1c9ee7ca945ea659d6347b4c6259f648.ppt
- Количество слайдов: 33
 IHE USA Presents the North American IHE Connectathon 17 -21 January, 2011 Chicago The first formal activity of the newly incorporated non-profit IHE USA, the official IHE International National Deployment Committee for the United States! Please set your cell phones on “stun!”
IHE USA Presents the North American IHE Connectathon 17 -21 January, 2011 Chicago The first formal activity of the newly incorporated non-profit IHE USA, the official IHE International National Deployment Committee for the United States! Please set your cell phones on “stun!”
 Elliot B. Sloane, Ph. D, CCE, FHIMSS Drexel University, Health Systems Engineering Director Founder, Center for Healthcare Information Research and Policy Board of Directors, IHE-USA Co-Chair, IHE International Board of Directors, Delaware Valley HIMSS Past Chair, HIMSS Security and Privacy Steering Committee Sponsor, IEEE 11073 Medical Informatics Standards Interoperability, Health Information Security, and Medical Devices,
Elliot B. Sloane, Ph. D, CCE, FHIMSS Drexel University, Health Systems Engineering Director Founder, Center for Healthcare Information Research and Policy Board of Directors, IHE-USA Co-Chair, IHE International Board of Directors, Delaware Valley HIMSS Past Chair, HIMSS Security and Privacy Steering Committee Sponsor, IEEE 11073 Medical Informatics Standards Interoperability, Health Information Security, and Medical Devices,
 IHE USA • Incorporated in 2010 as an independent non-profit agency in the state of Illinois, and is filing with the IRS as a 501(c)(3) educational foundation • Legally independent, and separate Board of Directors, from HIMSS, RSNA, and IHE International • Receives donations, tuition, membership and Connectathon fees, and grant funding • Provides educational, teaching, and research grants and scholarships, and • Pays for the development and dissemination of critical Open Source, free testing and implementation tools for hospitals, government agencies, and manufacturers • The first IHE USA membership drive is just starting! – Small and large institutions, plus individuals
IHE USA • Incorporated in 2010 as an independent non-profit agency in the state of Illinois, and is filing with the IRS as a 501(c)(3) educational foundation • Legally independent, and separate Board of Directors, from HIMSS, RSNA, and IHE International • Receives donations, tuition, membership and Connectathon fees, and grant funding • Provides educational, teaching, and research grants and scholarships, and • Pays for the development and dissemination of critical Open Source, free testing and implementation tools for hospitals, government agencies, and manufacturers • The first IHE USA membership drive is just starting! – Small and large institutions, plus individuals
 IHE International oversees the creation of detailed guidelines that use multiple standards to build interoperable health IT systems. IHE International also coordinates the activities of national and regional deployment committees worldwide, such as IHE USA is recognized by IHE International as the national deployment committee for the United States. IHE USA's role is to serve as the voice for US health IT interests and foster national adoption of information standards to enable interoperability
IHE International oversees the creation of detailed guidelines that use multiple standards to build interoperable health IT systems. IHE International also coordinates the activities of national and regional deployment committees worldwide, such as IHE USA is recognized by IHE International as the national deployment committee for the United States. IHE USA's role is to serve as the voice for US health IT interests and foster national adoption of information standards to enable interoperability
 IHE USA supports HHS & ONC IHE USA exists to serve America’s healthcare needs. It does its very best to discover and support synergies and efficiencies in HIT standards development and deployment, and to eliminate waste, redundancy, and unnecessary and wasteful conflict.
IHE USA supports HHS & ONC IHE USA exists to serve America’s healthcare needs. It does its very best to discover and support synergies and efficiencies in HIT standards development and deployment, and to eliminate waste, redundancy, and unnecessary and wasteful conflict.
 IHE USA is NOT the same as IHE Europe or IHE Oceania-Asia The US healthcare market uses different coding standards, is based on a privatized business and insurance models, has very different clinical delivery processes, and has its own unique legal and regulatory framework. e. g. , IHE USA still supports ICD-9 coding because that is stipulated in the HIPAA 4010 standards. US laws and practices drive IHE USA, not IHE International or other National Domains
IHE USA is NOT the same as IHE Europe or IHE Oceania-Asia The US healthcare market uses different coding standards, is based on a privatized business and insurance models, has very different clinical delivery processes, and has its own unique legal and regulatory framework. e. g. , IHE USA still supports ICD-9 coding because that is stipulated in the HIPAA 4010 standards. US laws and practices drive IHE USA, not IHE International or other National Domains
 IHE USA adapts well developed global standards to solve US challenges where efficient and appropriate • e. g. , DICOM, IEEE 11073, HL-7, ICD-9, ICD-10 are all well-recognized international standards. • CPT, LOINC, SNOMED, and UMLS are US-centric • Though many countries have national patient identifiers, but IHE USA supports the US patientmatching model using multi-factor patient identification
IHE USA adapts well developed global standards to solve US challenges where efficient and appropriate • e. g. , DICOM, IEEE 11073, HL-7, ICD-9, ICD-10 are all well-recognized international standards. • CPT, LOINC, SNOMED, and UMLS are US-centric • Though many countries have national patient identifiers, but IHE USA supports the US patientmatching model using multi-factor patient identification
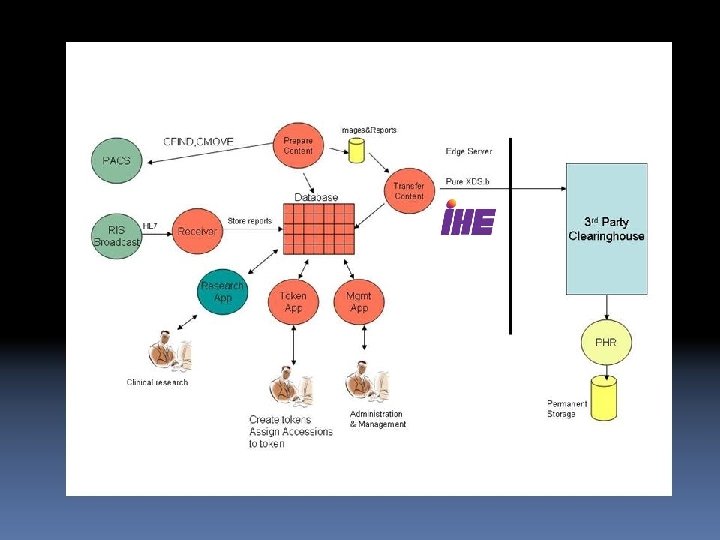
 IHE USA leverages well-tested modular core IHE services to meet US needs • e. g. , IHE’s “Consistent Time” Integration Profile assures that all records in and from all computer systems inside and outside of the hospital can synchronize the timestamps each clinical and business document • IHE’s XDS (Cross Enterprise Document Sharing) Integration Profile allows documents to be reliably and securely shared anywhere in the healthcare enterprise.
IHE USA leverages well-tested modular core IHE services to meet US needs • e. g. , IHE’s “Consistent Time” Integration Profile assures that all records in and from all computer systems inside and outside of the hospital can synchronize the timestamps each clinical and business document • IHE’s XDS (Cross Enterprise Document Sharing) Integration Profile allows documents to be reliably and securely shared anywhere in the healthcare enterprise.
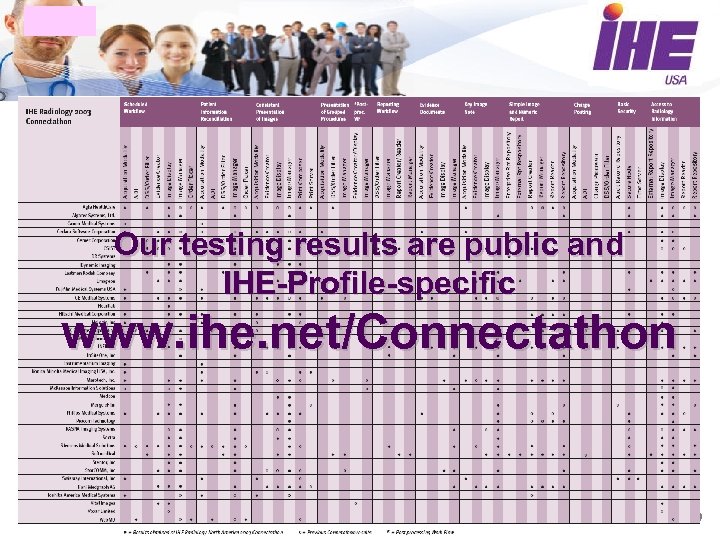 Our testing results are public and IHE-Profile-specific www. ihe. net/Connectathon 9
Our testing results are public and IHE-Profile-specific www. ihe. net/Connectathon 9
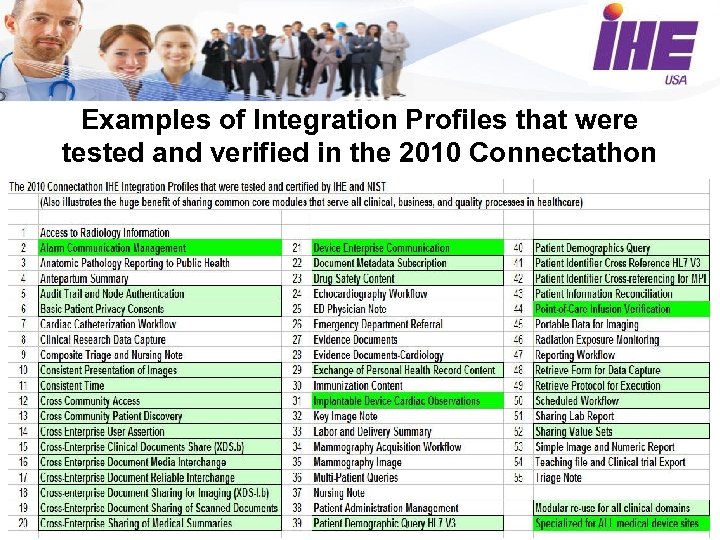 Examples of Integration Profiles that were tested and verified in the 2010 Connectathon
Examples of Integration Profiles that were tested and verified in the 2010 Connectathon
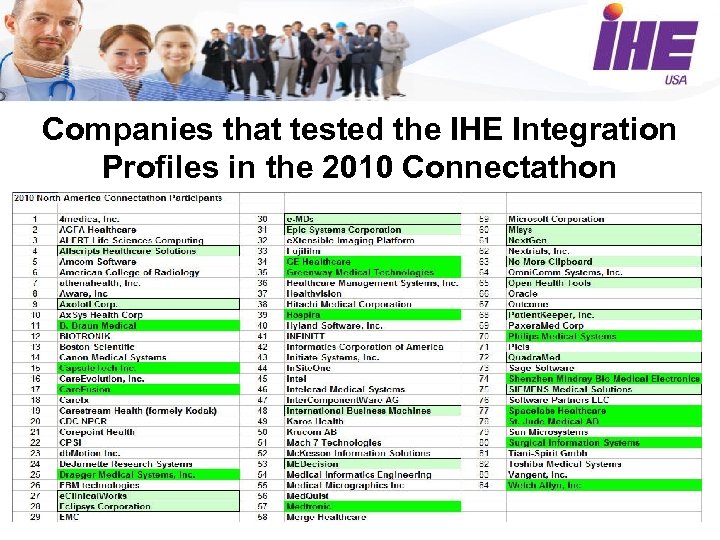 Companies that tested the IHE Integration Profiles in the 2010 Connectathon
Companies that tested the IHE Integration Profiles in the 2010 Connectathon
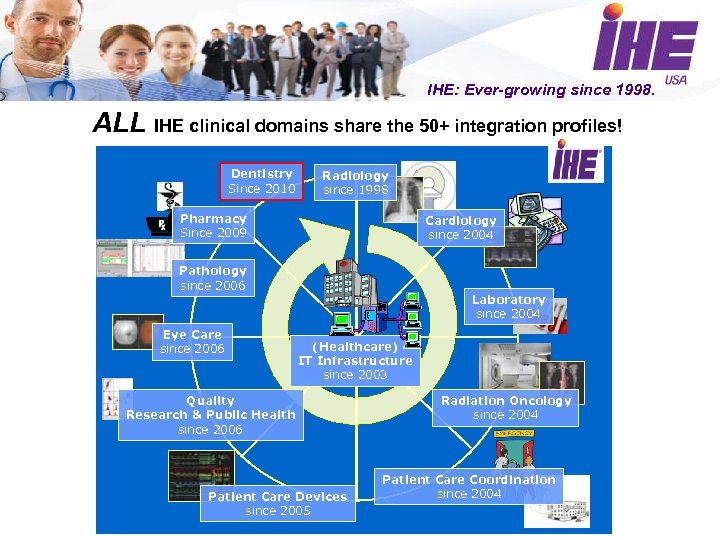 IHE: Ever-growing since 1998. ALL IHE clinical domains share the 50+ integration profiles! Dentistry Since 2010 Radiology since 1998 Pharmacy Since 2009 Cardiology since 2004 Pathology since 2006 Eye Care since 2006 Laboratory since 2004 (Healthcare) IT Infrastructure since 2003 Quality Research & Public Health since 2006 Patient Care Devices since 2005 Radiation Oncology since 2004 Patient Care Coordination since 2004
IHE: Ever-growing since 1998. ALL IHE clinical domains share the 50+ integration profiles! Dentistry Since 2010 Radiology since 1998 Pharmacy Since 2009 Cardiology since 2004 Pathology since 2006 Eye Care since 2006 Laboratory since 2004 (Healthcare) IT Infrastructure since 2003 Quality Research & Public Health since 2006 Patient Care Devices since 2005 Radiation Oncology since 2004 Patient Care Coordination since 2004
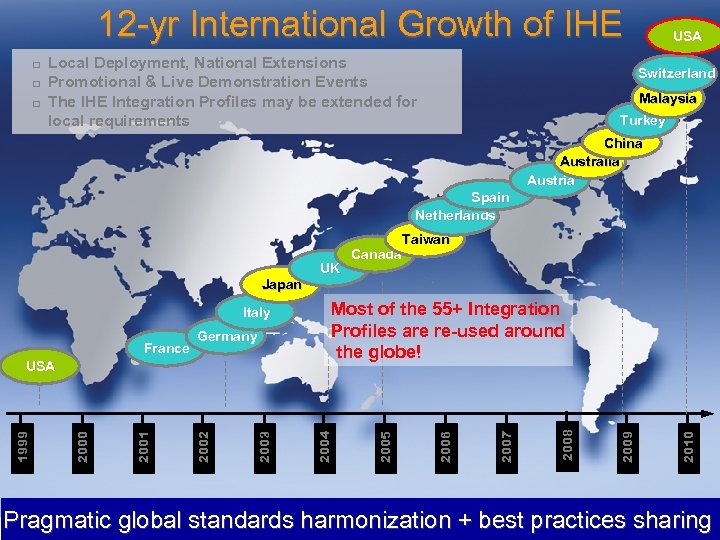 12 -yr International Growth of IHE q q q Local Deployment, National Extensions Promotional & Live Demonstration Events The IHE Integration Profiles may be extended for local requirements USA Switzerland Malaysia Turkey China Australia Austria Spain Netherlands UK Canada Taiwan Japan 2010 2009 2008 2007 2003 2002 2001 2000 1999 USA 2006 Germany 2005 France Most of the 55+ Integration Profiles are re-used around the globe! 2004 Italy 13 Pragmatic global standards harmonization + best practices sharing
12 -yr International Growth of IHE q q q Local Deployment, National Extensions Promotional & Live Demonstration Events The IHE Integration Profiles may be extended for local requirements USA Switzerland Malaysia Turkey China Australia Austria Spain Netherlands UK Canada Taiwan Japan 2010 2009 2008 2007 2003 2002 2001 2000 1999 USA 2006 Germany 2005 France Most of the 55+ Integration Profiles are re-used around the globe! 2004 Italy 13 Pragmatic global standards harmonization + best practices sharing
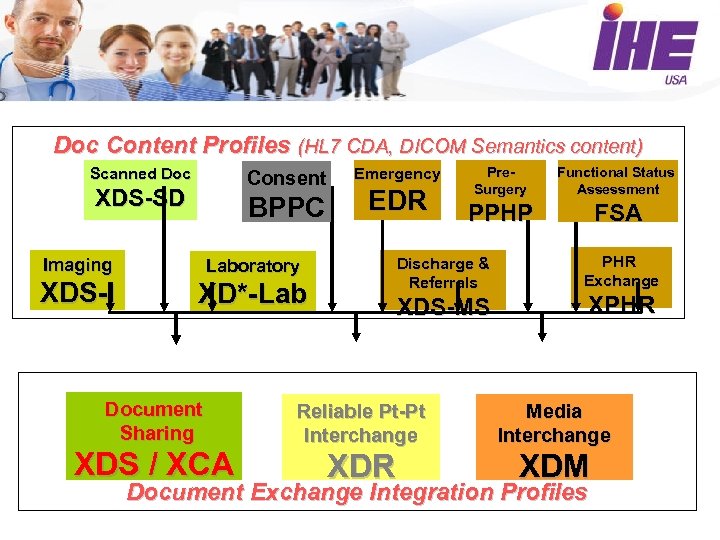 Doc Content Profiles (HL 7 CDA, DICOM Semantics content) Scanned Doc Consent XDS-SD BPPC Imaging EDR XD*-Lab Document Sharing XDS / XCA Pre. Surgery Functional Status Assessment PPHP FSA Discharge & Referrals Laboratory XDS-I Emergency XDS-MS PHR Exchange XPHR Reliable Pt-Pt Interchange Media Interchange XDR XDM Document Exchange Integration Profiles
Doc Content Profiles (HL 7 CDA, DICOM Semantics content) Scanned Doc Consent XDS-SD BPPC Imaging EDR XD*-Lab Document Sharing XDS / XCA Pre. Surgery Functional Status Assessment PPHP FSA Discharge & Referrals Laboratory XDS-I Emergency XDS-MS PHR Exchange XPHR Reliable Pt-Pt Interchange Media Interchange XDR XDM Document Exchange Integration Profiles
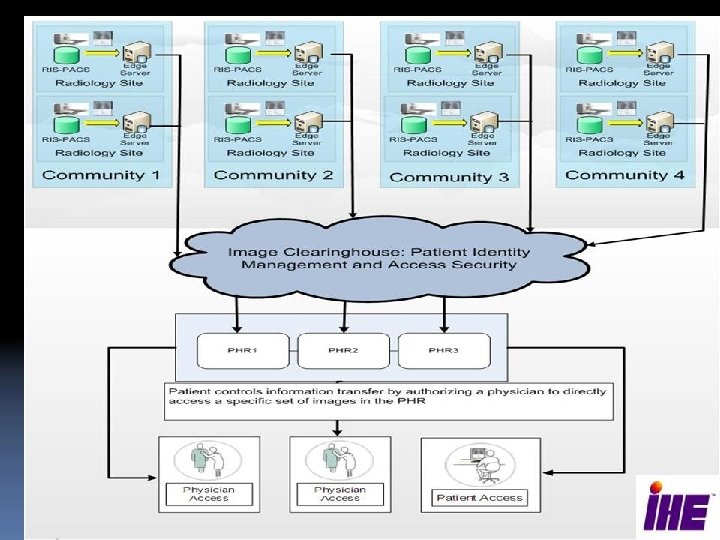
 How real is IHE XDS ? • • • Stable specification, tested by 100 vendors First implementation in clinical use in region of Genoa Italy) since early 2006. Several since: Three Austria regions, Applications in Vermont, Pennsylvania, Nagoya city, South Africa region, Italian & Dutch regions, France, etc. Adopted by several national programs world-wide 4 open source toolkits available, numerous product implementations in EMRs and Infrastructure offerings. 15
How real is IHE XDS ? • • • Stable specification, tested by 100 vendors First implementation in clinical use in region of Genoa Italy) since early 2006. Several since: Three Austria regions, Applications in Vermont, Pennsylvania, Nagoya city, South Africa region, Italian & Dutch regions, France, etc. Adopted by several national programs world-wide 4 open source toolkits available, numerous product implementations in EMRs and Infrastructure offerings. 15
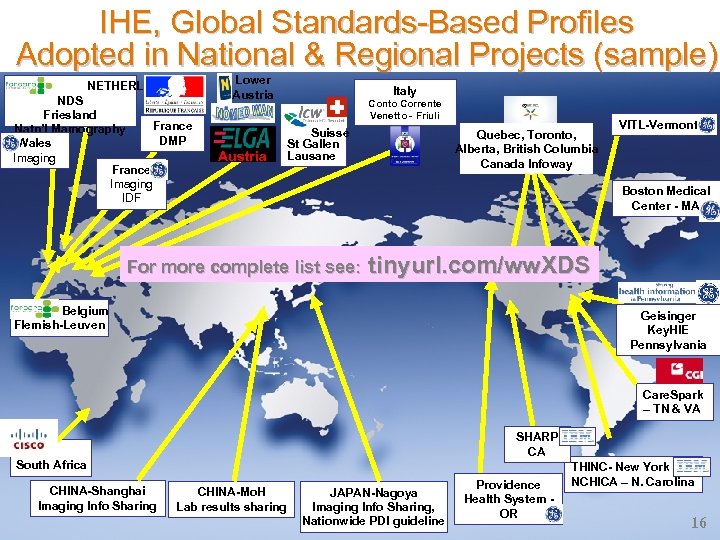 IHE, Global Standards-Based Profiles Adopted in National & Regional Projects (sample) NETHERLA NDS Friesland France Natn’l Mamography DMP Wales Imaging France Imaging IDF Lower Austria Italy Conto Corrente Venetto - Friuli Suisse St Gallen Lausane Quebec, Toronto, Alberta, British Columbia Canada Infoway VITL-Vermont Boston Medical Center - MA For more complete list see: tinyurl. com/ww. XDS Belgium Flemish-Leuven Geisinger Key. HIE Pennsylvania Care. Spark – TN & VA SHARP CA South Africa CHINA-Shanghai Imaging Info Sharing CHINA-Mo. H Lab results sharing JAPAN-Nagoya Imaging Info Sharing, Nationwide PDI guideline Providence Health System OR THINC- New York NCHICA – N. Carolina 16 1
IHE, Global Standards-Based Profiles Adopted in National & Regional Projects (sample) NETHERLA NDS Friesland France Natn’l Mamography DMP Wales Imaging France Imaging IDF Lower Austria Italy Conto Corrente Venetto - Friuli Suisse St Gallen Lausane Quebec, Toronto, Alberta, British Columbia Canada Infoway VITL-Vermont Boston Medical Center - MA For more complete list see: tinyurl. com/ww. XDS Belgium Flemish-Leuven Geisinger Key. HIE Pennsylvania Care. Spark – TN & VA SHARP CA South Africa CHINA-Shanghai Imaging Info Sharing CHINA-Mo. H Lab results sharing JAPAN-Nagoya Imaging Info Sharing, Nationwide PDI guideline Providence Health System OR THINC- New York NCHICA – N. Carolina 16 1
 At IHE, progress for patient care never stops!
At IHE, progress for patient care never stops!
 Connecting Medical Devices to the Electronic Health Record is my personal passion • IHE-PCD: IHE Patient Care Device Domain Committee • Have spent almost a decade developing integration profiles that allow medical and personal health devices to send vital signs data automatically, accurately, and securely in a medical record that can be read and stored in any EMR that can interpret an XML HL-7 v 2. 6 document In 2010, the IHE-PCD and Continua-WAN specifications were finally aligned, and a SINGLE data standard is now used by ALL devices to load clinical data directly from any device into the EMR
Connecting Medical Devices to the Electronic Health Record is my personal passion • IHE-PCD: IHE Patient Care Device Domain Committee • Have spent almost a decade developing integration profiles that allow medical and personal health devices to send vital signs data automatically, accurately, and securely in a medical record that can be read and stored in any EMR that can interpret an XML HL-7 v 2. 6 document In 2010, the IHE-PCD and Continua-WAN specifications were finally aligned, and a SINGLE data standard is now used by ALL devices to load clinical data directly from any device into the EMR
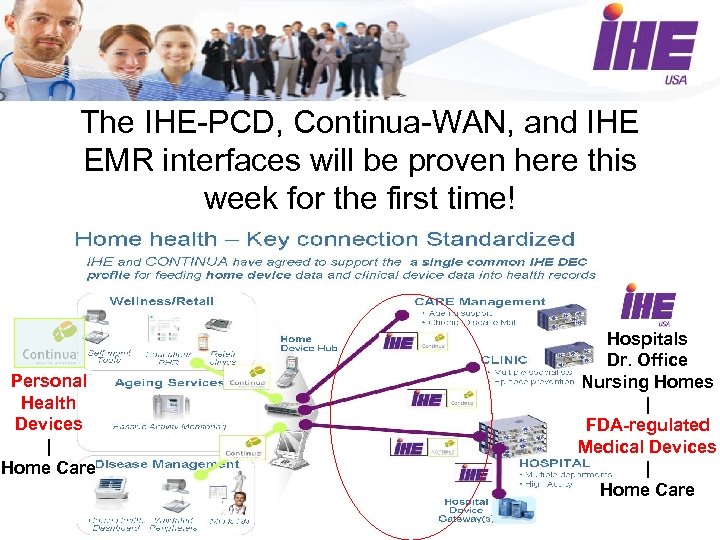 The IHE-PCD, Continua-WAN, and IHE EMR interfaces will be proven here this week for the first time! Personal Health Devices | Home Care Hospitals Dr. Office Nursing Homes | FDA-regulated Medical Devices | Home Care
The IHE-PCD, Continua-WAN, and IHE EMR interfaces will be proven here this week for the first time! Personal Health Devices | Home Care Hospitals Dr. Office Nursing Homes | FDA-regulated Medical Devices | Home Care
 Finally, a cross-enterprise labor-, error-, and delay-saving breakthrough for patient monitoring! The new IHE-PCD and Continua-WAN compatible interface allows flexible home monitoring, hospital monitoring, EMS monitoring, and alternate-site care monitoring with interchangeable, interoperable devices that can ALL load clinical data DIRECTLY to the EMR. We have ONC and Dr. John Halamka to thank: they asked for a single solution in 2008, so we developed one!
Finally, a cross-enterprise labor-, error-, and delay-saving breakthrough for patient monitoring! The new IHE-PCD and Continua-WAN compatible interface allows flexible home monitoring, hospital monitoring, EMS monitoring, and alternate-site care monitoring with interchangeable, interoperable devices that can ALL load clinical data DIRECTLY to the EMR. We have ONC and Dr. John Halamka to thank: they asked for a single solution in 2008, so we developed one!

 We are pleased to have Dr. Doug Fridsma with us today • Gives us the opportunity to expand the discussion about how IHE USA can best support him and his colleagues at ONC. • e. g. , how can IHE’s resources best support Meaningful Use Phases 1, 2, and 3? • e. g. , how can IHE’s resources best support the newly emerging S&I Framework?
We are pleased to have Dr. Doug Fridsma with us today • Gives us the opportunity to expand the discussion about how IHE USA can best support him and his colleagues at ONC. • e. g. , how can IHE’s resources best support Meaningful Use Phases 1, 2, and 3? • e. g. , how can IHE’s resources best support the newly emerging S&I Framework?
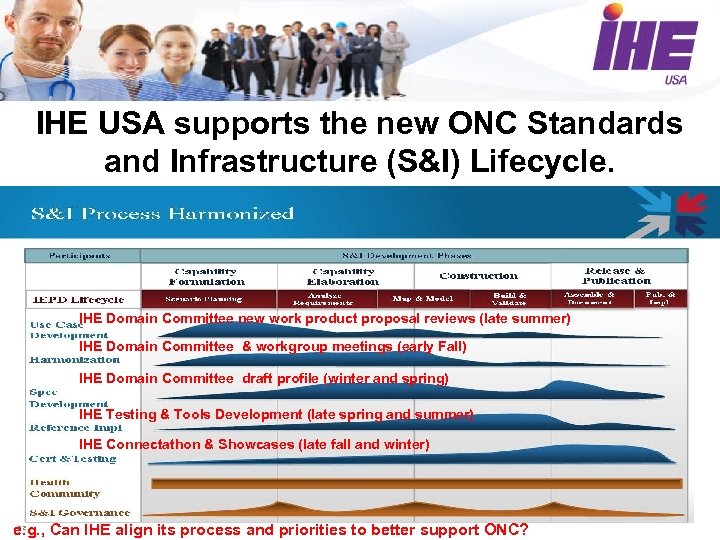 IHE USA supports the new ONC Standards and Infrastructure (S&I) Lifecycle. IHE Domain Committee new work product proposal reviews (late summer) IHE Domain Committee & workgroup meetings (early Fall) IHE Domain Committee draft profile (winter and spring) IHE Testing & Tools Development (late spring and summer) IHE Connectathon & Showcases (late fall and winter) e. g. , Can IHE align its process and priorities to better support ONC?
IHE USA supports the new ONC Standards and Infrastructure (S&I) Lifecycle. IHE Domain Committee new work product proposal reviews (late summer) IHE Domain Committee & workgroup meetings (early Fall) IHE Domain Committee draft profile (winter and spring) IHE Testing & Tools Development (late spring and summer) IHE Connectathon & Showcases (late fall and winter) e. g. , Can IHE align its process and priorities to better support ONC?
 IHE supports the new ONC IEPD artifacts: can some of IHE’s artifacts be useful? Information Exchange Package Documention
IHE supports the new ONC IEPD artifacts: can some of IHE’s artifacts be useful? Information Exchange Package Documention
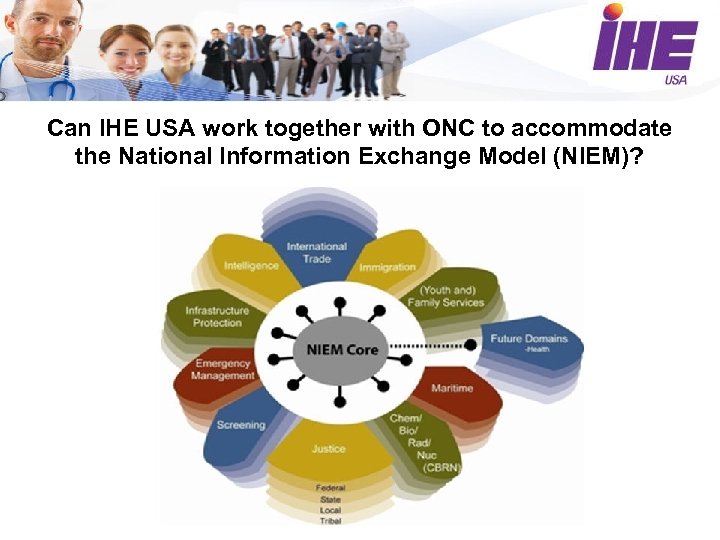 Can IHE USA work together with ONC to accommodate the National Information Exchange Model (NIEM)?
Can IHE USA work together with ONC to accommodate the National Information Exchange Model (NIEM)?
 IHE is Powered by Volunteers The nation salutes Dr. King each year with a Day of Service. IHE’s Volunteers donate tens of thousands of hours, day by day, year by year, to one shared dream: to create a world that will improve patient care by ensuring health data is secure, interoperable, complete, and readily available at the point and moment of care, regardless of location. Thank you to ALL of our volunteers! The Connectathon monitors you will meet today are all volunteers.
IHE is Powered by Volunteers The nation salutes Dr. King each year with a Day of Service. IHE’s Volunteers donate tens of thousands of hours, day by day, year by year, to one shared dream: to create a world that will improve patient care by ensuring health data is secure, interoperable, complete, and readily available at the point and moment of care, regardless of location. Thank you to ALL of our volunteers! The Connectathon monitors you will meet today are all volunteers.
 Rich Set of FREE Open Source Tools and Resources! 1 Implementation Guidance 2 Implementation Tools 2. 1 Development Tools 2. 1. 1 IHE Integration Profiles 2. 1. 2 OHT IHE Profiles 2. 2 Test Tools 2. 2. 1 MESA 2. 2. 2 Laika 2. 2. 3 Gazelle Test Management Tool 2. 2. 4 Patient Care Device Testing Tools 2. 2. 5 HL 7 V 2 Toolkit 2. 2. 6 EVS Message Validation Toolkit 2. 2. 7 XDS Toolkit 2. 2. 8 XDS-I and PIX/PDQv 3 Testing Tools 2. 2. 9 DICOMScope Visualization Tools (MESA Consistent Image Presentation) 2. 2. 10 Portable Data for Imaging Disk Validator 2. 2. 11 IHE-RO Test Tools 2. 3 Test Tools by Profile and Actor 3 Sample Files/Objects/Messages/Data http: //wiki. ihe. net/index. php? title=Implementation
Rich Set of FREE Open Source Tools and Resources! 1 Implementation Guidance 2 Implementation Tools 2. 1 Development Tools 2. 1. 1 IHE Integration Profiles 2. 1. 2 OHT IHE Profiles 2. 2 Test Tools 2. 2. 1 MESA 2. 2. 2 Laika 2. 2. 3 Gazelle Test Management Tool 2. 2. 4 Patient Care Device Testing Tools 2. 2. 5 HL 7 V 2 Toolkit 2. 2. 6 EVS Message Validation Toolkit 2. 2. 7 XDS Toolkit 2. 2. 8 XDS-I and PIX/PDQv 3 Testing Tools 2. 2. 9 DICOMScope Visualization Tools (MESA Consistent Image Presentation) 2. 2. 10 Portable Data for Imaging Disk Validator 2. 2. 11 IHE-RO Test Tools 2. 3 Test Tools by Profile and Actor 3 Sample Files/Objects/Messages/Data http: //wiki. ihe. net/index. php? title=Implementation
 FREE Open Source medical device IHE-ready XML test tools from the US Government - NIST has developed an Implementation Conformance Statement (ICS) generator tool called ICSGenerator to facilitate creation of vendor conformance statements that would be applicable to testing a particular ISO/IEEE 11073 (X 73) device. Additionally, NIST has completed initial development of a Validate. PDU tool, a tool that provides basic syntax and structure check and low level semantic checks for one or more captured messages. Both tools utilize the electronic representation of the X 73 standard's information model implemented by NIST researchers in the form of an XML schema. . Medical device test message generation is also possible to enable future manager/agent conformance test scenarios. http: //xw 2 k. nist. gov/medicaldevices/testtools. html
FREE Open Source medical device IHE-ready XML test tools from the US Government - NIST has developed an Implementation Conformance Statement (ICS) generator tool called ICSGenerator to facilitate creation of vendor conformance statements that would be applicable to testing a particular ISO/IEEE 11073 (X 73) device. Additionally, NIST has completed initial development of a Validate. PDU tool, a tool that provides basic syntax and structure check and low level semantic checks for one or more captured messages. Both tools utilize the electronic representation of the X 73 standard's information model implemented by NIST researchers in the form of an XML schema. . Medical device test message generation is also possible to enable future manager/agent conformance test scenarios. http: //xw 2 k. nist. gov/medicaldevices/testtools. html
 Large Library of Free IHE Educational Webinars Each month since 2008 were – and are – presented on topics including IHE, Interoperability, the IHE North America Connectathon, the HIMSS Interoperability Showcase, and a detailed review of profile supplements developed this year in IHE's twelve active domains! The Webinar schedule and links to free registration for upcoming sessions are below. Copies of slides are provided for sessions already presented. Video recordings of the sessions will be made available shortly. Additional Webinar sessions will be posted throughout the summer as dates are confirmed. Past IHE Webinar Series http: //www. ihe. net/Events/webinars 2010. cfm
Large Library of Free IHE Educational Webinars Each month since 2008 were – and are – presented on topics including IHE, Interoperability, the IHE North America Connectathon, the HIMSS Interoperability Showcase, and a detailed review of profile supplements developed this year in IHE's twelve active domains! The Webinar schedule and links to free registration for upcoming sessions are below. Copies of slides are provided for sessions already presented. Video recordings of the sessions will be made available shortly. Additional Webinar sessions will be posted throughout the summer as dates are confirmed. Past IHE Webinar Series http: //www. ihe. net/Events/webinars 2010. cfm
 We are IHE USA, and we’re here to serve. Welcome to the IHE Connectathon, and welcome to the ongoing conversation with our vibrant IHE USA professional community. Devoted to changing the way the healthcare world connects!
We are IHE USA, and we’re here to serve. Welcome to the IHE Connectathon, and welcome to the ongoing conversation with our vibrant IHE USA professional community. Devoted to changing the way the healthcare world connects!


