ea68c765d8c1cc4cd143c5e195bf93b5.ppt
- Количество слайдов: 70
 Implementing a Pharmaceutical Waste Management System Compliance and Pollution Prevention Workshop Kansas City December 6 th, 2007 John Brenna, R. Ph. Hospital Consultant Pharm. Ecology Associates, LLC jbrenna@pharmecology. com www. pharmecology. com 414 -292 -3959 Copyright © 2007 by Pharm. Ecology Associates, LLC
Implementing a Pharmaceutical Waste Management System Compliance and Pollution Prevention Workshop Kansas City December 6 th, 2007 John Brenna, R. Ph. Hospital Consultant Pharm. Ecology Associates, LLC jbrenna@pharmecology. com www. pharmecology. com 414 -292 -3959 Copyright © 2007 by Pharm. Ecology Associates, LLC
 Goals To develop a better understanding of the regulatory and environmental reasons for managing pharmaceutical waste more stringently Ø To review the definitions of a hazardous waste as they apply to waste pharmaceuticals Ø To explore implementation models for the management of hazardous pharmaceutical waste Ø Copyright 2007 Pharm. Ecology Associates, LLC
Goals To develop a better understanding of the regulatory and environmental reasons for managing pharmaceutical waste more stringently Ø To review the definitions of a hazardous waste as they apply to waste pharmaceuticals Ø To explore implementation models for the management of hazardous pharmaceutical waste Ø Copyright 2007 Pharm. Ecology Associates, LLC
 Who Are the Regulators? Ø USEPA & 10 Regions l l Ø Authorized State Hazardous Waste Programs l Ø Every state except Iowa and Alaska Local Publicly Owned Treatment Works (POTWs) l Ø Office of Solid Waste, Hazardous Waste Division Office of Water Must meet federal effluent guidelines The Joint Commission l l Primary accrediting body for many hospitals Federal funding dependent on accreditation Copyright 2007 Pharm. Ecology Associates, LLC
Who Are the Regulators? Ø USEPA & 10 Regions l l Ø Authorized State Hazardous Waste Programs l Ø Every state except Iowa and Alaska Local Publicly Owned Treatment Works (POTWs) l Ø Office of Solid Waste, Hazardous Waste Division Office of Water Must meet federal effluent guidelines The Joint Commission l l Primary accrediting body for many hospitals Federal funding dependent on accreditation Copyright 2007 Pharm. Ecology Associates, LLC
 USGS Water Quality Study* First nationwide reconnaissance of occurrence of pharmaceuticals, hormones, other organic wastewater contaminants – March, 2002 Ø 139 streams in 30 states, analyzed for 95 different OWCs Ø 82 of the 95 detected in at least one sample Ø One or more OWCs found in 80% of stream samples Ø 13% of sites had more than 20 OWCs Ø Minnesota Study: Found 79 out of 92; 23 were pharmaceuticals *http: //toxics. usgs. gov/pubs/OFR-02 -94/index. html Ø Copyright 2007 Pharm. Ecology Associates, LLC
USGS Water Quality Study* First nationwide reconnaissance of occurrence of pharmaceuticals, hormones, other organic wastewater contaminants – March, 2002 Ø 139 streams in 30 states, analyzed for 95 different OWCs Ø 82 of the 95 detected in at least one sample Ø One or more OWCs found in 80% of stream samples Ø 13% of sites had more than 20 OWCs Ø Minnesota Study: Found 79 out of 92; 23 were pharmaceuticals *http: //toxics. usgs. gov/pubs/OFR-02 -94/index. html Ø Copyright 2007 Pharm. Ecology Associates, LLC
 Below the Dose/Response Curve: Endocrine Disruptors Ø Ø Ø Endocrine Disruptors: chemicals that interfere with the normal function of the endocrine system (glands including thyroid, adrenals, ovaries, testicles) Mimic hormone, trigger identical response, block a hormone Do not follow the normal dose/response curve Active at much lower doses, especially in the fetus and newborn Estradiols, progesterone, testosterone Lindane Copyright 2007 Pharm. Ecology Associates, LLC
Below the Dose/Response Curve: Endocrine Disruptors Ø Ø Ø Endocrine Disruptors: chemicals that interfere with the normal function of the endocrine system (glands including thyroid, adrenals, ovaries, testicles) Mimic hormone, trigger identical response, block a hormone Do not follow the normal dose/response curve Active at much lower doses, especially in the fetus and newborn Estradiols, progesterone, testosterone Lindane Copyright 2007 Pharm. Ecology Associates, LLC
 The Faroes Statement Ø 200 environmental scientists from five continents met at the Faroes Islands in the North Atlantic –May 24, 2007 ØWarned of fetal exposure to toxic substances resulting in “fetal programming” to the 2 nd and 3 rd generation ØLifelong effects: obesity, diabetes, cancers, ADHD, Parkinson’s, Alzheimer’s, reduced immune system Ø“The dose makes the poison” replaced by “The timing makes the poison” ØNew approach to testing of chemicals strongly advocated; 80% of major chemicals never tested for damage to early development Øhttp: //www. precaution. org/lib/rpr-html. htm Copyright © 2007 by Pharm. Ecology Associates, LLC
The Faroes Statement Ø 200 environmental scientists from five continents met at the Faroes Islands in the North Atlantic –May 24, 2007 ØWarned of fetal exposure to toxic substances resulting in “fetal programming” to the 2 nd and 3 rd generation ØLifelong effects: obesity, diabetes, cancers, ADHD, Parkinson’s, Alzheimer’s, reduced immune system Ø“The dose makes the poison” replaced by “The timing makes the poison” ØNew approach to testing of chemicals strongly advocated; 80% of major chemicals never tested for damage to early development Øhttp: //www. precaution. org/lib/rpr-html. htm Copyright © 2007 by Pharm. Ecology Associates, LLC
 "When an activity raises threats of harm to human health or the environment, precautionary measures should be taken even if some cause and effect relationships are not fully established scientifically. " Wingspread Conference, Racine, WI 1998 Copyright 2007 Pharm. Ecology Associates, LLC
"When an activity raises threats of harm to human health or the environment, precautionary measures should be taken even if some cause and effect relationships are not fully established scientifically. " Wingspread Conference, Racine, WI 1998 Copyright 2007 Pharm. Ecology Associates, LLC
 Preliminary 2008 Effluent Guidelines Program Plan (Pre-publication version) Published in Federal Register Oct. 18 th, 2007 Ø Major pollutants of concern in discharges include pharmaceuticals and endocrine-disrupting compounds (EDCs) Ø Focus area: Unused pharmaceuticals Ø l Ø Physician offices, long-term care facilities, veterinary care services, hospitals and clinics Soliciting data, information, and comments on a variety of questions relating to disposal of unused pharmaceuticals Copyright 2007 Pharm. Ecology Associates, LLC
Preliminary 2008 Effluent Guidelines Program Plan (Pre-publication version) Published in Federal Register Oct. 18 th, 2007 Ø Major pollutants of concern in discharges include pharmaceuticals and endocrine-disrupting compounds (EDCs) Ø Focus area: Unused pharmaceuticals Ø l Ø Physician offices, long-term care facilities, veterinary care services, hospitals and clinics Soliciting data, information, and comments on a variety of questions relating to disposal of unused pharmaceuticals Copyright 2007 Pharm. Ecology Associates, LLC
 EPA’s Clean Water Act Review of the Management of Unused Pharmaceuticals for the Health Services Industrial Sector Ø What is the Scope of the Study? l l Ø What are They Studying? l l Ø Highlight good voluntary practices on an industry that may have significant discharges of consequence to the environment Focus include unused or expired pharmaceutical discharges to municipal wastewater treatment plants from hospitals, long-term care facilities, and veterinarians Current industry practices, guidance and regulatory requirements Source, and pass through or inhibition, associated with these discharges at municipal wastewater treatment plants Schedule: l l CY 2007: Complete data collection for identifying current industry practices, existing guidance/requirements, and possible BMPs FY 2008: Estimate pollutant loadings, identify possible best practices for controlling pollutant discharges and associated costs, and final report. Copyright 2007 Pharm. Ecology Associates, LLC
EPA’s Clean Water Act Review of the Management of Unused Pharmaceuticals for the Health Services Industrial Sector Ø What is the Scope of the Study? l l Ø What are They Studying? l l Ø Highlight good voluntary practices on an industry that may have significant discharges of consequence to the environment Focus include unused or expired pharmaceutical discharges to municipal wastewater treatment plants from hospitals, long-term care facilities, and veterinarians Current industry practices, guidance and regulatory requirements Source, and pass through or inhibition, associated with these discharges at municipal wastewater treatment plants Schedule: l l CY 2007: Complete data collection for identifying current industry practices, existing guidance/requirements, and possible BMPs FY 2008: Estimate pollutant loadings, identify possible best practices for controlling pollutant discharges and associated costs, and final report. Copyright 2007 Pharm. Ecology Associates, LLC
 Increasing USEPA Regulatory Activity Ø Region 1(New England) l Ø Veterans Administration Hospital, White River, Vermont, August 5 th, 2005 cited and fined $372, 254 for hazardous waste violations Region 2 (NY, NJ): l l North Shore University Hospital, Manhasset, NY fined $40, 000 (July 2003) Nassau University Medical Center, East Meadow, NY fined $279, 900 (Oct. 2003) Mountainside Hospital, Montclair, NJ fined $64, 349 (Nov. 2003) Memorial Sloan Kettering Cancer Center, New York , NY, fined $214, 420 Copyright 2007 Pharm. Ecology Associates, LLC
Increasing USEPA Regulatory Activity Ø Region 1(New England) l Ø Veterans Administration Hospital, White River, Vermont, August 5 th, 2005 cited and fined $372, 254 for hazardous waste violations Region 2 (NY, NJ): l l North Shore University Hospital, Manhasset, NY fined $40, 000 (July 2003) Nassau University Medical Center, East Meadow, NY fined $279, 900 (Oct. 2003) Mountainside Hospital, Montclair, NJ fined $64, 349 (Nov. 2003) Memorial Sloan Kettering Cancer Center, New York , NY, fined $214, 420 Copyright 2007 Pharm. Ecology Associates, LLC
 Additional USEPA Regional Activity Ø Region 3: PA, VA, WV, DE, MD, DC l Ø Region 4: KY, TN, NC, SC, FL, GA, MS, AL l Ø Sponsored seminars in 2006 & 2007; have been inspecting hospitals in Kansas City and St. Louis Region 8: ND, SD, MT, WY, UT, CO l Ø Site visits to hospitals in MN and WI Region 7: IA, MO, KS, NE l Ø Inspections in GA, AL, possibly additional states Region 5: OH, IN, IL, WI, MN, MI l Ø Located in Philadelphia, sponsored workshops in 2006 Sponsored SD DENR workshop Region 9: AZ, NV, CA l Inspecting hospitals in California Copyright 2007 Pharm. Ecology Associates, LLC
Additional USEPA Regional Activity Ø Region 3: PA, VA, WV, DE, MD, DC l Ø Region 4: KY, TN, NC, SC, FL, GA, MS, AL l Ø Sponsored seminars in 2006 & 2007; have been inspecting hospitals in Kansas City and St. Louis Region 8: ND, SD, MT, WY, UT, CO l Ø Site visits to hospitals in MN and WI Region 7: IA, MO, KS, NE l Ø Inspections in GA, AL, possibly additional states Region 5: OH, IN, IL, WI, MN, MI l Ø Located in Philadelphia, sponsored workshops in 2006 Sponsored SD DENR workshop Region 9: AZ, NV, CA l Inspecting hospitals in California Copyright 2007 Pharm. Ecology Associates, LLC
 Relationship to The Joint Commission Standards: Environment of Care Ø Ø Standard EC. 3. 10 The organization manages its hazardous materials and waste[1] risks. [1] Hazardous materials (HAZMAT) and waste: Materials whose handling, use, and storage are guided or regulated by local, state, or federal regulation. Examples include OSHA’s Regulations for Bloodborne Pathogens (regarding the blood, other infectious materials, contaminated items which would release blood or other infectious materials, or contaminated sharps), the Nuclear Regulatory Commission's regulations for handling and disposal of radioactive waste, management of hazardous vapors (such as glutaraldehyde, ethylene oxide, and nitrous oxide), chemicals regulated by the EPA, Department of Transportation requirements, and hazardous energy sources (for example, ionizing or non-ionizing radiation, lasers, microwaves, and ultrasound. ) Copyright 2007 Pharm. Ecology Associates, LLC
Relationship to The Joint Commission Standards: Environment of Care Ø Ø Standard EC. 3. 10 The organization manages its hazardous materials and waste[1] risks. [1] Hazardous materials (HAZMAT) and waste: Materials whose handling, use, and storage are guided or regulated by local, state, or federal regulation. Examples include OSHA’s Regulations for Bloodborne Pathogens (regarding the blood, other infectious materials, contaminated items which would release blood or other infectious materials, or contaminated sharps), the Nuclear Regulatory Commission's regulations for handling and disposal of radioactive waste, management of hazardous vapors (such as glutaraldehyde, ethylene oxide, and nitrous oxide), chemicals regulated by the EPA, Department of Transportation requirements, and hazardous energy sources (for example, ionizing or non-ionizing radiation, lasers, microwaves, and ultrasound. ) Copyright 2007 Pharm. Ecology Associates, LLC
 OSHA Hazardous Drugs Ø NIOSH Hazardous Drug Alert l Ø Hazardous drugs as defined by OSHA/NIOSH intersect but are not the same as EPA hazardous wastes ASHP Guidelines on Handling Hazardous Drugs l Deal primarily with OSHA employee exposure issues but also refer to required or recommended hazardous pharmaceutical waste management practices Copyright 2007 Pharm. Ecology Associates, LLC
OSHA Hazardous Drugs Ø NIOSH Hazardous Drug Alert l Ø Hazardous drugs as defined by OSHA/NIOSH intersect but are not the same as EPA hazardous wastes ASHP Guidelines on Handling Hazardous Drugs l Deal primarily with OSHA employee exposure issues but also refer to required or recommended hazardous pharmaceutical waste management practices Copyright 2007 Pharm. Ecology Associates, LLC
 Hazardous Drugs vs. Hazardous Waste Where OSHA & EPA Meet OSHA HAZARDOUS DRUGS - Genotoxicity - Teratogenicity - Reproductive toxicity - Carcinogenicity - Organ toxicity at low doses Examples: - Chemotherapy agents - Endocrine disruptors EPA TOXIC HAZARDOUS DRUG EXAMPLES EPA HAZARDOUS WASTE - Arsenic trioxide - Cyclophosphamide - Mitomycin - Melphalan P&U Listed Examples: - Epinephrine base - Warfarin - Nicotine EPA IGNITABLE HAZARDOUS DRUG EXAMPLES Characteristic Examples: - Formulations containing greater than or equal to 24% alcohol - Formulations containing heavy metals - Strong acids & bases - Paclitaxel - Valrubicin - Etoposide Copyright 2007 Pharm. Ecology Associates, LLC
Hazardous Drugs vs. Hazardous Waste Where OSHA & EPA Meet OSHA HAZARDOUS DRUGS - Genotoxicity - Teratogenicity - Reproductive toxicity - Carcinogenicity - Organ toxicity at low doses Examples: - Chemotherapy agents - Endocrine disruptors EPA TOXIC HAZARDOUS DRUG EXAMPLES EPA HAZARDOUS WASTE - Arsenic trioxide - Cyclophosphamide - Mitomycin - Melphalan P&U Listed Examples: - Epinephrine base - Warfarin - Nicotine EPA IGNITABLE HAZARDOUS DRUG EXAMPLES Characteristic Examples: - Formulations containing greater than or equal to 24% alcohol - Formulations containing heavy metals - Strong acids & bases - Paclitaxel - Valrubicin - Etoposide Copyright 2007 Pharm. Ecology Associates, LLC
 How is Pharmaceutical Waste Generated at the Healthcare Facility? IV Preparation Ø General Compounding Ø Spills/Breakage Ø Partially Used Vials Ø Partially Used Syringes/IVs l If Contaminated, Biohazardous Ø Discontinued, Unused Preparations Ø Unused Repacks (Unit Dose) Ø Patients’ Personal Medications Ø Outdated Pharmaceuticals Ø Copyright 2007 Pharm. Ecology Associates, LLC
How is Pharmaceutical Waste Generated at the Healthcare Facility? IV Preparation Ø General Compounding Ø Spills/Breakage Ø Partially Used Vials Ø Partially Used Syringes/IVs l If Contaminated, Biohazardous Ø Discontinued, Unused Preparations Ø Unused Repacks (Unit Dose) Ø Patients’ Personal Medications Ø Outdated Pharmaceuticals Ø Copyright 2007 Pharm. Ecology Associates, LLC
 When is an Outdated Drug a Waste? At the time and place the decision is made to discard it Ø Two EPA guidance letters to the industry: l Merck & Co. , 1981 l BFI Pharmaceutical, 1991 Ø Enables shipping of potentially usable outdates to a reverse distributor as product Ø PROHIBITS the shipping of waste-like items, such as unused IVs, partial vials, expired repacks, samples Ø Hospital is liable for using due diligence in selecting a vendor Ø Copyright 2007 Pharm. Ecology Associates, LLC
When is an Outdated Drug a Waste? At the time and place the decision is made to discard it Ø Two EPA guidance letters to the industry: l Merck & Co. , 1981 l BFI Pharmaceutical, 1991 Ø Enables shipping of potentially usable outdates to a reverse distributor as product Ø PROHIBITS the shipping of waste-like items, such as unused IVs, partial vials, expired repacks, samples Ø Hospital is liable for using due diligence in selecting a vendor Ø Copyright 2007 Pharm. Ecology Associates, LLC
 Non-returnable Waste Returnable Expired Product Copyright 2007 Pharm. Ecology Associates, LLC
Non-returnable Waste Returnable Expired Product Copyright 2007 Pharm. Ecology Associates, LLC
 RCRA: The Defining Regulation Resource Conservation & Recovery Act l Enacted in 1976, enforced by the EPA l Federal regulation of the disposal of solid wastes l Encourages the minimization of waste generation Ø Defines “hazardous waste” Ø “Cradle to Grave” tracking of hazardous waste Ø Copyright 2007 Pharm. Ecology Associates, LLC
RCRA: The Defining Regulation Resource Conservation & Recovery Act l Enacted in 1976, enforced by the EPA l Federal regulation of the disposal of solid wastes l Encourages the minimization of waste generation Ø Defines “hazardous waste” Ø “Cradle to Grave” tracking of hazardous waste Ø Copyright 2007 Pharm. Ecology Associates, LLC
 RCRA Risk Management & Liability Ø Civil and criminal liability l l Civil: State/USEPA enforcement Criminal: FBI, Attorney General, Grand Jury Ø Corporate fines: $32, 500/violation/day Ø Personal liability: fines and/or imprisonment Ø No statute of limitations Ø Managers up through CEO liable http: //www. epa. gov/compliance/resources/policies/criminal/exercise. pdf Copyright 2007 Pharm. Ecology Associates, LLC
RCRA Risk Management & Liability Ø Civil and criminal liability l l Civil: State/USEPA enforcement Criminal: FBI, Attorney General, Grand Jury Ø Corporate fines: $32, 500/violation/day Ø Personal liability: fines and/or imprisonment Ø No statute of limitations Ø Managers up through CEO liable http: //www. epa. gov/compliance/resources/policies/criminal/exercise. pdf Copyright 2007 Pharm. Ecology Associates, LLC
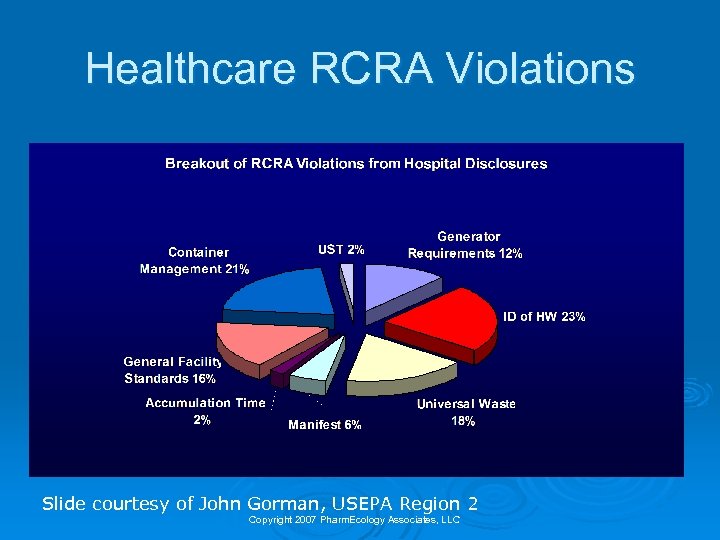 Healthcare RCRA Violations Slide courtesy of John Gorman, USEPA Region 2 Copyright 2007 Pharm. Ecology Associates, LLC
Healthcare RCRA Violations Slide courtesy of John Gorman, USEPA Region 2 Copyright 2007 Pharm. Ecology Associates, LLC
 Which Discarded Drugs Become RCRA Hazardous Waste? P-listed chemicals Ø l Sole active ingredient; unused; and empty containers U-listed chemicals Ø l Sole active ingredient; unused Characteristic of hazardous waste Ø l l Ignitability Toxicity Corrosivity Reactivity Copyright 2007 Pharm. Ecology Associates, LLC
Which Discarded Drugs Become RCRA Hazardous Waste? P-listed chemicals Ø l Sole active ingredient; unused; and empty containers U-listed chemicals Ø l Sole active ingredient; unused Characteristic of hazardous waste Ø l l Ignitability Toxicity Corrosivity Reactivity Copyright 2007 Pharm. Ecology Associates, LLC
 Examples of P-Listed Pharmaceutical Waste Ø Ø Ø Ø Arsenic trioxide Epinephrine base* Nicotine Nitroglycerin** (weak) Phentermine (CIV) Physostigmine Salicylate Warfarin >0. 3% P 012 P 042 P 075 P 081 P 046 P 204 P 188 P 001 *Salts excluded federally as of Oct. 16 th, 2007; States may or may not accept this position ** Excluded from the P list federally and in a number of states Copyright 2007 Pharm. Ecology Associates, LLC
Examples of P-Listed Pharmaceutical Waste Ø Ø Ø Ø Arsenic trioxide Epinephrine base* Nicotine Nitroglycerin** (weak) Phentermine (CIV) Physostigmine Salicylate Warfarin >0. 3% P 012 P 042 P 075 P 081 P 046 P 204 P 188 P 001 *Salts excluded federally as of Oct. 16 th, 2007; States may or may not accept this position ** Excluded from the P list federally and in a number of states Copyright 2007 Pharm. Ecology Associates, LLC
 Examples of P-Listed Pharmaceuticals Copyright 2007 Pharm. Ecology Associates, LLC
Examples of P-Listed Pharmaceuticals Copyright 2007 Pharm. Ecology Associates, LLC
 Examples of U-listed Pharmaceutical Waste Ø Chloral Hydrate(CIV) U 034 Ø Streptozotocin U 206 Ø Chlorambucil U 035 Ø Lindane U 129 Ø Cyclophosphamide U 058 Ø Saccharin U 202 Ø Daunomycin Ø Diethylstilbestrol Ø Melphalan Ø Mitomycin C U 059 Ø Selenium Sulfide U 205 U 089 Ø Uracil Mustard U 237 U 150 Ø Warfarin<0. 3% U 248 U 010 Copyright 2007 Pharm. Ecology Associates, LLC
Examples of U-listed Pharmaceutical Waste Ø Chloral Hydrate(CIV) U 034 Ø Streptozotocin U 206 Ø Chlorambucil U 035 Ø Lindane U 129 Ø Cyclophosphamide U 058 Ø Saccharin U 202 Ø Daunomycin Ø Diethylstilbestrol Ø Melphalan Ø Mitomycin C U 059 Ø Selenium Sulfide U 205 U 089 Ø Uracil Mustard U 237 U 150 Ø Warfarin<0. 3% U 248 U 010 Copyright 2007 Pharm. Ecology Associates, LLC
 Examples of U-Listed Pharmaceuticals Copyright 2007 Pharm. Ecology Associates, LLC
Examples of U-Listed Pharmaceuticals Copyright 2007 Pharm. Ecology Associates, LLC
 Characteristic of Ignitability Ø Ø Ø Ø Aqueous Solution containing 24% alcohol or more by volume & flash point<140° F Non-aqueous solutions with flash points <140 ° F Oxidizers Flammable aerosols Hazardous Waste Number: D 001 Rubbing Alcohol Topical Preparations Injections Copyright 2007 Pharm. Ecology Associates, LLC
Characteristic of Ignitability Ø Ø Ø Ø Aqueous Solution containing 24% alcohol or more by volume & flash point<140° F Non-aqueous solutions with flash points <140 ° F Oxidizers Flammable aerosols Hazardous Waste Number: D 001 Rubbing Alcohol Topical Preparations Injections Copyright 2007 Pharm. Ecology Associates, LLC
 Characteristic of Corrosivity Ø An aqueous solution having a p. H < or = 2 or > or = to 12. 5 Ø Examples: Primarily compounding chemicals l l Glacial Acetic Acid Sodium Hydroxide Ø Hazardous waste number: D 002 Copyright 2007 Pharm. Ecology Associates, LLC
Characteristic of Corrosivity Ø An aqueous solution having a p. H < or = 2 or > or = to 12. 5 Ø Examples: Primarily compounding chemicals l l Glacial Acetic Acid Sodium Hydroxide Ø Hazardous waste number: D 002 Copyright 2007 Pharm. Ecology Associates, LLC
 Characteristic of Toxicity 40 chemicals which must be below specific leaching concentrations Ø Must pass the Toxicity Characteristic Leaching Procedure (TCLP) Ø Must evaluate IVs, such as TPN (total parenteral nutrition)– may come out of regulation due to dilution Ø Examples of potential toxic pharmaceuticals: Ø Ø Ø Arsenic Barium Cadmium Chromium Lindane m-Cresol Mercury (thimerosal, phenylmercuric acetate) Selenium Silver Copyright 2007 Pharm. Ecology Associates, LLC
Characteristic of Toxicity 40 chemicals which must be below specific leaching concentrations Ø Must pass the Toxicity Characteristic Leaching Procedure (TCLP) Ø Must evaluate IVs, such as TPN (total parenteral nutrition)– may come out of regulation due to dilution Ø Examples of potential toxic pharmaceuticals: Ø Ø Ø Arsenic Barium Cadmium Chromium Lindane m-Cresol Mercury (thimerosal, phenylmercuric acetate) Selenium Silver Copyright 2007 Pharm. Ecology Associates, LLC
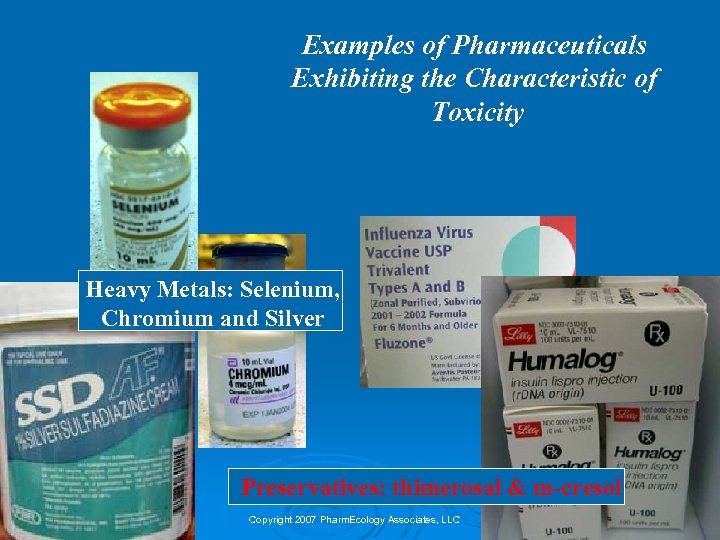 Examples of Pharmaceuticals Exhibiting the Characteristic of Toxicity Heavy Metals: Selenium, Chromium and Silver Preservatives: thimerosal & m-cresol Copyright 2007 Pharm. Ecology Associates, LLC
Examples of Pharmaceuticals Exhibiting the Characteristic of Toxicity Heavy Metals: Selenium, Chromium and Silver Preservatives: thimerosal & m-cresol Copyright 2007 Pharm. Ecology Associates, LLC
 Characteristic of Reactivity Ø Meet eight separate criteria identifying certain explosive and water reactive wastes Ø Nitroglycerin formulations may be considered excluded federally from the P 081 listing as non-reactive as of August 14, 2001 under FR: May 16, 2001, unless they exhibit another characteristics, such as ignitability. Ø Many, but not all states, have adopted the federal exclusion for nitroglycerin. Waste must still be evaluated for ignitability. Ø Hazardous Waste Number for reactives: D 003 Copyright 2007 Pharm. Ecology Associates, LLC
Characteristic of Reactivity Ø Meet eight separate criteria identifying certain explosive and water reactive wastes Ø Nitroglycerin formulations may be considered excluded federally from the P 081 listing as non-reactive as of August 14, 2001 under FR: May 16, 2001, unless they exhibit another characteristics, such as ignitability. Ø Many, but not all states, have adopted the federal exclusion for nitroglycerin. Waste must still be evaluated for ignitability. Ø Hazardous Waste Number for reactives: D 003 Copyright 2007 Pharm. Ecology Associates, LLC
 Chemotherapy Agents: Many Are Not Regulated by RCRA Eight chemotherapy agents are U-listed; one is P -listed Ø About 100 chemotherapy agents not regulated by EPA Ø Examples: Ø l l l Alkylating agents: Cisplatin, Thiotepa Antimetabolites: Fluorouracil, Methotrexate Hormonal (antiandrogen): Lupron® (leuprolide) Hormonal (antiestrogen): Tamoxifen Mitotic Inhibitor: Taxol® (paclitaxol) Copyright 2007 Pharm. Ecology Associates, LLC
Chemotherapy Agents: Many Are Not Regulated by RCRA Eight chemotherapy agents are U-listed; one is P -listed Ø About 100 chemotherapy agents not regulated by EPA Ø Examples: Ø l l l Alkylating agents: Cisplatin, Thiotepa Antimetabolites: Fluorouracil, Methotrexate Hormonal (antiandrogen): Lupron® (leuprolide) Hormonal (antiestrogen): Tamoxifen Mitotic Inhibitor: Taxol® (paclitaxol) Copyright 2007 Pharm. Ecology Associates, LLC
 Two Types of Chemotherapy Waste Ø Trace Chemotherapy Waste l l l Ø Medical waste hauler protocols for “Chemo Waste” Empty vials, syringes, IV’s Treated as infectious medical waste preferably through regulated medical waste incineration “Bulk” Chemotherapy Waste l If not empty, should be placed into Hazardous Waste container Copyright 2007 Pharm. Ecology Associates, LLC
Two Types of Chemotherapy Waste Ø Trace Chemotherapy Waste l l l Ø Medical waste hauler protocols for “Chemo Waste” Empty vials, syringes, IV’s Treated as infectious medical waste preferably through regulated medical waste incineration “Bulk” Chemotherapy Waste l If not empty, should be placed into Hazardous Waste container Copyright 2007 Pharm. Ecology Associates, LLC
 Definition of “Empty” Ø “P” List Containers of “P” listed chemicals are considered hazardous waste, unless they have been rinsed three times and the rinsate discarded as hazardous waste. Ø “U” List Containers of “U” listed chemicals are empty only when l All contents removed that can be removed through normal means l And no more than 3% by weight remains l Example: “Empty” Cytoxan vial would be “trace” chemotherapy Copyright 2007 Pharm. Ecology Associates, LLC
Definition of “Empty” Ø “P” List Containers of “P” listed chemicals are considered hazardous waste, unless they have been rinsed three times and the rinsate discarded as hazardous waste. Ø “U” List Containers of “U” listed chemicals are empty only when l All contents removed that can be removed through normal means l And no more than 3% by weight remains l Example: “Empty” Cytoxan vial would be “trace” chemotherapy Copyright 2007 Pharm. Ecology Associates, LLC
 What Is Pharm. E Hazardous® Waste? Ø Drugs which may cause harm to human health or the environment and need to be managed according to BMPs l l NIOSH Hazardous Drug Alert Appendix A The US Department of Health and Human Services National Toxicology Program's Report on Carcinogens (11 th Edition) l l Ø Drugs with LD 50 s at or below 50 mg/kg Endocrine disruptors BMP recommendation is to segregate at least chemo agents into RCRA toxic hazardous waste containers and to dispose of other agents through incineration Copyright 2007 Pharm. Ecology Associates, LLC
What Is Pharm. E Hazardous® Waste? Ø Drugs which may cause harm to human health or the environment and need to be managed according to BMPs l l NIOSH Hazardous Drug Alert Appendix A The US Department of Health and Human Services National Toxicology Program's Report on Carcinogens (11 th Edition) l l Ø Drugs with LD 50 s at or below 50 mg/kg Endocrine disruptors BMP recommendation is to segregate at least chemo agents into RCRA toxic hazardous waste containers and to dispose of other agents through incineration Copyright 2007 Pharm. Ecology Associates, LLC
 Federal Waste Generation Status Ø Large Quantity Generator (LQG): generates more than 1000 kg/month of hazardous waste or >1 kg/month “P” listed waste. Ø Small Quantity Generator (SQG): Generates <1000 kg/month but >100 kg/month of hazardous waste & < or = 1 kg/month “P” listed waste. Ø Conditionally Exempt Small Quantity Generator (CESQG) : Generates < or = 100 kg haz waste/month, < or = 1 kg P listed waste/month Copyright 2007 Pharm. Ecology Associates, LLC
Federal Waste Generation Status Ø Large Quantity Generator (LQG): generates more than 1000 kg/month of hazardous waste or >1 kg/month “P” listed waste. Ø Small Quantity Generator (SQG): Generates <1000 kg/month but >100 kg/month of hazardous waste & < or = 1 kg/month “P” listed waste. Ø Conditionally Exempt Small Quantity Generator (CESQG) : Generates < or = 100 kg haz waste/month, < or = 1 kg P listed waste/month Copyright 2007 Pharm. Ecology Associates, LLC
 Documenting Generator Status Ø Large quantity generator: no need to record P waste separately. Ø Small quantity generator or CESQG: need to segregate all P-listed including empty containers and document weights per calendar month Ø Cannot exceed 1 kg or 2. 2 lbs/month for any given month Copyright 2007 Pharm. Ecology Associates, LLC
Documenting Generator Status Ø Large quantity generator: no need to record P waste separately. Ø Small quantity generator or CESQG: need to segregate all P-listed including empty containers and document weights per calendar month Ø Cannot exceed 1 kg or 2. 2 lbs/month for any given month Copyright 2007 Pharm. Ecology Associates, LLC
 How Should RCRA Hazardous Waste be Handled? Ø Need one or two new waste streams in Pharmacy, certain Patient Care Areas, Oncology Clinics Ø RCRA Hazardous Waste: Toxic l P, U, toxic Ds, (all Chemotherapy Residues, Chemo Spills) Ø RCRA Hazardous Waste: Ignitable (D 001) Ø May be able to combine these into one waste stream based on state and waste vendor requirements Copyright 2007 Pharm. Ecology Associates, LLC
How Should RCRA Hazardous Waste be Handled? Ø Need one or two new waste streams in Pharmacy, certain Patient Care Areas, Oncology Clinics Ø RCRA Hazardous Waste: Toxic l P, U, toxic Ds, (all Chemotherapy Residues, Chemo Spills) Ø RCRA Hazardous Waste: Ignitable (D 001) Ø May be able to combine these into one waste stream based on state and waste vendor requirements Copyright 2007 Pharm. Ecology Associates, LLC
 Traditional Chemo Waste Containers New Hazardous Waste Containers Bulk chemo in vials, unused IV’s, P, U. toxic D Empty vials, syringes, IVs, tubing, gowns, gloves, etc. Covidien/Kendall Copyright 2007 Pharm. Ecology Associates, LLC
Traditional Chemo Waste Containers New Hazardous Waste Containers Bulk chemo in vials, unused IV’s, P, U. toxic D Empty vials, syringes, IVs, tubing, gowns, gloves, etc. Covidien/Kendall Copyright 2007 Pharm. Ecology Associates, LLC
 How Should RCRA Hazardous Waste Be Disposed? Ø Either contract with a hazardous waste broker or develop internal expertise for: l Labeling l Waste profiling l Manifest preparation l Land ban preparation Ø Contract with a federally permitted RCRA hazardous waste incineration facility (TSDF: Treatment, Storage & Disposal Facility) Copyright 2007 Pharm. Ecology Associates, LLC
How Should RCRA Hazardous Waste Be Disposed? Ø Either contract with a hazardous waste broker or develop internal expertise for: l Labeling l Waste profiling l Manifest preparation l Land ban preparation Ø Contract with a federally permitted RCRA hazardous waste incineration facility (TSDF: Treatment, Storage & Disposal Facility) Copyright 2007 Pharm. Ecology Associates, LLC
 How Should Non-hazardous Drugs be Handled, Stored and Disposed? Ø Ø Ø BMPs strongly discourage sewering and landfilling of non -hazardous drugs Organization can minimize risks by adopting BMPs Possible exception: controlled substances due to difficulty in rendering non-recoverable under Drug Enforcement Administration (DEA) regulations Consider segregating into white Covidien container with blue top Label “Incinerate Only” Dispose at a regulated medical waste incinerator or municipal incinerator that is permitted to accept nonhazardous pharmaceutical waste Copyright 2007 Pharm. Ecology Associates, LLC
How Should Non-hazardous Drugs be Handled, Stored and Disposed? Ø Ø Ø BMPs strongly discourage sewering and landfilling of non -hazardous drugs Organization can minimize risks by adopting BMPs Possible exception: controlled substances due to difficulty in rendering non-recoverable under Drug Enforcement Administration (DEA) regulations Consider segregating into white Covidien container with blue top Label “Incinerate Only” Dispose at a regulated medical waste incinerator or municipal incinerator that is permitted to accept nonhazardous pharmaceutical waste Copyright 2007 Pharm. Ecology Associates, LLC
 A Quick Primer on Incinerators Ø Municipal l Ø Medical Waste l l Ø Permitted to burn municipal “garbage” Usually not permitted to handle infectious waste May be permitted to handle non-hazardous pharmaceuticals, with certain volume restrictions Permitted by USEPA and the state to accept pathology waste, red bag and red sharps waste, trace chemo waste May be permitted to accept non-hazardous pharmaceutical waste Hazardous Waste l l l Permitted by USEPA, known as a Treatment, Storage and Disposal Facility (TSDF) High temperature, molecular bonds broken Authorized to accept the “worst of the worst” hazardous chemicals, shipped on a 6 -part Uniform Manifest Copyright 2007 Pharm. Ecology Associates, LLC
A Quick Primer on Incinerators Ø Municipal l Ø Medical Waste l l Ø Permitted to burn municipal “garbage” Usually not permitted to handle infectious waste May be permitted to handle non-hazardous pharmaceuticals, with certain volume restrictions Permitted by USEPA and the state to accept pathology waste, red bag and red sharps waste, trace chemo waste May be permitted to accept non-hazardous pharmaceutical waste Hazardous Waste l l l Permitted by USEPA, known as a Treatment, Storage and Disposal Facility (TSDF) High temperature, molecular bonds broken Authorized to accept the “worst of the worst” hazardous chemicals, shipped on a 6 -part Uniform Manifest Copyright 2007 Pharm. Ecology Associates, LLC
 Common Pharmaceutical Waste Stream Management Type of Waste Container Color code Contents Treatment Method Red bag (nonpathology) Red Biohazardous (RMW) + Rx Autoclave/ Landfill Red sharps/ needlebox Red Biohazardous; needles, etc. + Rx Autoclave/ Landfill Trace chemo Rx Yellow or White Bulk & Trace Chemo, needles, tubing RMW Incineration Sewer Unused IVs, tablets, etc. Wastewater Treatment Plant Municipal Trash Unused ointments, etc. Landfill Copyright 2007 Pharm. Ecology Associates, LLC
Common Pharmaceutical Waste Stream Management Type of Waste Container Color code Contents Treatment Method Red bag (nonpathology) Red Biohazardous (RMW) + Rx Autoclave/ Landfill Red sharps/ needlebox Red Biohazardous; needles, etc. + Rx Autoclave/ Landfill Trace chemo Rx Yellow or White Bulk & Trace Chemo, needles, tubing RMW Incineration Sewer Unused IVs, tablets, etc. Wastewater Treatment Plant Municipal Trash Unused ointments, etc. Landfill Copyright 2007 Pharm. Ecology Associates, LLC
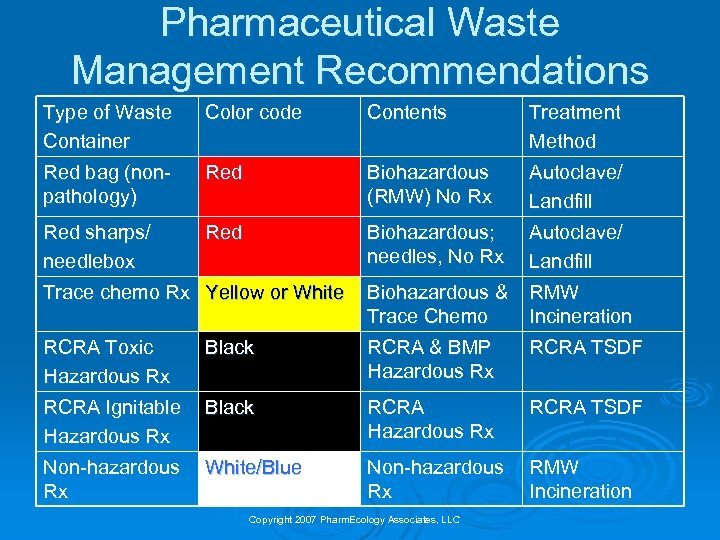 Pharmaceutical Waste Management Recommendations Type of Waste Container Color code Contents Treatment Method Red bag (nonpathology) Red Biohazardous (RMW) No Rx Autoclave/ Landfill Red sharps/ needlebox Red Biohazardous; needles, No Rx Autoclave/ Landfill Trace chemo Rx Yellow or White Biohazardous & Trace Chemo RMW Incineration RCRA Toxic Hazardous Rx Black RCRA & BMP Hazardous Rx RCRA TSDF RCRA Ignitable Hazardous Rx Black RCRA Hazardous Rx RCRA TSDF Non-hazardous Rx White/Blue Non-hazardous Rx RMW Incineration Copyright 2007 Pharm. Ecology Associates, LLC
Pharmaceutical Waste Management Recommendations Type of Waste Container Color code Contents Treatment Method Red bag (nonpathology) Red Biohazardous (RMW) No Rx Autoclave/ Landfill Red sharps/ needlebox Red Biohazardous; needles, No Rx Autoclave/ Landfill Trace chemo Rx Yellow or White Biohazardous & Trace Chemo RMW Incineration RCRA Toxic Hazardous Rx Black RCRA & BMP Hazardous Rx RCRA TSDF RCRA Ignitable Hazardous Rx Black RCRA Hazardous Rx RCRA TSDF Non-hazardous Rx White/Blue Non-hazardous Rx RMW Incineration Copyright 2007 Pharm. Ecology Associates, LLC
 Satellite Accumulation Ø Ø Ø Segregated, labeled and contained in areas where it is generated Available in all units in which hazardous waste is generated & must be under the control of the operator Label each container as “Hazardous Waste” with the appropriate waste stream noted No time limit to fill the container No more than 55 gallons of U listed and characteristic waste or 1 quart of P listed waste may be accumulated Must be moved to storage accumulation within 3 days after these quantities are reached (federal regulations) l Some states are stricter Copyright 2007 Pharm. Ecology Associates, LLC
Satellite Accumulation Ø Ø Ø Segregated, labeled and contained in areas where it is generated Available in all units in which hazardous waste is generated & must be under the control of the operator Label each container as “Hazardous Waste” with the appropriate waste stream noted No time limit to fill the container No more than 55 gallons of U listed and characteristic waste or 1 quart of P listed waste may be accumulated Must be moved to storage accumulation within 3 days after these quantities are reached (federal regulations) l Some states are stricter Copyright 2007 Pharm. Ecology Associates, LLC
 Storage Accumulation Hazardous Waste Storage Accumulation Site: Ø Provides a safe and secure storage area for hazardous waste while it awaits shipping. Ø Same locked area as mercury, xylene, formaldehyde, lab chemicals Ø Maximum storage time: Ø 90 days if LQG; 180 days if SQG Copyright 2007 Pharm. Ecology Associates, LLC
Storage Accumulation Hazardous Waste Storage Accumulation Site: Ø Provides a safe and secure storage area for hazardous waste while it awaits shipping. Ø Same locked area as mercury, xylene, formaldehyde, lab chemicals Ø Maximum storage time: Ø 90 days if LQG; 180 days if SQG Copyright 2007 Pharm. Ecology Associates, LLC
 What Departments Get Involved in Managing Pharmaceutical Waste? Pharmacy Ø Nursing Ø Infection Control Ø Environmental Services Ø Safety Ø Facility Management Ø Risk Management Ø Materials Management Ø Copyright 2007 Pharm. Ecology Associates, LLC
What Departments Get Involved in Managing Pharmaceutical Waste? Pharmacy Ø Nursing Ø Infection Control Ø Environmental Services Ø Safety Ø Facility Management Ø Risk Management Ø Materials Management Ø Copyright 2007 Pharm. Ecology Associates, LLC
 Considering the Optimal Management Options l l l Need to label items that need segregation in a manner that makes it easy for pharmacy and nursing personnel Shelf stickers in pharmacy Data Applied to Dispensing Software and/or Message inserted into automated dispensing machines, etc. and MAR (Medication Administration Record) and/or Stickers Applied Manually Copyright 2007 Pharm. Ecology Associates, LLC
Considering the Optimal Management Options l l l Need to label items that need segregation in a manner that makes it easy for pharmacy and nursing personnel Shelf stickers in pharmacy Data Applied to Dispensing Software and/or Message inserted into automated dispensing machines, etc. and MAR (Medication Administration Record) and/or Stickers Applied Manually Copyright 2007 Pharm. Ecology Associates, LLC
 Getting Implementation Done: Choosing a Model Ø Model 1: Automated sorting device l In development Ø Model 2: Labeling electronically Ø Model 3: Labeling manually Ø Model 4: Central collection and sorting Ø Model 5: Manage all as hazardous waste Copyright 2007 Pharm. Ecology Associates, LLC
Getting Implementation Done: Choosing a Model Ø Model 1: Automated sorting device l In development Ø Model 2: Labeling electronically Ø Model 3: Labeling manually Ø Model 4: Central collection and sorting Ø Model 5: Manage all as hazardous waste Copyright 2007 Pharm. Ecology Associates, LLC
 Model 2: Electronic Labeling Entire inventory is analyzed Shelf stickers in pharmacy Data is entered into the dispensing software at the NDC or “pneumonic” level Ø Label prints with pre-determined code Ø Ø Ø l HW 1, RCRA 1, Black Bin, etc. Nursing staff are trained on waste segregation based on codes Ø Black “satellite accumulation” containers in soiled utility rooms Ø Hybrid Model: North Memorial Health Care Ø l Programmed automated dispensing machines (e. g. , Pyxis) Copyright 2007 Pharm. Ecology Associates, LLC
Model 2: Electronic Labeling Entire inventory is analyzed Shelf stickers in pharmacy Data is entered into the dispensing software at the NDC or “pneumonic” level Ø Label prints with pre-determined code Ø Ø Ø l HW 1, RCRA 1, Black Bin, etc. Nursing staff are trained on waste segregation based on codes Ø Black “satellite accumulation” containers in soiled utility rooms Ø Hybrid Model: North Memorial Health Care Ø l Programmed automated dispensing machines (e. g. , Pyxis) Copyright 2007 Pharm. Ecology Associates, LLC
 Model 3: Manual Labeling of Hazardous Waste Entire inventory is analyzed Shelf stickers in pharmacy Items are stickered upon dispensing Nursing staff are trained on waste segregation based on stickers Ø Black “satellite accumulation” containers in soiled utility rooms Ø Model program: North Memorial Health Care l http: //www. hospitalconnect. com/hfmmagazine/jsp/arti cledisplay. jsp? dcrpath=HFMMAGAZINE/Pubs. News. Ar ticle. Gen/data/2006 March/0603 HFM_DEPT_Envir. Ser &domain=HFMMAGAZINE Ø Ø Copyright 2007 Pharm. Ecology Associates, LLC
Model 3: Manual Labeling of Hazardous Waste Entire inventory is analyzed Shelf stickers in pharmacy Items are stickered upon dispensing Nursing staff are trained on waste segregation based on stickers Ø Black “satellite accumulation” containers in soiled utility rooms Ø Model program: North Memorial Health Care l http: //www. hospitalconnect. com/hfmmagazine/jsp/arti cledisplay. jsp? dcrpath=HFMMAGAZINE/Pubs. News. Ar ticle. Gen/data/2006 March/0603 HFM_DEPT_Envir. Ser &domain=HFMMAGAZINE Ø Ø Copyright 2007 Pharm. Ecology Associates, LLC
 North Memorial Health Care Robbinsdale, MN SPECIAL DISPOSAL REQUIRED Photos courtesy of North Memorial Health Care Copyright 2007 Pharm. Ecology Associates, LLC
North Memorial Health Care Robbinsdale, MN SPECIAL DISPOSAL REQUIRED Photos courtesy of North Memorial Health Care Copyright 2007 Pharm. Ecology Associates, LLC
 Model 4: Centralizing Segregation Ø All pharmaceutical waste is collected in hazardous waste containers in the pharmacy and in the nursing units Ø The mixed waste is removed to the central hazardous waste storage accumulation area Ø Sorting is done by hazardous waste vendor or trained hospital staff based on an analysis of the inventory Ø Hazardous waste and related items are manifested and disposed as such Ø Model: Abbott Northwestern Hospital Copyright 2007 Pharm. Ecology Associates, LLC
Model 4: Centralizing Segregation Ø All pharmaceutical waste is collected in hazardous waste containers in the pharmacy and in the nursing units Ø The mixed waste is removed to the central hazardous waste storage accumulation area Ø Sorting is done by hazardous waste vendor or trained hospital staff based on an analysis of the inventory Ø Hazardous waste and related items are manifested and disposed as such Ø Model: Abbott Northwestern Hospital Copyright 2007 Pharm. Ecology Associates, LLC
 Abbott Northwestern Hospital Minneapolis, MN Copyright 2007 Pharm. Ecology Associates, LLC
Abbott Northwestern Hospital Minneapolis, MN Copyright 2007 Pharm. Ecology Associates, LLC
 Model 5: Managing All Pharmaceutical Waste as Hazardous Ø Easiest, most expensive Ø May still need to sort out ignitables Ø Still need to do analysis of inventory to determine waste codes for manifesting Ø Hybrid Model: UW Health, Madison, WI l l All tablets/capsules/solids hazardous IVs hazardous if RCRA, Pharm. E Hazardous® (BMP) Copyright 2007 Pharm. Ecology Associates, LLC
Model 5: Managing All Pharmaceutical Waste as Hazardous Ø Easiest, most expensive Ø May still need to sort out ignitables Ø Still need to do analysis of inventory to determine waste codes for manifesting Ø Hybrid Model: UW Health, Madison, WI l l All tablets/capsules/solids hazardous IVs hazardous if RCRA, Pharm. E Hazardous® (BMP) Copyright 2007 Pharm. Ecology Associates, LLC
 UW Health Madison, WI Copyright 2007 Pharm. Ecology Associates, LLC
UW Health Madison, WI Copyright 2007 Pharm. Ecology Associates, LLC
 Summary Managing hazardous pharmaceutical waste is an emerging and rapidly evolving management area Ø Several implementation models are available and more may evolve Ø Successful implementation will require an interdisciplinary team committed to moving forward Ø The rewards include cost-effective compliance and evidence of a commitment to environmental excellence Ø Copyright 2007 Pharm. Ecology Associates, LLC
Summary Managing hazardous pharmaceutical waste is an emerging and rapidly evolving management area Ø Several implementation models are available and more may evolve Ø Successful implementation will require an interdisciplinary team committed to moving forward Ø The rewards include cost-effective compliance and evidence of a commitment to environmental excellence Ø Copyright 2007 Pharm. Ecology Associates, LLC
 Resources Ø NIOSH Hazardous Drug Alert l Ø ASHP Guidance on Handling Hazardous Drugs l Ø l l http: //www. h 2 e-online. org/ Pharmaceutical waste webpage: http: //www. h 2 e-online. org/hazmat/pharma. html Managing Pharmaceutical Waste: A 10 -Step Blueprint for Health Care Facilities In the United States: http: //www. h 2 e-online. org/docs/h 2 epharmablueprint 41506. pdf Pharm. Ecology Associates, LLC § l l Ø http: //www. osha-slc. gov/dts/osta/otm_vi/otm_vi_2. html Hospitals for a Healthy Environment l Ø http: //www. ashp. org/s_ashp/bin. asp? CID=6&DID=5420&DOC=FILE. PDF OSHA Technical Manual l Ø http: //www. cdc. gov/niosh/docs/2004 -165/#sum www. pharmecology. com FAQs, state and federal waste regulations, subscription search engine Pharm. E™ Waste Wizard identifies RCRA hazardous waste plus NIOSH hazardous drugs, among additional criteria Pharmaceuticals and Personal Care Products as Environmental Pollutants: § http: //www. epa. gov/nerlesd 1/chemistry/pharma/index. htm Copyright 2007 Pharm. Ecology Associates, LLC
Resources Ø NIOSH Hazardous Drug Alert l Ø ASHP Guidance on Handling Hazardous Drugs l Ø l l http: //www. h 2 e-online. org/ Pharmaceutical waste webpage: http: //www. h 2 e-online. org/hazmat/pharma. html Managing Pharmaceutical Waste: A 10 -Step Blueprint for Health Care Facilities In the United States: http: //www. h 2 e-online. org/docs/h 2 epharmablueprint 41506. pdf Pharm. Ecology Associates, LLC § l l Ø http: //www. osha-slc. gov/dts/osta/otm_vi/otm_vi_2. html Hospitals for a Healthy Environment l Ø http: //www. ashp. org/s_ashp/bin. asp? CID=6&DID=5420&DOC=FILE. PDF OSHA Technical Manual l Ø http: //www. cdc. gov/niosh/docs/2004 -165/#sum www. pharmecology. com FAQs, state and federal waste regulations, subscription search engine Pharm. E™ Waste Wizard identifies RCRA hazardous waste plus NIOSH hazardous drugs, among additional criteria Pharmaceuticals and Personal Care Products as Environmental Pollutants: § http: //www. epa. gov/nerlesd 1/chemistry/pharma/index. htm Copyright 2007 Pharm. Ecology Associates, LLC
 Questions? John Brenna, R. Pharm. Ecology Associates, LLC info@pharmecology. com www. pharmecology. com 414 -292 -3959 Copyright 2007 Pharm. Ecology Associates, LLC
Questions? John Brenna, R. Pharm. Ecology Associates, LLC info@pharmecology. com www. pharmecology. com 414 -292 -3959 Copyright 2007 Pharm. Ecology Associates, LLC
 Pharmaceutical Waste Management In which waste container should you discard each of the following items? What is the appropriate waste stream designation for each? How should each item be destroyed? Copyright 2007 Pharm. Ecology Associates, LLC
Pharmaceutical Waste Management In which waste container should you discard each of the following items? What is the appropriate waste stream designation for each? How should each item be destroyed? Copyright 2007 Pharm. Ecology Associates, LLC
 Empty cyclophosphamide IV bag Yellow chemotherapy container Regulated Medical Waste Incinerate only Copyright © 2007 by Pharm. Ecology Associates, LLC
Empty cyclophosphamide IV bag Yellow chemotherapy container Regulated Medical Waste Incinerate only Copyright © 2007 by Pharm. Ecology Associates, LLC
 Partial vial of paclitaxel Black hazardous waste container– ignitable RCRA Hazardous Waste Federally permitted Treatment, Storage and Disposal Facility Copyright © 2007 by Pharm. Ecology Associates, LLC
Partial vial of paclitaxel Black hazardous waste container– ignitable RCRA Hazardous Waste Federally permitted Treatment, Storage and Disposal Facility Copyright © 2007 by Pharm. Ecology Associates, LLC
 Used chemo gown Yellow chemotherapy container Regulated Medical Waste Incinerate only Copyright © 2007 by Pharm. Ecology Associates, LLC
Used chemo gown Yellow chemotherapy container Regulated Medical Waste Incinerate only Copyright © 2007 by Pharm. Ecology Associates, LLC
 Empty arsenic trioxide ampule Black hazardous waste container – toxic RCRA Hazardous Waste Federally permitted Treatment, Storage and Disposal Facility Copyright © 2007 by Pharm. Ecology Associates, LLC
Empty arsenic trioxide ampule Black hazardous waste container – toxic RCRA Hazardous Waste Federally permitted Treatment, Storage and Disposal Facility Copyright © 2007 by Pharm. Ecology Associates, LLC
 Contents of spill kit after a hazardous spill clean-up Black hazardous waste container – toxic RCRA Hazardous Waste Federally permitted Treatment, Storage and Disposal Facility Copyright © 2007 by Pharm. Ecology Associates, LLC
Contents of spill kit after a hazardous spill clean-up Black hazardous waste container – toxic RCRA Hazardous Waste Federally permitted Treatment, Storage and Disposal Facility Copyright © 2007 by Pharm. Ecology Associates, LLC
 Partial Methotrexate IV Black hazardous waste container – toxic Best Management Practice RCRA Hazardous Waste Federally permitted Treatment, Storage and Disposal Facility Copyright © 2007 by Pharm. Ecology Associates, LLC
Partial Methotrexate IV Black hazardous waste container – toxic Best Management Practice RCRA Hazardous Waste Federally permitted Treatment, Storage and Disposal Facility Copyright © 2007 by Pharm. Ecology Associates, LLC
 Expired Methotrexate tablets (Original container) Reverse distribution Copyright © 2007 by Pharm. Ecology Associates, LLC
Expired Methotrexate tablets (Original container) Reverse distribution Copyright © 2007 by Pharm. Ecology Associates, LLC
 Ziplock bag used to transport chemo Yellow chemotherapy container Regulated Medical Waste Incinerate only Copyright © 2007 by Pharm. Ecology Associates, LLC
Ziplock bag used to transport chemo Yellow chemotherapy container Regulated Medical Waste Incinerate only Copyright © 2007 by Pharm. Ecology Associates, LLC
 Needle & syringe used in IV chemo preparation Yellow chemotherapy container Regulated Medical Waste Incinerate only Copyright © 2007 by Pharm. Ecology Associates, LLC
Needle & syringe used in IV chemo preparation Yellow chemotherapy container Regulated Medical Waste Incinerate only Copyright © 2007 by Pharm. Ecology Associates, LLC
 Empty lindane bottle Municipal trash Landfilled Copyright © 2007 by Pharm. Ecology Associates, LLC
Empty lindane bottle Municipal trash Landfilled Copyright © 2007 by Pharm. Ecology Associates, LLC
 Copyright 2007 Pharm. Ecology Associates, LLC
Copyright 2007 Pharm. Ecology Associates, LLC


