3a5d231e3f72794b500b8a98bc5f735f.ppt
- Количество слайдов: 32

Implementation of Quality by Design: An Office of Biotechnology Products Perspective Steven Kozlowski, M. D. , Director Office of Biotechnology Products OPS/CDER ACPS 10/5/06

Overview • • OBP Products & Quality by Design Relevant Product Attributes Manufacturing Process Implementation Plans
Office of Biotechnology Products • Therapeutic Proteins – Growth Factors – Enzymes – Cytokines – Chemokines – Angiogenic factors – Toxins – Soluble Receptors/Receptor antagonists • Monoclonal antibodies –Related Products
Office of Biotechnology Products • These proteins are usually produced from: –Cell culture • Recombinant & non-recombinant • Mammalian, bacterial, yeast, etc. –Transgenic plants & animals • Products transferred from CBER to CDER • in October 2003 ONDQA regulates some protein products
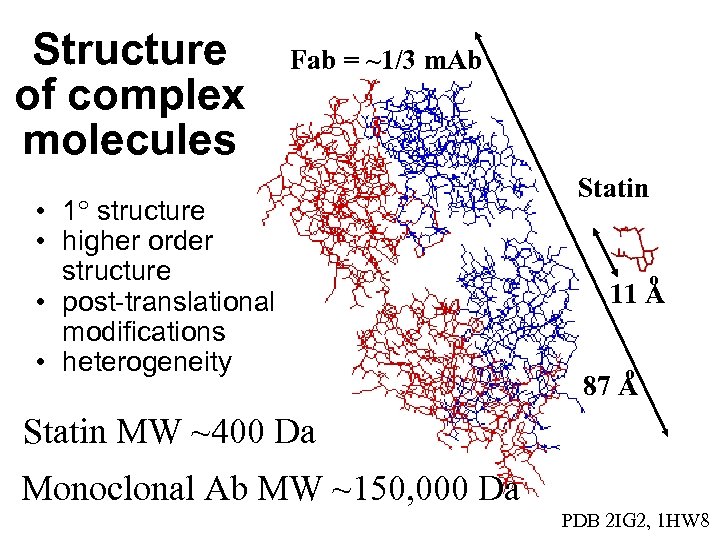
Structure of complex molecules Fab = ~1/3 m. Ab • 1 structure • higher order structure • post-translational modifications • heterogeneity Statin o 11 A o 87 A Statin MW ~400 Da Monoclonal Ab MW ~150, 000 Da PDB 2 IG 2, 1 HW 8
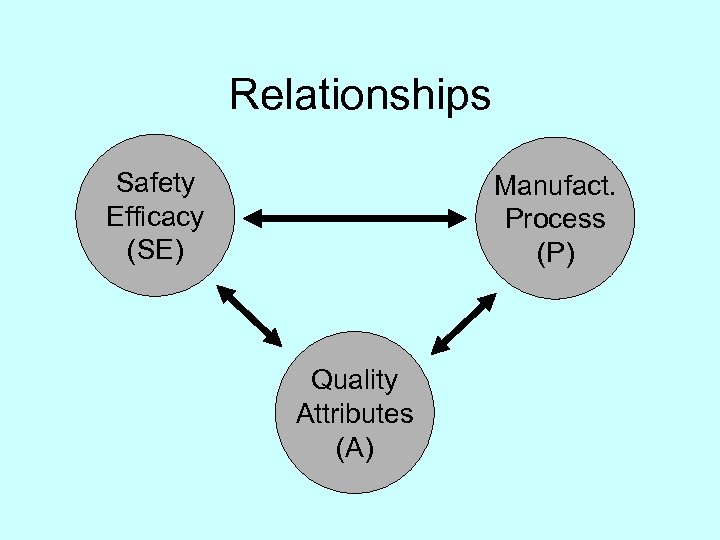
Relationships Safety Efficacy (SE) Manufact. Process (P) Quality Attributes (A)
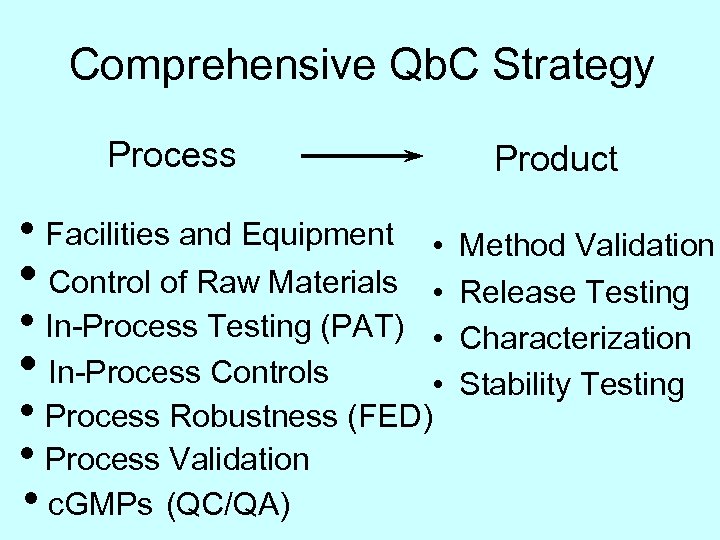
Comprehensive Qb. C Strategy Process • Facilities and Equipment • Control of Raw Materials • • In-Process Testing (PAT) • In-Process Controls • • Process Robustness (FED) • Process Validation • c. GMPs (QC/QA) • • Product Method Validation Release Testing Characterization Stability Testing

What is “Quality by Design”? • Quality – “Good pharmaceutical quality represents an acceptably low risk of failing to achieve the desired clinical attributes. ” • Quality by Design (Qb. D) – “Means that product and process performance characteristics are scientifically designed to meet specific objectives, not merely empirically derived from performance of test batches. ” Janet Woodcock (2004)
SE P A Quality by Design SE P A Moheb Nasr

Overview • • OBP Products & Quality by Design Relevant Product Attributes Manufacturing Process Implementation Plans
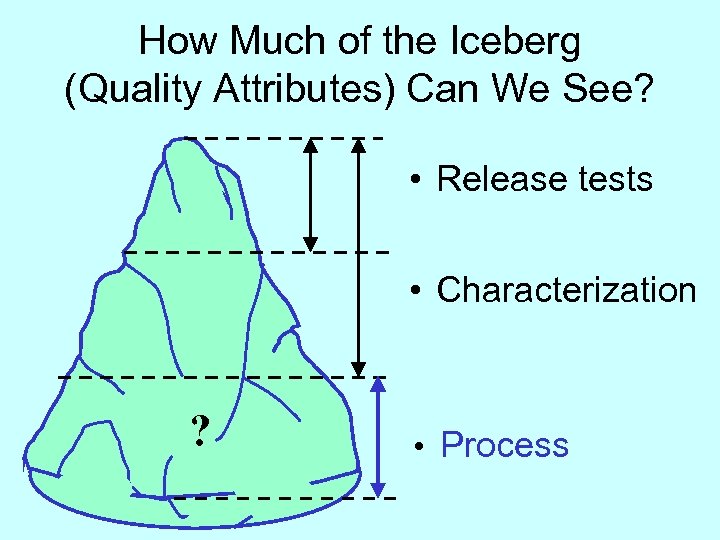
How Much of the Iceberg (Quality Attributes) Can We See? • Release tests • Characterization ? • Process
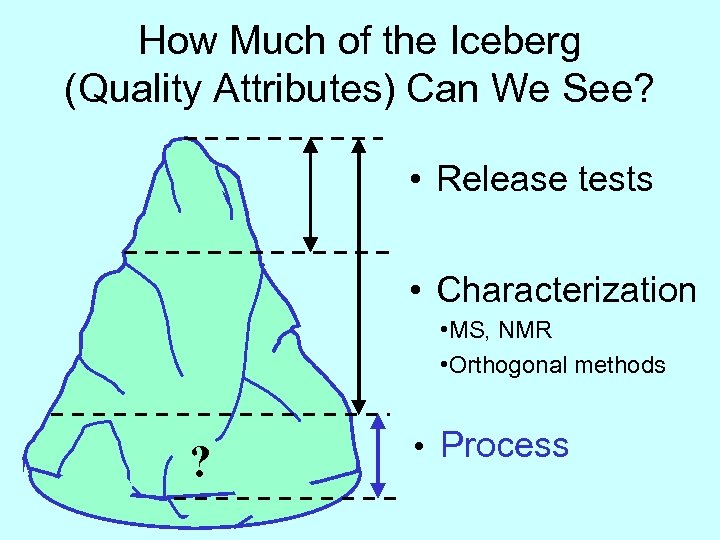
How Much of the Iceberg (Quality Attributes) Can We See? • Release tests • Characterization • MS, NMR • Orthogonal methods ? • Process
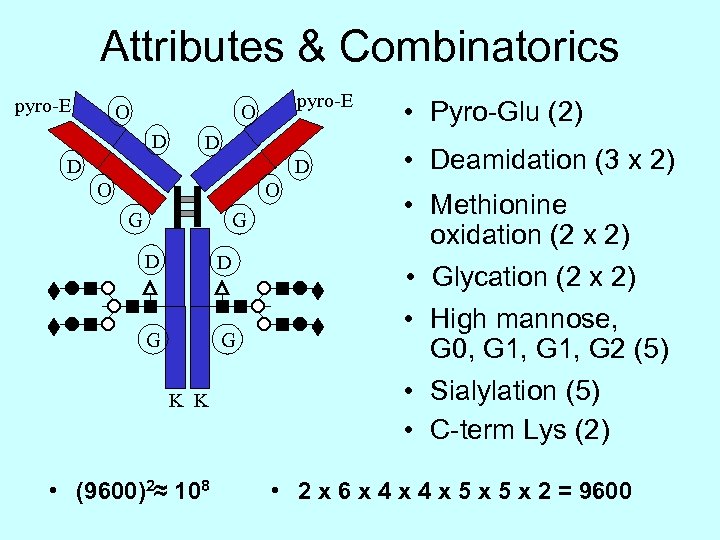
Attributes & Combinatorics pyro-E O D D O O G G D D G G K K • (9600)2≈ 108 • Pyro-Glu (2) • Deamidation (3 x 2) • Methionine oxidation (2 x 2) • Glycation (2 x 2) • High mannose, G 0, G 1, G 2 (5) • Sialylation (5) • C-term Lys (2) • 2 x 6 x 4 x 5 x 2 = 9600
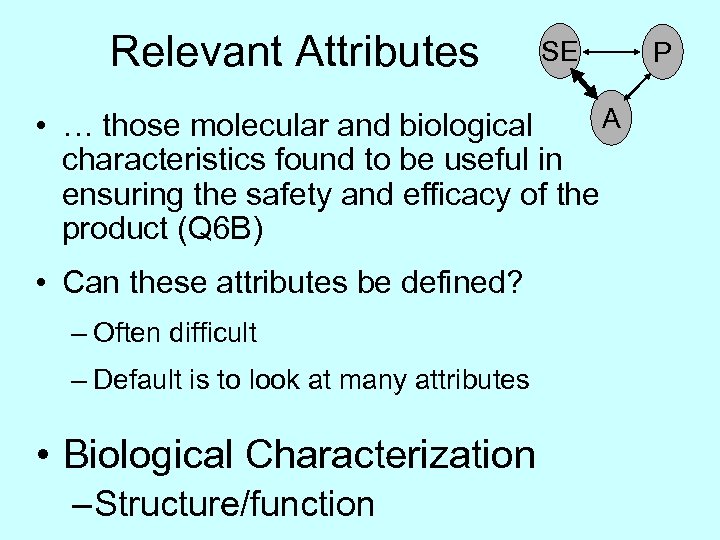
Relevant Attributes SE A • … those molecular and biological characteristics found to be useful in ensuring the safety and efficacy of the product (Q 6 B) • Can these attributes be defined? – Often difficult – Default is to look at many attributes • Biological Characterization – Structure/function P
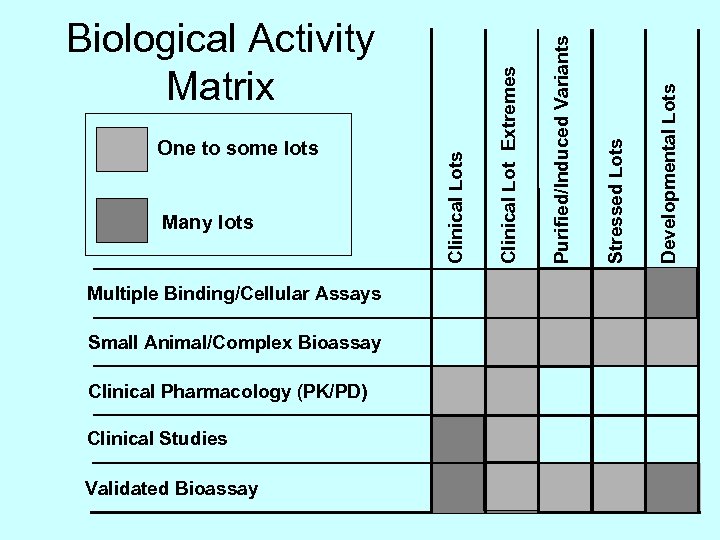
Multiple Binding/Cellular Assays Small Animal/Complex Bioassay Clinical Pharmacology (PK/PD) Clinical Studies Validated Bioassay Developmental Lots Stressed Lots Purified/Induced Variants Many lots Clinical Lot Extremes One to some lots Clinical Lots Biological Activity Matrix
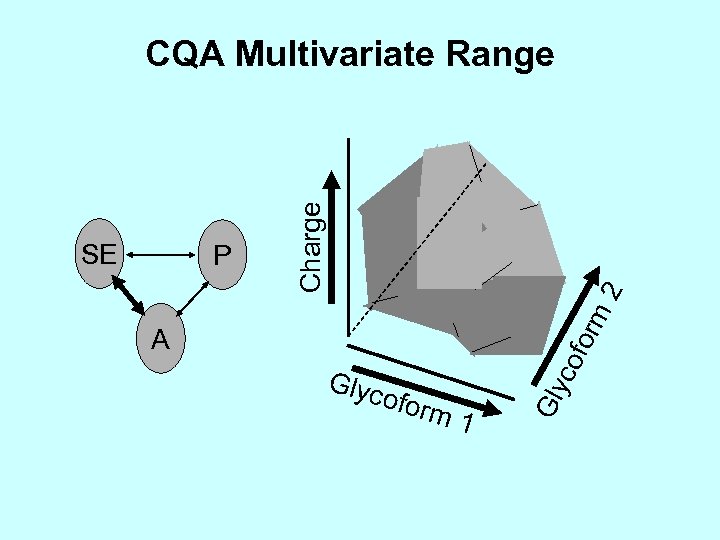
2 for m P Glyc ofor co A m 1 Gly SE Charge CQA Multivariate Range
The Desired Product • Desired attributes of the API (Focus for Biotech) ─ Opportunity for rational protein engineering • Customize quality ─ Increase attributes that are desirable ─ Limit attributes that negatively impact product quality (via process or product) • Understanding structure/function relationships is critical for this approach
Protein Engineering • Reduce tendency for aggregation ─ Block free sulfhydryl groups ─ Alter amino acid sequence based on computational predictions v Glycine repeats or proline insertions v Maintenance of net charge v Alternate polar and non polar residues v Clusters of hydrophobic residues −Human Calcitonin (Zurdo et al PNAS 2005) −Immunogenicity considerations

QSE…by Design • Safety & Efficacy by Design • • • Improving function/new properties Increasing Bioavailability Reducing immunogenicity – Selective technologies in development such as phage, ribosome and yeast display • Quality by design – These strategies can also be used to select for product quality and manufacturability

Overview • • OBP Products & Quality by Design Relevant Product Attributes Manufacturing Process Implementation Plans
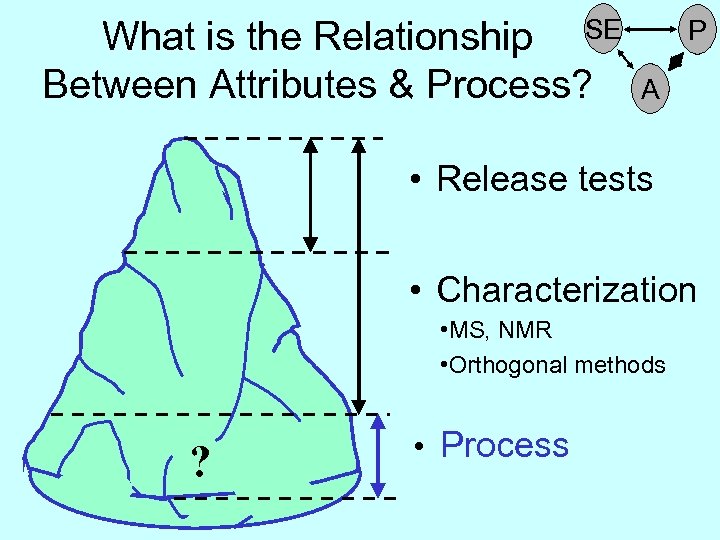
What is the Relationship SE Between Attributes & Process? P A • Release tests • Characterization • MS, NMR • Orthogonal methods ? • Process
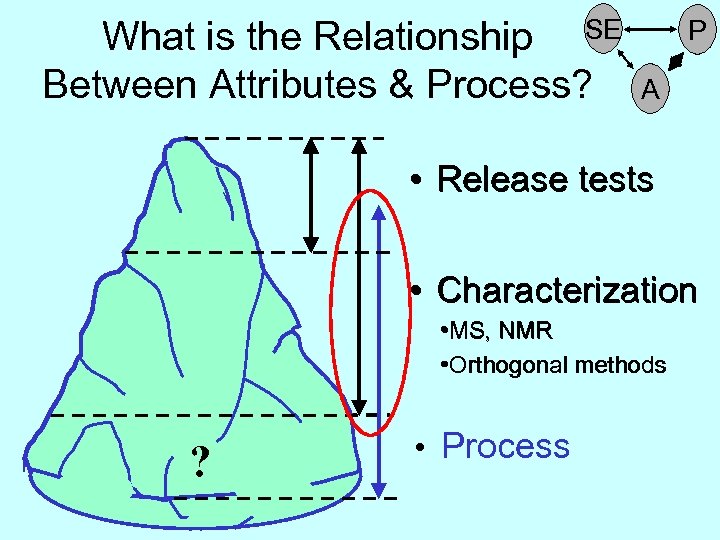
What is the Relationship SE Between Attributes & Process? P A • Release tests • Characterization • MS, NMR • Orthogonal methods ? • Process
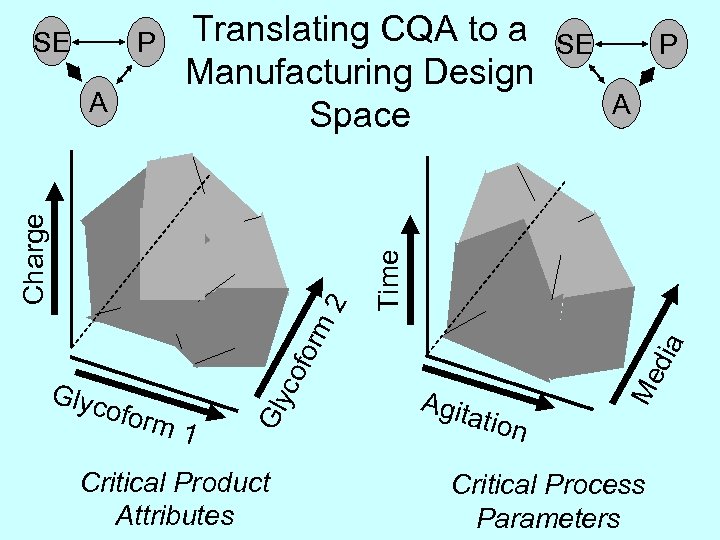
for co ofor m 1 Glyc Critical Product Attributes SE P A dia m 2 Charge A Translating CQA to a Manufacturing Design Space Agit ation Me P Time SE Critical Process Parameters

The Desired Process • Desired attributes of the API or drug product – Opportunity for rational process design • Choose unit operations that select for – select for desired product attributes – minimize impurities/contaminants – are robust • Order unit operations to maximize efficiency – minimize buffer changes – consider impurities introduced by each step • Process control – consider impact of variable inputs – parameters set based on maximizing multiple variables – for critical steps- ideally real time & based on a knowledge base (PAT)
Examples of Problematic Process Designs • Processes that promote product variability • Heat treatment step added after aggregate clearance step • Process performed at room temperature with negative impact on quality • Recloning is used to establish new WCB • Processes that are difficult to control • Roller bottle processes (open, multiple fermentations difficult to control) • Poor formulation (stability & interactions)

Overview • • OBP Products & Quality by Design Relevant Product Attributes Manufacturing Process Implementation Plans
Implementation of Qb. D • Benefit from other OPS knowledge on implementation • • OBP participation in agency CRADAs to facilitate Qb. D OBP Structure • – e. g. ONDQA pilot • • • Mixture of research/reviewers and full time reviewers Experience with new technologies, fermentation/purification Expertise in biological characterization Encourage industry to engineer proteins for quality as well as safety and efficacy – “desired product” • Encourage industry to consider “desired process” • Focus on “small steps” for biotech………………….

Small Steps • Product Testing – If no likely impact on S and E don’t include as a specification (no rejection limit) § use as a process consistency measure, where exceeding a limit initiates an investigation § if not a consistency measure, drop the test entirely – Need to discuss more extensive in-house reviewer training • Process Changes – Strategy to assess risk – Select classes of change based on • supplement load • variable risk to product – FDA/industry forums targeted to create risk map for a single class of change – Publication as white papers
Small Steps (cont. ) • Complex API pilot – Encourage manufacturers to generate and submit data on characterization of structural attributes • the use of multiple biological assays for characterization § the generation of a product attribute space § consideration of associated relief (e. g. greater flexibility for comparability protocols) – Need to consider & discuss further • Platform Strategies – monoclonal antibodies may share a great deal of structure – efficient use of prior knowledge – long history of a regulatory path for modular/generic validation (Mab PTC) • underutilized – currently of interest to many biotech companies – conference to put interested parties together
Question to ACPS • Should FDA develop a pilot program and/or initiative to explore specific Qb. D issues that are important for biotechnology products? • Such a program could focus on issues such as: – (1) general approaches to CQA for complex API – (2) platform approaches/extrapolation for highly related complex APIs.
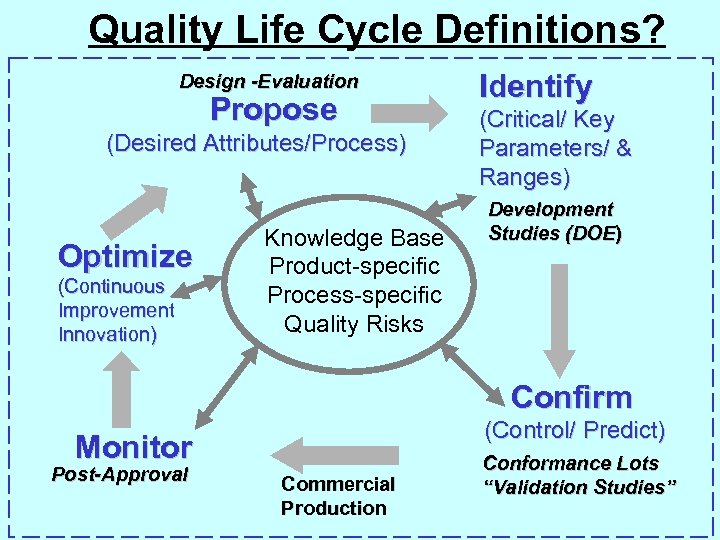
Quality Life Cycle Definitions? Design -Evaluation Propose (Desired Attributes/Process) Optimize (Continuous Improvement Innovation) Knowledge Base Product-specific Process-specific Quality Risks Identify (Critical/ Key Parameters/ & Ranges) Development Studies (DOE) Confirm (Control/ Predict) Monitor Post-Approval Commercial Production Conformance Lots “Validation Studies”

Credits • • • Barry Cherney Patrick Swann Moheb Nasr Keith Webber Ajaz Hussain
3a5d231e3f72794b500b8a98bc5f735f.ppt