drug Substance Characterization Property 1. Purity 2. Solubility/dissolution 1. 2. 3. Partitioning 4. Stability 3. 4. 5. Solid state form/shape 5. 6. Hygroscopicity 6. Theory-method Chemistry - HPLC Thermodynamics, Kinetics - traditional and automated measurement Thermo - various Chemistry and HPLC - SS methods Crystallography SS physics - screening, prediction control BET - Automated systems
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Initial Drug Substance Characterization Property 1. Purity 2. Solubility/dissolution 1. 2. 3. Partitioning 4. Stability 3. 4. 5. Solid state form/shape 5. 6. Hygroscopicity 6. Theory-method Chemistry - HPLC Thermodynamics, Kinetics - traditional and automated measurement Thermo - various Chemistry and HPLC - SS methods Crystallography SS physics - screening, prediction control BET - Automated systems
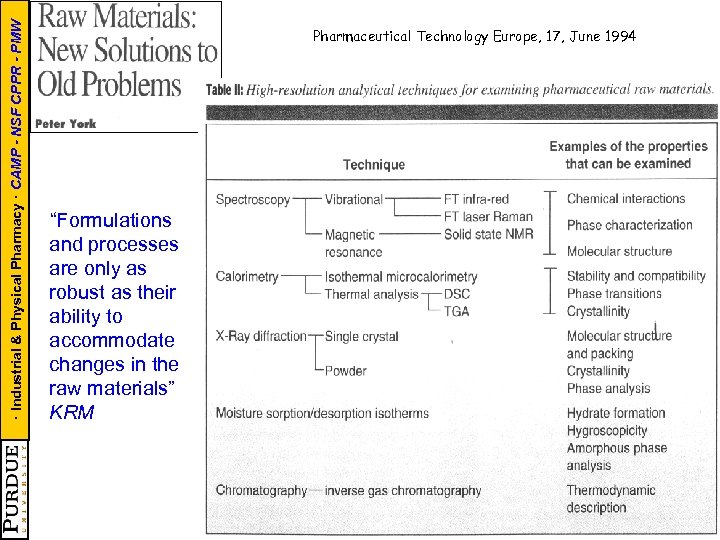 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Pharmaceutical Technology Europe, 17, June 1994 “Formulations and processes are only as robust as their ability to accommodate changes in the raw materials” KRM
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Pharmaceutical Technology Europe, 17, June 1994 “Formulations and processes are only as robust as their ability to accommodate changes in the raw materials” KRM
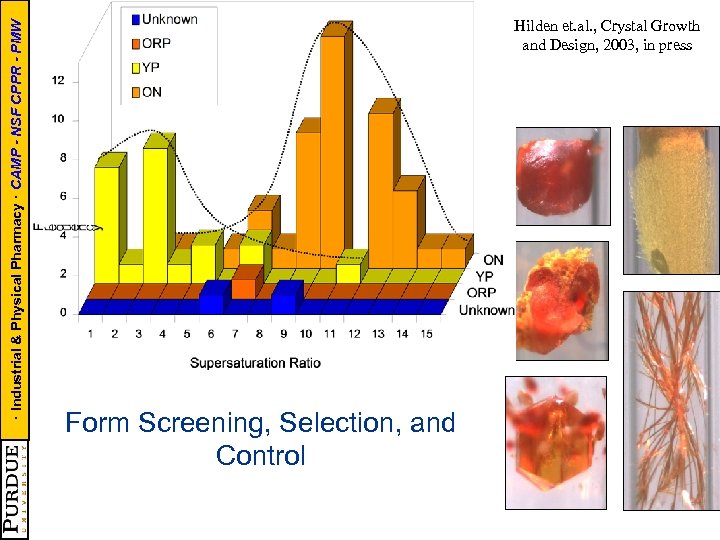 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Hilden et. al. , Crystal Growth and Design, 2003, in press Form Screening, Selection, and Control
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Hilden et. al. , Crystal Growth and Design, 2003, in press Form Screening, Selection, and Control
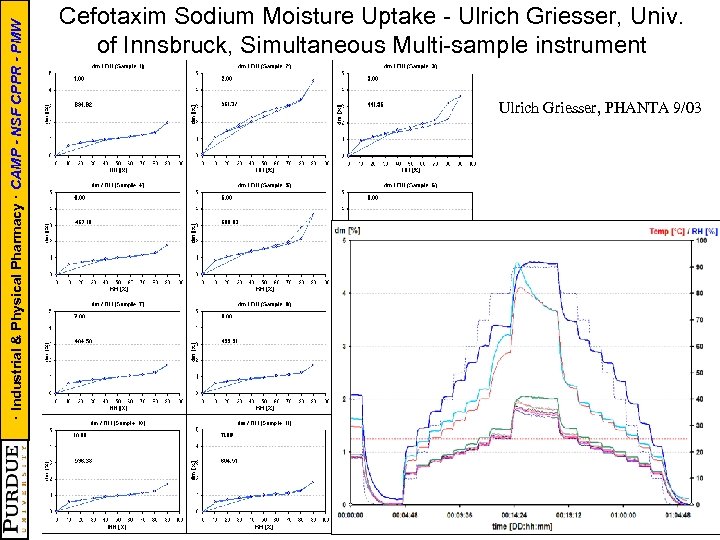 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Cefotaxim Sodium Moisture Uptake - Ulrich Griesser, Univ. of Innsbruck, Simultaneous Multi-sample instrument Ulrich Griesser, PHANTA 9/03
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Cefotaxim Sodium Moisture Uptake - Ulrich Griesser, Univ. of Innsbruck, Simultaneous Multi-sample instrument Ulrich Griesser, PHANTA 9/03
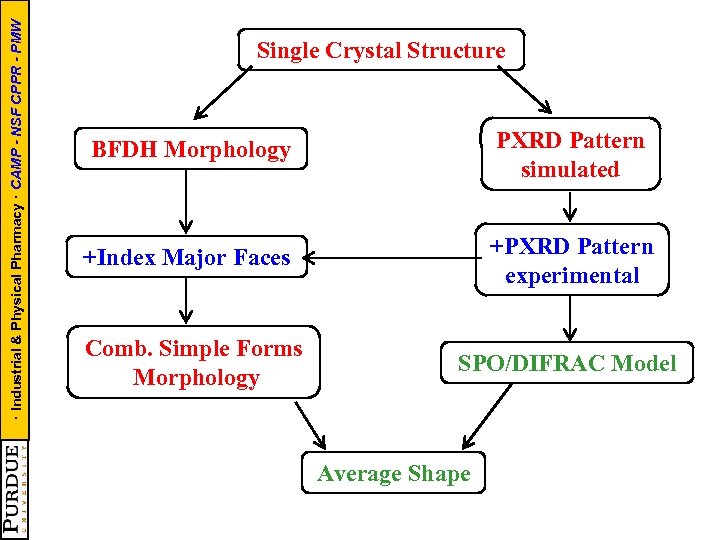 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Single Crystal Structure BFDH Morphology PXRD Pattern simulated +Index Major Faces +PXRD Pattern experimental Comb. Simple Forms Morphology SPO/DIFRAC Model Average Shape
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Single Crystal Structure BFDH Morphology PXRD Pattern simulated +Index Major Faces +PXRD Pattern experimental Comb. Simple Forms Morphology SPO/DIFRAC Model Average Shape
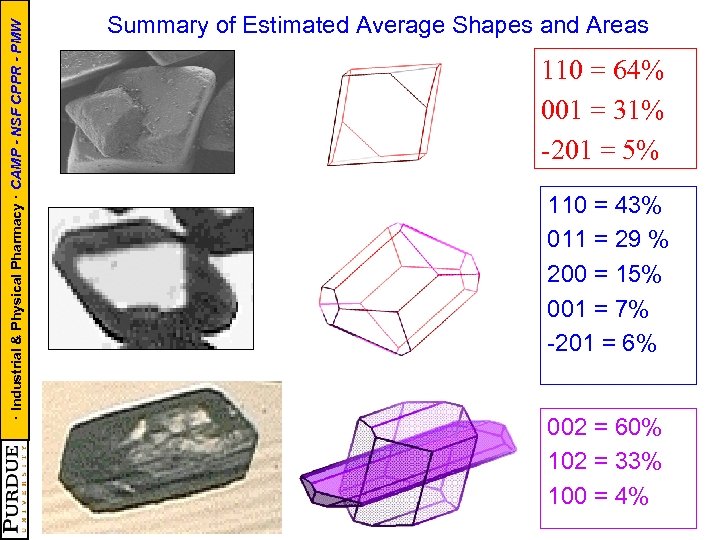 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Summary of Estimated Average Shapes and Areas 110 = 64% 001 = 31% -201 = 5% 110 = 43% 011 = 29 % 200 = 15% 001 = 7% -201 = 6% 002 = 60% 102 = 33% 100 = 4%
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Summary of Estimated Average Shapes and Areas 110 = 64% 001 = 31% -201 = 5% 110 = 43% 011 = 29 % 200 = 15% 001 = 7% -201 = 6% 002 = 60% 102 = 33% 100 = 4%
 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Formulation Design and API Process Development Formulation element 1. Dosage form selection 2. Excipient selection 3. Stability to processing 4. Mechanical properties 1. Flow 2. Compaction 5. Initial processing 1. 2. 3. 4. Theory-method Medical processability Excipient properties – interaction studies, phsico-chemical properties PIT – ME/MSE – 1. flow correlations, 2. heckel analysis 5. Process models – prototypes and PAT
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Formulation Design and API Process Development Formulation element 1. Dosage form selection 2. Excipient selection 3. Stability to processing 4. Mechanical properties 1. Flow 2. Compaction 5. Initial processing 1. 2. 3. 4. Theory-method Medical processability Excipient properties – interaction studies, phsico-chemical properties PIT – ME/MSE – 1. flow correlations, 2. heckel analysis 5. Process models – prototypes and PAT
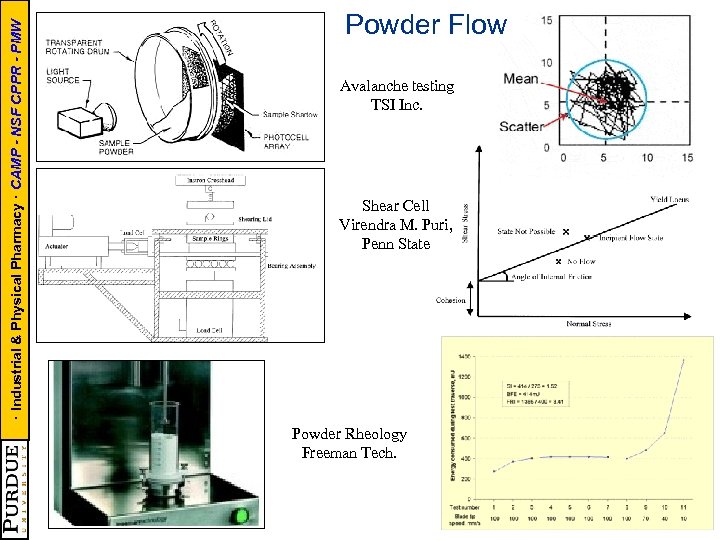 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Powder Flow Avalanche testing TSI Inc. Shear Cell Virendra M. Puri, Penn State Powder Rheology Freeman Tech.
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Powder Flow Avalanche testing TSI Inc. Shear Cell Virendra M. Puri, Penn State Powder Rheology Freeman Tech.
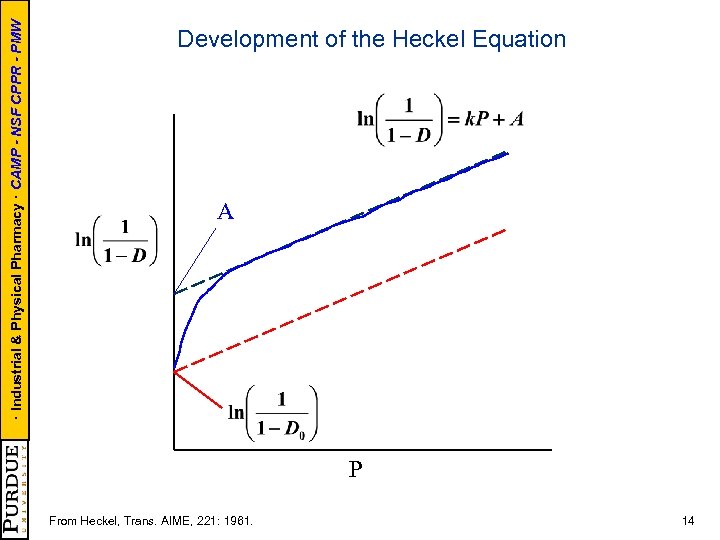 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Development of the Heckel Equation A P From Heckel, Trans. AIME, 221: 1961. 14
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Development of the Heckel Equation A P From Heckel, Trans. AIME, 221: 1961. 14
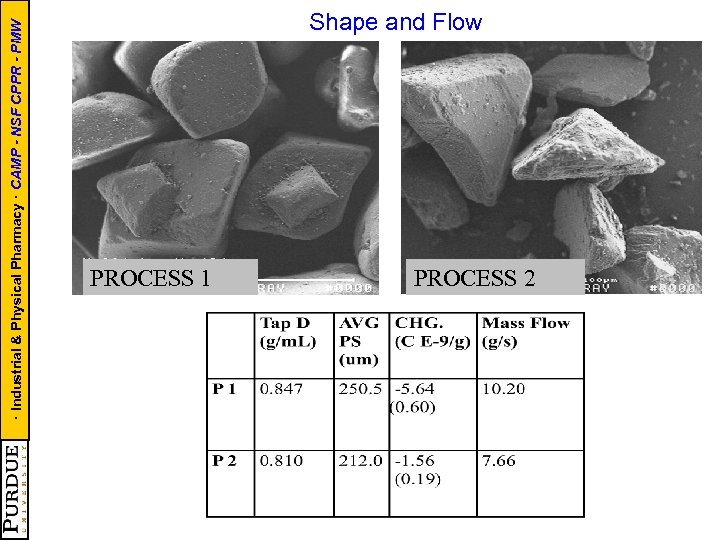 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Shape and Flow PROCESS 1 PROCESS 2
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Shape and Flow PROCESS 1 PROCESS 2
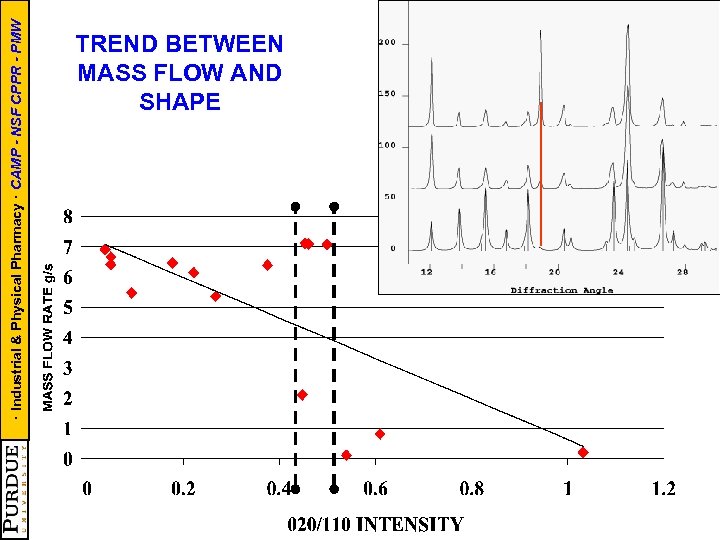 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW TREND BETWEEN MASS FLOW AND SHAPE
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW TREND BETWEEN MASS FLOW AND SHAPE
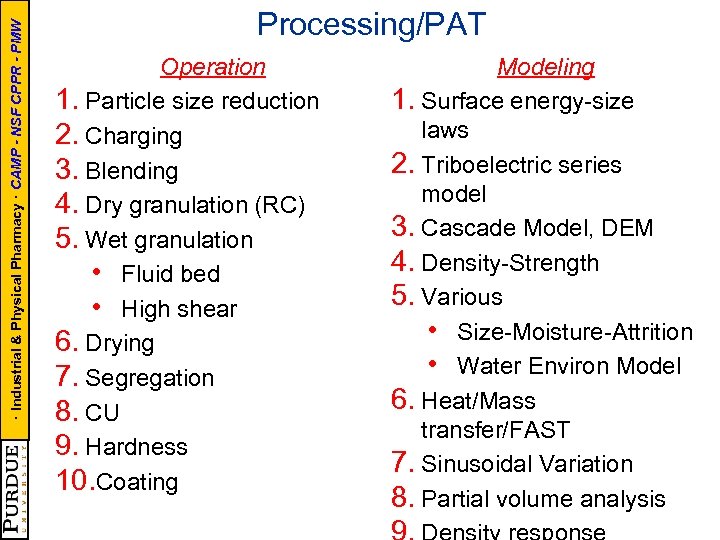 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Processing/PAT Operation 1. Particle size reduction 2. Charging 3. Blending 4. Dry granulation (RC) 5. Wet granulation • Fluid bed • High shear 6. Drying 7. Segregation 8. CU 9. Hardness 10. Coating Modeling 1. Surface energy-size laws 2. Triboelectric series model 3. Cascade Model, DEM 4. Density-Strength 5. Various • Size-Moisture-Attrition • Water Environ Model 6. Heat/Mass transfer/FAST 7. Sinusoidal Variation 8. Partial volume analysis
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Processing/PAT Operation 1. Particle size reduction 2. Charging 3. Blending 4. Dry granulation (RC) 5. Wet granulation • Fluid bed • High shear 6. Drying 7. Segregation 8. CU 9. Hardness 10. Coating Modeling 1. Surface energy-size laws 2. Triboelectric series model 3. Cascade Model, DEM 4. Density-Strength 5. Various • Size-Moisture-Attrition • Water Environ Model 6. Heat/Mass transfer/FAST 7. Sinusoidal Variation 8. Partial volume analysis
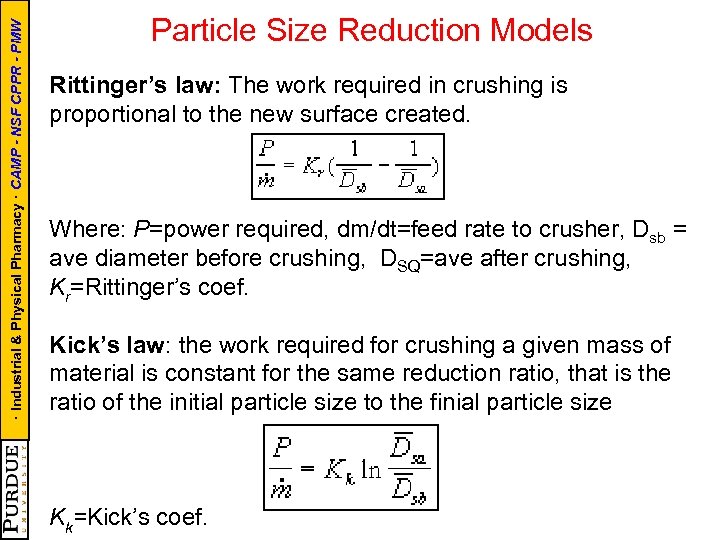 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Particle Size Reduction Models Rittinger’s law: The work required in crushing is proportional to the new surface created. Where: P=power required, dm/dt=feed rate to crusher, Dsb = ave diameter before crushing, DSQ=ave after crushing, Kr=Rittinger’s coef. Kick’s law: the work required for crushing a given mass of material is constant for the same reduction ratio, that is the ratio of the initial particle size to the finial particle size Kk=Kick’s coef.
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Particle Size Reduction Models Rittinger’s law: The work required in crushing is proportional to the new surface created. Where: P=power required, dm/dt=feed rate to crusher, Dsb = ave diameter before crushing, DSQ=ave after crushing, Kr=Rittinger’s coef. Kick’s law: the work required for crushing a given mass of material is constant for the same reduction ratio, that is the ratio of the initial particle size to the finial particle size Kk=Kick’s coef.
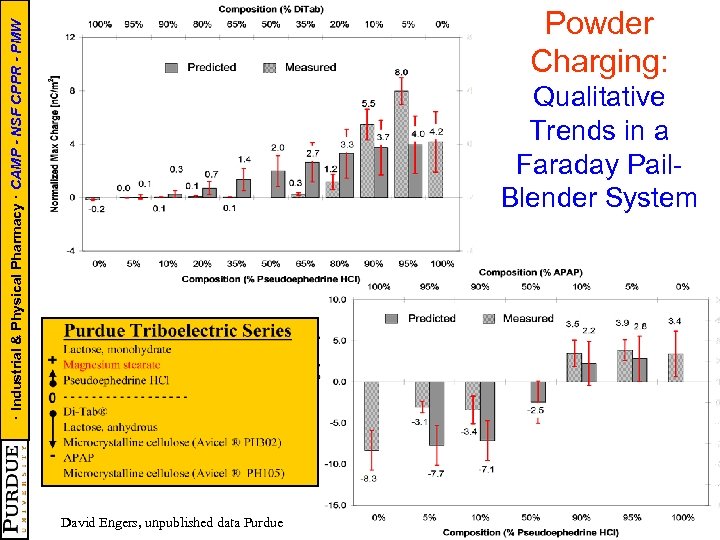 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Powder Charging: Qualitative Trends in a Faraday Pail. Blender System David Engers, unpublished data Purdue
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Powder Charging: Qualitative Trends in a Faraday Pail. Blender System David Engers, unpublished data Purdue
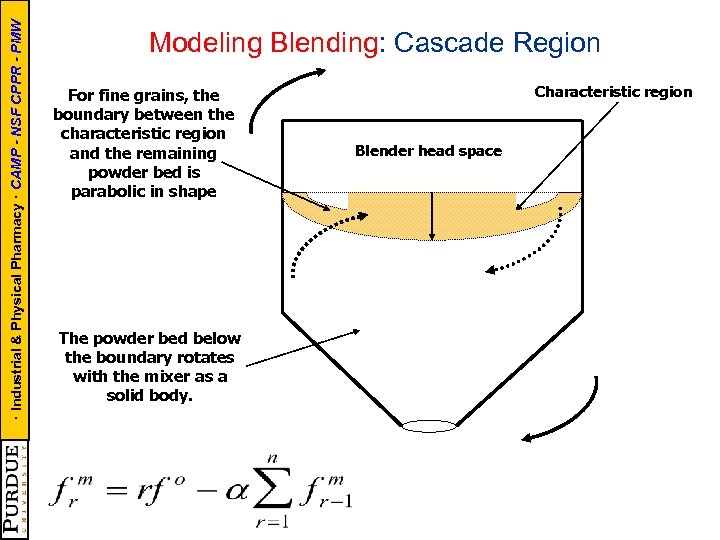 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Modeling Blending: Cascade Region For fine grains, the boundary between the characteristic region and the remaining powder bed is parabolic in shape The powder bed below the boundary rotates with the mixer as a solid body. Characteristic region Blender head space
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Modeling Blending: Cascade Region For fine grains, the boundary between the characteristic region and the remaining powder bed is parabolic in shape The powder bed below the boundary rotates with the mixer as a solid body. Characteristic region Blender head space
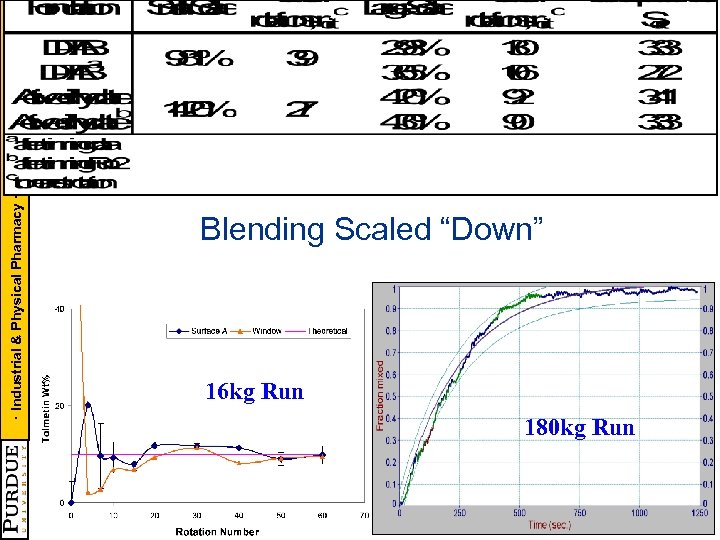 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Blending Scaled “Down” 16 kg Run 180 kg Run
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Blending Scaled “Down” 16 kg Run 180 kg Run
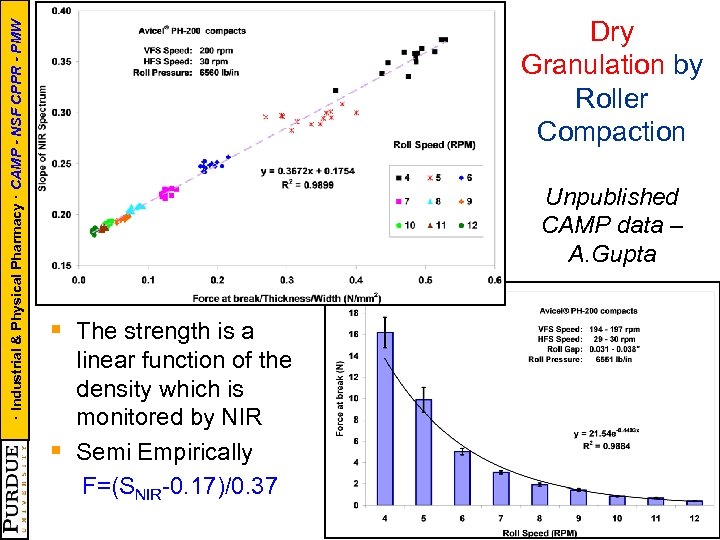 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Dry Granulation by Roller Compaction Unpublished CAMP data – A. Gupta § The strength is a § linear function of the density which is monitored by NIR Semi Empirically F=(SNIR-0. 17)/0. 37
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Dry Granulation by Roller Compaction Unpublished CAMP data – A. Gupta § The strength is a § linear function of the density which is monitored by NIR Semi Empirically F=(SNIR-0. 17)/0. 37
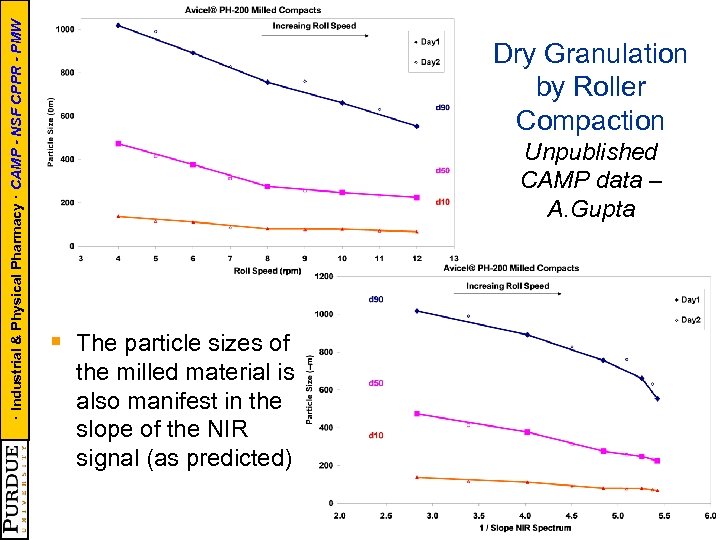 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Dry Granulation by Roller Compaction Unpublished CAMP data – A. Gupta § The particle sizes of the milled material is also manifest in the slope of the NIR signal (as predicted)
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Dry Granulation by Roller Compaction Unpublished CAMP data – A. Gupta § The particle sizes of the milled material is also manifest in the slope of the NIR signal (as predicted)
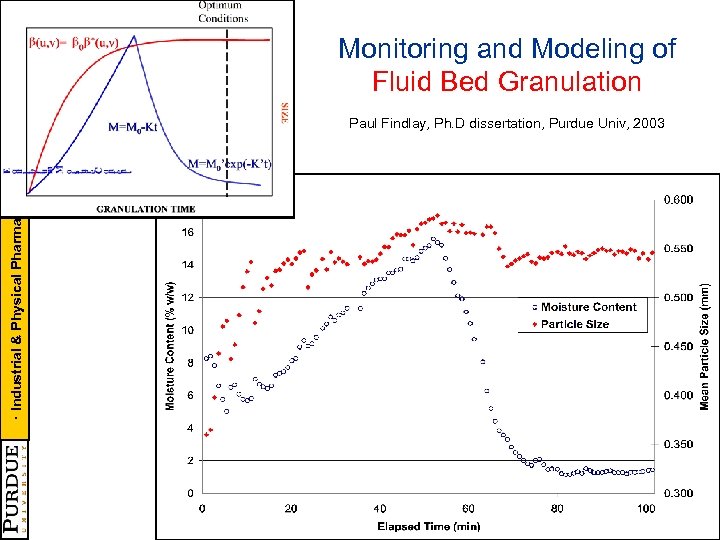 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Monitoring and Modeling of Fluid Bed Granulation Paul Findlay, Ph. D dissertation, Purdue Univ, 2003
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Monitoring and Modeling of Fluid Bed Granulation Paul Findlay, Ph. D dissertation, Purdue Univ, 2003
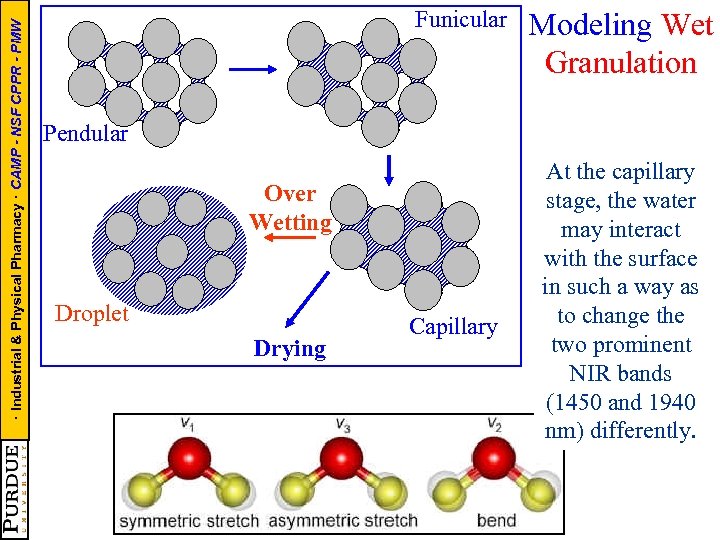 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Funicular Modeling Wet Granulation Pendular Over Wetting Droplet Drying Capillary At the capillary stage, the water may interact with the surface in such a way as to change the two prominent NIR bands (1450 and 1940 nm) differently.
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Funicular Modeling Wet Granulation Pendular Over Wetting Droplet Drying Capillary At the capillary stage, the water may interact with the surface in such a way as to change the two prominent NIR bands (1450 and 1940 nm) differently.
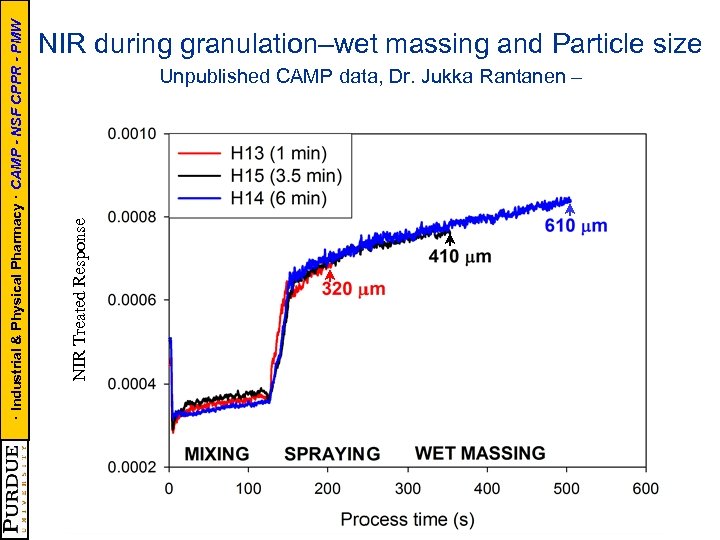 Unpublished CAMP data, Dr. Jukka Rantanen – (=X 3) NIR Treated Response · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW NIR during granulation–wet massing and Particle size X 1=110 g X 2=255 rpm
Unpublished CAMP data, Dr. Jukka Rantanen – (=X 3) NIR Treated Response · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW NIR during granulation–wet massing and Particle size X 1=110 g X 2=255 rpm
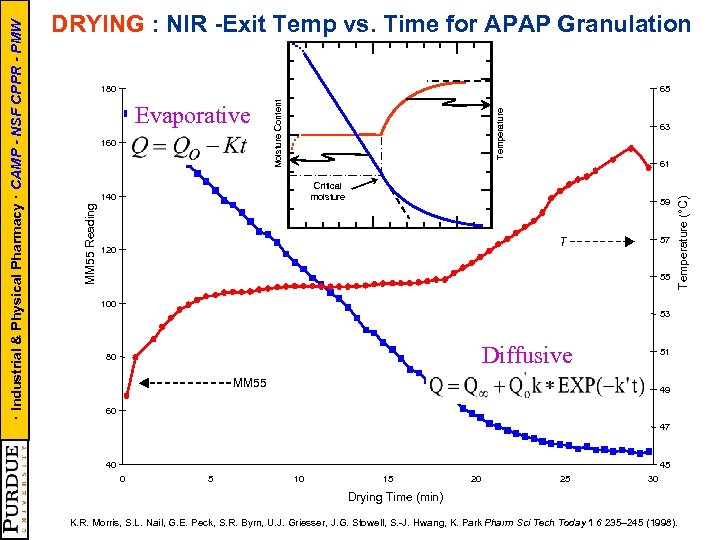 65 63 61 Critical moisture 140 59 57 T 120 55 Temperature (°C) 160 Temperature Evaporative Moisture Content 180 MM 55 Reading · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW DRYING : NIR -Exit Temp vs. Time for APAP Granulation 100 53 Diffusive 80 51 MM 55 49 60 47 40 45 0 5 10 15 20 25 30 Drying Time (min) K. R. Morris, S. L. Nail, G. E. Peck, S. R. Byrn, U. J. Griesser, J. G. Stowell, S. -J. Hwang, K. Park Pharm Sci Tech Today 1 6 235– 245 (1998).
65 63 61 Critical moisture 140 59 57 T 120 55 Temperature (°C) 160 Temperature Evaporative Moisture Content 180 MM 55 Reading · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW DRYING : NIR -Exit Temp vs. Time for APAP Granulation 100 53 Diffusive 80 51 MM 55 49 60 47 40 45 0 5 10 15 20 25 30 Drying Time (min) K. R. Morris, S. L. Nail, G. E. Peck, S. R. Byrn, U. J. Griesser, J. G. Stowell, S. -J. Hwang, K. Park Pharm Sci Tech Today 1 6 235– 245 (1998).
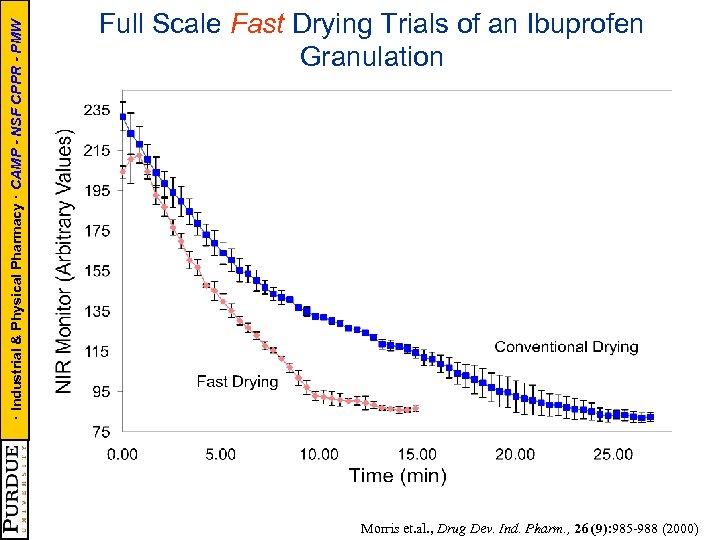 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Full Scale Fast Drying Trials of an Ibuprofen Granulation Morris et. al. , Drug Dev. Ind. Pharm. , 26 (9): 985 -988 (2000)
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Full Scale Fast Drying Trials of an Ibuprofen Granulation Morris et. al. , Drug Dev. Ind. Pharm. , 26 (9): 985 -988 (2000)
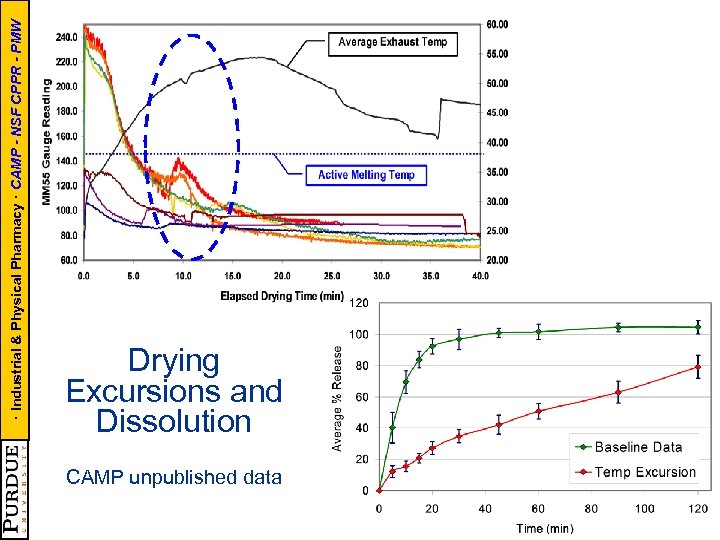 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Drying Excursions and Dissolution CAMP unpublished data
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Drying Excursions and Dissolution CAMP unpublished data
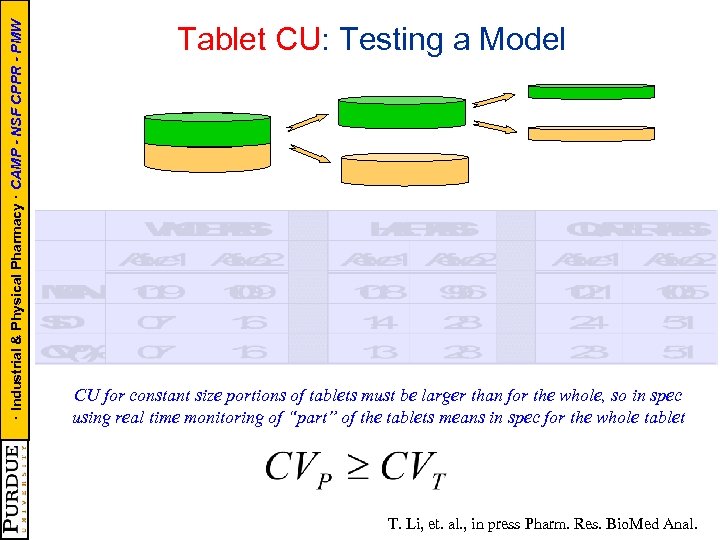 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Tablet CU: Testing a Model CU for constant size portions of tablets must be larger than for the whole, so in spec using real time monitoring of “part” of the tablets means in spec for the whole tablet T. Li, et. al. , in press Pharm. Res. Bio. Med Anal.
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Tablet CU: Testing a Model CU for constant size portions of tablets must be larger than for the whole, so in spec using real time monitoring of “part” of the tablets means in spec for the whole tablet T. Li, et. al. , in press Pharm. Res. Bio. Med Anal.
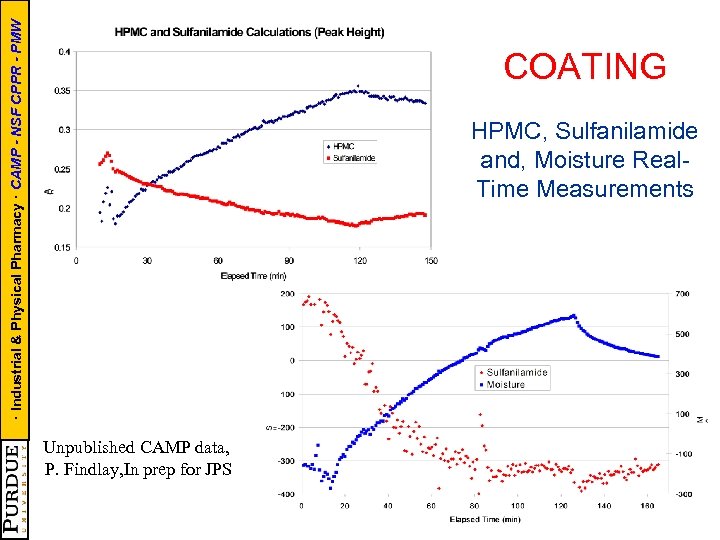 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW COATING HPMC, Sulfanilamide and, Moisture Real. Time Measurements Unpublished CAMP data, P. Findlay, In prep for JPS
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW COATING HPMC, Sulfanilamide and, Moisture Real. Time Measurements Unpublished CAMP data, P. Findlay, In prep for JPS
 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Where do we stand? § Taken individually these theories and techniques § § § look independent Together, however, they show a concerted effort to describe contributions to the overall process of drug development. These principles and techniques are applicable to batch and continuous processing and may be linked by multi-variate (chemometric) methods after univariate conformation. Ultimately this give us the ability to understand how development variables interact to influence the final product and to design in the quality.
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW Where do we stand? § Taken individually these theories and techniques § § § look independent Together, however, they show a concerted effort to describe contributions to the overall process of drug development. These principles and techniques are applicable to batch and continuous processing and may be linked by multi-variate (chemometric) methods after univariate conformation. Ultimately this give us the ability to understand how development variables interact to influence the final product and to design in the quality.
 · Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW The Business Case § Using existing scientific principles, monitoring and § § § modeling capabilities one will understand more about processes and be able to detect variations quickly • The earlier you start collecting information the more you’ll know the more comfortable everyone will be Given this level of knowledge and communication with FDA, you will be at the lowest risk (as proposed) possible for your product/process If your studies show up variability, the sooner you know the better. There is no such thing as what you don’t know won’t hurt you in science based development. The companies have many of the tools to lower their risk levels RIGHT NOW This will only improve with more research.
· Industrial & Physical Pharmacy · CAMP - NSF CPPR - PMW The Business Case § Using existing scientific principles, monitoring and § § § modeling capabilities one will understand more about processes and be able to detect variations quickly • The earlier you start collecting information the more you’ll know the more comfortable everyone will be Given this level of knowledge and communication with FDA, you will be at the lowest risk (as proposed) possible for your product/process If your studies show up variability, the sooner you know the better. There is no such thing as what you don’t know won’t hurt you in science based development. The companies have many of the tools to lower their risk levels RIGHT NOW This will only improve with more research.


