Immunity A-gen A body.pptx
- Количество слайдов: 25

Immunity A-gen, A-body G. Abdulina
Immunity is the ability of the body to specifically counteract foreign organism or substances. 1. Innate resistance is genetically predetermined resistance of an individuals to certain diseases. 2. Acquired immunity is specific resistance to infection developed during the life of the individuals. Naturally acquired immunity active and passive • Active is Immunity resulting from infection is called; this type of immunity may be long lasting. • Passive is transferred from antibodies a mother to a fetus (placental transfer) or to a newborn in colostrum; this type of immunity can last up to a few month

Artificially acquired immunity active and passive • Active is Immunity resulting from vaccination and can be long lasting. Vaccines can be prepared attenuated, inactivated or killed microorganisms. • Passive is immunity refers to humoral antibodies acquired by injection; this type of immunity can last up to a few weeks.
2


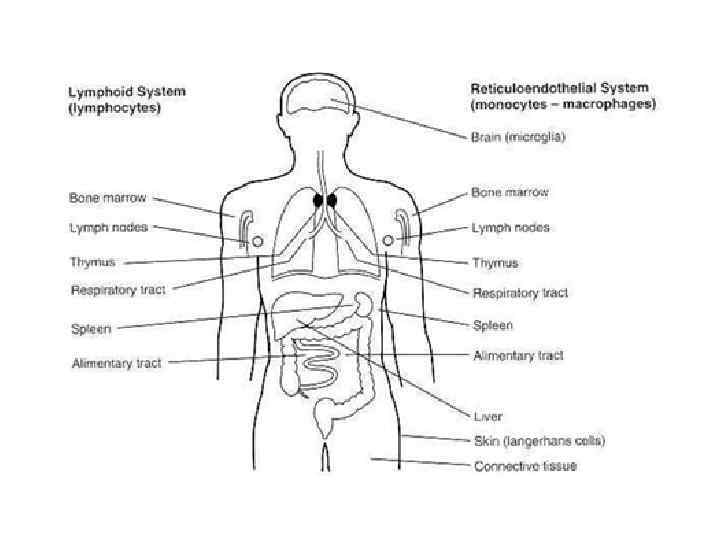
Primarily they can be divided into two major categories Non-specific or innate immunity. Anatomical barriers ● Skin ● Intestinal movement ● Oscillation of broncho-pulmonary cilia Secretory molecules ● Transferrin and lactoferrin deprive organisms of iron. ● Interferon inhibits viral replication ● Lysozyme, in serum and tears, breaks down the bacterial cell wall (peptidoglycan) ● Fibronectin coats (opsonizes) bacteria and promotes their rapid phagocytosis.
● Complement cause destruction of microorganism directly or with the help of phagocytic cells. ● TNF-alpha suppresses viral replication and activates phagocytes. Cellular Components Phagocytic cells: Neutrophils (PMN) and macrophages and monocytes are the most important cellular components of the non-specific immune system. OTHER CELLS: NK cells are large granular lymphocytes that nonspecifically kill certain types of tumor cells and virus-infected cells.

Specific or adaptive immunity. This is a response to a specific immune stimulus (antigen) that involves cells of the immune system and frequently leads to a state of immune memory. Immunogen- A substance that induces a specific immune response. Antigen (Ag)- A substance that reacts with the products of a specific immune response. Superantigens which polyclonally activate a large fraction of the T cells (up to 25%). Hapten- A substance that is non-immunogenic but which can react with the products of a specific immune response. Haptens have the property of antigenicity but not immunogenicity.
Contribution of the Immunogen 1. Foreignness - The immune system normally discriminates between self and non-self such that only foreign molecules are immunogenic. 2. Size- the larger the molecule the more immunogenic it is likely to be. 3. Chemical Composition- In general, the more complex the substance is chemically the more immunogenic it will be. 4. Physical form- In general particulate antigens are more immunogenic than soluble ones and denatured antigens more immunogenic than the native form.
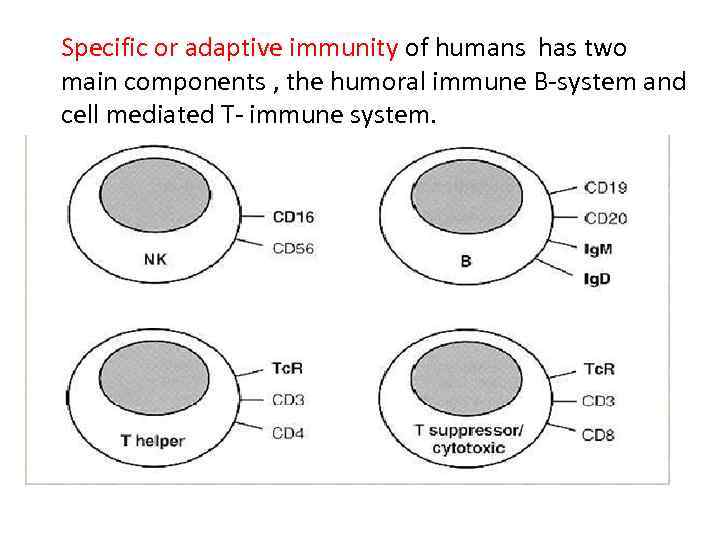
Specific or adaptive immunity of humans has two main components , the humoral immune B-system and cell mediated T- immune system.
T Helper Cells: These cells are the primary regulators of T cell- and B cell-mediated responses. 1) aid antigenstimulated subsets of B lymphocytes to proliferate and differentiate toward antibody producing cells; 2) express the CD 4 molecule; 3) recognize foreign antigen complexed with MHC class II T Cytotoxic Cells: These cells are cytotoxic against tumor cells and host cells infected with intracellular pathogens. These cells 1) usually express CD 8, 2) destroy infected cells in an antigen-specific manner that is dependent upon the expression of MHC class I molecules. T Suppressor Cells: These cells suppress the T and B cell responses and express CD 8 molecules.
B Lymphocytes: These cells differentiate into plasma cells to secrete antibodies and are involved in processing proteins and presenting the resultant peptide antigen fragments in the context of MHC molecule to T cells. The genesis of μ and delta chain-positive, mature B cells from pre-B cells is antigen independent Immunoglobulins (Ig) Glycoprotein molecules that are produced by plasma cells in response to an immunogen and which function as antibodies. (The immunoglobulins derive their name from the finding that they migrate with globular proteins when antibody-containing serum is placed in an electrical field)
. GENERAL FUNCTIONS OF IMMUNOGLOBULINS (Ig) Antigen binding by antibodies is the primary function of antibodies and can result in protection of the host. The valency of antibody refers to the number of antigenic determinants that an individual antibody molecule can bind. Effector functions. Such effector functions include: 1. Fixation of complement - This results in lysis of cells and release of biologically active molecules 2. Binding to various cell types - Phagocytic cells, lymphocytes, platelets, mast cells, and basophils. Ig. G also bind to receptors on placental trophoblasts, which results in transfer of the Ig across the placenta. As a result, the transferred maternal antibodies provide immunity to the fetus and newborn
BASIC STRUCTURE OF IMMUNOGLOBULINS A. Heavy and Light Chains IG-s are composed of two identical light chains (23 k. D) and two identical heavy chains (50 -70 k. D) B. Disulfide bonds 1. Inter-chain - The heavy and light chains and the two heavy chains are held together by inter-chain disulfide bonds and by non-covalent interactions 2. Intra-chain Within each of the polypeptide chains there also intrachain disulfide bonds. C. Variable (V) and Constant (C) Regions 1. Light Chain - VL (110 amino acids) and CL (110 amino acids) 2. Heavy Chain - VH (110 amino acids) and CH (330 -440 amino acids)

D. Hinge Region This is the region at which the arms of the antibody molecule forms a Y. It is called the hinge region because there is some flexibility in the molecule at this point. E. Domains These regions are called domains. 1. Light Chain Domains VL and CL 2. Heavy Chain Domains - VH, CH 1 - CH 3 (or CH 4)


Immunoglobulin classes The immunoglobulins can be divided into five different classes, based on differences in the amino acid sequences in the constant region of the heavy chains 1. Ig. G - Gamma heavy chains 2. Ig. M - Mu heavy chains 3. Ig. A - Alpha heavy chains 4. Ig. D - Delta heavy chains 5. Ig. E - Epsilon heavy chains
Immunoglobulin Subclasses The classes of immunoglobulins can de divided into subclasses based on small differences in the amino acid sequences in the constant region of the heavy chains. These differences are most commonly detected by serological means. 1. Ig. G Subclasses a) Ig. G 1 - Gamma 1 heavy chains b) Ig. G 2 - Gamma 2 heavy chains c) Ig. G 3 - Gamma 3 heavy chains d) Ig. G 4 - Gamma 4 heavy chains 2. Ig. A Subclasses a) Ig. A 1 - Alpha 1 heavy chainsb) b) Ig. A 2 - Alpha 2 heavy chains

Immunoglobulin Types Ig-s can also be classified by the type of light chain that they have. 1. Kappa light chains 2. Lambda light chains D. Immunoglobulin Subtypes: The light chains can also be divided into subtypes based on differences in the amino acid sequences in the constant region of the light chain. 1. Lambda subtypes a) Lambda 1 b) Lambda 2 c) Lambda 3 d) Lambda 4

Properties Ig. G a) Ig. G is the major Ig in serum - 75% of serum Ig is Ig. G b) Ig. G is the major Ig in extra vascular spaces c) Placental transfer - Ig. G is the only class of Ig that crosses the placenta ( Ig. G 2 does not cross well). d) Fixes complement – (Ig. G 4 does not fix complement e) Binding to cells - Macrophages, monocytes, PMN's and some lymphocytes have Fc receptors for the Fc region of Ig. G(Ig. G 2 and Ig. G 4 do not bind to Fc receptors). The antibody has prepared the antigen for eating by the phagocytic cells. The term opsonin is used to describe substances that enhance phagocytosis. Ig. G is a good opsonin.
Properties Ig. M 1. Ig. M normally exists as a pentamer (19 S immunoglobulin) but it can also exist as a monomer. 2. Ig. M has an extra domain on the mu chain (CH 4 a) 3. Ig. M has another protein covalently bound via a S-S bond called the J chain. 4. Ig. M is the third most common serum Ig. 5. Ig. M is the first Ig to be made by the fetus and the first Ig to be made by a virgin B cells when it is stimulated by antigen. 6. Ig. M is a good complement fixing Ig. 7. The valence is theoretically 10. 8. Ig. M antibodies are very efficient in leading to the lysis of microorganisms. and, Ig. M is also a good agglutinating Ig.

Ig. A 1. Structure Serum Ig. A is a monomer but Ig. A found in secretions is a dimer. When Ig. A exits as a dimer, a J chain is associated with it. The secretory piece is made in epithelial cells and is added to the Ig. A as it passes into the secretions 2. Ig. A is the 2 nd most common serum Ig. 3. Ig. A is the major class of Ig in secretions - tears, saliva, colostrum, mucus. Since it is found in secretions secretory Ig. A is important in local (mucosal) immunity. 4. Normally Ig. A does not fix complement, unless aggregated. 5. Ig. A can binding to some cells - PMN's and some lymphocytes.
Ig. D 1. Structure: Ig. D exists only as a monomer. 2. Properties a) Ig. D is found in low levels in serum; its role in serum uncertain. b) Ig. D is primarily found on B cell surfaces where it functions as a receptor for antigen Ig. E 1. Structure Ig. E exists as a monomer and has an extra domain in the constant region. 2. Properties • Ig. E is the least common serum Ig • it binds to Fc receptors on basophils and mast cells • Involved in allergic reactions • Ig. E also plays a role in parasitic helminth diseases. • Ig. E does not fix complement.

Immunity A-gen A body.pptx