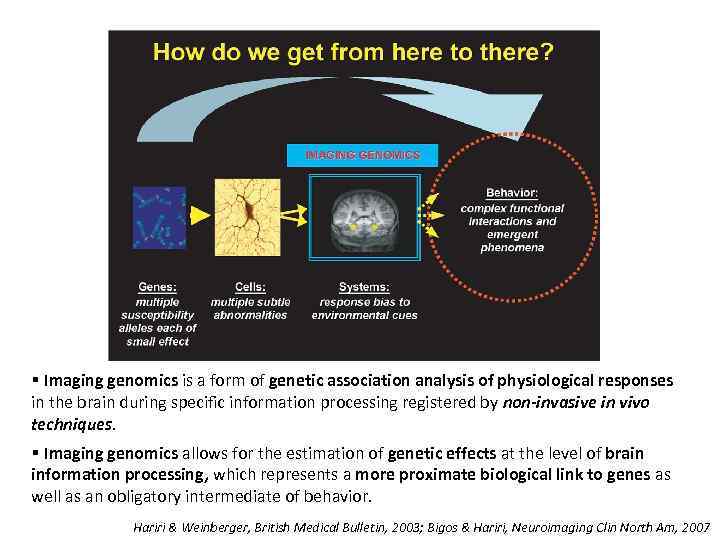
 § Imaging genomics is a form of genetic association analysis of physiological responses in the brain during specific information processing registered by non-invasive in vivo techniques. § Imaging genomics allows for the estimation of genetic effects at the level of brain information processing, which represents a more proximate biological link to genes as well as an obligatory intermediate of behavior. Hariri & Weinberger, British Medical Bulletin, 2003; Bigos & Hariri, Neuroimaging Clin North Am, 2007
§ Imaging genomics is a form of genetic association analysis of physiological responses in the brain during specific information processing registered by non-invasive in vivo techniques. § Imaging genomics allows for the estimation of genetic effects at the level of brain information processing, which represents a more proximate biological link to genes as well as an obligatory intermediate of behavior. Hariri & Weinberger, British Medical Bulletin, 2003; Bigos & Hariri, Neuroimaging Clin North Am, 2007
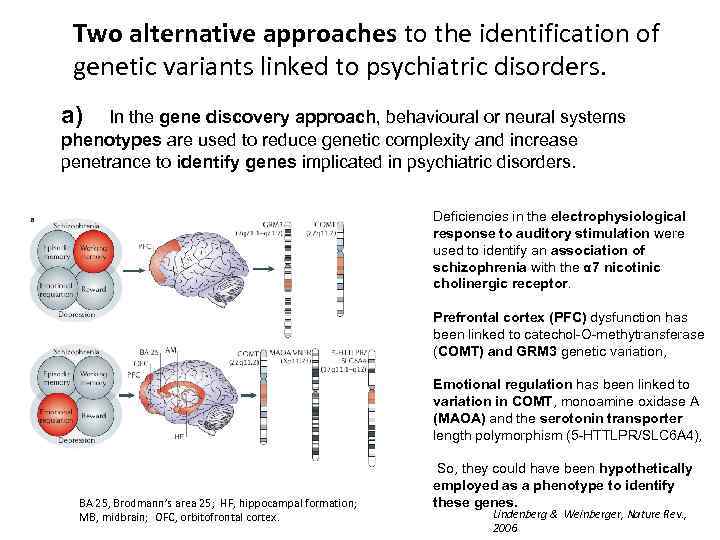 Two alternative approaches to the identification of genetic variants linked to psychiatric disorders. a) In the gene discovery approach, behavioural or neural systems phenotypes are used to reduce genetic complexity and increase penetrance to identify genes implicated in psychiatric disorders. Deficiencies in the electrophysiological response to auditory stimulation were used to identify an association of schizophrenia with the α 7 nicotinic cholinergic receptor. Prefrontal cortex (PFC) dysfunction has been linked to catechol-O-methytransferase (COMT) and GRM 3 genetic variation, Emotional regulation has been linked to variation in COMT, monoamine oxidase A (MAOA) and the serotonin transporter length polymorphism (5 -HTTLPR/SLC 6 A 4), BA 25, Brodmann’s area 25; HF, hippocampal formation; MB, midbrain; OFC, orbitofrontal cortex. So, they could have been hypothetically employed as a phenotype to identify these genes. Lindenberg & Weinberger, Nature Rev. , 2006
Two alternative approaches to the identification of genetic variants linked to psychiatric disorders. a) In the gene discovery approach, behavioural or neural systems phenotypes are used to reduce genetic complexity and increase penetrance to identify genes implicated in psychiatric disorders. Deficiencies in the electrophysiological response to auditory stimulation were used to identify an association of schizophrenia with the α 7 nicotinic cholinergic receptor. Prefrontal cortex (PFC) dysfunction has been linked to catechol-O-methytransferase (COMT) and GRM 3 genetic variation, Emotional regulation has been linked to variation in COMT, monoamine oxidase A (MAOA) and the serotonin transporter length polymorphism (5 -HTTLPR/SLC 6 A 4), BA 25, Brodmann’s area 25; HF, hippocampal formation; MB, midbrain; OFC, orbitofrontal cortex. So, they could have been hypothetically employed as a phenotype to identify these genes. Lindenberg & Weinberger, Nature Rev. , 2006
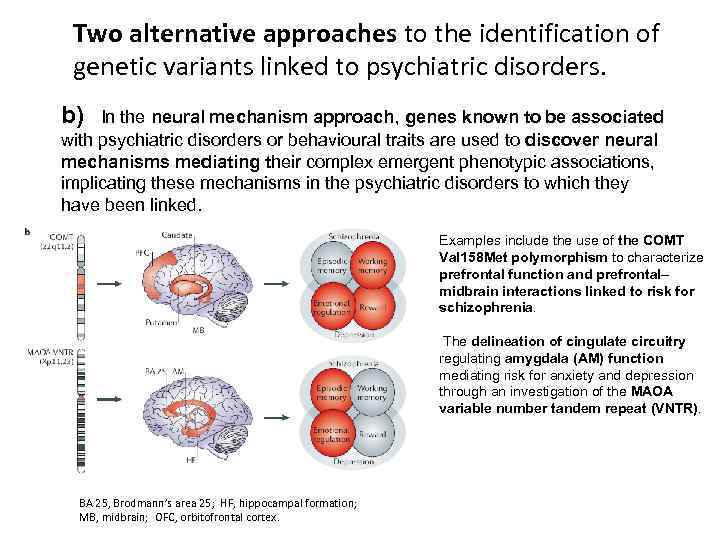 Two alternative approaches to the identification of genetic variants linked to psychiatric disorders. b) In the neural mechanism approach, genes known to be associated with psychiatric disorders or behavioural traits are used to discover neural mechanisms mediating their complex emergent phenotypic associations, implicating these mechanisms in the psychiatric disorders to which they have been linked. Examples include the use of the COMT Val 158 Met polymorphism to characterize prefrontal function and prefrontal– midbrain interactions linked to risk for schizophrenia. The delineation of cingulate circuitry regulating amygdala (AM) function mediating risk for anxiety and depression through an investigation of the MAOA variable number tandem repeat (VNTR). BA 25, Brodmann’s area 25; HF, hippocampal formation; MB, midbrain; OFC, orbitofrontal cortex.
Two alternative approaches to the identification of genetic variants linked to psychiatric disorders. b) In the neural mechanism approach, genes known to be associated with psychiatric disorders or behavioural traits are used to discover neural mechanisms mediating their complex emergent phenotypic associations, implicating these mechanisms in the psychiatric disorders to which they have been linked. Examples include the use of the COMT Val 158 Met polymorphism to characterize prefrontal function and prefrontal– midbrain interactions linked to risk for schizophrenia. The delineation of cingulate circuitry regulating amygdala (AM) function mediating risk for anxiety and depression through an investigation of the MAOA variable number tandem repeat (VNTR). BA 25, Brodmann’s area 25; HF, hippocampal formation; MB, midbrain; OFC, orbitofrontal cortex.
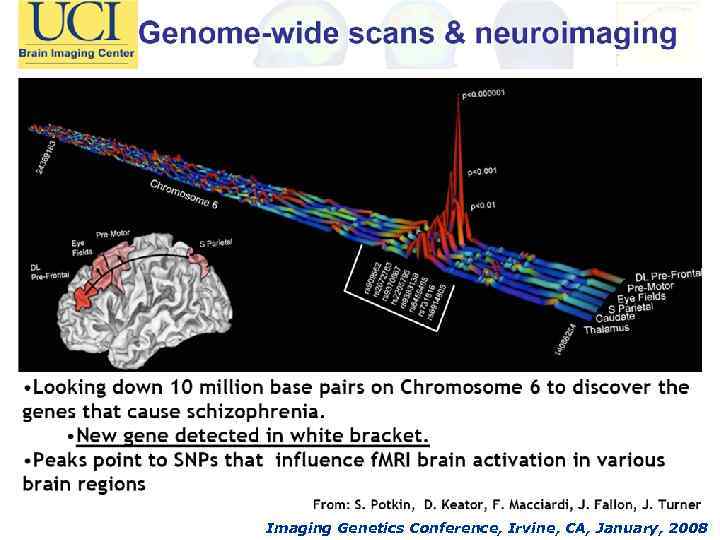 Imaging Genetics Conference, Irvine, CA, January, 2008
Imaging Genetics Conference, Irvine, CA, January, 2008
 Imaging genetics: III. Common polymorphisms having an impact on the hippocampus and episodic memory KIBRA (kidney brain). § The newly discovered brain protein, KIBRA, is associated significantly with normal variability in memory performance. A common thymine (T) to cytosine substitution within intron 9 of the human KIBRA gene is associated significantly with differential memory performance. Carriers of the KIBRA T allele exhibit greater memory scores in tests of episodic memory. § KIBRA is highly expressed in brain regions involved in memory (e. g. , hippocampus) that is critical in allowing KIBRA to interact with other systems to regulate synaptic plasticity. § f. MRI was used to explore the impact of the KIBRA T allele on memory-related brain activity. Papassotiropoulos et al. , Science, 2006
Imaging genetics: III. Common polymorphisms having an impact on the hippocampus and episodic memory KIBRA (kidney brain). § The newly discovered brain protein, KIBRA, is associated significantly with normal variability in memory performance. A common thymine (T) to cytosine substitution within intron 9 of the human KIBRA gene is associated significantly with differential memory performance. Carriers of the KIBRA T allele exhibit greater memory scores in tests of episodic memory. § KIBRA is highly expressed in brain regions involved in memory (e. g. , hippocampus) that is critical in allowing KIBRA to interact with other systems to regulate synaptic plasticity. § f. MRI was used to explore the impact of the KIBRA T allele on memory-related brain activity. Papassotiropoulos et al. , Science, 2006
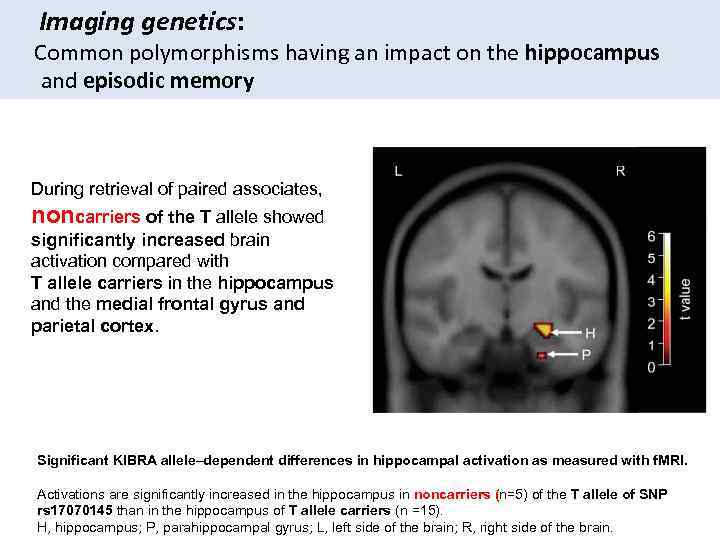 Imaging genetics: Common polymorphisms having an impact on the hippocampus and episodic memory During retrieval of paired associates, noncarriers of the T allele showed significantly increased brain activation compared with T allele carriers in the hippocampus and the medial frontal gyrus and parietal cortex. Significant KIBRA allele–dependent differences in hippocampal activation as measured with f. MRI. Activations are significantly increased in the hippocampus in noncarriers (n=5) of the T allele of SNP rs 17070145 than in the hippocampus of T allele carriers (n =15). H, hippocampus; P, parahippocampal gyrus; L, left side of the brain; R, right side of the brain.
Imaging genetics: Common polymorphisms having an impact on the hippocampus and episodic memory During retrieval of paired associates, noncarriers of the T allele showed significantly increased brain activation compared with T allele carriers in the hippocampus and the medial frontal gyrus and parietal cortex. Significant KIBRA allele–dependent differences in hippocampal activation as measured with f. MRI. Activations are significantly increased in the hippocampus in noncarriers (n=5) of the T allele of SNP rs 17070145 than in the hippocampus of T allele carriers (n =15). H, hippocampus; P, parahippocampal gyrus; L, left side of the brain; R, right side of the brain.
 Imaging genetics: Common polymorphisms having an impact on the hippocampus and episodic memory As memory performance did not differ between these groups, the data suggest that noncarriers of the T allele need greater activation in these memory-related brain regions to reach the same level of retrieval performance as T allele carriers. Thus, the potential impact of the KIBRA T allele on synaptic plasticity manifest as more efficient processing of information in memory-related neural circuits and subsequently superior memory ability.
Imaging genetics: Common polymorphisms having an impact on the hippocampus and episodic memory As memory performance did not differ between these groups, the data suggest that noncarriers of the T allele need greater activation in these memory-related brain regions to reach the same level of retrieval performance as T allele carriers. Thus, the potential impact of the KIBRA T allele on synaptic plasticity manifest as more efficient processing of information in memory-related neural circuits and subsequently superior memory ability.
 Brain-computer interface A brain-computer interface (BCI), or a brain-machine interface, is a direct communication pathway between a human or animal brain (or brain cell culture) and an external device. In one-way BCIs, computers either accept commands from the brain or send signals to it but not both. Two-way BCIs would allow brains and external devices to exchange information in both directions but have yet to be successfully implanted in animals or humans. Research on BCIs began in the 1970 s, but it wasn't until the mid-1990 s that the first working experimental implants in humans appeared. Following years of animal experimentation, early working implants in humans now exist, designed to restore damaged hearing, sight and movement.
Brain-computer interface A brain-computer interface (BCI), or a brain-machine interface, is a direct communication pathway between a human or animal brain (or brain cell culture) and an external device. In one-way BCIs, computers either accept commands from the brain or send signals to it but not both. Two-way BCIs would allow brains and external devices to exchange information in both directions but have yet to be successfully implanted in animals or humans. Research on BCIs began in the 1970 s, but it wasn't until the mid-1990 s that the first working experimental implants in humans appeared. Following years of animal experimentation, early working implants in humans now exist, designed to restore damaged hearing, sight and movement.
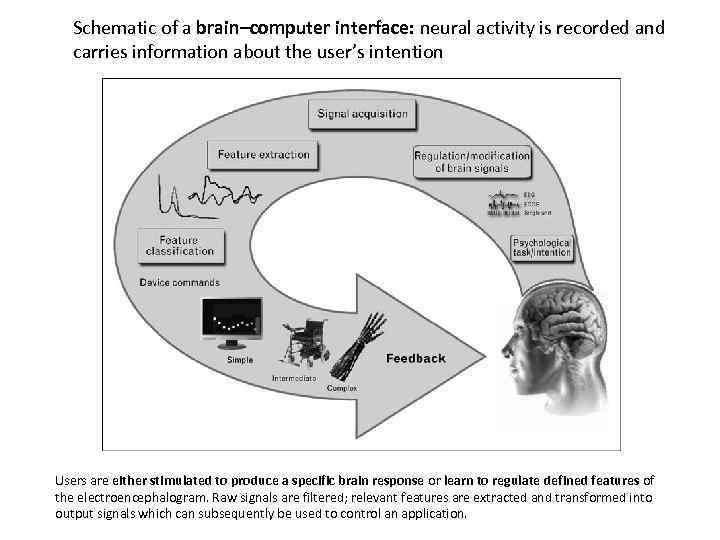 Schematic of a brain–computer interface: neural activity is recorded and carries information about the user’s intention Users are either stimulated to produce a specific brain response or learn to regulate defined features of the electroencephalogram. Raw signals are filtered; relevant features are extracted and transformed into output signals which can subsequently be used to control an application.
Schematic of a brain–computer interface: neural activity is recorded and carries information about the user’s intention Users are either stimulated to produce a specific brain response or learn to regulate defined features of the electroencephalogram. Raw signals are filtered; relevant features are extracted and transformed into output signals which can subsequently be used to control an application.
 BCI vs. Neuroprosthetics is an area of neuroscience concerned with neural prostheses — using artificial devices to replace the function of impaired nervous systems or sensory organs. The most widely used neuroprosthetic device is the cochlear implant, which was implanted in approximately 100, 000 people worldwide as of 2006. There also several neuroprosthetic devices that aim to restore vision, including retinal implants. The differences between BCIs and neuroprosthetics are mostly in the ways the terms are used: neuroprosthetics typically connect the nervous system, to a device, whereas the term "BCIs" usually connect the brain (or nervous system) with a computer system. Neuroprosthetics and BCI seek to achieve the same aims, such as restoring sight, hearing, movement, ability to communicate, and even cognitive function. Both use similar experimental methods and surgical techniques.
BCI vs. Neuroprosthetics is an area of neuroscience concerned with neural prostheses — using artificial devices to replace the function of impaired nervous systems or sensory organs. The most widely used neuroprosthetic device is the cochlear implant, which was implanted in approximately 100, 000 people worldwide as of 2006. There also several neuroprosthetic devices that aim to restore vision, including retinal implants. The differences between BCIs and neuroprosthetics are mostly in the ways the terms are used: neuroprosthetics typically connect the nervous system, to a device, whereas the term "BCIs" usually connect the brain (or nervous system) with a computer system. Neuroprosthetics and BCI seek to achieve the same aims, such as restoring sight, hearing, movement, ability to communicate, and even cognitive function. Both use similar experimental methods and surgical techniques.
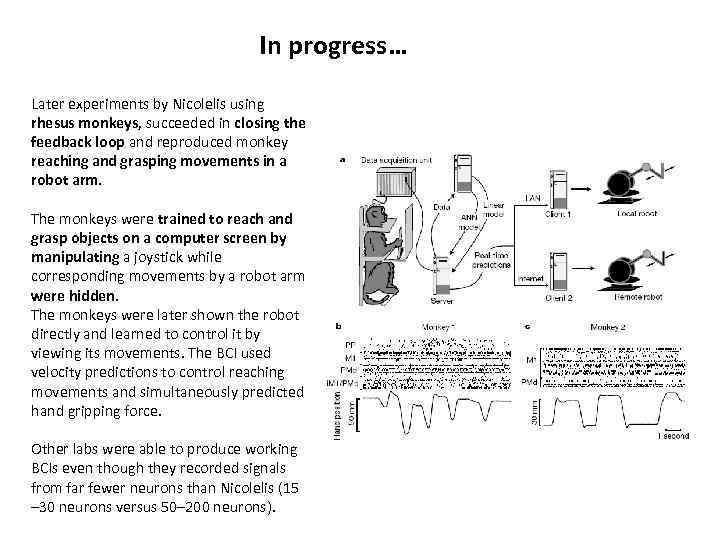 In progress… Later experiments by Nicolelis using rhesus monkeys, succeeded in closing the feedback loop and reproduced monkey reaching and grasping movements in a robot arm. The monkeys were trained to reach and grasp objects on a computer screen by manipulating a joystick while corresponding movements by a robot arm were hidden. The monkeys were later shown the robot directly and learned to control it by viewing its movements. The BCI used velocity predictions to control reaching movements and simultaneously predicted hand gripping force. Other labs were able to produce working BCIs even though they recorded signals from far fewer neurons than Nicolelis (15 – 30 neurons versus 50– 200 neurons).
In progress… Later experiments by Nicolelis using rhesus monkeys, succeeded in closing the feedback loop and reproduced monkey reaching and grasping movements in a robot arm. The monkeys were trained to reach and grasp objects on a computer screen by manipulating a joystick while corresponding movements by a robot arm were hidden. The monkeys were later shown the robot directly and learned to control it by viewing its movements. The BCI used velocity predictions to control reaching movements and simultaneously predicted hand gripping force. Other labs were able to produce working BCIs even though they recorded signals from far fewer neurons than Nicolelis (15 – 30 neurons versus 50– 200 neurons).
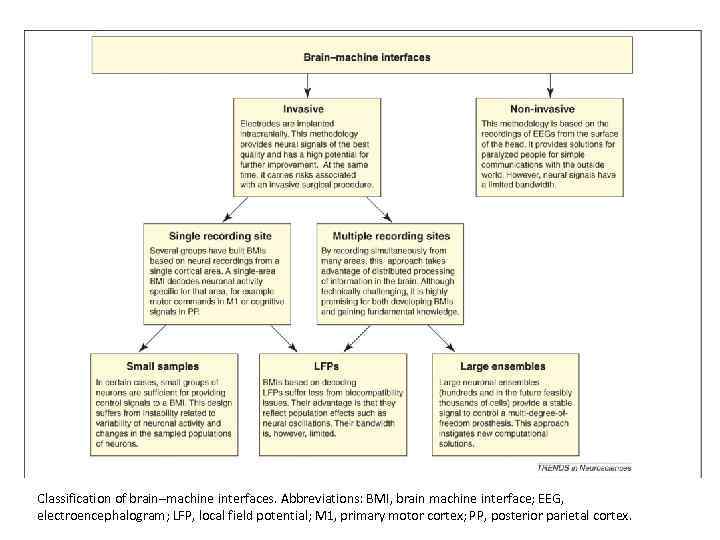 Classification of brain–machine interfaces. Abbreviations: BMI, brain machine interface; EEG, electroencephalogram; LFP, local field potential; M 1, primary motor cortex; PP, posterior parietal cortex.
Classification of brain–machine interfaces. Abbreviations: BMI, brain machine interface; EEG, electroencephalogram; LFP, local field potential; M 1, primary motor cortex; PP, posterior parietal cortex.
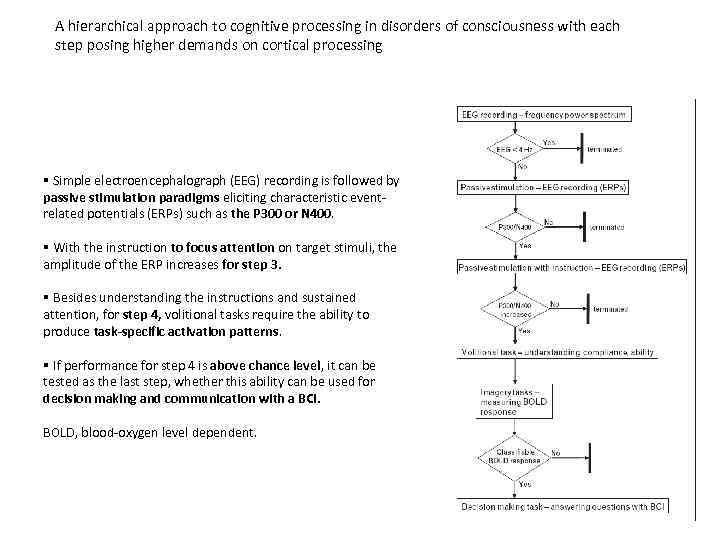 A hierarchical approach to cognitive processing in disorders of consciousness with each step posing higher demands on cortical processing § Simple electroencephalograph (EEG) recording is followed by passive stimulation paradigms eliciting characteristic eventrelated potentials (ERPs) such as the P 300 or N 400. § With the instruction to focus attention on target stimuli, the amplitude of the ERP increases for step 3. § Besides understanding the instructions and sustained attention, for step 4, volitional tasks require the ability to produce task-specific activation patterns. § If performance for step 4 is above chance level, it can be tested as the last step, whether this ability can be used for decision making and communication with a BCI. BOLD, blood-oxygen level dependent.
A hierarchical approach to cognitive processing in disorders of consciousness with each step posing higher demands on cortical processing § Simple electroencephalograph (EEG) recording is followed by passive stimulation paradigms eliciting characteristic eventrelated potentials (ERPs) such as the P 300 or N 400. § With the instruction to focus attention on target stimuli, the amplitude of the ERP increases for step 3. § Besides understanding the instructions and sustained attention, for step 4, volitional tasks require the ability to produce task-specific activation patterns. § If performance for step 4 is above chance level, it can be tested as the last step, whether this ability can be used for decision making and communication with a BCI. BOLD, blood-oxygen level dependent.
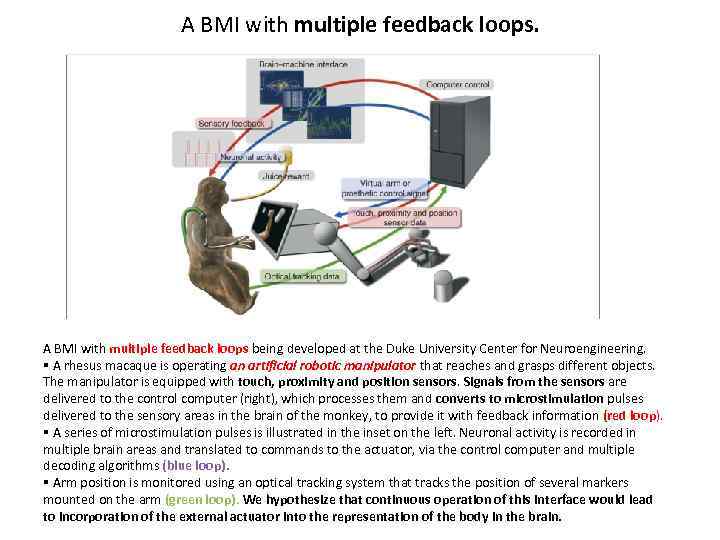 A BMI with multiple feedback loops. A BMI with multiple feedback loops being developed at the Duke University Center for Neuroengineering. § A rhesus macaque is operating an artificial robotic manipulator that reaches and grasps different objects. The manipulator is equipped with touch, proximity and position sensors. Signals from the sensors are delivered to the control computer (right), which processes them and converts to microstimulation pulses delivered to the sensory areas in the brain of the monkey, to provide it with feedback information (red loop). § A series of microstimulation pulses is illustrated in the inset on the left. Neuronal activity is recorded in multiple brain areas and translated to commands to the actuator, via the control computer and multiple decoding algorithms (blue loop). § Arm position is monitored using an optical tracking system that tracks the position of several markers mounted on the arm (green loop). We hypothesize that continuous operation of this interface would lead to incorporation of the external actuator into the representation of the body in the brain.
A BMI with multiple feedback loops. A BMI with multiple feedback loops being developed at the Duke University Center for Neuroengineering. § A rhesus macaque is operating an artificial robotic manipulator that reaches and grasps different objects. The manipulator is equipped with touch, proximity and position sensors. Signals from the sensors are delivered to the control computer (right), which processes them and converts to microstimulation pulses delivered to the sensory areas in the brain of the monkey, to provide it with feedback information (red loop). § A series of microstimulation pulses is illustrated in the inset on the left. Neuronal activity is recorded in multiple brain areas and translated to commands to the actuator, via the control computer and multiple decoding algorithms (blue loop). § Arm position is monitored using an optical tracking system that tracks the position of several markers mounted on the arm (green loop). We hypothesize that continuous operation of this interface would lead to incorporation of the external actuator into the representation of the body in the brain.
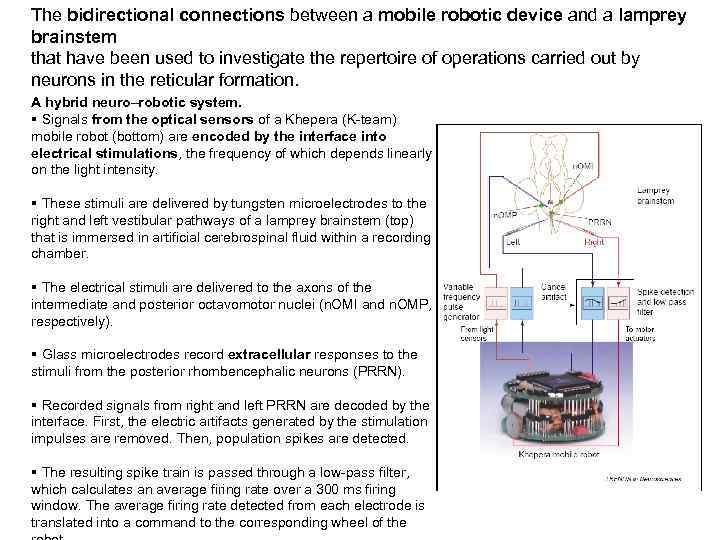 The bidirectional connections between a mobile robotic device and a lamprey brainstem that have been used to investigate the repertoire of operations carried out by neurons in the reticular formation. A hybrid neuro–robotic system. § Signals from the optical sensors of a Khepera (K-team) mobile robot (bottom) are encoded by the interface into electrical stimulations, the frequency of which depends linearly on the light intensity. § These stimuli are delivered by tungsten microelectrodes to the right and left vestibular pathways of a lamprey brainstem (top) that is immersed in artificial cerebrospinal fluid within a recording chamber. § The electrical stimuli are delivered to the axons of the intermediate and posterior octavomotor nuclei (n. OMI and n. OMP, respectively). § Glass microelectrodes record extracellular responses to the stimuli from the posterior rhombencephalic neurons (PRRN). § Recorded signals from right and left PRRN are decoded by the interface. First, the electric artifacts generated by the stimulation impulses are removed. Then, population spikes are detected. § The resulting spike train is passed through a low-pass filter, which calculates an average firing rate over a 300 ms firing window. The average firing rate detected from each electrode is translated into a command to the corresponding wheel of the
The bidirectional connections between a mobile robotic device and a lamprey brainstem that have been used to investigate the repertoire of operations carried out by neurons in the reticular formation. A hybrid neuro–robotic system. § Signals from the optical sensors of a Khepera (K-team) mobile robot (bottom) are encoded by the interface into electrical stimulations, the frequency of which depends linearly on the light intensity. § These stimuli are delivered by tungsten microelectrodes to the right and left vestibular pathways of a lamprey brainstem (top) that is immersed in artificial cerebrospinal fluid within a recording chamber. § The electrical stimuli are delivered to the axons of the intermediate and posterior octavomotor nuclei (n. OMI and n. OMP, respectively). § Glass microelectrodes record extracellular responses to the stimuli from the posterior rhombencephalic neurons (PRRN). § Recorded signals from right and left PRRN are decoded by the interface. First, the electric artifacts generated by the stimulation impulses are removed. Then, population spikes are detected. § The resulting spike train is passed through a low-pass filter, which calculates an average firing rate over a 300 ms firing window. The average firing rate detected from each electrode is translated into a command to the corresponding wheel of the
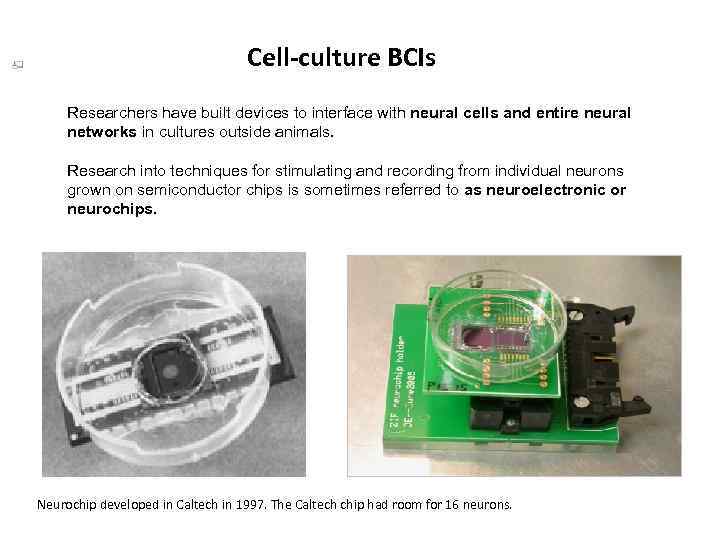 Cell-culture BCIs Researchers have built devices to interface with neural cells and entire neural networks in cultures outside animals. Research into techniques for stimulating and recording from individual neurons grown on semiconductor chips is sometimes referred to as neuroelectronic or neurochips. Neurochip developed in Caltech in 1997. The Caltech chip had room for 16 neurons.
Cell-culture BCIs Researchers have built devices to interface with neural cells and entire neural networks in cultures outside animals. Research into techniques for stimulating and recording from individual neurons grown on semiconductor chips is sometimes referred to as neuroelectronic or neurochips. Neurochip developed in Caltech in 1997. The Caltech chip had room for 16 neurons.
 Neuroinformatics combines neuroscience and informatics research to develop and apply advanced tools and approaches essential for a major advancement in understanding the structure and function of the brain. The field covers three primary areas: • neuroscience data and knowledge bases, from molecular to behavioral levels • tools for data-acquisition, analysis, visualization and distribution of nervous system data • theoretical, computational and simulation environments for modeling and understanding the brain Neuroinformatics research is uniquely placed at the intersections of medical and behavioral sciences, biology, physical and mathematical sciences, computer science and engineering. The synergy from combining these approaches will accelerate scientific and technological progress, resulting in major medical, social, and economic benefits.
Neuroinformatics combines neuroscience and informatics research to develop and apply advanced tools and approaches essential for a major advancement in understanding the structure and function of the brain. The field covers three primary areas: • neuroscience data and knowledge bases, from molecular to behavioral levels • tools for data-acquisition, analysis, visualization and distribution of nervous system data • theoretical, computational and simulation environments for modeling and understanding the brain Neuroinformatics research is uniquely placed at the intersections of medical and behavioral sciences, biology, physical and mathematical sciences, computer science and engineering. The synergy from combining these approaches will accelerate scientific and technological progress, resulting in major medical, social, and economic benefits.


