47a44690a0d3721ff2b971e51c91fe84.ppt
- Количество слайдов: 25
 Immunoassay Enhanced LC/MS/MS Method for the Determination of Nucleotides Van Damme Thomas, Jenny Zhang, Frederic Lynen and Pat Sandra
Immunoassay Enhanced LC/MS/MS Method for the Determination of Nucleotides Van Damme Thomas, Jenny Zhang, Frederic Lynen and Pat Sandra
 Presentation Outline 1. Introduction • Oligonucleotides • ELISA 2. Experimental work and results 3. Conclusion
Presentation Outline 1. Introduction • Oligonucleotides • ELISA 2. Experimental work and results 3. Conclusion
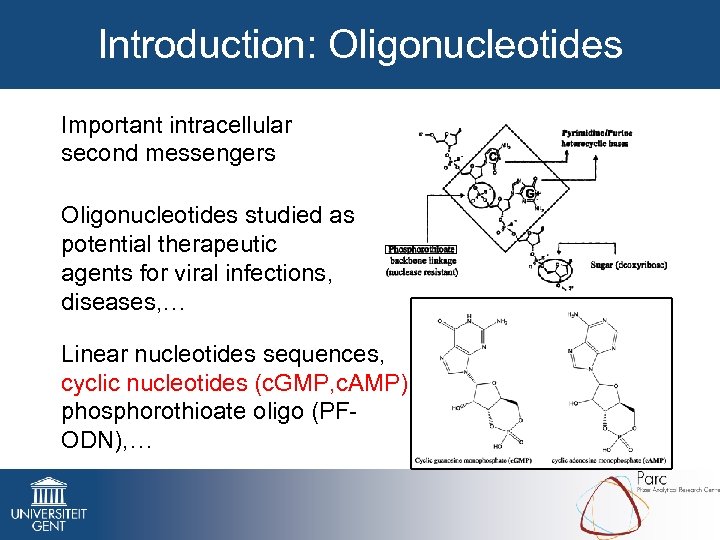 Introduction: Oligonucleotides Important intracellular second messengers Oligonucleotides studied as potential therapeutic agents for viral infections, diseases, … Linear nucleotides sequences, cyclic nucleotides (c. GMP, c. AMP), phosphorothioate oligo (PFODN), …
Introduction: Oligonucleotides Important intracellular second messengers Oligonucleotides studied as potential therapeutic agents for viral infections, diseases, … Linear nucleotides sequences, cyclic nucleotides (c. GMP, c. AMP), phosphorothioate oligo (PFODN), …
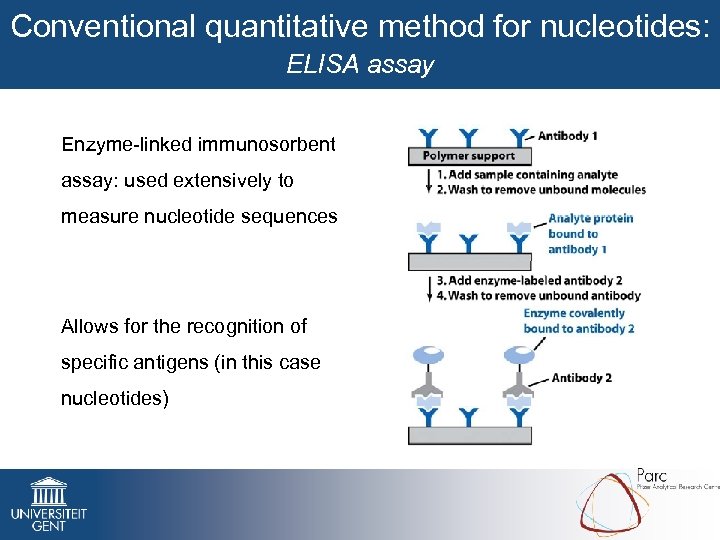 Conventional quantitative method for nucleotides: ELISA assay Enzyme-linked immunosorbent assay: used extensively to measure nucleotide sequences Allows for the recognition of specific antigens (in this case nucleotides)
Conventional quantitative method for nucleotides: ELISA assay Enzyme-linked immunosorbent assay: used extensively to measure nucleotide sequences Allows for the recognition of specific antigens (in this case nucleotides)
 Conventional quantitative method for nucleotides: ELISA assay → problems: - high cost of an ELISA kit - not sufficiently specific for al nucleotides of interest ( immunological cross-reactions ) → possible solution: tandem mass spectrometry (LC-MS/MS) or immunoassay + tandem MS
Conventional quantitative method for nucleotides: ELISA assay → problems: - high cost of an ELISA kit - not sufficiently specific for al nucleotides of interest ( immunological cross-reactions ) → possible solution: tandem mass spectrometry (LC-MS/MS) or immunoassay + tandem MS
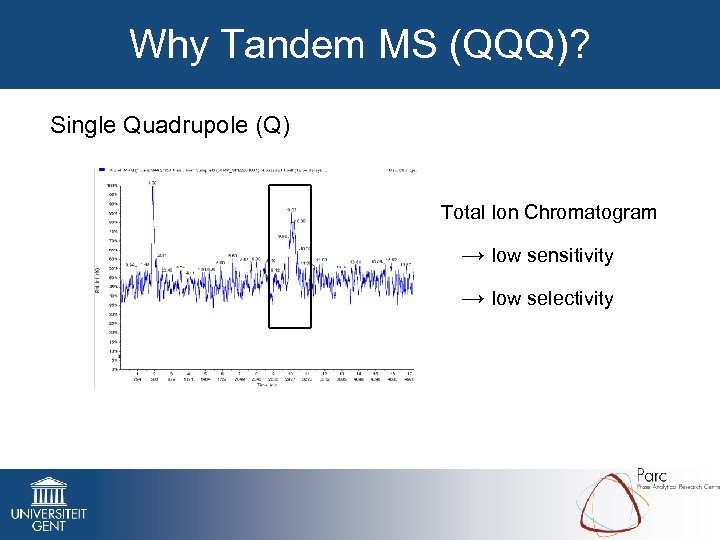 Why Tandem MS (QQQ)? Single Quadrupole (Q) Total Ion Chromatogram → low sensitivity → low selectivity
Why Tandem MS (QQQ)? Single Quadrupole (Q) Total Ion Chromatogram → low sensitivity → low selectivity
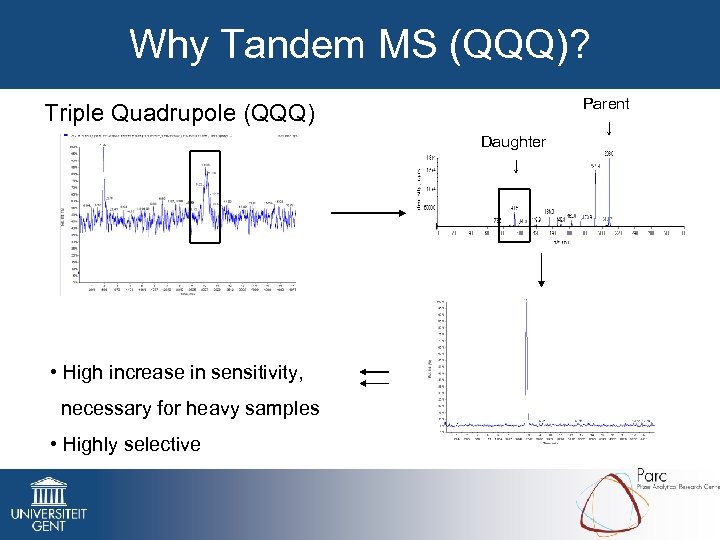 Why Tandem MS (QQQ)? Triple Quadrupole (QQQ) Daughter ↓ • High increase in sensitivity, necessary for heavy samples • Highly selective Parent ↓
Why Tandem MS (QQQ)? Triple Quadrupole (QQQ) Daughter ↓ • High increase in sensitivity, necessary for heavy samples • Highly selective Parent ↓
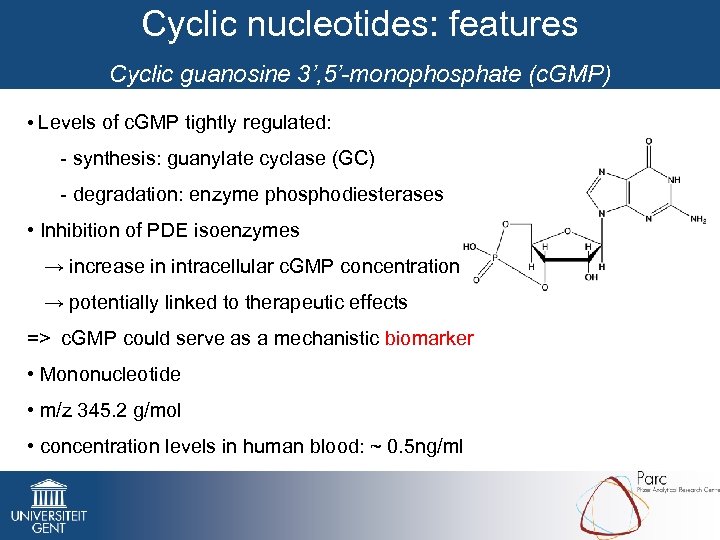 Cyclic nucleotides: features Cyclic guanosine 3’, 5’-monophosphate (c. GMP) • Levels of c. GMP tightly regulated: - synthesis: guanylate cyclase (GC) - degradation: enzyme phosphodiesterases • Inhibition of PDE isoenzymes → increase in intracellular c. GMP concentration → potentially linked to therapeutic effects => c. GMP could serve as a mechanistic biomarker • Mononucleotide • m/z 345. 2 g/mol • concentration levels in human blood: ~ 0. 5 ng/ml
Cyclic nucleotides: features Cyclic guanosine 3’, 5’-monophosphate (c. GMP) • Levels of c. GMP tightly regulated: - synthesis: guanylate cyclase (GC) - degradation: enzyme phosphodiesterases • Inhibition of PDE isoenzymes → increase in intracellular c. GMP concentration → potentially linked to therapeutic effects => c. GMP could serve as a mechanistic biomarker • Mononucleotide • m/z 345. 2 g/mol • concentration levels in human blood: ~ 0. 5 ng/ml
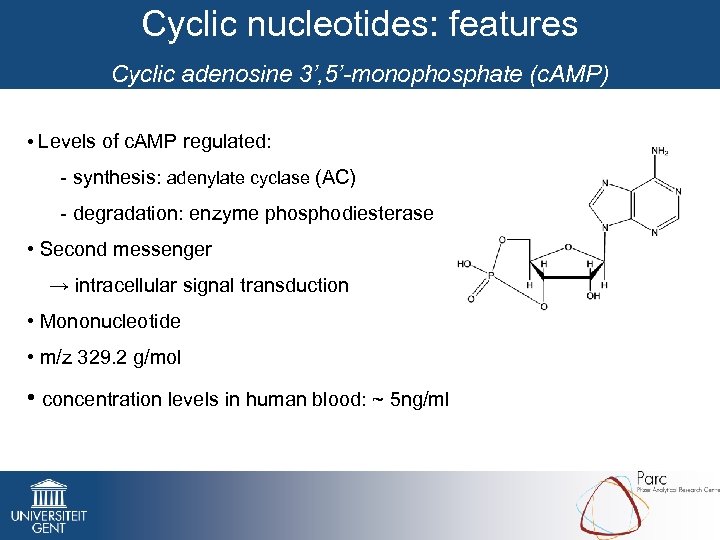 Cyclic nucleotides: features Cyclic adenosine 3’, 5’-monophosphate (c. AMP) • Levels of c. AMP regulated: - synthesis: adenylate cyclase (AC) - degradation: enzyme phosphodiesterase • Second messenger → intracellular signal transduction • Mononucleotide • m/z 329. 2 g/mol • concentration levels in human blood: ~ 5 ng/ml
Cyclic nucleotides: features Cyclic adenosine 3’, 5’-monophosphate (c. AMP) • Levels of c. AMP regulated: - synthesis: adenylate cyclase (AC) - degradation: enzyme phosphodiesterase • Second messenger → intracellular signal transduction • Mononucleotide • m/z 329. 2 g/mol • concentration levels in human blood: ~ 5 ng/ml
 Development of an LC/MS/MS Method for the Analysis of c. GMP & c. AMP • Stationary phase: Zorbax SB-C 18 column 3. 0 mm * 150 mm * 3. 5µm • Mobile phase: A/ 0, 1% Formic Acid B/ ACN/Water/Me. OH 1: 4: 2 • Negative mode (mass spectrometer) • Chromatographic conditions: - 0. 3 m. L/min flow rate - 0% A → 50% B in 10 min
Development of an LC/MS/MS Method for the Analysis of c. GMP & c. AMP • Stationary phase: Zorbax SB-C 18 column 3. 0 mm * 150 mm * 3. 5µm • Mobile phase: A/ 0, 1% Formic Acid B/ ACN/Water/Me. OH 1: 4: 2 • Negative mode (mass spectrometer) • Chromatographic conditions: - 0. 3 m. L/min flow rate - 0% A → 50% B in 10 min
 Instrument used for Analysis: API 2000 ESI – negative mode
Instrument used for Analysis: API 2000 ESI – negative mode
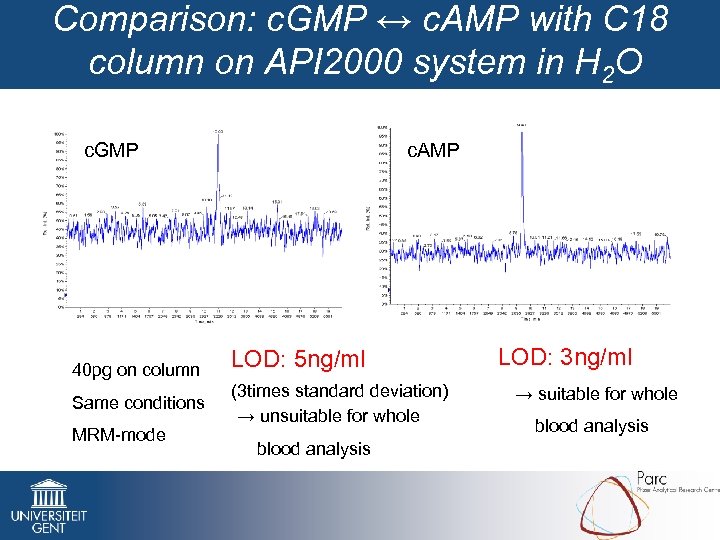 Comparison: c. GMP ↔ c. AMP with C 18 column on API 2000 system in H 2 O c. GMP 40 pg on column Same conditions MRM-mode c. AMP LOD: 5 ng/ml (3 times standard deviation) → unsuitable for whole blood analysis LOD: 3 ng/ml → suitable for whole blood analysis
Comparison: c. GMP ↔ c. AMP with C 18 column on API 2000 system in H 2 O c. GMP 40 pg on column Same conditions MRM-mode c. AMP LOD: 5 ng/ml (3 times standard deviation) → unsuitable for whole blood analysis LOD: 3 ng/ml → suitable for whole blood analysis
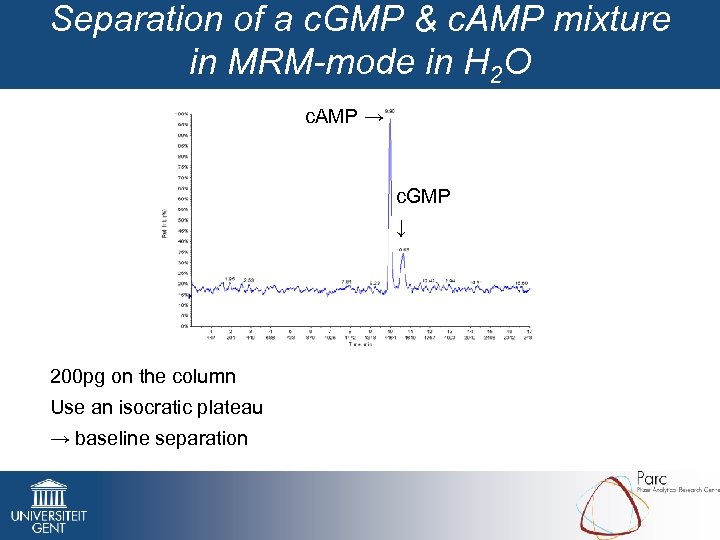 Separation of a c. GMP & c. AMP mixture in MRM-mode in H 2 O c. AMP → c. GMP ↓ 200 pg on the column Use an isocratic plateau → baseline separation
Separation of a c. GMP & c. AMP mixture in MRM-mode in H 2 O c. AMP → c. GMP ↓ 200 pg on the column Use an isocratic plateau → baseline separation
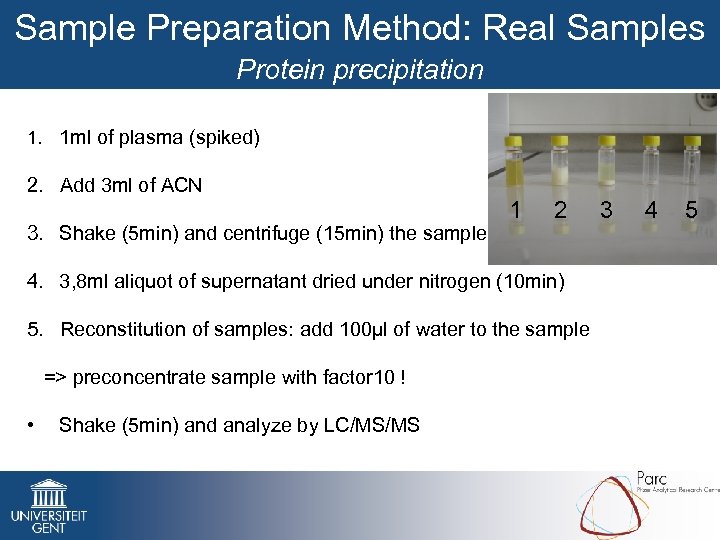 Sample Preparation Method: Real Samples Protein precipitation 1. 1 ml of plasma (spiked) 2. Add 3 ml of ACN 3. Shake (5 min) and centrifuge (15 min) the sample 1 2 4. 3, 8 ml aliquot of supernatant dried under nitrogen (10 min) 5. Reconstitution of samples: add 100µl of water to the sample => preconcentrate sample with factor 10 ! • Shake (5 min) and analyze by LC/MS/MS 3 4 5
Sample Preparation Method: Real Samples Protein precipitation 1. 1 ml of plasma (spiked) 2. Add 3 ml of ACN 3. Shake (5 min) and centrifuge (15 min) the sample 1 2 4. 3, 8 ml aliquot of supernatant dried under nitrogen (10 min) 5. Reconstitution of samples: add 100µl of water to the sample => preconcentrate sample with factor 10 ! • Shake (5 min) and analyze by LC/MS/MS 3 4 5
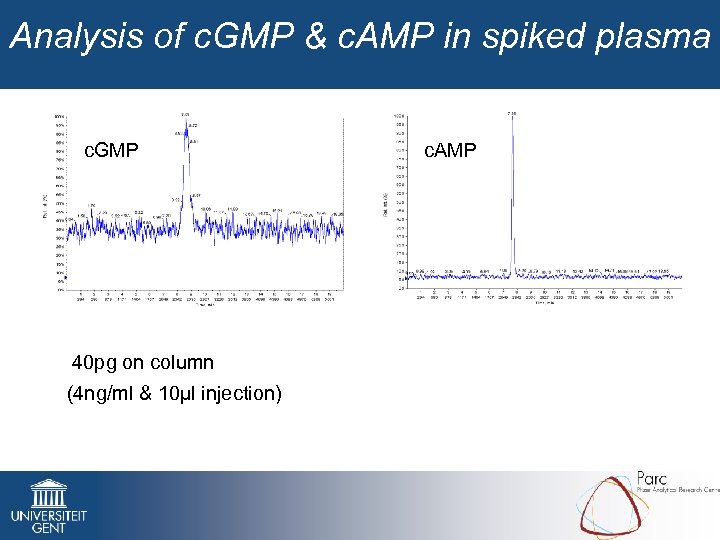 Analysis of c. GMP & c. AMP in spiked plasma c. GMP 40 pg on column (4 ng/ml & 10µl injection) c. AMP
Analysis of c. GMP & c. AMP in spiked plasma c. GMP 40 pg on column (4 ng/ml & 10µl injection) c. AMP
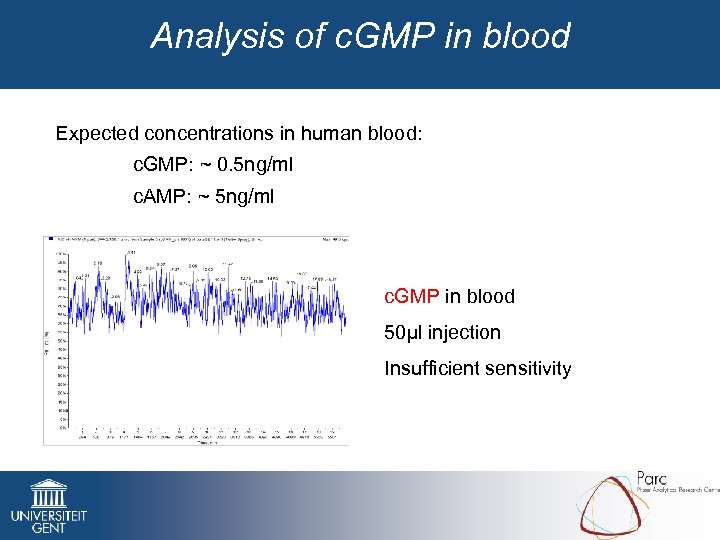 Analysis of c. GMP in blood Expected concentrations in human blood: c. GMP: ~ 0. 5 ng/ml c. AMP: ~ 5 ng/ml c. GMP in blood 50µl injection Insufficient sensitivity
Analysis of c. GMP in blood Expected concentrations in human blood: c. GMP: ~ 0. 5 ng/ml c. AMP: ~ 5 ng/ml c. GMP in blood 50µl injection Insufficient sensitivity
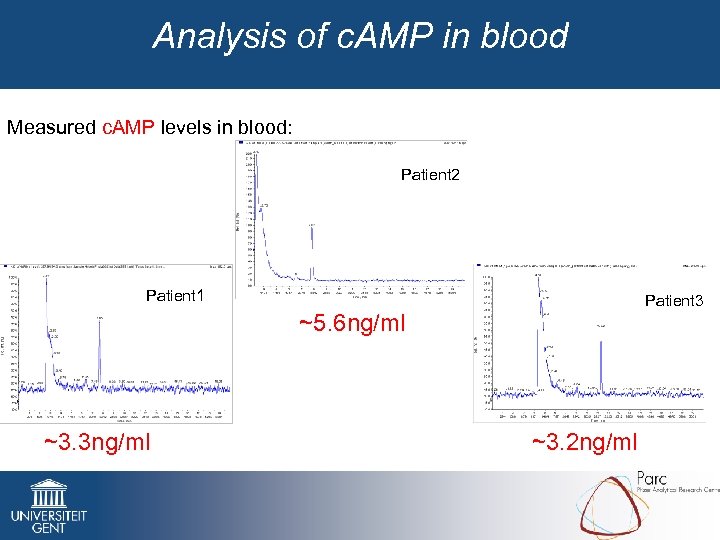 Analysis of c. AMP in blood Measured c. AMP levels in blood: Patient 2 Patient 1 Patient 3 ~5. 6 ng/ml ~3. 3 ng/ml ~3. 2 ng/ml
Analysis of c. AMP in blood Measured c. AMP levels in blood: Patient 2 Patient 1 Patient 3 ~5. 6 ng/ml ~3. 3 ng/ml ~3. 2 ng/ml
 Sample Preparation Method: HILIC SPE Alternative to immuno extraction? • Hydrophilic interaction liquid chromatography SPE: → normal phase conditions → especially for polar molecules • Try to purify the matrix and preconcentrate the sample • HILIC SPE cartridges were made in-house → Fill a glass cartridge with 500 mg of Silica 60µm particles
Sample Preparation Method: HILIC SPE Alternative to immuno extraction? • Hydrophilic interaction liquid chromatography SPE: → normal phase conditions → especially for polar molecules • Try to purify the matrix and preconcentrate the sample • HILIC SPE cartridges were made in-house → Fill a glass cartridge with 500 mg of Silica 60µm particles
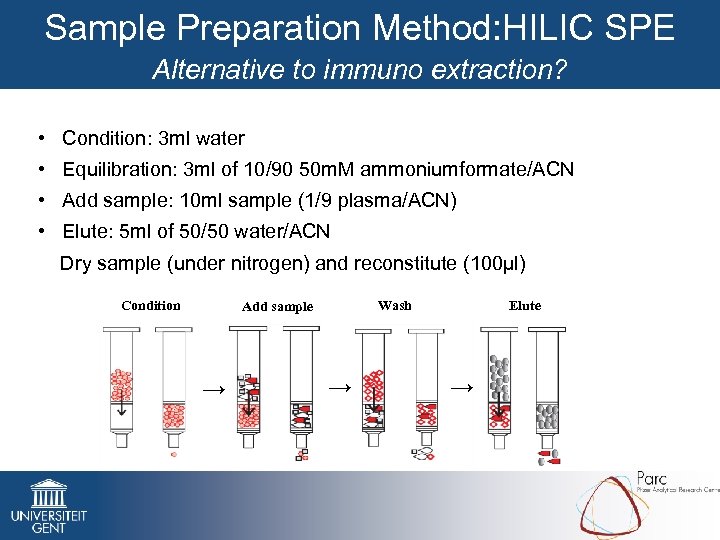 Sample Preparation Method: HILIC SPE Alternative to immuno extraction? • Condition: 3 ml water • Equilibration: 3 ml of 10/90 50 m. M ammoniumformate/ACN • Add sample: 10 ml sample (1/9 plasma/ACN) • Elute: 5 ml of 50/50 water/ACN Dry sample (under nitrogen) and reconstitute (100µl) Condition Wash Add sample → → Elute →
Sample Preparation Method: HILIC SPE Alternative to immuno extraction? • Condition: 3 ml water • Equilibration: 3 ml of 10/90 50 m. M ammoniumformate/ACN • Add sample: 10 ml sample (1/9 plasma/ACN) • Elute: 5 ml of 50/50 water/ACN Dry sample (under nitrogen) and reconstitute (100µl) Condition Wash Add sample → → Elute →
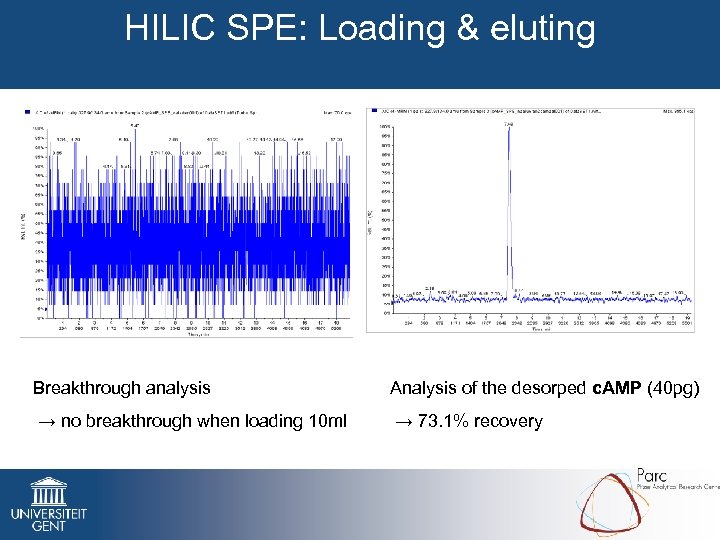 HILIC SPE: Loading & eluting Breakthrough analysis Analysis of the desorped c. AMP (40 pg) → no breakthrough when loading 10 ml → 73. 1% recovery
HILIC SPE: Loading & eluting Breakthrough analysis Analysis of the desorped c. AMP (40 pg) → no breakthrough when loading 10 ml → 73. 1% recovery
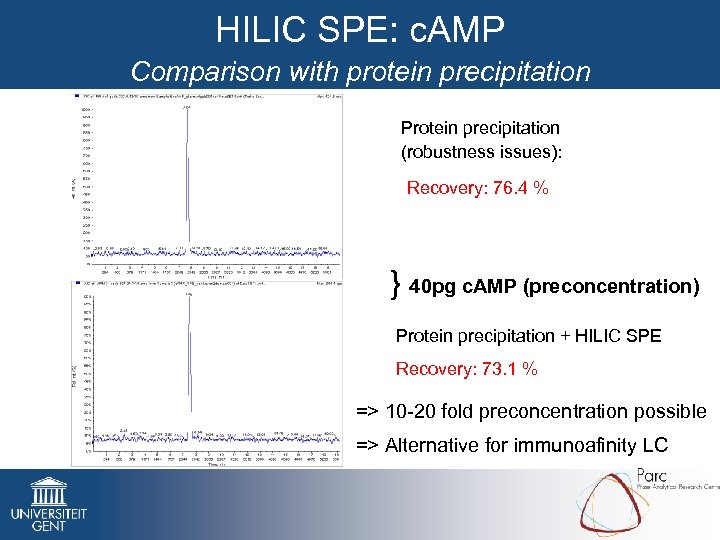 HILIC SPE: c. AMP Comparison with protein precipitation Protein precipitation (robustness issues): Recovery: 76. 4 % } 40 pg c. AMP (preconcentration) Protein precipitation + HILIC SPE Recovery: 73. 1 % => 10 -20 fold preconcentration possible => Alternative for immunoafinity LC
HILIC SPE: c. AMP Comparison with protein precipitation Protein precipitation (robustness issues): Recovery: 76. 4 % } 40 pg c. AMP (preconcentration) Protein precipitation + HILIC SPE Recovery: 73. 1 % => 10 -20 fold preconcentration possible => Alternative for immunoafinity LC
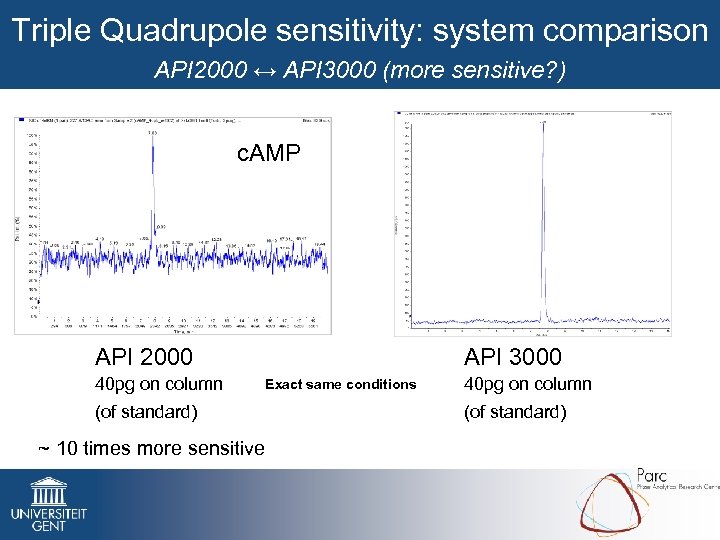 Triple Quadrupole sensitivity: system comparison API 2000 ↔ API 3000 (more sensitive? ) c. AMP API 2000 40 pg on column API 3000 Exact same conditions (of standard) ~ 10 times more sensitive 40 pg on column (of standard)
Triple Quadrupole sensitivity: system comparison API 2000 ↔ API 3000 (more sensitive? ) c. AMP API 2000 40 pg on column API 3000 Exact same conditions (of standard) ~ 10 times more sensitive 40 pg on column (of standard)
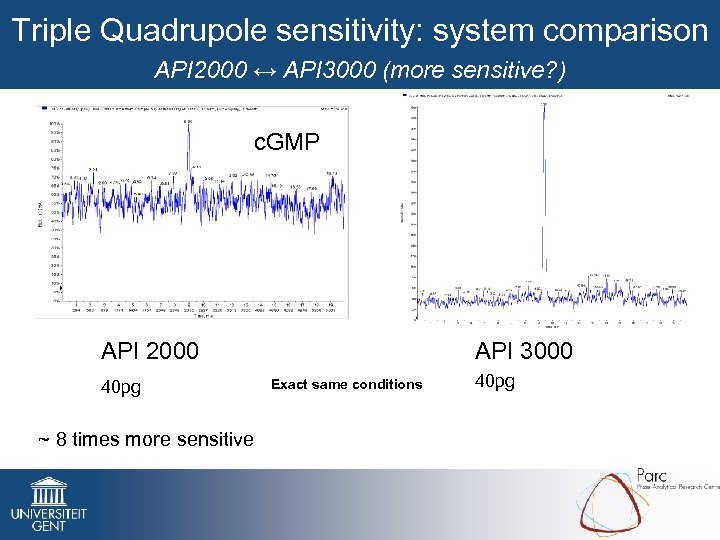 Triple Quadrupole sensitivity: system comparison API 2000 ↔ API 3000 (more sensitive? ) c. GMP API 2000 40 pg ~ 8 times more sensitive API 3000 Exact same conditions 40 pg
Triple Quadrupole sensitivity: system comparison API 2000 ↔ API 3000 (more sensitive? ) c. GMP API 2000 40 pg ~ 8 times more sensitive API 3000 Exact same conditions 40 pg
 Conclusion • Combination of protein precipitation + HILIC SPE + API 3000 instrument allow for c. GMP and c. AMP determination (ppt level) in blood in a routine way
Conclusion • Combination of protein precipitation + HILIC SPE + API 3000 instrument allow for c. GMP and c. AMP determination (ppt level) in blood in a routine way
 Thank you for your attention
Thank you for your attention


