908e0426894a19398a9b97e71a1ec932.ppt
- Количество слайдов: 27
 IHE – Radiation Oncology
IHE – Radiation Oncology
 What is IHE? • Standards-based, • Global Initiative generating • Real-world Implementations in patient care “Defining, testing, and implementing standards-based interoperability for EHR’s” 2 2
What is IHE? • Standards-based, • Global Initiative generating • Real-world Implementations in patient care “Defining, testing, and implementing standards-based interoperability for EHR’s” 2 2
 IHE brings reality to workflow 3 3
IHE brings reality to workflow 3 3
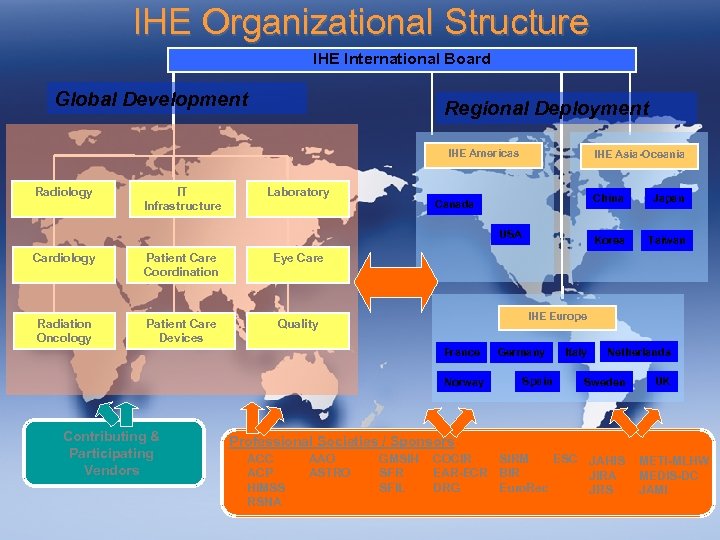 IHE Organizational Structure IHE International Board Global Development Regional Deployment IHE Americas Radiology IT Infrastructure Laboratory IHE Asia-Oceania China USA Cardiology Patient Care Coordination Patient Care Devices Quality Taiwan Eye Care Radiation Oncology Japan Korea Canada IHE Europe France Norway Contributing & Participating Vendors Germany Spain Italy Netherlands Sweden UK Professional Societies / Sponsors ACC ACP HIMSS RSNA AAO ASTRO GMSIH SFR SFIL COCIR SIRM ESC JAHIS EAR-ECR BIR JIRA DRG Euro. Rec JRS METI-MLHW MEDIS-DC JAMI 4
IHE Organizational Structure IHE International Board Global Development Regional Deployment IHE Americas Radiology IT Infrastructure Laboratory IHE Asia-Oceania China USA Cardiology Patient Care Coordination Patient Care Devices Quality Taiwan Eye Care Radiation Oncology Japan Korea Canada IHE Europe France Norway Contributing & Participating Vendors Germany Spain Italy Netherlands Sweden UK Professional Societies / Sponsors ACC ACP HIMSS RSNA AAO ASTRO GMSIH SFR SFIL COCIR SIRM ESC JAHIS EAR-ECR BIR JIRA DRG Euro. Rec JRS METI-MLHW MEDIS-DC JAMI 4
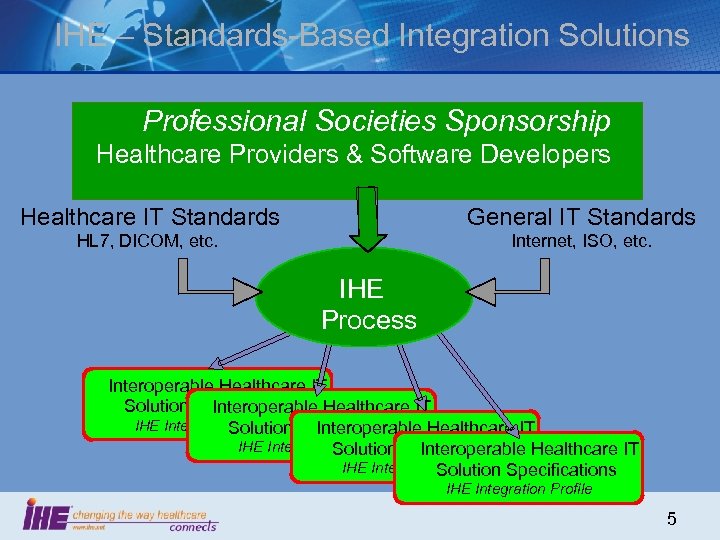 IHE – Standards-Based Integration Solutions Professional Societies Sponsorship Healthcare Providers & Software Developers Healthcare IT Standards General IT Standards HL 7, DICOM, etc. Internet, ISO, etc. IHE Process Interoperable Healthcare IT Solution Specifications Healthcare IT Interoperable IHE Integration Profile Specifications Healthcare IT Solution Interoperable IHE Integration Profile Specifications Solution IHE Integration Profile 5
IHE – Standards-Based Integration Solutions Professional Societies Sponsorship Healthcare Providers & Software Developers Healthcare IT Standards General IT Standards HL 7, DICOM, etc. Internet, ISO, etc. IHE Process Interoperable Healthcare IT Solution Specifications Healthcare IT Interoperable IHE Integration Profile Specifications Healthcare IT Solution Interoperable IHE Integration Profile Specifications Solution IHE Integration Profile 5
 What are these Standards? • DICOM (Digital Imaging and Communications in Medicine) – DICOM is a standard for handling, storing, printing, and transmitting information in medical imaging. – DICOM enables the integration of scanners, servers, workstations, printers, and network hardware from multiple manufacturers – http: //medical. nema. org • HL 7 (Health Level 7) – HL 7 is an international community of healthcare subject matter experts and information scientists collaborating to create standards for the exchange, management and integration of electronic healthcare information. – HL 7 promotes the use of such standards within and among healthcare organizations to increase the effectiveness and efficiency of healthcare delivery for the benefit of all. – http: //www. HL 7. org Parts from http: //www. wikipedia. org 6
What are these Standards? • DICOM (Digital Imaging and Communications in Medicine) – DICOM is a standard for handling, storing, printing, and transmitting information in medical imaging. – DICOM enables the integration of scanners, servers, workstations, printers, and network hardware from multiple manufacturers – http: //medical. nema. org • HL 7 (Health Level 7) – HL 7 is an international community of healthcare subject matter experts and information scientists collaborating to create standards for the exchange, management and integration of electronic healthcare information. – HL 7 promotes the use of such standards within and among healthcare organizations to increase the effectiveness and efficiency of healthcare delivery for the benefit of all. – http: //www. HL 7. org Parts from http: //www. wikipedia. org 6
 IHE – Domain Sponsors • Domain Sponsors shall be Member Organizations in the User Category with no direct or indirect responsibilities in the acquisition decision of systems implementing IHE specified capabilities. Domain Sponsors generally come from Healthcare Professional Organizations (clinical, IT, administrative, public health, etc. ) or Governmental Agencies. In supporting development activities of IHE International in the domain (or domains) relevant to their membership and mission, they shall adhere to the principles of IHE and promote the broad success of the initiative worldwide. • Domain Sponsors take responsibility for development of IHE Technical Frameworks and associated documents in the domain (or domains) relevant to their membership and mission. 7
IHE – Domain Sponsors • Domain Sponsors shall be Member Organizations in the User Category with no direct or indirect responsibilities in the acquisition decision of systems implementing IHE specified capabilities. Domain Sponsors generally come from Healthcare Professional Organizations (clinical, IT, administrative, public health, etc. ) or Governmental Agencies. In supporting development activities of IHE International in the domain (or domains) relevant to their membership and mission, they shall adhere to the principles of IHE and promote the broad success of the initiative worldwide. • Domain Sponsors take responsibility for development of IHE Technical Frameworks and associated documents in the domain (or domains) relevant to their membership and mission. 7
 IHE – Domain Secretary • Organizing and hosting domain planning and technical committees composed of volunteer domain experts and IHE Member representatives. • Coordinating with the IHE International Sponsors and Domain Coordination Committee to publish the work of their domain committees under IHE International Copyright; • Providing information for publication about their membership, plans, process and work product (e. g. , via the www. ihe. net Website). 8
IHE – Domain Secretary • Organizing and hosting domain planning and technical committees composed of volunteer domain experts and IHE Member representatives. • Coordinating with the IHE International Sponsors and Domain Coordination Committee to publish the work of their domain committees under IHE International Copyright; • Providing information for publication about their membership, plans, process and work product (e. g. , via the www. ihe. net Website). 8
 Why is IHE-RO Important? • ASTRO’s 6 -point patient protection plan – 5) Further developing our Integrating the Healthcare Enterprise – Radiation Oncology (IHE-RO) connectivity compliance program to ensure that medical technologies from different manufacturers can safely transfer information to reduce the chance of a medical error. • Promotes discussion and correction of protocols / standards for data communication to improve the reliability and safety of data exchange in radiation oncology • Provides a mechanism for inter-manufacturer testing of radiation oncology products prior to delivery – Domain Pre-testing – Connectathon 9
Why is IHE-RO Important? • ASTRO’s 6 -point patient protection plan – 5) Further developing our Integrating the Healthcare Enterprise – Radiation Oncology (IHE-RO) connectivity compliance program to ensure that medical technologies from different manufacturers can safely transfer information to reduce the chance of a medical error. • Promotes discussion and correction of protocols / standards for data communication to improve the reliability and safety of data exchange in radiation oncology • Provides a mechanism for inter-manufacturer testing of radiation oncology products prior to delivery – Domain Pre-testing – Connectathon 9
 The Connectathon: IHE’s Conformance Testing Process
The Connectathon: IHE’s Conformance Testing Process
 Proven Standards Adoption Process Develop technical specifications Testing at Connectathons Identify available standards (e. g. HL 7, Products with IHE DICOM, IETF, OASIS) Document Use Case Requirements IHE Demonstrations Timely access to information Easy to integrate products 11
Proven Standards Adoption Process Develop technical specifications Testing at Connectathons Identify available standards (e. g. HL 7, Products with IHE DICOM, IETF, OASIS) Document Use Case Requirements IHE Demonstrations Timely access to information Easy to integrate products 11
 What is a Connectathon? Cross-vendor, live, supervised, structured tests • All participating vendors’ products tested together in the same place/time • Experts from each vendor available for immediate problem resolution… fixes are done in minutes, not months!! • Each vendor tests with multiple trading partners (actual product to product) • Testing of real-world clinical scenarios with IHE Integration Profiles 12
What is a Connectathon? Cross-vendor, live, supervised, structured tests • All participating vendors’ products tested together in the same place/time • Experts from each vendor available for immediate problem resolution… fixes are done in minutes, not months!! • Each vendor tests with multiple trading partners (actual product to product) • Testing of real-world clinical scenarios with IHE Integration Profiles 12
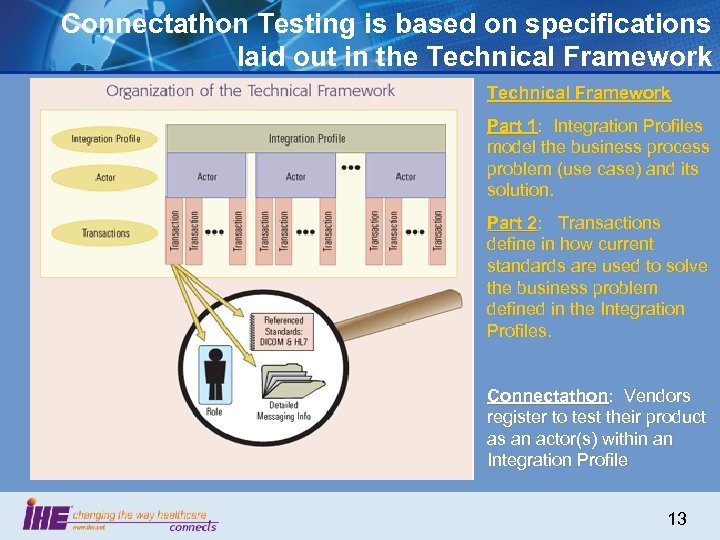 Connectathon Testing is based on specifications laid out in the Technical Framework Part 1: Integration Profiles model the business process problem (use case) and its solution. Part 2: Transactions define in how current standards are used to solve the business problem defined in the Integration Profiles. Connectathon: Vendors register to test their product as an actor(s) within an Integration Profile 13
Connectathon Testing is based on specifications laid out in the Technical Framework Part 1: Integration Profiles model the business process problem (use case) and its solution. Part 2: Transactions define in how current standards are used to solve the business problem defined in the Integration Profiles. Connectathon: Vendors register to test their product as an actor(s) within an Integration Profile 13
 What happens after the Connectathon? • Successful results (specific by IHE profile/actor) are published by the sponsors (www. ihe. net/connectahons) • Vendors self-certify, by publishing IHE Integration Statements: Precise and explicit public interoperability commitment fro a specific commercial product. • Only vendors who are successful are entitled to participate in Interoperability Showcase demonstrations: – – – HIMSS Annual Conference (February 26 th – March 1 st, New Orleans) ACC Annual Conference (March 24 th – 27 th, New Orleans) Canada e-Health 2007 Conference (May 27 th – 30 th, Quebec City) RSNA Annual Conference (November 25 th – 30 th, Chicago) others… 14
What happens after the Connectathon? • Successful results (specific by IHE profile/actor) are published by the sponsors (www. ihe. net/connectahons) • Vendors self-certify, by publishing IHE Integration Statements: Precise and explicit public interoperability commitment fro a specific commercial product. • Only vendors who are successful are entitled to participate in Interoperability Showcase demonstrations: – – – HIMSS Annual Conference (February 26 th – March 1 st, New Orleans) ACC Annual Conference (March 24 th – 27 th, New Orleans) Canada e-Health 2007 Conference (May 27 th – 30 th, Quebec City) RSNA Annual Conference (November 25 th – 30 th, Chicago) others… 14
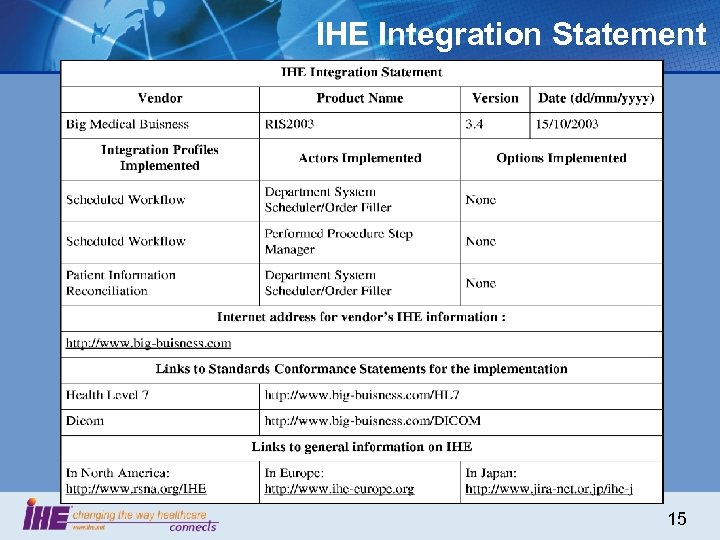 IHE Integration Statement 15
IHE Integration Statement 15
 RFPs & Integration Statements • Be Brief ? – “The system must support HL 7” • Be Effective ? – “The system must support the following HL 7 V 2 messages according to the following 100 pages of specifications” • Be Both: – “The system must support IHE Patient Id Cross-reference as a Patient Identifier Source actor” • Integration Statement – Version 2. 1 of the Enterprise HIS supports IHE Patient Id Cross-referencing as Patient Identifier Source actor Requiring vendors to publish their products IHE Integration Statements provide a very effective pressure point for market-driven interoperability 16
RFPs & Integration Statements • Be Brief ? – “The system must support HL 7” • Be Effective ? – “The system must support the following HL 7 V 2 messages according to the following 100 pages of specifications” • Be Both: – “The system must support IHE Patient Id Cross-reference as a Patient Identifier Source actor” • Integration Statement – Version 2. 1 of the Enterprise HIS supports IHE Patient Id Cross-referencing as Patient Identifier Source actor Requiring vendors to publish their products IHE Integration Statements provide a very effective pressure point for market-driven interoperability 16
 RFP Language (actual example) 17
RFP Language (actual example) 17
 How Does IHE-RO Function? • Planning Committee • Technical Committee – Identifies information systems or components of information systems that produce, manage, or act on information associated with clinical and operational activities that require integration. – Identifies and implements standards for interactions between actors that communicate the required information through standards-based messages. The PC and TC work together to Prioritize Efforts, Propose Solutions, and Eventually Demonstrate Connectivity 18
How Does IHE-RO Function? • Planning Committee • Technical Committee – Identifies information systems or components of information systems that produce, manage, or act on information associated with clinical and operational activities that require integration. – Identifies and implements standards for interactions between actors that communicate the required information through standards-based messages. The PC and TC work together to Prioritize Efforts, Propose Solutions, and Eventually Demonstrate Connectivity 18
 IHE-RO Progress • Profiles – 2007 • Basic Interoperability – 2008 • Multi-Modality Image Registration – 2009 • Basic Worklist / Workflow Integration • Advanced Interoperability – 2010 • Dose Compositing • Treatment Delivery Workflow 19
IHE-RO Progress • Profiles – 2007 • Basic Interoperability – 2008 • Multi-Modality Image Registration – 2009 • Basic Worklist / Workflow Integration • Advanced Interoperability – 2010 • Dose Compositing • Treatment Delivery Workflow 19
 Domain Pre-Testing 2006 • 25+ participants, 9 Vendors, 10+ systems • 95+ monitored test cases 20
Domain Pre-Testing 2006 • 25+ participants, 9 Vendors, 10+ systems • 95+ monitored test cases 20
 IHE-RO 2007 Formal Connect-a-thon ASTRO HQ Elekta Impac CMS Brainlab Nucletron Tomotherapy Philips Varian Courtesy of ASTRO HQ 21
IHE-RO 2007 Formal Connect-a-thon ASTRO HQ Elekta Impac CMS Brainlab Nucletron Tomotherapy Philips Varian Courtesy of ASTRO HQ 21
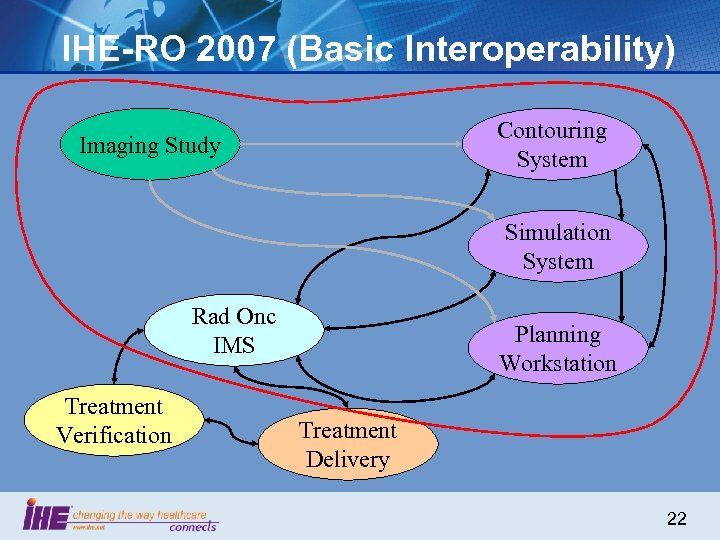 IHE-RO 2007 (Basic Interoperability) Contouring System Imaging Study Simulation System Rad Onc IMS Treatment Verification Planning Workstation Treatment Delivery 22
IHE-RO 2007 (Basic Interoperability) Contouring System Imaging Study Simulation System Rad Onc IMS Treatment Verification Planning Workstation Treatment Delivery 22
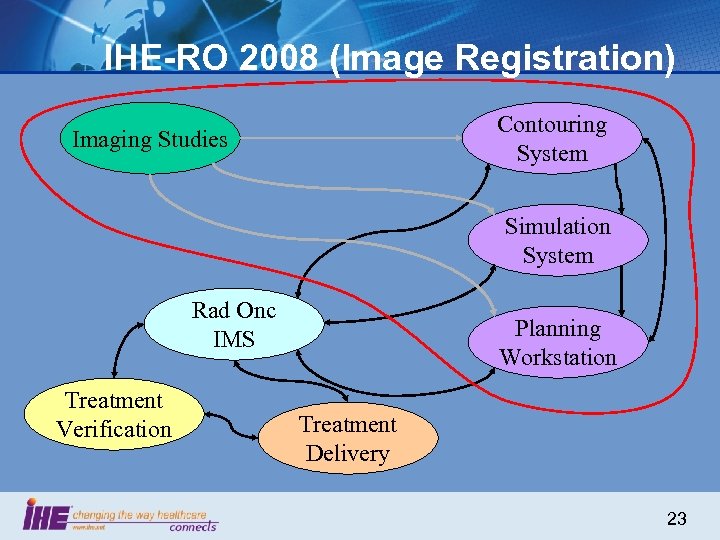 IHE-RO 2008 (Image Registration) Contouring System Imaging Studies Simulation System Rad Onc IMS Treatment Verification Planning Workstation Treatment Delivery 23
IHE-RO 2008 (Image Registration) Contouring System Imaging Studies Simulation System Rad Onc IMS Treatment Verification Planning Workstation Treatment Delivery 23
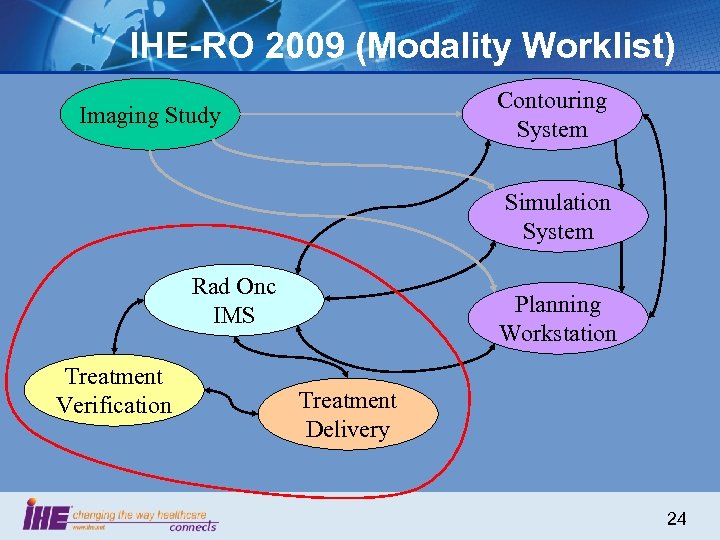 IHE-RO 2009 (Modality Worklist) Contouring System Imaging Study Simulation System Rad Onc IMS Treatment Verification Planning Workstation Treatment Delivery 24
IHE-RO 2009 (Modality Worklist) Contouring System Imaging Study Simulation System Rad Onc IMS Treatment Verification Planning Workstation Treatment Delivery 24
 Where can you learn more? http: //wiki. ihe. net 25
Where can you learn more? http: //wiki. ihe. net 25
 Where can you learn more? http: //www. ihe. net 26
Where can you learn more? http: //www. ihe. net 26



