c048378ebc9cfe28b015a61295d71942.ppt
- Количество слайдов: 27

II Workshop on Medicines Regulation in the Caribbean Region Regulatory Function of DRUG REGISTRATION Celeste Sánchez González, Ph. D. Adviser. CECMED/Cuba sanchez. celeste@gmail. com/evareg@cecmed. sld. cu II Workshop on Medicines Regulationthin the Caribbean Region th Barbados, September 8 and 9 , 2009 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 1
Content of the Presentation 1. Drug registration as a regulatory function 2. What does drug registration is? 3. Regulatory requirements for drug registration 4. Ways to implement registration 5. Steps of the registration process 6. Drug registration requirements according to the Pan American Network for Drug Regulation Harmonization (PANDRH) II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 2
An approach of Regulatory Functions of Medicines National Regulatory Authorities (1) Ratanawijitrasin S. & Wondemagegnehu E. Effective drug regulation. A multicountry study. WHO, 2002 II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 3
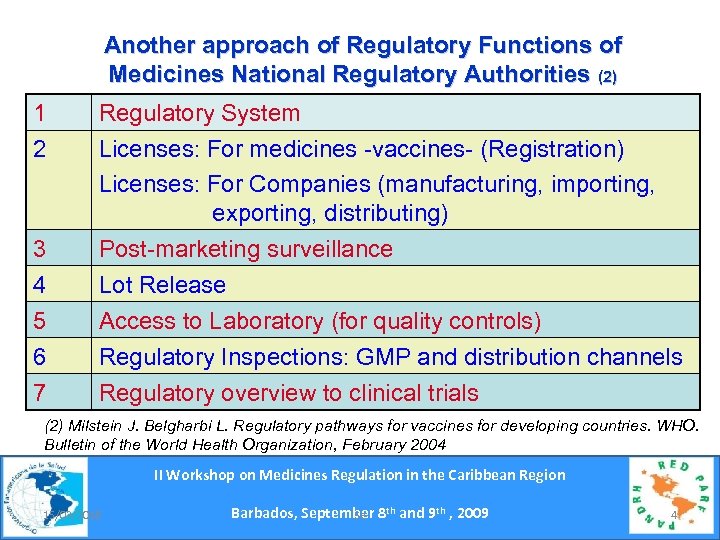
Another approach of Regulatory Functions of Medicines National Regulatory Authorities (2) 1 2 Regulatory System Licenses: For medicines -vaccines- (Registration) Licenses: For Companies (manufacturing, importing, exporting, distributing) 3 4 5 6 7 Post-marketing surveillance Lot Release Access to Laboratory (for quality controls) Regulatory Inspections: GMP and distribution channels Regulatory overview to clinical trials (2) Milstein J. Belgharbi L. Regulatory pathways for vaccines for developing countries. WHO. Bulletin of the World Health Organization, February 2004 II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 4

Drug Registration An important task for a Drug National Regulatory Authority (NRA) is to institute a system which subjects all pharmaceutical products to: • Pre marketing evaluation; • Marketing authorization (registration); • Postmarketing review To ensure that they conform to required standards of quality, safety and efficacy (3) Guiding Principles for small national drug regulatory authorities. Quality Assurance of pharmaceuticals: a compendium of gudelines and related materials, Vol. Geneva, WHO, 1997: 18 -30. II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 5

Drug Registration Drug registration a function of regulation and control within NRAs that deals with this objectives interrelated with the other regulatory functions It has been defined as: “a system that subjects all pharmaceutical products (under the scope of the NRA) to pre-marketing evaluation, marketing authorization (registration), and post-marketing review to ensure that they conform to required standards of quality, safety and efficacy established by NRA” (4) How to implement Computer-Assisted Drug Registration. Annex 2. Regulatory Support Series, No. 2. Geneva, WHO/MSH, 1998. II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 6

Drug Registration Results The outcome of the drug registration process is the issuance or denial of a pharmaceutical product marketing authorization or license (registry) The registry holder is obliges the registry holder to commercialize the product for therapeutic conditions indicated, with the specifications, pharmaceutical form, presentations, manufacturer, storage conditions, etc. Information for patients, health professionals are also results of this regulatory function II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 7
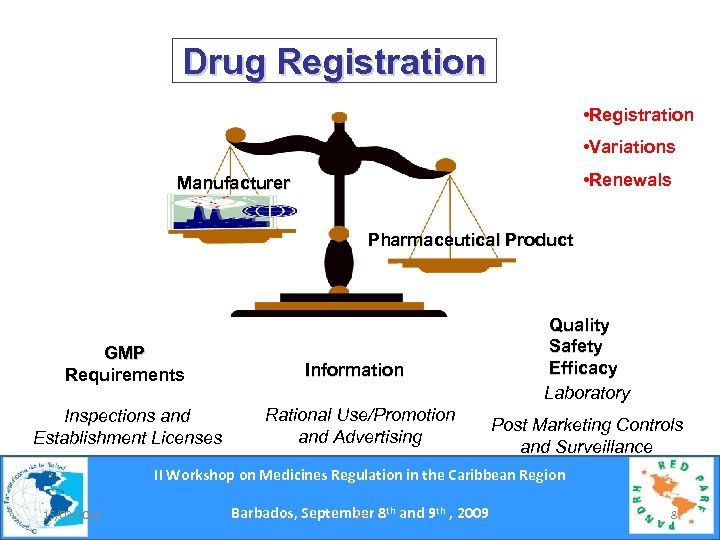
Drug Registration • Variations • Renewals Manufacturer Pharmaceutical Product GMP Requirements Inspections and Establishment Licenses Information Rational Use/Promotion and Advertising Quality Safety Efficacy Laboratory Post Marketing Controls and Surveillance II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 8

Drug Registration Requirements in Terms of Regulation for a NRA • Legal Bases • Guidelines • Assessment procedures • Human resources and others • Records • Availability of the information (5) WHO DATA COLLECTION TOOL (To be used jointly with the Guidance for the assessment of Drug Regulatory Systems). WHO, 2009 II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 9
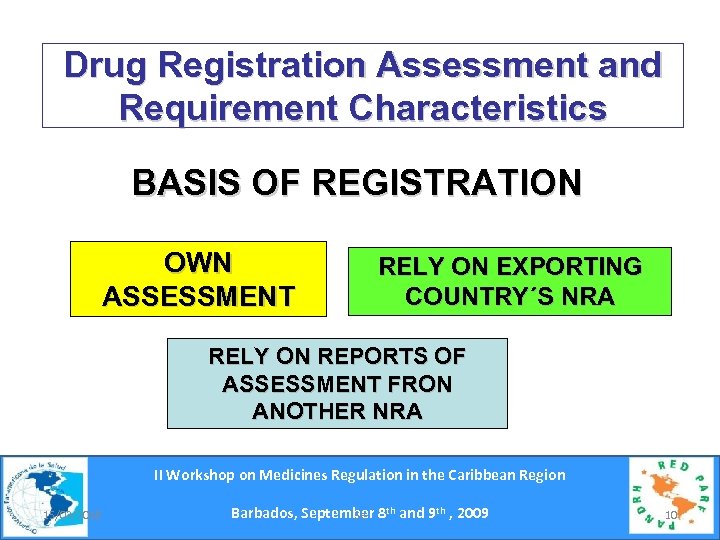
Drug Registration Assessment and Requirement Characteristics BASIS OF REGISTRATION OWN ASSESSMENT RELY ON EXPORTING COUNTRY´S NRA RELY ON REPORTS OF ASSESSMENT FRON ANOTHER NRA II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 10
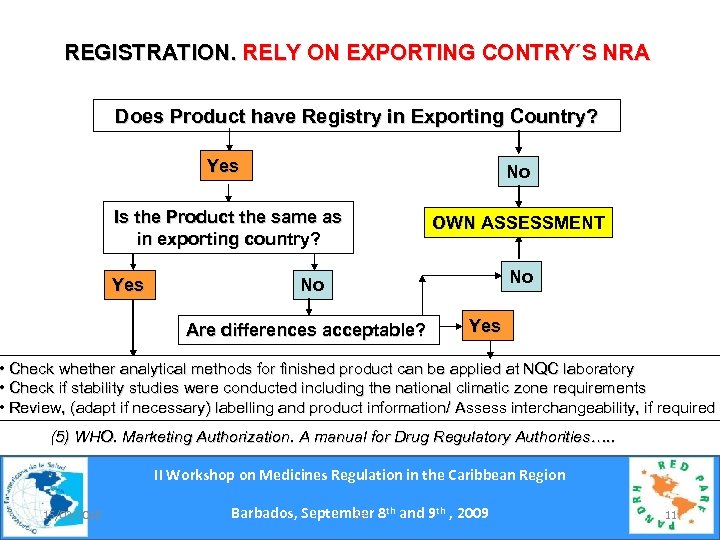
REGISTRATION. RELY ON EXPORTING CONTRY´S NRA Does Product have Registry in Exporting Country? Yes No Is the Product the same as in exporting country? OWN ASSESSMENT Yes No No Are differences acceptable? Yes • Check whether analytical methods for finished product can be applied at NQC laboratory • Check if stability studies were conducted including the national climatic zone requirements • Review, (adapt if necessary) labelling and product information/ Assess interchangeability, if required (5) WHO. Marketing Authorization. A manual for Drug Regulatory Authorities…. . II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 11
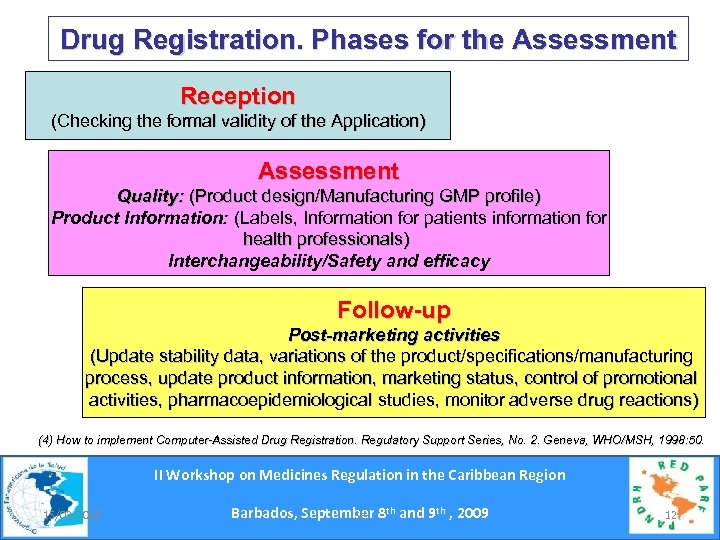
Drug Registration. Phases for the Assessment Reception (Checking the formal validity of the Application) Assessment Quality: (Product design/Manufacturing GMP profile) Product Information: (Labels, Information for patients information for health professionals) Interchangeability/Safety and efficacy Follow-up Post-marketing activities (Update stability data, variations of the product/specifications/manufacturing process, update product information, marketing status, control of promotional activities, pharmacoepidemiological studies, monitor adverse drug reactions) (4) How to implement Computer-Assisted Drug Registration. Regulatory Support Series, No. 2. Geneva, WHO/MSH, 1998: 50. II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 12
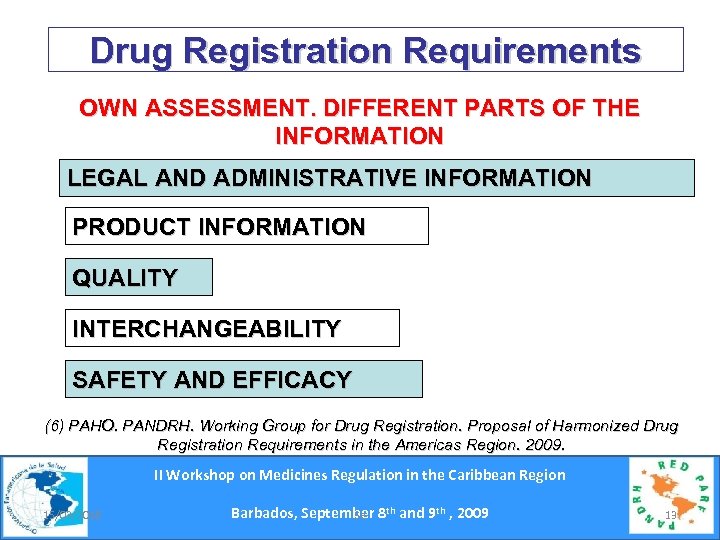
Drug Registration Requirements OWN ASSESSMENT. DIFFERENT PARTS OF THE INFORMATION LEGAL AND ADMINISTRATIVE INFORMATION PRODUCT INFORMATION QUALITY INTERCHANGEABILITY SAFETY AND EFFICACY (6) PAHO. PANDRH. Working Group for Drug Registration. Proposal of Harmonized Drug Registration Requirements in the Americas Region. 2009. II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 13

PANDRH* DRUG REGISTRATION REQUIREMENTS LEGAL AND ADMINISTRATIVE INFORMATION • • • Trademark INN (7) or generic name Concentration or strength Pharmaceutical form Technical Director Country Legal Representative International License Holder. Manufacturer of Active Pharmaceutical Ingredient (s) (API): Name, address, telephone, fax, mail Manufacturer (s) of the Finished Pharmaceutical Product (FPP): Name, address, telephone, fax, mail Another manufacturers of the FPP: Name, address, telephone, fax, mail of other manufacturers in case they participate in any stage of the FPP. For freeze dried products also should be declared the manufacturer of diluents Commercial presentation (Primary container) Route of Administration (7) WHO Guidelines on the Useon Medicines Regulation in the Caribbean Region for Pharmaceutical II Workshop of International Nonproprietary Names (INNs) Substances, 1997, WHO MD. Available at: http: //www. who. int/druginformation/general/innlists-shtml. Barbados, September 8 th and 9 th , 2009 15/03/2018 Se 14

PANDRH REGISTRATION REQUIREMENTS LEGAL AND ADMINISTRATIVE INFORMATION • • Shelf life and storage conditions Dispensing category Quali-quantitative formulation per dosage unite and %. Legal documentation for: Technical Director Representative Certificate of a Pharmaceutical Product (CPP) Certificate of GMP for manufacturers taking part in any step of the FPP (including activities authorized) Trade mark certificate Information of the product: . Labelling (inner container and secondary package) Insert Information for health professionals Samples or Mock ups of the final package for marketing (including accessories) Samples of the finished products Quality Analysis Certificate (corresponding to the bath of the samples) II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 15

PANDRH REGISTRATION REQUIREMENTS BASIC INFORMATION FOR INNER CONTAINERS Trademark INN or generic name Pharmaceutical form Concentration or strength Content/Volume Number of dosages per vial (for multidose presentations) Route of Administration Storage conditions (if container size is big enough) Warnings Batch number Expiry date Manufacturer Registration Number (according to the country legislation/container size) II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 16

PANDRH REGISTRATION REQUIREMENTS BASIC INFORMATION FOR SECONDARY PACKAGE Distribution level (according Trademark to country legislation) INN or generic name Special signals (according to Pharmaceutical form country legislation) Concentration or strength Batch number Content/Volume Expiry date Number of dosages per vial Name and address of the (for multidose manufacturing of the FPP presentations) Name and address of the Composition packer of the FPP (if Declaration of excipients different) (according to country Name of the Technical legislation) Responsible (according to Storage conditions country legislation) Route of Administration Registration Number Instructions for preparation (according to country Instructions. Workshop on Medicines Regulation in the Caribbean Region II for use legislation) Warnings (according to the package size) Barbados, September 8 th and 9 th , 2009 15/03/2018 Se 17

PANDRH REGISTRATION REQUIREMENTS BASIC INFORMATION FOR INSERTS Trademark INN or generic name Pharmaceutical form Concentration or strength Content/Volume Number of dosages per vial (for multidose presentations) Composition Declaration of excipients Route of Administration Indications Instructions for use Dosage/ Posology Maximal dosage in 24 hours Precautions Warnings Contraindications Over dosage Use in pregnancy and lactation Shelf life and storage conditions Name and address of the manufacturing of the FPP Name and address of the packer of the FPP (if different) II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 18

PANDRH REGISTRATION REQUIREMENTS BASIC INFORMATION FOR QUALITY Active Pharmaceutical Ingredient (s): • • • Chemical name (WHO/Relevant Pharmacopoeia) Manufacturer (s): (According to country legislation). Note: For fixed drug combinations (FDC), this information applies for each API Characteristics: Complete description (odor, taste, flavor, etc. ) Physical Chemical Specifications of API Analytical Method: Validation of the Analytical method (According to country legislation). Validity period For New Molecular Entities: Structural and molecular formula/ Molecular weight/ Synthesis/ Source II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 19

PANDRH REGISTRATION REQUIREMENTS BASIC INFORMATION FOR QUALITY Finished Pharmaceutical Product: • • • Description and composition: Full description of the FPP, detailing API (s), preservatives, stabilizers and other excipients and their function, For freeze dried products should be included description and close-container system for dissolvent. Pharmaceutical development: Studies for establishing the pharmaceutical form, formulation, manufacturing process and close-container system Manufacturing of the FPP: Batch formula (List of all components according country legislation) Manufacturing process (Flow with critical steps, in process controls, intermediate products and FPP) Physical and chemical characteristics for excipients (according country legislation) II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 20

PANDRH REGISTRATION REQUIREMENTS BASIC INFORMATION FOR QUALITY FPP Control • Specifications • Analytical Methods • Validation of analytical methods of the FPP including experimental data • Standards and reference materials information • Description of the close-container system (including specifications of component materials) • Stability studies (according to the country legislation and climatic zones): Protocols and results of stability studies justifying validity period: (According to country legislation including: study protocol, specifications, analytical methods, II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 21

PANDRH REGISTRATION REQUIREMENTS BASIC INFORMATION FOR QUALITY FPP Control description of close-container system, storage conditions (temperature and humidity) results of 3 batches minimal manufactured with 3 different batches of API, conclusions and proposed validity period). For freeze dried products should be demonstrated compatibility between lyophilized and diluents • Program of stability studies post registration: Program or commitment including number of batches to be included annually and analytical tests to be performed. Each NRA will establish mechanisms for checking updating of this information • Validity Period and storage conditions • Description of procedures for assuring cold chain: For products to be refrigerated detailed measures for assuring adequate temperature and humidity trough storage and distribution chain indicating controls II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 22

PANDRH REGISTRATION REQUIREMENTS BASIC INFORMATION FOR INTERCHANGEABILITY Biopharmaceutical Information – In Vitro Equivalence Studies (dissolution profiles/SBC System) – In Vivo Equivalence Studies (Pharmacokinetics studies, Bioequivalence studies; Pharmacodinamic studies, Clinical trials) II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 23

PANDRH REGISTRATION REQUIREMENTS BASIC INFORMATION FOR NON CLINICAL STUDIES Applicable for New Molecular Entities • Pharmacodinamics studies • Pharmacokinetics studies II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 24

PANDRH REGISTRATION REQUIREMENTS BASIC INFORMATION FOR NON CLINICAL STUDIES Applicable for New Molecular Entities • Pharmacodinamic studies • Pharmacokinetics studies • Toxicology studies General Toxicology Special toxicology • New Fixed Dose Combinations: According WHO Technical Report Series Nº 929, Annex 5 • For New excipients, new administration routes and FDCs are necessary appropriate toxicological studies II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 25

PANDRH REGISTRATION REQUIREMENTS BASIC INFORMATION FOR CLINICAL STUDIES Applicable for New Molecular Entities Summary of Clinical Studies • Phase I studies (also apply for new concentration/strengths) • Phase II studies • Phase III studies (also apply for new concentration/strengths, new combinations, new formulations) • Phase IV studies (Pharmacovigilance Plan) • Studies in special populations II Workshop on Medicines Regulation in the Caribbean Region 15/03/2018 Barbados, September 8 th and 9 th , 2009 Se 26

II Workshop on Medicines Regulation in the Caribbean Region THANK YOU VERY MUCH 15/03/2018 MUCHAS Se GRACIAS Barbados, September 8 th and 9 th , 2009 27
c048378ebc9cfe28b015a61295d71942.ppt