fe770237728c9ef564a51ed9e5904242.ppt
- Количество слайдов: 43
Immunoassay / Immunochemical assay A laboratory technique that makes use of the binding between an antigen and its homologous antibody in order to identify and quantify the specific antigen or antibody in a sample. The technique identifies and quantifies usually in minute amounts, a protein such as a hormone or an enzyme, based on its ability to act as an antigen or antibody in a chemical reaction. I. Radioimmunoassay (RIA) II. Enzyme-linked Immunosorbent assay (ELISA)
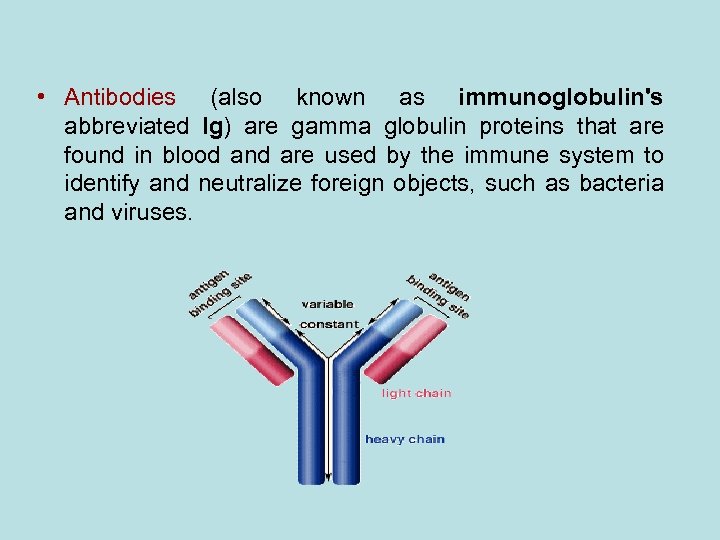
• Antibodies (also known as immunoglobulin's abbreviated Ig) are gamma globulin proteins that are found in blood and are used by the immune system to identify and neutralize foreign objects, such as bacteria and viruses.

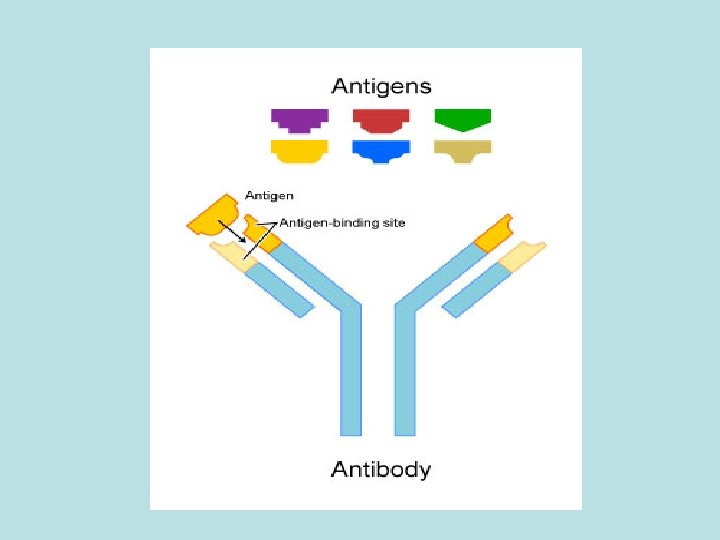
Antigens A substance that when introduced into the body stimulates the production of an antibody. • Analyte The sample being analyzed and in immunoassays the analyte is either Antibody or Antigen.

• The Antibody: An immunoglobulin, a specialized immune protein, produced because of the introduction of an antigen into the body, and which possesses the remarkable ability to combine with the very antigen that triggered its production (specific affinity). The antibody recognises and bind to the antigenic determinant region of the antigen.
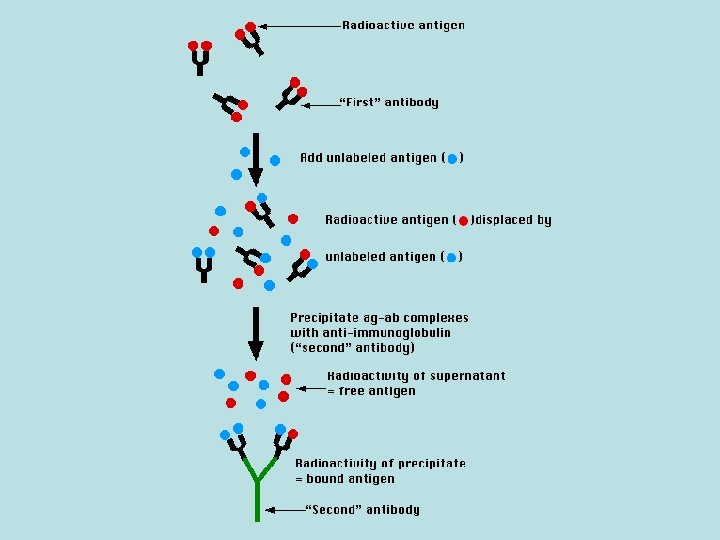
• Radioimmunoassay(RIA) involves the separation of a protein (from a mixture) using the specificity of antibodyantigen binding and quantitation using radioactivity. • The technique of radioimmunoassay has revolutionized research and clinical practice in many areas, e. g. , – blood banking – diagnosis of allergies – endocrinology The technique was introduced in 1960 by Berson and Yalow as an assay for the concentration of insulin in plasma. It represented the first time that hormone levels in the blood could be detected by an in vitro assay.
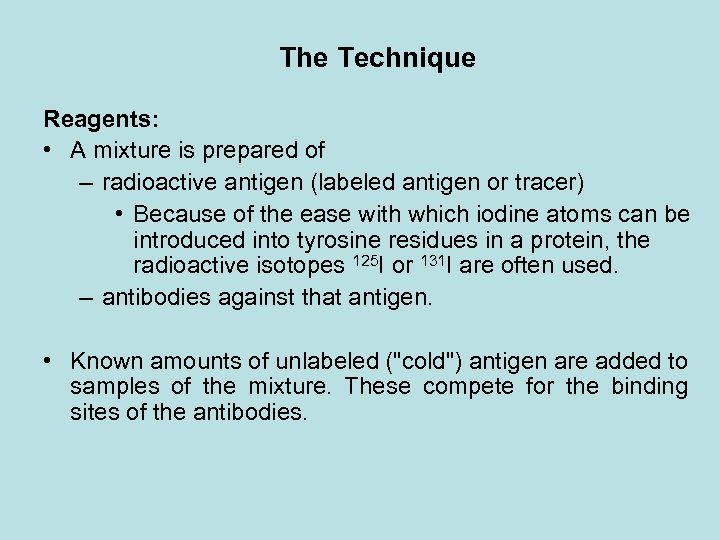
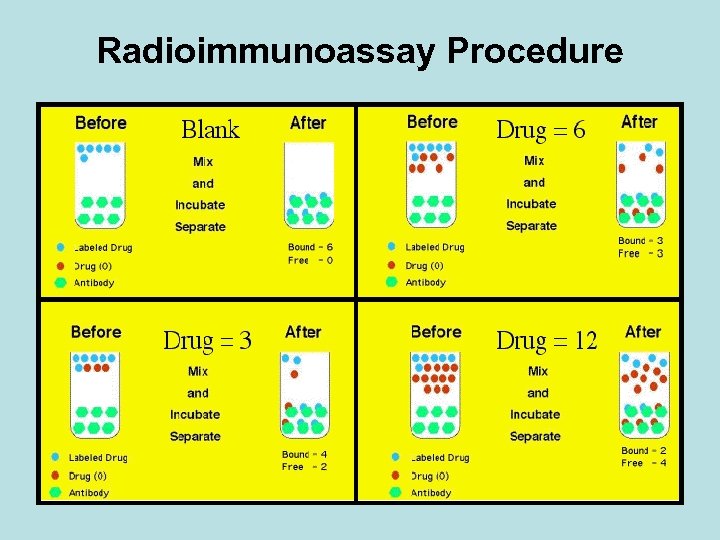
The Technique Reagents: • A mixture is prepared of – radioactive antigen (labeled antigen or tracer) • Because of the ease with which iodine atoms can be introduced into tyrosine residues in a protein, the radioactive isotopes 125 I or 131 I are often used. – antibodies against that antigen. • Known amounts of unlabeled ("cold") antigen are added to samples of the mixture. These compete for the binding sites of the antibodies.

• At increasing concentrations of unlabeled antigen, an increasing amount of radioactive antigen is displaced from the antibody molecules. • The antibody-bound antigen is separated from the free antigen in the supernatant fluid, and the radioactivity of each is measured.
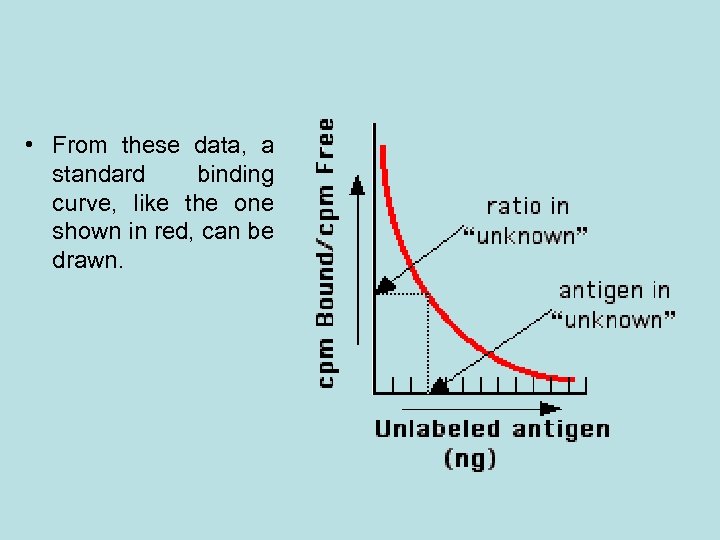
• From these data, a standard binding curve, like the one shown in red, can be drawn.

• The samples to be assayed (the unknowns) are run in parallel. • After determining the ratio of bound to free antigen in each unknown, the antigen concentrations can be read directly from the standard curve.
Count gamma emission • Counts per minute (CPM) for each tube • A sample containing a higher concentration of the unknown antigen will have a lower CPM.

Gamma Counter
Radioimmunoassay Procedure

Separating Bound from Free Antigen Precipitate the antigen-antibody complexes by adding a "second" antibody directed against the first. For example, if a rabbit Ig. G is used to bind the antigen, the complex can be precipitated by adding an anti-rabbit-Ig. G antiserum (e. g. , raised by immunizing a goat with rabbit Ig. G).

• Radioimmunoassay is widely-used because of its greater sensitivity. Using antibodies of high affinity, it is possible to detect a few picograms (10− 12 g) of antigen in the tube. The greater the specificity of the antiserum, the greater the specificity of the assay. • The main drawbacks to radioimmunoassay are the expense and hazards of preparing and handling the radioactive antigen. Both 125 I or 131 I emit gamma radiation that requires special counting equipment. The body concentrates iodine atoms — radioactive or not — in the thyroid gland where they are incorporated in thyroxine (T 4).

Applications: • Despite these drawbacks, RIA has become a major tool in the clinical laboratory where it is used to assay • plasma levels of: – most of our hormones; – digitoxin or digoxin in patients receiving these drugs; – certain abused drugs • for the presence of hepatitis B surface antigen (HBs. Ag) in donated blood;
Enzyme-Linked Immunosorbent Assay (ELISA)

• ELISA is a widely-used method for measuring the concentration of a particular molecule (e. g. , a hormone or drug) in a fluid such as serum or urine. It is also known as enzyme immunoassay or EIA. • ELISA has many of the advantages (e. g. , sensitivity, ease of handling of multiple samples) without the disadvantages of dealing with radioactivity (like in RIA).

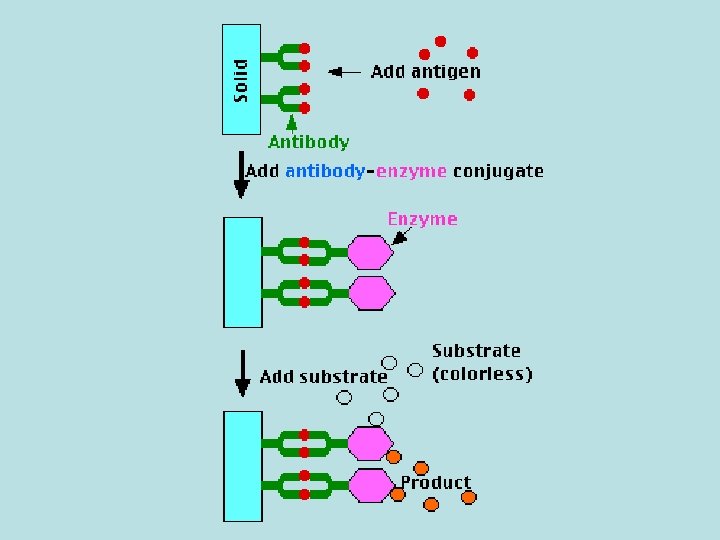
• The molecule is detected by antibodies that have been made against it; that is, for which it is the antigen. Monoclonal antibodies are often used. • The test requires: – the antibodies fixed to a solid surface, such as the inner surface of a test tube; – a preparation of the same antibodies coupled to an enzyme. This is one (e. g. , β-galactosidase) that produces a colored product from a colorless substrate.
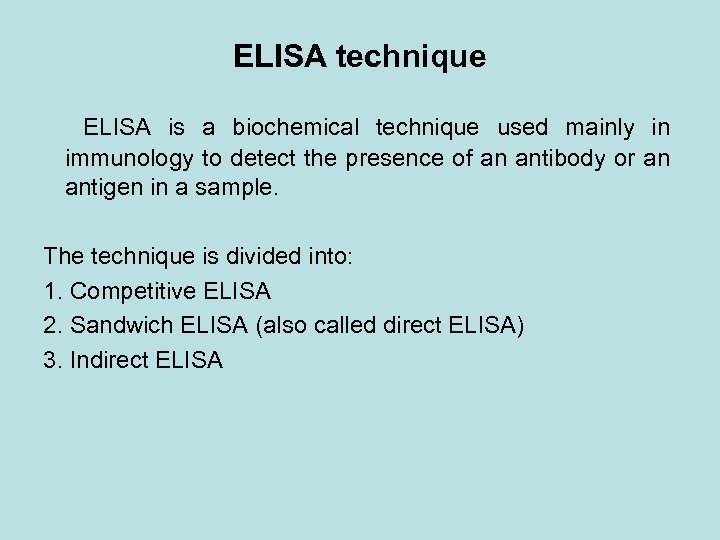
ELISA technique ELISA is a biochemical technique used mainly in immunology to detect the presence of an antibody or an antigen in a sample. The technique is divided into: 1. Competitive ELISA 2. Sandwich ELISA (also called direct ELISA) 3. Indirect ELISA

Performing the Test • The tubes are filled with the antigen solution (e. g. , urine) to be assayed. Any antigen molecules present bind to the immobilized antibody molecules. • The same antibody-enzyme conjugate is added to the reaction mixture. The antibody part of the conjugate binds to any antigen molecules that were bound previously, creating an antibody-antigen-antibody "sandwich". • After washing away any unbound conjugate, the substrate solution which is specific for the enzyme is added. • After a set interval, the reaction is stopped (e. g. , by adding 1 N Na. OH) and the concentration of colored product formed is measured in a spectrophotometer (or plate reader). The intensity of color is proportional to the concentration of bound antigen.


• Literally hundreds of ELISA kits are manufactured for research and human / veterinary disease diagnosis. Some examples: • Screening donated blood for evidence of viral contamination by. HIV-1 and HIV-2 (presence of anti-HIV antibodies) hepatitis C (presence of antibodies) hepatitis B (testing for both antibodies and a viral antigen, HBs. Ag) • Measuring hormone levels – LH (determining the time of ovulation) – TSH, T 3 and T 4 (for thyroid function) – hormones (e. g. , anabolic steroids, HGH) that may have been used illicitly by athletes

• Detecting infections – sexually-transmitted agents like HIV, syphilis, and chlamydia – hepatitis B and C • detecting allergens in food and house dust. • measuring "rheumatoid factors" and other auto antibodies in autoimmune diseases. • measuring toxins in contaminated food. • detecting illicit drugs, e. g. , – cocaine – opiates – Δ-9 -tetrahydrocannabinol, the active ingredient in marijuana.
An example of an ELISA experiment • Before starting the work read kit instruction carefully • 1 - The 96 well plate is labeled carefully and the first wells are used to draw the standard curve.
An example of an ELISA experiment • The sample is added to plate in duplicate or triplicate and then the mean result is calculated. • The quality control sample which is provided with the kit is treated as the test samples.
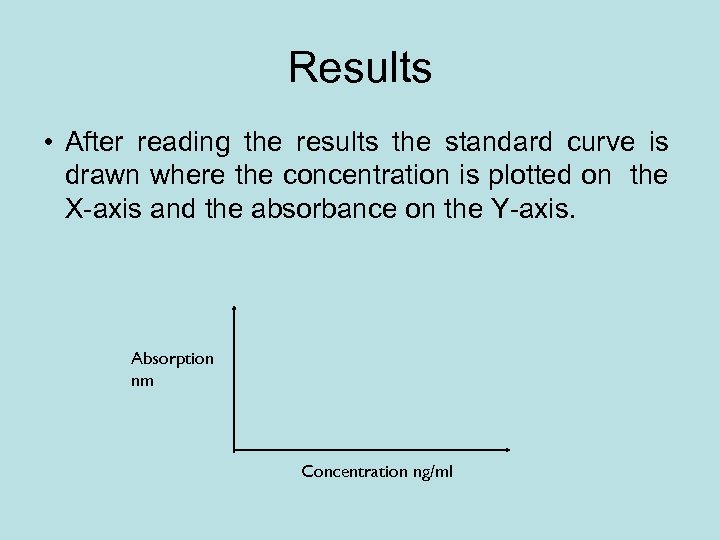
Results • After reading the results the standard curve is drawn where the concentration is plotted on the X-axis and the absorbance on the Y-axis. Absorption nm Concentration ng/ml
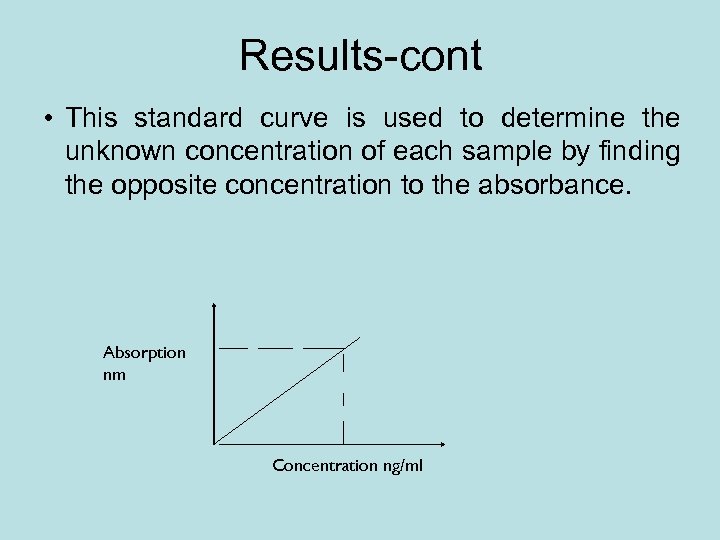
Results-cont • This standard curve is used to determine the unknown concentration of each sample by finding the opposite concentration to the absorbance. Absorption nm Concentration ng/ml
Results-cont • The quality control sample concentration is determined from the standard curve and if the result is in the range given by the kit manufacturer the results could be accepted.

Validation of Analytical Methods
What is Method Validation? • Method validation is the process of proving that an analytical method is acceptable for its intended purpose. • In order to validate any method, the method performance parameters are determined using equipment that is: – Within specification – Working correctly – Adequately calibrated – Being handled by competent operators

Why is Method Validation Necessary? • • To increase the value of test results To justify customer’s trust To trace criminals To prove what we claim is true Examples – To value goods for trade purposes – To support health care – To check the quality of drinking water

The Professional Duty of the Analytical Chemist • To increase reliability of laboratory results • To increase trust of laboratory customers • To prove the truth
When should Methods be Validated • New method development • Revision of established methods • When established methods are used in different laboratories/different analysts etc. • QC indicates method changes • Comparison of methods

Who Carries out Method Validation • Validation in a group of laboratories – Collaborative studies – Inter-laboratory comparisons

What to Check (Performance Characteristics) • • • Selectivity/Specificity Limit of Detection Limit of Quantitation Linearity Accuracy Trueness Sensitivity Ruggedness (or Robustness) Recovery
Identity and Selectivity/Specificity • Identity: Signal to be attributed to the analyte – GLC (change column/polarity) • Selectivity: The ability of the method to determine accurately the analyte of interest in the presence of other components in a sample matrix under the stated conditions of the test. • Specificity is a state of perfect selectivity

• Confirmation versus repeatability Confirmation: Measure by more than one technique. Repeatability: Measure several times by one technique. • How to establish selectivity: Compare the response of the analyte in a test mixture with the response of a solution containing only the analyte.
• The procedure to establish selectivity: – Analyze samples and reference materials. – Assess the ability of the methods to confirm identity and measure the analyte. – Choose the more appropriate method. – Analyze samples. – Examine the effect of interferences.
Accuracy / Trueness • Accuracy: the closeness of a result to a true value (= trueness+precision). • Trueness: The closeness of agreement between the average value obtained from a large set of test results and an accepted reference value. • Precision: how close results are to one another.
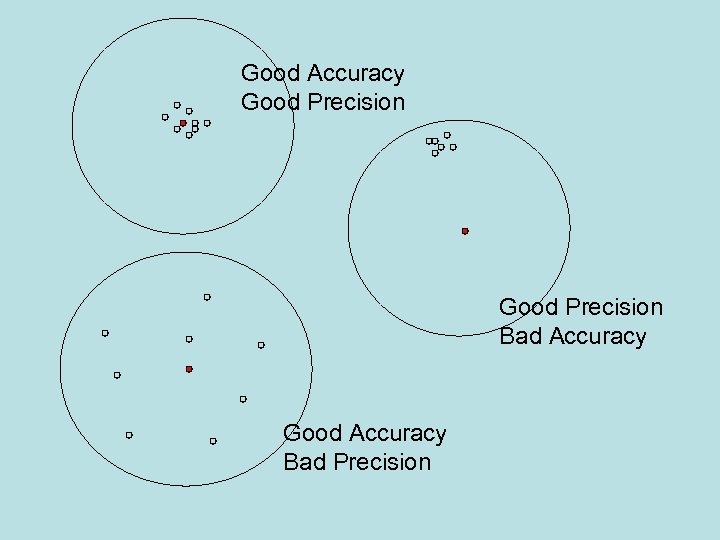
Good Accuracy Good Precision Bad Accuracy Good Accuracy Bad Precision
fe770237728c9ef564a51ed9e5904242.ppt